Evaluation of the Antibacterial Activities of Mangrove Honeybee Propolis Extract and the Identification of Transpeptidase and Transglycosylase as Targets for New Antibiotics Using Molecular Docking
Abstract
1. Introduction
2. Results
2.1. Evaluating the Antibacterial Activity of Mangrove Honeybee Propolis Extract
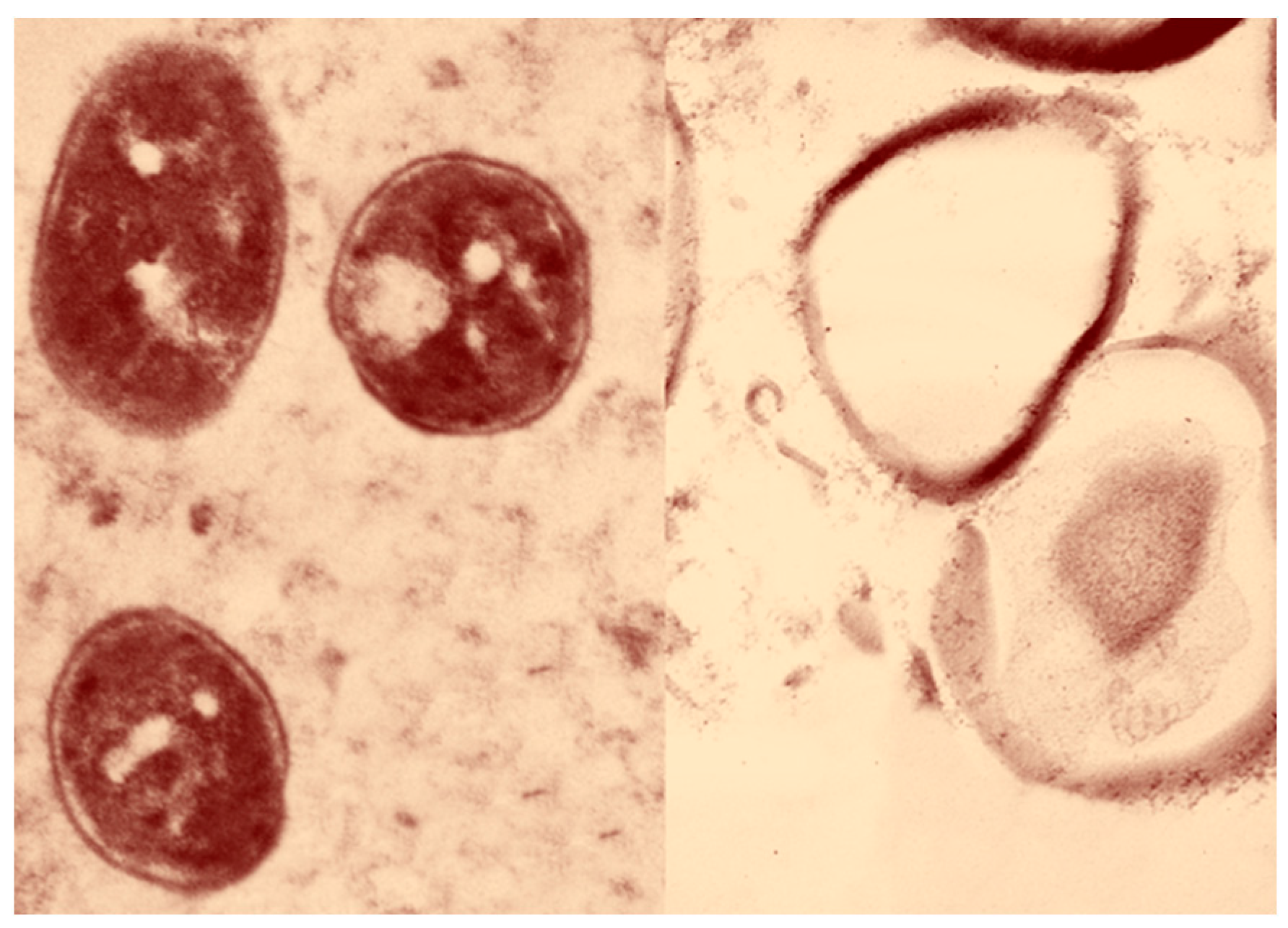
2.2. Ultrastructural Changes of the Tested Staphylococcus aureus
2.3. LC-MS Analysis of Propolis Methanolic Extracts
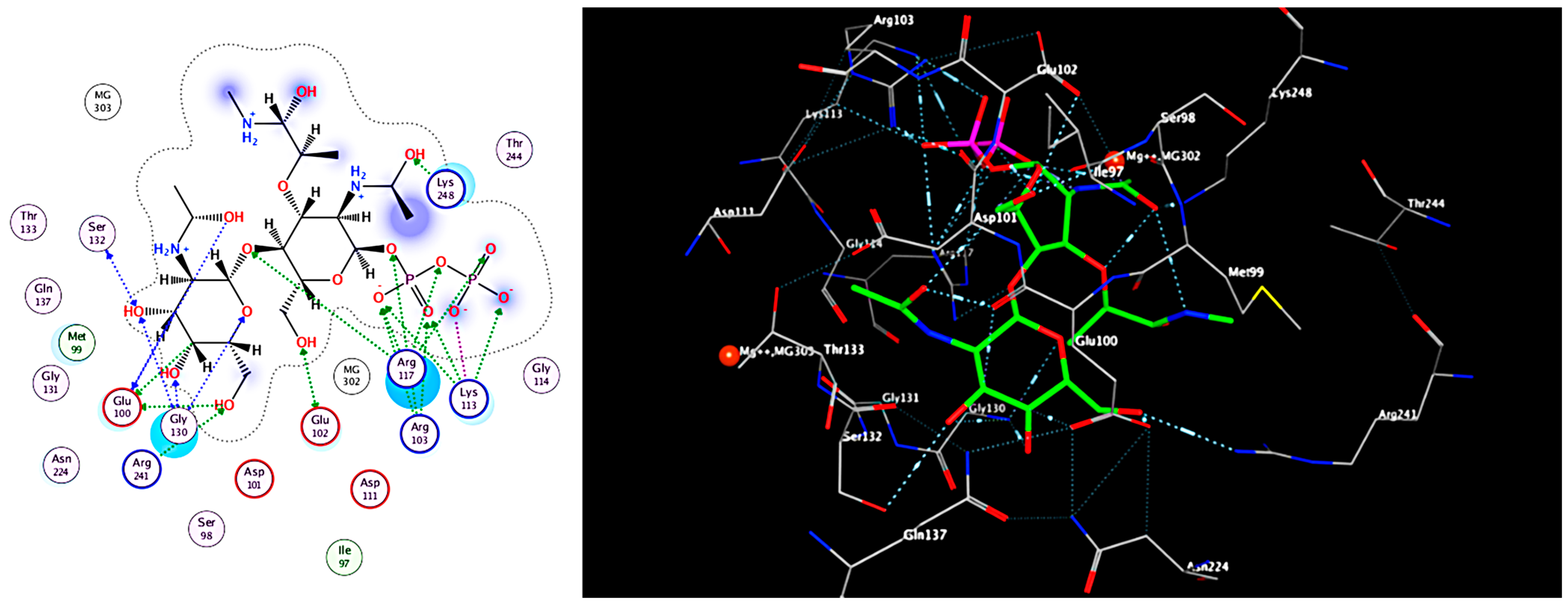
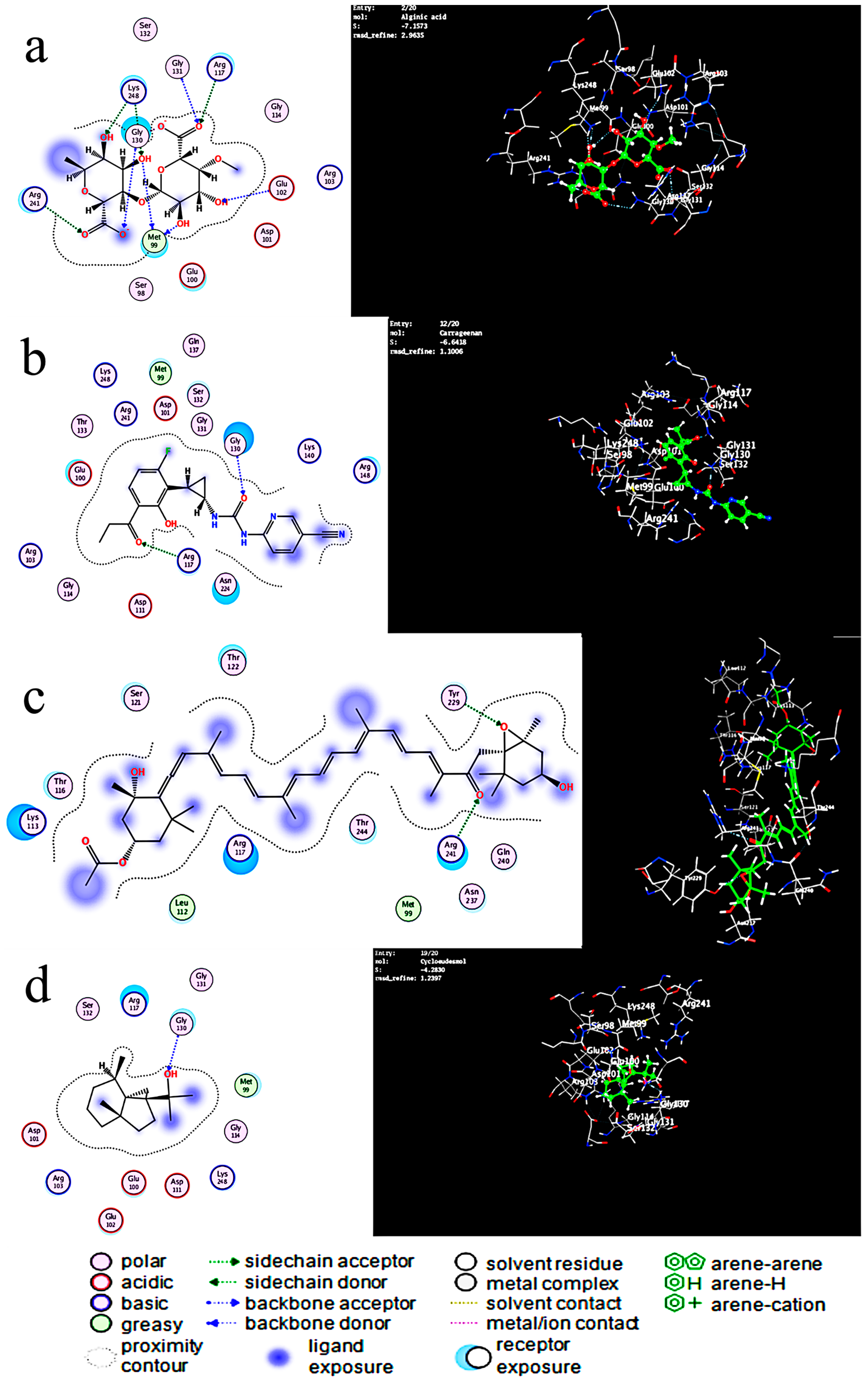
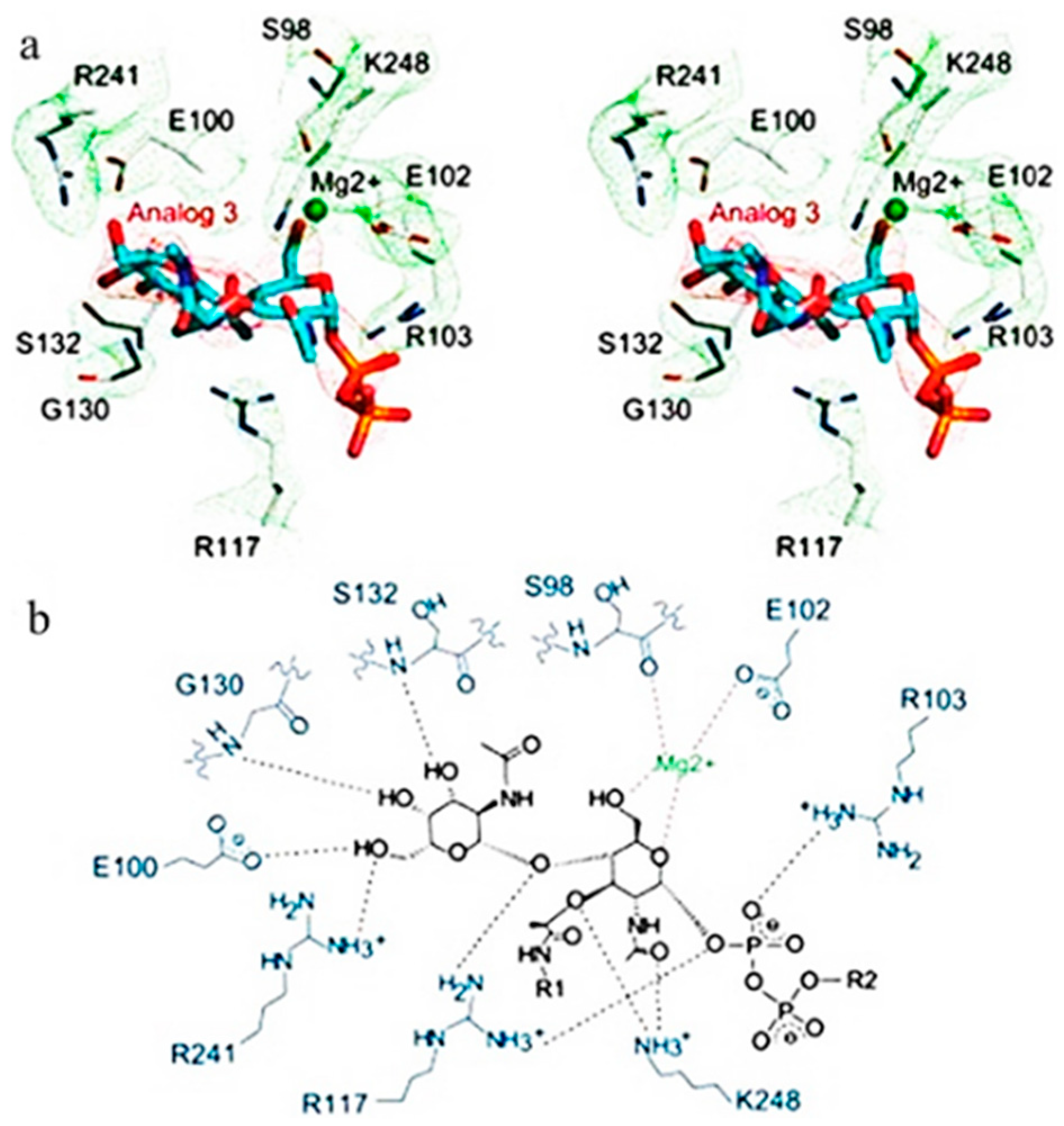
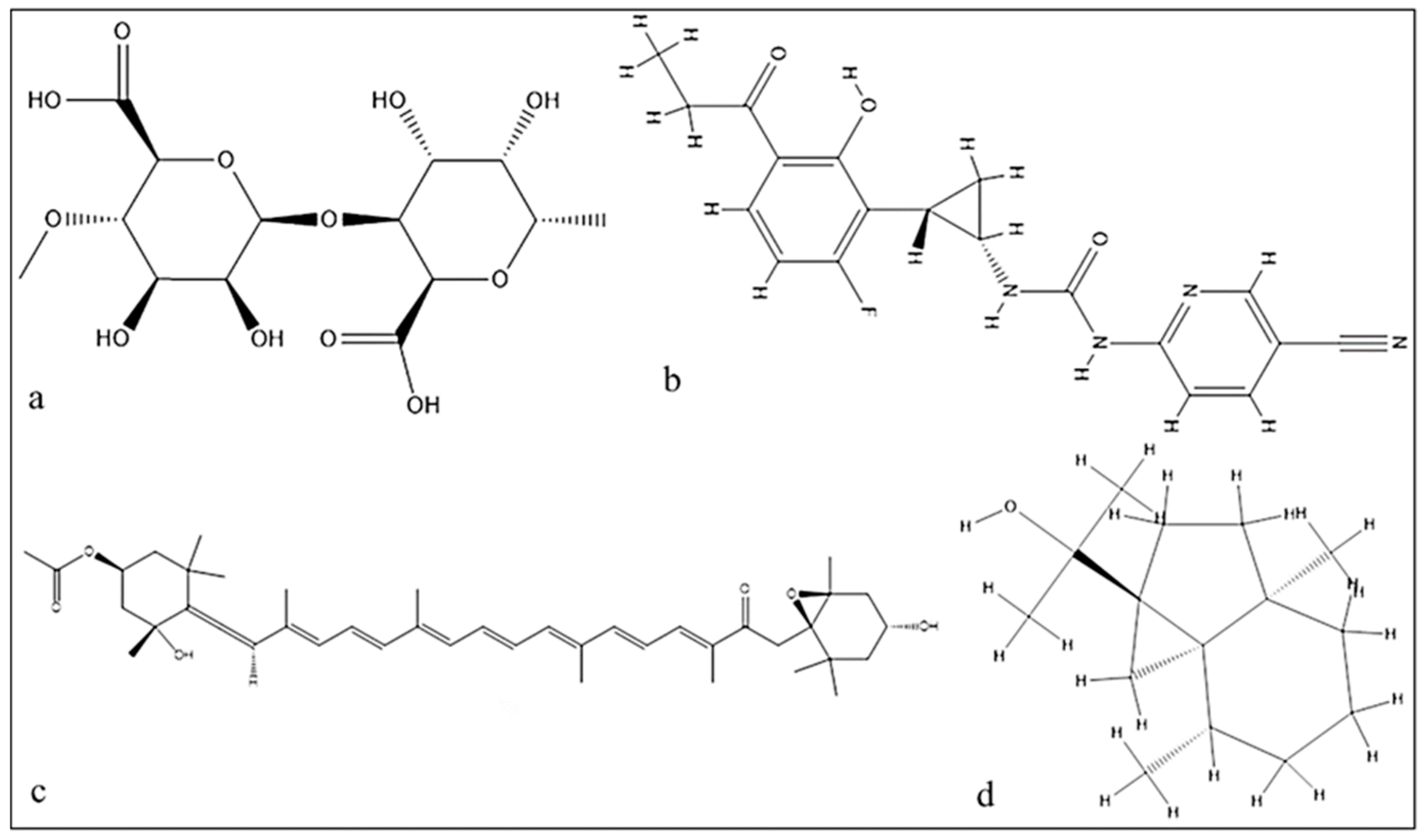
2.4. Molecular Docking of the Mangrove honeybee Propolis Extracted Compounds
3. Discussion
4. Materials and Methods
4.1. Propolis Sample Collection
4.2. Antibacterial Activity of the Mangrove Honeybee Propolis Extract
4.3. Transmission Electron Microscopy (TEM)
4.4. Chemical Analysis of Mangrove Honeybee Propolis Extract
4.5. Molecular Modelling Study
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Bin Emran, T.; Dhama, K.; Ripon, K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Church, N.A.; McKillip, J.L. Antibiotic resistance crisis: Challenges and imperatives. Biologia 2021, 76, 1535–1550. [Google Scholar] [CrossRef]
- Hamdani, S.S.; Bhat, B.A.; Tariq, L.; Yaseen, S.I.; Ara, I.; Rafi, B.; Hamdani, S.N.; Hassan, T.; Rashid, O. Antibiotic resistance: The future disaster. Int. J. Res. Appl. Sci. Biotechnol. 2020, 7, 13. [Google Scholar]
- Malik, B.; Bhattacharyya, S. Antibiotic drug-resistance as a complex system driven by socio-economic growth and antibiotic misuse. Sci. Rep. 2019, 9, 9788. [Google Scholar] [CrossRef] [PubMed]
- Abu Al-Halawa, D.; Abu Seir, R.; Qasrawi, R. Antibiotic Resistance Knowledge, Attitudes, and Practices among Pharmacists: A Cross-Sectional Study in West Bank, Palestine. J. Environ. Public Health 2023, 2023, 2294048. [Google Scholar] [CrossRef] [PubMed]
- Atif, M.; Asghar, S.; Mushtaq, I.; Malik, I.; Amin, A.; Babar, Z.-U.; Scahill, S. What drives inappropriate use of antibiotics? A mixed methods study from Bahawalpur, Pakistan. Infect. Drug Resist. 2019, 12, 687–699. [Google Scholar] [CrossRef]
- Alnasser, A.H.A.; Al-Tawfiq, J.A.; Ahmed, H.A.A.; Alqithami, S.M.H.; Alhaddad, Z.M.A.; Rabiah, A.S.M.; Albrahim, M.A.A.; Al Kalif, M.S.H.; Barry, M.; Temsah, M.-H.; et al. Public knowledge, attitude and practice towards antibiotics use and antimicrobial resistance in Saudi Arabia: A web-based cross-sectional survey. J. Public Health Res. 2021, 10, 2276. [Google Scholar] [CrossRef]
- Jamrozik, E.; Selgelid, M. Ethics and Drug Resistance: Collective Responsibility for Global Public Health; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Qadri, H.; Shah, A.H.; Mir, M. Novel strategies to combat the emerging drug resistance in human pathogenic microbes. Curr. Drug Targets 2021, 22, 1424–1436. [Google Scholar]
- Douglas, E.J.A.; Wulandari, S.W.; Lovell, S.D.; Laabei, M. Novel antimicrobial strategies to treat multi-drug resistant Staphylococcus aureus infections. Microb. Biotechnol. 2023, 16, 1456–1474. [Google Scholar] [CrossRef]
- Weis, W.A.; Ripari, N.; Conte, F.L.; da Silva Honorio, M.; Sartori, A.A.; Matucci, R.H.; Sforcin, J.M. An overview about apitherapy and its clinical applications. Phytomedicine Plus 2022, 2, 100239. [Google Scholar] [CrossRef]
- Vogt, N.A.; Vriezen, E.; Nwosu, A.; Sargeant, J.M. A scoping review of the evidence for the medicinal use of natural honey in animals. Front. Vet. Sci. 2021, 7, 618301. [Google Scholar] [CrossRef] [PubMed]
- Nainu, F.; Masyita, A.; Bahar, M.A.; Raihan, M.; Prova, S.R.; Mitra, S.; Bin Emran, T.; Simal-Gandara, J. Pharmaceutical prospects of bee products: Special focus on anticancer, antibacterial, antiviral, and antiparasitic properties. Antibiotics 2021, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Choudhary, P.; Singh, S. Antimicrobial Peptides: Mechanism of Action. Insights Antimicrob. Pept. 2022, 23, 1417. [Google Scholar]
- Tran, T.D.; Ogbourne, S.M.; Brooks, P.R.; Sánchez-Cruz, N.; Medina-Franco, J.L.; Quinn, R.J. Lessons from exploring chemical space and chemical diversity of propolis components. Int. J. Mol. Sci. 2020, 21, 4988. [Google Scholar] [CrossRef] [PubMed]
- Simone-Finstrom, M.; Spivak, M. Propolis and Bee Health: The Natural History and Significance of Resin Use by Honey Bees. Antibiotics 2019, 8, 13. [Google Scholar] [CrossRef]
- Bankova, V. Chemical Diversity of Propolis and the Problem of Standardization. Antibiotics 2020, 9, 410. [Google Scholar] [CrossRef]
- Sforcin, J.M. Propolis and the Immune System: A Review. Antibiotics 2020, 9, 60. [Google Scholar] [CrossRef]
- Cunha, L.K.H.; de Santana Santos, T.; da Silva, C.F.; da Conceição Santos, A.V.; Dos Santos, H.G.; de Araújo, A.M.S.; de Oliveira, J.S. Antimicrobial and Immunomodulatory Activities of Propolis: A Review. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef]
- Yosri, N.; Abd El-Wahed, A.A.; Ghonaim, R.; Khattab, O.M.; Sabry, A.; Ibrahim, M.A.A.; Moustafa, M.F.; Guo, Z.; Zou, X.; Algethami, A.F.M.; et al. Anti-Viral and Immunomodulatory Properties of Propolis: Chemical Diversity, Pharmacological Properties, Preclinical and Clinical Applications, and In Silico Potential against SARS-CoV-2. Foods 2021, 10, 1776. [Google Scholar] [CrossRef]
- Salatino, A.; Salatino, M.L.F.; Negri, G. How diverse is the chemistry and plant origin of Brazilian propolis? Apidologie 2021, 52, 1075–1097. [Google Scholar] [CrossRef]
- Falcão, S.I.; Lopes, M.; Vilas-Boas, M. A first approach to the chemical composition and antioxidant potential of Guinea-Bissau propolis. Nat. Prod. Commun. 2019, 14, 1934578X19844138. [Google Scholar] [CrossRef]
- Hou, Y.; Huang, Q.; Luo, W. Chemical compositions and antimicrobial activities of propolis from the mangrove forests of China. J. Chem. Ecol. 2014, 40, 610–622. [Google Scholar]
- Berretta, A.A.; Arruda, C.; Miguel, F.G.; Baptista, N.; Nascimento, A.P.; Marquele-Oliveira, F.; Hori, J.I.; Barud, H.; Damaso, B.; Ramos, C.; et al. Functional Properties of Brazilian Propolis: From Chemical Composition Until the Market. In Superfood and Functional Food—An Overview of Their Processing and Utilization; Waisundara, V., Ed.; Intech Open: London, UK, 2017. [Google Scholar]
- Sforcin, J.M. Biological properties and therapeutic applications of propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Goffin, C.; Ghuysen, J.M. Multimodular penicillin-binding proteins: An enigmatic family of orthologs and paralogs. Microbiol. Mol. Biol. Rev. 1998, 62, 1079–1093. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalaf, A.A.; Alabdelkareem, I.; Al-Rejaie, S.S.; Mohany, M.; Hozzein, W.N. Molecular Docking Studies on Methanolic Propolis Extracts Collected from Different Regions in Saudi Arabia as a Potential Inhibitor of Topoisomerase IIβ. Separations 2022, 9, 392. [Google Scholar] [CrossRef]
- Wiklund, S.; Fagerberg, I.; Örtqvist, Å.; Vading, M.; Giske, C.G.; Broliden, K.; Tammelin, A. Knowledge and understanding of antibiotic resistance and the risk of becoming a carrier when travelling abroad: A qualitative study of Swedish travellers. Scand. J. Public Health 2015, 43, 302–308. [Google Scholar] [CrossRef]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef]
- Landecker, H. Antibiotic resistance and the biology of history. Body Soc. 2016, 22, 19–52. [Google Scholar] [CrossRef]
- Balagurunathan, R.; Radhakrishnan, M.; Shanmugasundaram, T.; Gopikrishnan, V.; Jerrine, J. Protocols in Actinobacterial Research; Springer: New York, NY, USA, 2020. [Google Scholar]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; Eid, N.; Abd El-Wahed, A.A.; Rateb, M.E.; Afifi, H.S.; Algethami, A.F.; Khalifa, S.A. Honey bee products: Preclinical and clinical studies of their anti-inflammatory and immunomodulatory properties. Front. Nutr. 2022, 8, 761267. [Google Scholar] [PubMed]
- Nader, R.A.; Mackieh, R.; Wehbe, R.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Beehive products as antibacterial agents: A review. Antibiotics 2021, 10, 717. [Google Scholar] [CrossRef]
- Annavajhala, M.K.; Gomez-Simmonds, A.; Uhlemann, A.-C. Multidrug-resistant Enterobacter cloacae complex emerging as a global, diversifying threat. Front. Microbiol. 2019, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Negri, G.; Silva, C.C.; Coelho, G.R.; Nascimento, R.M.; Mendonça, R.Z. Cardanols detected in non-polar propolis extracts from Scaptotrigona aff. postica (Hymenoptera, Apidae, Meliponini). Braz. J. Food Technol. 2019, 22, e2018265. [Google Scholar] [CrossRef]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 7. [Google Scholar]
- Howden, B.P.; Giulieri, S.G.; Lung, T.W.F.; Baines, S.L.; Sharkey, L.K.; Lee, J.Y.H.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus aureus host interactions and adaptation. Nat. Rev. Microbiol. 2023, 21, 380–395. [Google Scholar]
- Zhang, W.; Margarita, G.E.; Wu, D.; Yuan, W.; Yan, S.; Qi, S.; Xue, X.; Wang, K.; Wu, L. Antibacterial activity of Chinese red propolis against Staphylococcus aureus and MRSA. Molecules 2022, 27, 1693. [Google Scholar] [CrossRef]
- Audah, K.A.; Ettin, J.; Darmadi, J.; Azizah, N.N.; Anisa, A.S.; Hermawan, T.D.F.; Tjampakasari, C.R.; Heryanto, R.; Ismail, I.S.; Batubara, I. Indonesian mangrove Sonneratia caseolaris leaves ethanol extract is a potential super antioxidant and anti methicillin-resistant Staphylococcus aureus drug. Molecules 2022, 27, 8369. [Google Scholar] [CrossRef]
- Remya, R.R.; Samrot, A.V.; Kumar, S.S.; Mohanavel, V.; Karthick, A.; Chinnaiyan, V.K.; Umapathy, D.; Muhibbullah, M. Bioactive potential of brown algae. Adsorpt. Sci. Technol. 2022, 2022, 9104835. [Google Scholar] [CrossRef]
- Naveen, J.; Baskaran, R.; Baskaran, V. Profiling of bioactives and in vitro evaluation of antioxidant and antidiabetic property of polyphenols of marine algae Padina tetrastromatica. Algal Res. 2021, 55, 102250. [Google Scholar] [CrossRef]
- Giriwono, P.E.; Iskandriati, D.; Tan, C.P.; Andarwulan, N. Sargassum seaweed as a source of anti-inflammatory substances and the potential insight of the tropical species: A review. Mar. Drugs 2019, 17, 590. [Google Scholar] [PubMed]
- Zango, U.U.; Ibrahim, M.; Shawai, S.A.A.; Shamsuddin, I.M. A review on β-lactam antibiotic drug resistance. MOJ Drug Des. Develop. Ther. 2019, 3, 52–58. [Google Scholar]
- Buommino, E.; Vollaro, A.; Nocera, F.; Lembo, F.; DellaGreca, M.; De Martino, L.; Catania, M. Synergistic effect of abietic acid with oxacillin against methicillin-resistant Staphylococcus pseudintermedius. Antibiotics 2021, 10, 80. [Google Scholar] [CrossRef]
- Bates, M.K.; Phillips, D.S.; O’Bryan, J. Shaker. Agitation rate and orbit affect growth of cultured bacteria. Thermo. Fish. Sci. Anshorbgrt 2016, 816. [Google Scholar]
- Amin, B. Isolation and characterization of antiprotozoal and antimicrobial metabolite from Penicillium roqueforti. Afr. J. Mycol. Biotech. 2016, 21, 13–26. [Google Scholar]
- Amin, B.H.; Abou-Dobara, M.I.; Diab, M.A.; Gomaa, E.A.; El-Mogazy, M.A.; El-Sonbati, A.Z.; El-Ghareib, M.S.; Hussien, M.A.; Salama, H.M. Synthesis, characterization, and biological investigation of new mixed-ligand complexes. Appl. Organomet. Chem. 2020, 34, 5689. [Google Scholar] [CrossRef]
- Claeson, P.; Tuchinda, P.; Reutrakuv, V. Some empirical aspects on the practical use of flash chromatography and medium pressure uquid chromatography for the isolation of biologically active compounds from plants. Sci. Asia 1993, 19, 73. [Google Scholar] [CrossRef]
- Jabborova, D.; Annapurna, K.; Paul, S.; Kumar, S.; Saad, H.A.; Desouky, S.; Ibrahim, M.F.M.; Elkelish, A. Beneficial Features of Biochar and Arbuscular Mycorrhiza for Improving Spinach Plant Growth, Root Morphological Traits, Physiological Properties, and Soil Enzymatic Activities. J. Fungi 2021, 7, 571. [Google Scholar] [CrossRef]
- Huang, C.Y.; Shih, H.W.; Lin, L.Y.; Tien, Y.W.; Cheng, T.J.; Cheng, W.C.; Wong, C.H.; Ma, C. Crystal structure of Staphylococcus aureus transglycosylase in complex with a lipid II analog and elucidation of peptidoglycan synthesis mechanism. Proc. Natl. Acad. Sci. USA 2012, 109, 6496–6501. [Google Scholar]
- Corbeil, C.R.; Williams, C.I.; Labute, P. Variability in docking success rates due to dataset preparation. J. Comput. Mol. Des. 2012, 26, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Heaslet, H.; Shaw, B.; Mistry, A.; Miller, A.A. Characterization of the active site of S. aureus monofunctional glycosyltransferase (Mtg) by site-directed mutation and structural analysis of the protein complexed with moenomycin. J. Struct. Biol. 2009, 167, 129–135. [Google Scholar] [CrossRef] [PubMed]


| Growth Inhibition Zone (mm) | ||
|---|---|---|
| Enterobacter cloacae | Streptococcus mutans | Staphylococcus aureus |
| 20 ± 0.2 | 18 ± 0.2 | 35 ± 0.2 |
| Compound | (a) Binding Energy (Score) (E) | (b) Amino Acids Involved in the Receptor | (c) RMSD Refine Å | Interaction |
|---|---|---|---|---|
| Alginic Acid | −7.447 | Arg241, Arg117, Gly130, Lys248, and Glu102 | 1.22 | H acceptor, ionic |
| Carrageenan | −6.64 | Gly130-Gly117 | 1.1 | H acceptor |
| Fucoxanthin | −6.57 | Arg241 | 1.75 | H-acceptor |
| Cycloeudesmol | −4.6 | Arg117 | 1.55 | H-acceptor |
| (Ref. Ligand) (Lipid II Analog) | −6.8 | Arg241, Arg 103, Glu100, Lys248, Glu102, Ser132, Gly130, and Arg117 | 1.55 | H donor, H acceptor, ionic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshiekheid, M.A. Evaluation of the Antibacterial Activities of Mangrove Honeybee Propolis Extract and the Identification of Transpeptidase and Transglycosylase as Targets for New Antibiotics Using Molecular Docking. Antibiotics 2023, 12, 1197. https://doi.org/10.3390/antibiotics12071197
Alshiekheid MA. Evaluation of the Antibacterial Activities of Mangrove Honeybee Propolis Extract and the Identification of Transpeptidase and Transglycosylase as Targets for New Antibiotics Using Molecular Docking. Antibiotics. 2023; 12(7):1197. https://doi.org/10.3390/antibiotics12071197
Chicago/Turabian StyleAlshiekheid, Maha A. 2023. "Evaluation of the Antibacterial Activities of Mangrove Honeybee Propolis Extract and the Identification of Transpeptidase and Transglycosylase as Targets for New Antibiotics Using Molecular Docking" Antibiotics 12, no. 7: 1197. https://doi.org/10.3390/antibiotics12071197
APA StyleAlshiekheid, M. A. (2023). Evaluation of the Antibacterial Activities of Mangrove Honeybee Propolis Extract and the Identification of Transpeptidase and Transglycosylase as Targets for New Antibiotics Using Molecular Docking. Antibiotics, 12(7), 1197. https://doi.org/10.3390/antibiotics12071197




