Clinical and Microbiological Outcomes and Follow-Up of Secondary Bacterial and Fungal Infections among Critically Ill COVID-19 Adult Patients Treated with and without Immunomodulation: A Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Inclusion
2.3. Data Collection
2.4. Diagnostic Evaluation
2.5. Treatment Allocation, Follow-Up
2.6. Outcomes
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Outcome Characteristics
3.3. Microbiological Characteristics
4. Discussion
4.1. Present Study
4.2. Previous Studies
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARDS | acute respiratory distress syndrome |
| BAL | bronchoalveolar lavage |
| CAPA | COVID-19-associated invasive pulmonary aspergillosis |
| COVID-19 | coronavirus disease-19 |
| CRP | C-reactive protein |
| CT | computed tomography |
| ECDC | European Centre for Disease Prevention and Control |
| EIA | enzyme-immunoassay |
| IL-6 | interleukin-6 |
| IL-6R | IL-6-receptor |
| ICU | intensive care unit |
| IVIG | intravenous immunoglobuline |
| IQR | interquartile region |
| JAK | Janus kinase |
| LDH | lactate dehydrogenase |
| LOS | length of stay |
| NIV | non-invasive ventilation |
| PCR | polymerase chain reaction |
| RCT | randomized clinical trials |
| RPT | reconvalescent plasmatherapy |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus-2 |
| SOC | standard-of-care |
| VAP | ventilator-associated bacterial pneumonia |
| WHO | World Health Organization |
References
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; de Silva, T.I.; Egan, C.; et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: A multicentre, prospective cohort study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef] [PubMed]
- Ripa, M.; Galli, L.; Poli, A.; Oltolini, C.; Spagnuolo, V.; Mastrangelo, A.; Muccini, C.; Monti, G.; De Luca, G.; Landoni, G.; et al. Secondary infections in patients hospitalized with COVID-19: Incidence and predictive factors. Clin. Microbiol. Infect. 2021, 27, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Gragueb-Chatti, I.; Lopez, A.; Hamidi, D.; Guervilly, C.; Loundou, A.; Daviet, F.; Cassir, N.; Papazian, L.; Forel, J.M.; Leone, M.; et al. Impact of dexamethasone on the incidence of ventilator-associated pneumonia and blood stream infections in COVID-19 patients requiring invasive mechanical ventilation: A multicenter retrospective study. Ann. Intensive Care 2021, 11, 87. [Google Scholar] [CrossRef]
- Lamouche-Wilquin, P.; Souchard, J.; Pere, M.; Raymond, M.; Asfar, P.; Darreau, C.; Reizine, F.; Hourmant, B.; Colin, G.; Rieul, G.; et al. Early steroids ventilator-associated pneumonia in COVID-19-related ARDS. Crit. Care 2022, 26, 233. [Google Scholar] [CrossRef]
- Tang, G.; Huang, M.; Luo, Y.; Liu, W.; Lin, Q.; Mao, L.; Wu, S.; Xiong, Z.; Hou, H.; Sun, Z.; et al. The Dynamic Immunological Parameter Landscape in Coronavirus Disease 2019 Patients with Different Outcomes. Front. Immunol. 2021, 12, 697622. [Google Scholar] [CrossRef]
- Ranieri, V.I.T.O.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Camou, F.; Issa, N.; Hessamfar, M.; Guisset, O.; Mourissoux, G.; Pedeboscq, S.; Minot, A.; Bonnet, F. Is Tocilizumab Plus Dexamethasone Associated with Superinfection in Critically Ill COVID-19 Patients? J. Clin. Med. 2022, 11, 5559. [Google Scholar] [CrossRef]
- Lepape, A.; Machut, A.; Bretonnière, C.; Friggeri, A.; Vacheron, C.H.; Savey, A. Effect of SARS-CoV-2 infection and pandemic period on healthcare-associated infections acquired in intensive care units. Clin. Microbiol. Infect. 2023, 29, 530–536. [Google Scholar] [CrossRef]
- Conway Morris, A.; Kohler, K.; De Corte, T.; Ercole, A.; De Grooth, H.J.; Elbers, P.W.; Povoa, P.; Morais, R.; Koulenti, D.; Jog, S.; et al. Co-infection and ICU-acquired infection in COIVD-19 ICU patients: A secondary analysis of the UNITE-COVID data set. Crit. Care 2022, 26, 236. [Google Scholar] [CrossRef]
- Lakatos, B.; Szabó, B.G.; Bobek, I.; Kiss-Dala, N.; Gáspár, Z.; Riczu, A.; Petrik, B.; Farkas, B.F.; Sebestyén, G.; Gopcsa, L.; et al. Baricitinib vs. tocilizumab treatment for hospitalized adult patients with severe COVID-19 and associated cytokine storm: A prospective, investigational, real-world study. Int. J. Infect. Dis. 2022, 125, 233–240. [Google Scholar] [CrossRef]
- European Centre for Disease Control and Prevention. Case Definition for COVID-19, as of 3 December 2020. 2020. Available online: https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition (accessed on 15 May 2023).
- World Health Organization. Clinical Management of COVID-19. 2023. Available online: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-clinical-2023 (accessed on 15 May 2023).
- Bartoletti, M.; Azap, O.; Barac, A.; Bussini, L.; Ergonul, O.; Krause, R.; Paño-Pardo, J.R.; Power, N.R.; Sibani, M.; Szabo, B.G.; et al. ESCMID COVID-19 living guidelines: Drug treatment and clinical management. Clin. Microbiol. Infect. 2022, 28, 222–238. [Google Scholar] [CrossRef]
- Kasler, M. (Ed.) Hungarian Coronavirus Handbook [Magyar Koronavírus Kézikönyv], 1st ed.; Ministry of Human Capacities: Budapest, Hungary, 2020. (In Hungarian)
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 18 (Suppl. 7), 19–37. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijnders, B.J.A.; Sherertz, R.J.; Warren, D.K. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 49, 1–45. [Google Scholar] [CrossRef]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Bassi, G.L.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT). Eur. Respir. J. 2017, 50, 1700582. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. 2023. Available online: www.eucast.org (accessed on 15 May 2023).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef]
- Reyes, L.F.; Rodriguez, A.; Bastidas, A.; Parra-Tanoux, D.; Fuentes, Y.V.; García-Gallo, E.; Moreno, G.; Ospina-Tascon, G.; Hernandez, G.; Silva, E.; et al. Dexamethasone as risk-factor for ICU-acquired respiratory tract infections in severe COVID-19. J. Crit. Care 2022, 69, 154014. [Google Scholar] [CrossRef]
- Raymond, M.; Le Thuaut, A.; Asfar, P.; Darreau, C.; Reizine, F.; Colin, G.; Dano, C.; Lorber, J.; Hourmant, B.; Delbove, A.; et al. Association of early dexamethasone therapy with mortality in critically Ill COVID-19 patients: A French multicenter study. Ann. Intensive Care 2022, 12, 102. [Google Scholar] [CrossRef]
- Dupuis, C.; de Montmollin, E.; Buetti, N.; Goldgran-Toledano, D.; Reignier, J.; Schwebel, C.; Domitile, J.; Neuville, M.; Ursino, M.; Siami, S.; et al. Impact of early corticosteroids on 60-day mortality in critically ill patients with COVID-19: A multicenter cohort study of the OUTCOMEREA network. PLoS ONE 2021, 16, e0255644. [Google Scholar] [CrossRef] [PubMed]
- Blonz, G.; Kouatchet, A.; Chudeau, N.; Pontis, E.; Lorber, J.; Lemeur, A.; Planche, L.; Lascarrou, J.-B.; Colin, G. Epidemiology and microbiology of ventilator-associated pneumonia in COVID-19 patients: A multicenter retrospective study in 188 patients in an un-inundated French region. Crit. Care 2021, 25, 72. [Google Scholar] [CrossRef] [PubMed]
- Vacheron, C.H.; Lepape, A.; Savey, A.; Machut, A.; Timsit, J.F.; Comparot, S.; Courno, G.; Vanhems, P.; Landel, V.; Lavigne, T.; et al. Attributable Mortality of Ventilator-associated Pneumonia Among Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2022, 206, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients With COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef]
- Vélez Pintado, M.; Camiro-Zúñiga, A.; Aguilar Soto, M.; Cuenca, D.; Mercado, M.; Crabtree-Ramirez, B.; ARMII Study Gruop. COVID-19-associated invasive pulmonary aspergillosis in a tertiary care center in Mexico City. Med. Mycol. 2021, 59, 828–833. [Google Scholar] [CrossRef]
- Rouzé, A.; Lemaitre, E.; Martin-Loeches, I.; Povoa, P.; Diaz, E.; Nyga, R.; Torres, A.; Metzelard, M.; Du Cheyron, D.; Lambiotte, F.; et al. Invasive pulmonary aspergillosis among intubated patients with SARS-CoV-2 or influenza pneumonia: A European multicenter comparative cohort study. Crit. Care 2022, 26, 11. [Google Scholar] [CrossRef]
| Parameter | Total (n = 379) | Immunomodulatory Treatment (n = 299) | No Immunomodulatory Treatment (n = 80) | p Value |
|---|---|---|---|---|
| Age (years, median ± IQR, min–max) | 69.0 ± 17.3 (24–95) | 69.0 ± 19.0 (24–92) | 72.5 ± 13.2 (41–95) | <0.01 |
| Male sex (n, %) | 234 (61.7) | 192 (64.2) | 42 (52.5) | 0.07 |
| Comorbidities (n, %): | ||||
| 275 (72.5) | 212 (70.9) | 63 (78.7) | 0.20 |
| 126 (33.2) | 92 (30.8) | 34 (42.5) | 0.06 |
| 98 (25.9) | 73 (24.4) | 25 (31.2) | 0.25 |
| 63 (16.6) | 54 (18.1) | 9 (11.3) | 0.18 |
| 69 (18.2) | 50 (16.7) | 19 (23.8) | 0.19 |
| 14 (3.7) | 13 (4.3) | 1 (1.3) | 0.32 |
| 55 (14.5) | 39 (13.0) | 16 (20.0) | 0.15 |
| 139 (36.7) | 105 (35.1) | 34 (42.5) | 0.24 |
| 33 (8.7) | 22 (7.4) | 11 (13.8) | 0.08 |
| 30 (7.9) | 24 (8.0) | 6 (7.5) | 1.00 |
| 13 (3.4) | 12 (4.0) | 1 (1.3) | 0.32 |
| 7 (1.8) | 5 (1.7) | 2 (2.5) | 0.64 |
| 12 (3.2) | 11 (3.7) | 1 (1.3) | 0.47 |
| 10 (2.6) | 9 (3.0) | 1 (1.3) | 0.70 |
| 16 (4.2) | 11 (3.7) | 5 (6.3) | 0.35 |
| COVID-19 vaccination status at baseline (n, %): | ||||
| 346 (91.3) | 268 (89.8) | 78 (97.5) | 0.02 |
| 20 (5.3) | 18 (6.0) | 2 (2.5) | 0.27 |
| 13 (3.4) | 13 (4.3) | 0 (0.0) | 0.08 |
| Clinical characteristics at baseline: | ||||
| 376 (99.2) | 297 (99.3) | 79 (98.8) | 0.51 |
| 273 (72.0) | 219 (73.2) | 54 (67.5) | 0.33 |
| 80 (21.1) | 62 (20.7) | 18 (22.5) | 0.76 |
| 144 (38.0) | 122 (40.8) | 22 (27.5) | 0.04 |
| 148 (39.1) | 131 (43.8) | 17 (21.2) | <0.01 |
| 196 (51.7) | 171 (57.2) | 25 (31.2) | <0.01 |
| Oxygen support initiated at baseline (n, %): | ||||
| 22 (5.8) | 13 (4.3) | 9 (11.3) | 0.03 |
| 80 (21.1) | 63 (21.1) | 17 (21.2) | 1.00 |
| 26 (6.9) | 26 (8.7) | 0 (0.0) | <0.01 |
| 249 (65.7) | 196 (65.6) | 53 (66.2) | 0.97 |
| Laboratory characteristics at baseline (median ± IQR, min–max): | ||||
| 0.8 ± 0.6 (0.0–21.4) | 0.8 ± 0.5 (0–21.4) | 0.8 ± 0.6 (0.0–10.8) | 0.80 |
| 204 ± 134 (16–694) | 202 ± 127 (17–694) | 205 ± 166 (16–633) | 0.75 |
| 140 ± 137 (0.4–456) | 140 ± 125 (0.4–456) | 132 ± 176 (0.6–404) | 0.49 |
| 62.7 ± 122.2 (2–55,000) | 62.7 ± 123.1 (2–55,000) | 59.1 ± 119.8 (2.7–55,000) | 0.58 |
| 1103 ± 1254 (18–34,650) | 1151 ± 1214 (39–34,650) | 968 ± 1822 (18–23,174) | 0.46 |
| 762 ± 448 (0–4890) | 793 ± 423 (0–4890) | 616 ± 421 (230–3302) | <0.001 |
| 1385 ± 1866 (114–140,786) | 1395 ± 1804 (203–137,997) | 1379 ± 1929 (114–140,786) | 0.85 |
| Parameter | Total (n = 379) | Immunomodulatory Treatment (n = 299) | No Immunomodulatory Treatment (n = 80) | p Value |
|---|---|---|---|---|
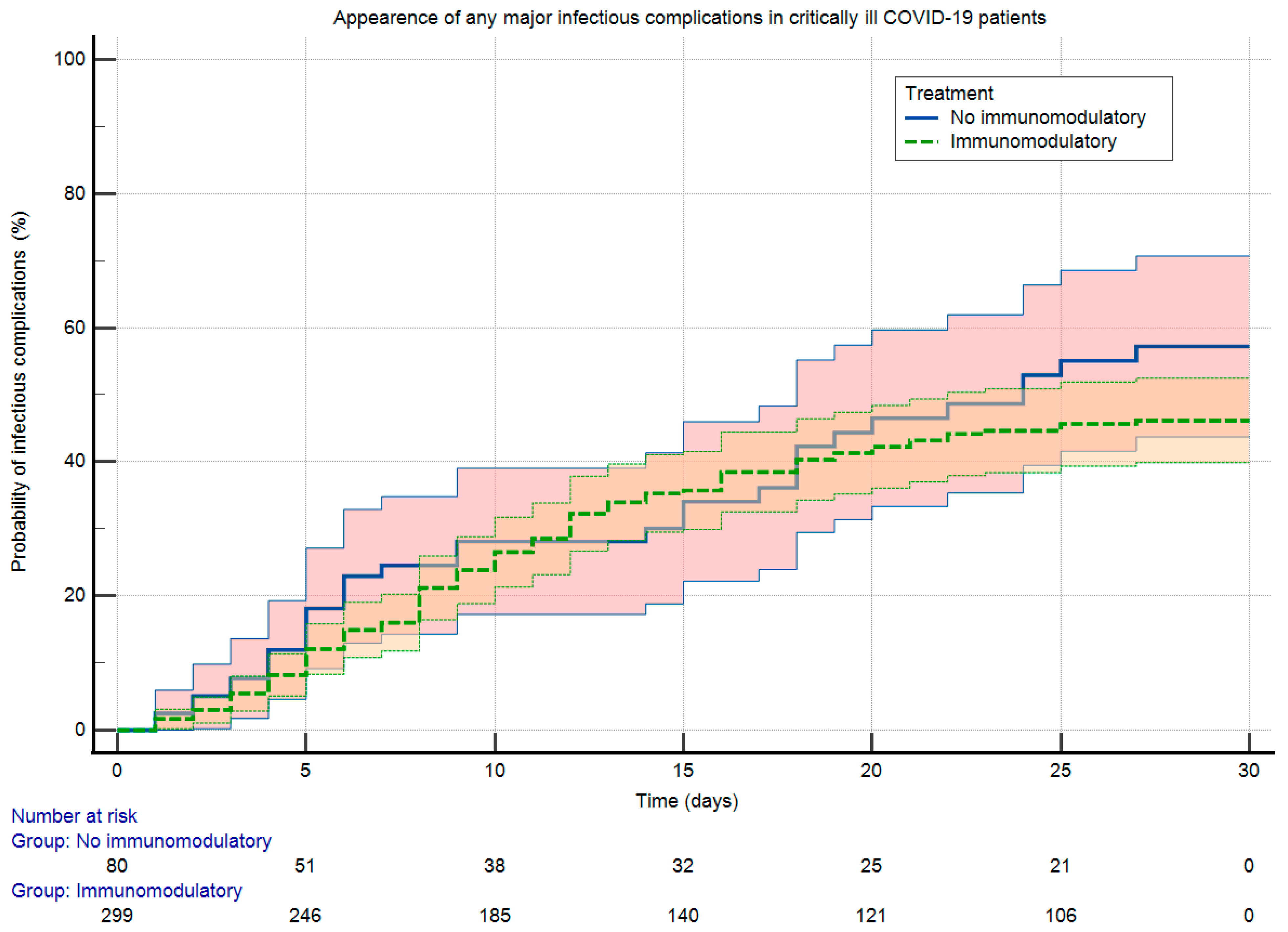
| Rate of any major infectious complication (n, %) | 151 (39.8) | 118 (39.5) | 33 (41.3) | 0.78 |
| Types of major infectious complications * (n, %): | ||||
| 94 (24.8) | 73 (24.4) | 21 (26.3) | 0.77 |
| 76 (20.1) | 60 (20.1) | 16 (20.0) | 1.00 |
| 20 (5.3) | 17 (5.7) | 3 (3.8) | 0.78 |
| Requirement of invasive mechanical ventilation (n, %) | 303 (79.9) | 241 (80.6) | 62 (77.5) | 0.53 |
| All-cause mortality (n, %) | 203 (53.6) | 160 (53.5) | 43 (53.8) | 1.00 |
| Antiviral treatment received (n, %) | ||||
| 9 (2.4) | 6 (2.0) | 3 (3.8) | 0.41 |
| 51 (13.5) | 40 (13.4) | 11 (13.8) | 1.00 |
| 216 (57.0) | 198 (66.2) | 18 (22.5) | <0.01 |
| Immunomodulatory treatment received (n, %) | n.a. | n.a. | ||
| 263 (69.4) | 263 (88.0) | ||
| 77 (20.3) | 77 (25.8) | ||
| 111 (29.3) | 111 (37.1) | ||
| 21 (5.5) | 21 (7.0) | ||
| Adjunctive treatment received (n, %): | ||||
| 45 (11.9) | 40 (13.4) | 5 (6.3) | 0.12 |
| 87 (23.0) | 65 (21.7) | 22 (27.5) | 0.30 |
| PARAMETER | With any Infection (n = 151) | Without any Infection (n = 228) | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |||
| Age (years, median ± IQR, min–max) | 70.4 ± 15.8 (29–91) | 68.9 ± 17.2 (24–95) | 1.02 (1.00–1.04) | 0.01 | 1.00 (0.99–1.03) | 0.52 |
| Male sex (n, %) | 91 (60.3) | 143 (62.7) | 0.90 (0.59–1.38) | 0.63 | ||
| Comorbidities (n, %): | ||||||
| 112 (74.1) | 163 (71.5) | 1.09 (0.70–1.70) | 0.71 | ||
| 53 (35.1) | 73 (32.0) | 1.15 (0.74–1.77) | 0.53 | ||
| 42 (27.8) | 56 (24.6) | 1.18 (0.74–1.89) | 0.48 | ||
| 26 (17.2) | 37 (16.2) | 1.07 (0.62–1.86) | 0.80 | ||
| 30 (19.9) | 39 (17.1) | 1.20 (0.71–2.04) | 0.49 | ||
| 8 (5.3) | 6 (2.6) | 2.07 (0.70–6.09) | 0.19 | ||
| 29 (19.2) | 26 (11.4) | 1.85 (1.04–3.28) | 0.04 | 1.62 (0.85–3.09) | 0.14 |
| 66 (43.7) | 73 (32.0) | 1.65 (1.08–2.52) | 0.02 | 1.33 (0.84–2.11) | 0.23 |
| 16 (10.6) | 17 (7.5) | 1.47 (0.72–3.01) | 0.29 | ||
| 11 (7.3) | 19 (8.3) | 0.86 (0.40–1.87) | 0.71 | ||
| 6 (4.0) | 7 (3.1) | 1.31 (0.43–3.97) | 0.64 | ||
| 8 (5.3) | 8 (3.5) | 1.54 (0.56–4.19) | 0.40 | ||
| 8 (5.3) | 2 (0.9) | 6.32 (1.32–30.19) | 0.02 | 4.82 (0.90–25.70) | 0.07 |
| Received ≥1 COVID-19 vaccine (n, %) | 11 (7.3) | 22 (9.6) | 0.74 (0.35–1.57) | 0.43 | ||
| Oxygen support initiated at baseline (n, %): | ||||||
| 8 (5.3) | 14 (6.1) | 0.86 (0.35–2.09) | 0.73 | ||
| 22 (14.6) | 58 (25.4) | 0.50 (0.29–0.86) | 0.01 | 0.62 (0.23–1.69) | 0.35 |
| 5 (3.3) | 21 (9.2) | 0.34 (0.12–0.92) | 0.03 | 0.56 (0.14–2.21) | 0.41 |
| 116 (76.8) | 133 (58.3) | 2.37 (1.49–3.75) | <0.001 | 0.99 (0.39–2.47) | 0.98 |
| Laboratory characteristics at baseline | ||||||
| (median ± IQR, min–max): | ||||||
| 0.8 ± 0.5 (0.1–10.8) | 0.8 ± 0.6 (0.0–21.4) | 0.95 (0.83–1.10) | 0.53 | ||
| 141 ± 133 (2.4–416) | 138 ± 141 (0.4–456) | 1.00 (1.00–1.00) | 0.84 | ||
| 85 ± 155 (3–55,000) | 54 ± 110 (2–55,000) | 1.00 (1.00–1.00) | 0.70 | ||
| 793 ± 510 (0–4135) | 747 ± 379 (69–4890) | 1.00 (1.00–1.00) | 0.56 | ||
| 1048 ± 1362 (18–21,595) | 1152 ± 1274 (39–34,650) | 1.00 (1.00–1.00) | 0.54 | ||
| Remdesivir treatment (n, %) | 81 (53.6) | 135 (59.2) | 0.80 (0.53–1.21) | 0.28 | ||
| Immunomodulatory treatment (n, %) | 118 (78.1) | 181 (79.4) | 0.93 (0.56–1.53) | 0.77 | 0.89 (0.51–1.54) | 0.67 |
| Requirement of pressoramines or inotropes (n, %) | 132 (87.4) | 141 (61.8) | 4.29 (2.47–7.43) | <0.001 | 2.78 (1.47–5.24) | <0.01 |
| Requrement of renal replacement therapy (n, %) | 45 (29.8) | 35 (15.4) | 2.34 (1.42–3.86) | <0.001 | 1.74 (1.01–2.98) | 0.04 |
| Requirement of prone positioning (n, %) | 73 (48.3) | 71 (31.1) | 2.07 (1.35–3.16) | <0.001 | 1.62 (1.01–2.61) | 0.05 |
| ARDS (n, %) | 68 (45.0) | 80 (35.1) | 1.52 (0.995–2.31) | 0.053 | ||
| Cytokine storm (n, %) | 73 (48.3) | 123 (53.9) | 0.80 (0.53–1.21) | 0.29 | ||
| SPECIES | Identified Causative Microorganism (n, % *) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| From BSI Episodes | From VAP Episodes | From CAPA Episodes | |||||||
| Total | IMT | No IMT | Total | IMT | No IMT | Total | IMT | No IMT | |
| Gram-negative bacteria: | n.a. | n.a. | n.a. | ||||||
| 1 (0.8) | 0 | 1 (3.3) | 0 | 0 | 0 | |||
| 0 | 0 | 0 | 1 (1.1) | 0 | 1 (5.9) | |||
| 19 (15.6) | 17 (18.1) | 2 (6.6) | 9 (9.9) | 7 (9.5) | 2 (11.8) | |||
| 1 (0.8) | 1 (1.1) | 0 | 0 | 0 | 0 | |||
| 1 (0.8) | 1 (1.1) | 0 | 0 | 0 | 0 | |||
| 5 (4.1) | 3 (3.2) | 2 (6.6) | 2 (2.2) | 2 (2.7) | 0 | |||
| 5 (4.1) | 5 (5.3) | 0 | 8 (8.8) | 8 (10.8) | 0 | |||
| 0 | 0 | 0 | 1 (1.1) | 0 | 1 (5.9) | |||
| 4 (3.3) | 1 (1.1) | 3 (10.0) | 0 | 0 | 0 | |||
| 0 | 0 | 0 | 1 (1.1) | 0 | 1 (5.9) | |||
| 2 | 2 (2.1) | 0 | 5 (5.5) | 3 (4.1) | 2 (11.8) | |||
| 0 | 0 | 0 | 3 (3.3) | 3 (4.1) | 0 | |||
| 0 | 0 | 0 | 1 (1.1) | 1 (1.4) | 0 | |||
| 1 (0.8) | 0 | 1 (3.3) | 0 | 0 | 0 | |||
| 14 (11.5) | 11 (11.7) | 3 (10.0) | 26 (28.6) | 17 (23.0) | 9 | |||
| 1 (0.8) | 1 (1.1) | 0 | 1 (1.1) | 1 (1.4) | 0 | |||
| 3 (2.5) | 1 (1.1) | 2 (6.6) | 4 (4.4) | 4 (5.4) | 0 | |||
| 1 (0.8) | 0 | 1 (3.3) | 0 | 0 | 0 | |||
| 4 (3.3) | 4 (4.3) | 0 | 6 (6.6) | 6 (8.1) | 0 | |||
| Gram-positive bacteria: | n.a. | n.a. | n.a. | ||||||
| 1 (0.8) | 1 (1.1) | 0 | 0 | 0 | 0 | |||
| 2 (1.6) | 1 (1.1) | 1 (3.3) | 0 | 0 | 0 | |||
| 27 (22.1) | 22 (23.4) | 5 | 0 | 0 | 0 | |||
| 8 (6.6) | 6 (6.4) | 2 (6.6) | 0 | 0 | 0 | |||
| 1 (0.8) | 0 | 1 (3.3) | 0 | 0 | 0 | |||
| 1 (0.8) | 1 (1.1) | 0 | 0 | 0 | 0 | |||
| 9 (7.4) | 6 (6.4) | 3 (10.0) | 22 (24.2) | 21 (28.4) | 1 (5.9) | |||
| 6 (4.9) | 3 (3.2) | 3 (10.0) | 0 | 0 | 0 | |||
| 1 (0.8) | 1 (1.1) | 0 | 0 | 0 | 0 | |||
| 1 (0.8) | 1 (1.1) | 0 | 0 | 0 | 0 | |||
| 1 (0.8) | 1 (1.1) | 0 | 0 | 0 | 0 | |||
| 1 (0.8) | 1 (1.1) | 0 | 0 | 0 | 0 | |||
| 1 (0.8) | 1 (1.1) | 0 | 0 | 0 | 0 | |||
| 1 (0.8) | 1 (1.1) | 0 | 0 | 0 | 0 | |||
| 1 (0.8) | 1 (1.1) | 0 | 1 (1.1) | 1 (1.4) | 0 | |||
| Molds: | |||||||||
| n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 20 (100) | 17 (100) | 3 (100) |
| Yeasts: | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |||
| 4 (57.1) | 2 (40.0) | 2 (100) | ||||||
| 1 (14.3) | 1 (20.0) | 0 | ||||||
| 1 (14.3) | 1 (20.0) | 0 | ||||||
| 1 (14.3) | 1 (20.0) | 0 | ||||||
| TOTAL | Bacterial: 124 | Bacterial: 94 | Bacterial: 30 | 91 | 74 | 17 | 20 | 17 | 3 |
| Fungal: 7 | Fungal: 5 | Fungal: 2 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, B.G.; Czél, E.; Nagy, I.; Korózs, D.; Petrik, B.; Marosi, B.; Gáspár, Z.; Rajmon, M.; Di Giovanni, M.; Vályi-Nagy, I.; et al. Clinical and Microbiological Outcomes and Follow-Up of Secondary Bacterial and Fungal Infections among Critically Ill COVID-19 Adult Patients Treated with and without Immunomodulation: A Prospective Cohort Study. Antibiotics 2023, 12, 1196. https://doi.org/10.3390/antibiotics12071196
Szabó BG, Czél E, Nagy I, Korózs D, Petrik B, Marosi B, Gáspár Z, Rajmon M, Di Giovanni M, Vályi-Nagy I, et al. Clinical and Microbiological Outcomes and Follow-Up of Secondary Bacterial and Fungal Infections among Critically Ill COVID-19 Adult Patients Treated with and without Immunomodulation: A Prospective Cohort Study. Antibiotics. 2023; 12(7):1196. https://doi.org/10.3390/antibiotics12071196
Chicago/Turabian StyleSzabó, Bálint Gergely, Eszter Czél, Imola Nagy, Dorina Korózs, Borisz Petrik, Bence Marosi, Zsófia Gáspár, Martin Rajmon, Márk Di Giovanni, István Vályi-Nagy, and et al. 2023. "Clinical and Microbiological Outcomes and Follow-Up of Secondary Bacterial and Fungal Infections among Critically Ill COVID-19 Adult Patients Treated with and without Immunomodulation: A Prospective Cohort Study" Antibiotics 12, no. 7: 1196. https://doi.org/10.3390/antibiotics12071196
APA StyleSzabó, B. G., Czél, E., Nagy, I., Korózs, D., Petrik, B., Marosi, B., Gáspár, Z., Rajmon, M., Di Giovanni, M., Vályi-Nagy, I., Sinkó, J., Lakatos, B., & Bobek, I. (2023). Clinical and Microbiological Outcomes and Follow-Up of Secondary Bacterial and Fungal Infections among Critically Ill COVID-19 Adult Patients Treated with and without Immunomodulation: A Prospective Cohort Study. Antibiotics, 12(7), 1196. https://doi.org/10.3390/antibiotics12071196






