Hamamelis virginiana L. Leaf Extracts Inhibit the Growth of Antibiotic-Resistant Gram-Positive and Gram-Negative Bacteria
Abstract
1. Introduction
2. Results
2.1. Antibacterial Activities
2.2. Fractional Inhibitory Concentration (FIC) Determinations
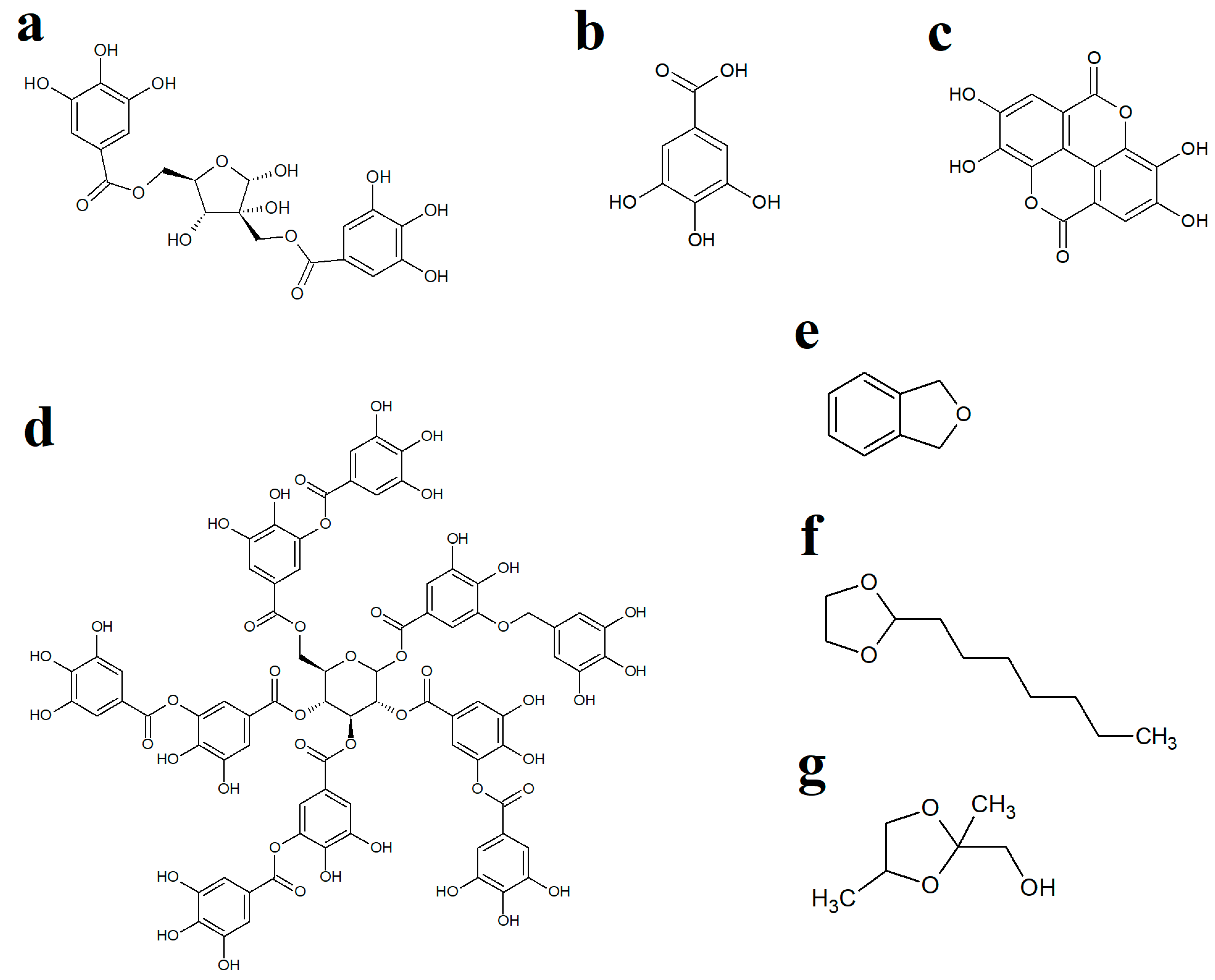
2.3. Qualitative GC-MS Profiling of Extracts
3. Discussion
4. Materials and Methods
4.1. Plant Sources and Extractions
4.2. Bacterial Cultures
4.3. Conventional Antibiotics
4.4. Tannins
4.5. Bacterial Growth Inhibition on Agar
4.6. Microplate Liquid Dilution MIC Assay
4.7. Fractional Inhibitory (FIC) and ΣFIC Assessment
4.8. GC-MS Profiling Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. The Review on Antimicrobial Resistance. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 22 May 2023).
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- D’Andrea, M.M.; Fraziano, M.; Thaller, M.C.; Rossolini, G.M. The urgent need for novel antimicrobial agents and strategies to fight antibiotic resistance. Antibiotics 2019, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, S.; Tschudin-Sutter, S.; Egli, A.; Osthoff, M. Optimizing antibiotic therapies to reduce the risk of bacterial resistance. Eur. J. Intern. Med. 2022, 99, 7–12. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. Levels and Trends in Child Mortality: Report 2019.; World Bank Group: Washington, DC, USA, 2019; p. 52. Available online: https://www.unicef.org/reports/levels-and-trends-child-mortality-report-2019 (accessed on 24 January 2023).
- Chaurasia, S.; Sivanandan, S.; Agarwal, R.; Ellis, S.; Sharland, M.; Sankar, M.J. Neonatal sepsis in South Asia: Huge burden and spiralling antimicrobial resistance. BMJ 2019, 364, k5314. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, A.; Fernández, L.; Gutiérrez, D.; Iglesias, B.; Rodríguez, A.; García, P. Methicillin-resistant Staphylococcus aureus in hospitals: Latest trends and treatments based on bacteriophages. J. Clin. Microbiol. 2019, 57, e01006-19. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, A.; Papucci, S.L.; Aletti, A.; Chiossone, A.; Pigozzi, F.; Sguassero, Y. Community-acquired methicillin-resistant Staphylococcus aureus pneumonia in a children’s hospital. Our ten-year experience. Arch. Argent. Pediatr. 2021, 119, 11–17. [Google Scholar]
- Ali, M.F.; Marzouq, M.A.; Hussein, S.A.; Salman, B.I. A bio-analytically validated HPLC-UV method for simultaneous determination of doripenem and ertapenem in pharmaceutical dosage forms and human plasma: A dual carbapenem regimen for treatment of drug-resistant strain of Klebsiella pneumoniae. RSC Adv. 2021, 11, 3125–3133. [Google Scholar] [CrossRef]
- Levine, M.M.; Nasrin, D.; Acácio, S.; Bassat, Q.; Powell, H.; Tennant, S.M.; Sow, S.O.; Sur, D.; Zaidi, A.K.; Faruque, A.S.; et al. Diarrhoeal disease and subsequent risk of death in infants and children residing in low-income and middle-income countries: Analysis of the GEMS case-control study and 12-month GEMS-1A follow-on study. Lancet Glob. Health. 2020, 8, e204–e214. [Google Scholar] [CrossRef]
- Yigit, H.; Queenan, A.M.; Anderson, G.J.; Domenech-Sanchez, A.; Biddle, J.W.; Steward, C.D.; Alberti, S.; Bush, K.; Tenover, F.C. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 1151–1161. [Google Scholar] [CrossRef]
- McDanel, J.; Schweizer, M.; Crabb, V.; Nelson, R.; Samore, M.; Khader, K.; Blevins, A.E.; Diekema, D.; Chiang, H.Y.; Nair, R.; et al. Incidence of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella infections in the United States: A systematic literature review. Infect. Control Hosp. Epidemiol. 2017, 38, 1209–1215. [Google Scholar] [CrossRef]
- Kawamura, K.; Nagano, N.; Suzuki, M.; Wachino, J.I.; Kimura, K.; Arakawa, Y. ESBL-producing Escherichia coli and its rapid rise among healthy people. Food Saf. 2017, 5, 122–150. [Google Scholar] [CrossRef]
- Lam, M.M.; Wyres, K.L.; Wick, R.R.; Judd, L.M.; Fostervold, A.; Holt, K.E.; Löhr, I.H. Convergence of virulence and MDR in a single plasmid vector in MDR Klebsiella pneumoniae ST15. J. Antimicrob. Chemother. 2019, 74, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, Y.; Hsia, Y.; Sharland, M.; Heath, P.T. Systematic review of carbapenem-resistant Enterobacteriaceae causing neonatal sepsis in China. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar]
- Cheesman, M.J.; Alcorn, S.; Verma, V.; Cock, I.E. An assessment of the growth inhibition profiles of Hamamelis virginiana L. extracts against Streptococcus and Staphylococcus spp. J. Tradit. Complement. Med. 2021, 11, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Cheesman, M.J.; Alcorn, S.R.; Cock, I.E. Effects of Hamamelis virginiana L. extracts on Pseudomonas aeruginosa growth and antagonism of ciprofloxacin. Pharmacogn. Commun. 2023, 13, 92–98. [Google Scholar] [CrossRef]
- Korting, H.C.; Schäfer-Korting, M.; Hart, H.; Laux, P.; Schmid, M. Anti-inflammatory activity of hamamelis distillate applied topically to the skin. Eur. J. Clin. Pharmacol. 1993, 44, 315–318. [Google Scholar] [CrossRef]
- Moerman, D.E. Medicinal Plants of Native America, Part 1; Museum Anthropology, University of Michigan: Ann Arbor, MI, USA, 1986. [Google Scholar]
- Wang, H.; Provan, G.J.; Helliwell, K. Determination of hamamelitannin, catechins and gallic acid in witch hazel bark, twig and leaf by HPLC. J. Pharm. Biomed. Anal. 2003, 33, 539–544. [Google Scholar] [CrossRef]
- Brackman, G.; Breyne, K.; De Rycke, R.; Vermote, A.; Van Nieuwerburgh, F.; Meyer, E.; Van Calenbergh, S.; Coenye, T. The quorum sensing inhibitor hamamelitannin increases antibiotic susceptibility of Staphylococcus aureus biofilms by affecting peptidoglycan biosynthesis and eDNA release. Sci. Rep. 2016, 6, 20321. [Google Scholar] [CrossRef]
- Rasooly, R.; Molnar, A.; Choi, H.Y.; Do, P.; Racicot, K.; Apostolidis, E. In-vitro inhibition of staphylococcal pathogenesis by witch-hazel and green tea extracts. Antibiotics 2019, 8, 244. [Google Scholar] [CrossRef]
- Kiran, M.D.; Adikesavan, N.V.; Cirioni, O.; Giacometti, A.; Silvestri, C.; Scalise, G.; Ghiselli, R.; Saba, V.; Orlando, F.; Shoham, M.; et al. Discovery of a quorum-sensing inhibitor of drug-resistant staphylococcal infections by structure-based virtual screening. Mol. Pharmacol. 2008, 73, 1578–1586. [Google Scholar] [CrossRef]
- Abbas, T.F.; Abbas, M.F.; Lafta, A.J. Antibacterial activity and medical properties of Witch Hazel Hamamelis virginiana. Ann. Trop. Med. Public Health 2020, 23, 46. [Google Scholar] [CrossRef]
- Eloff, J.N. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 1998, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Parekh, A.D.; Shaikh, O.A.; Khan, M.; Ochani, S. Acute liver failure secondary to the use of unmonitored drugs and herbal supplements: An underreported and serious issue. Ir. J. Med. Sci. 2023, 1–3. [Google Scholar] [CrossRef]
- Rasooly, R.; Molnar, A.; Do, P.; Morroni, G.; Brescini, L.; Cirioni, O.; Giacometti, A.; Apostolidis, E. Witch hazel significantly improves the efficacy of commercially available teat dips. Pathogens 2020, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Vennat, B.; Pourrat, H.; Pouget, M.P.; Gross, D.; Pourrat, A. Tannins from Hamamelis virginiana: Identification of proanthocyanidins and hamamelitannin quantification in leaf, bark, and stem extracts. Planta Med. 1988, 54, 454–457. [Google Scholar] [CrossRef]
- Piazza, S.; Martinelli, G.; Vrhovsek, U.; Masuero, D.; Fumagalli, M.; Magnavacca, A.; Pozzoli, C.; Canilli, L.; Terno, M.; Angarano, M.; et al. Anti-inflammatory and anti-acne effects of Hamamelis virginiana bark in human keratinocytes. Antioxidants 2022, 11, 1119. [Google Scholar] [CrossRef]
- Leong, C.; Schmid, B.; Buttafuoco, A.; Glatz, M.; Bosshard, P.P. In vitro efficacy of antifungal agents alone and in shampoo formulation against dandruff-associated Malassezia spp. and Staphylococcus spp. Int. J. Cosmet. Sci. 2019, 41, 221–227. [Google Scholar] [CrossRef]
- Akiyama, H.; Fujii, K.; Yamasaki, O.; Oono, T.; Iwatsuki, K. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef]
- Farha, A.K.; Yang, Q.Q.; Kim, G.; Li, H.B.; Zhu, F.; Liu, H.Y.; Gan, R.Y.; Corke, H. Tannins as an alternative to antibiotics. Food Biosci. 2020, 38, 100751. [Google Scholar] [CrossRef]
- Lu, L.L.; Lu, X.Y.; Ma, N. Kinetics of non-catalyzed hydrolysis of tannin in high temperature liquid water. J. Zhejiang Univ. Sci. B. 2008, 9, 401–406. [Google Scholar] [CrossRef]
- Sharma, A.; Biharee, A.; Kumar, A.; Jaitak, V. Antimicrobial terpenoids as a potential substitute in overcoming antimicrobial resistance. Curr. Drug Targets. 2020, 21, 1476–1494. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A. Isocoumarins, miraculous natural products blessed with diverse pharmacological activities. Eur. J. Med. Chem. 2016, 116, 290–317. [Google Scholar] [CrossRef] [PubMed]
- Shabir, G.; Saeed, A.; El-Seedi, H.R. Natural isocoumarins: Structural styles and biological activities, the revelations carry on…. Phytochemistry 2021, 181, 112568. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Y.; Liu, Z.; Cai, R.; Lu, Y.; Huang, X.; She, Z. Isocoumarins and benzofurans from the mangrove endophytic fungus Talaromyces amestolkiae possess α-glucosidase inhibitory and antibacterial activities. RSC Adv. 2016, 6, 26412–26420. [Google Scholar] [CrossRef]
- Park, H.B.; Perez, C.E.; Perry, E.K.; Crawford, J.M. Activating and attenuating the amicoumacin antibiotics. Molecules 2016, 21, 824. [Google Scholar] [CrossRef]
- Huang, G.L.; Zhou, X.M.; Bai, M.; Liu, Y.X.; Zhao, Y.L.; Luo, Y.P.; Niu, Y.Y.; Zheng, C.J.; Chen, G.Y. Dihydroisocoumarins from the mangrove-derived fungus Penicillium citrinum. Mar. Drugs 2016, 14, 177. [Google Scholar] [CrossRef]
- Lei, H.; Lin, X.; Han, L.; Ma, J.; Ma, Q.; Zhong, J.; Liu, Y.; Sun, T.; Wang, J.; Huang, X. New metabolites and bioactive chlorinated benzophenone derivatives produced by a marine-derived fungus Pestalotiopsis heterocornis. Mar. Drugs 2017, 15, 69. [Google Scholar] [CrossRef]
- Damasceno, J.P.L.; Rodrigues, R.P.; Goncalves, R.D.C.R.; Kitagawa, R.R. Anti-Helicobacter pylori activity of isocoumarin paepalantine: Morphological and molecular docking analysis. Molecules 2017, 22, 786. [Google Scholar] [CrossRef]
- Gu, B.B.; Tang, J.; Jiao, W.H.; Li, L.; Sun, F.; Wang, S.P.; Yang, F.; Lin, H.W. Azaphilone and isocoumarin derivatives from the sponge-derived fungus Eupenicillium sp. 6A-9. Tetrahedron Lett. 2018, 59, 3345–3348. [Google Scholar] [CrossRef]
- Devienne, K.F.; Raddi, M.G.; Coelho, R.G.; Vilegas, W. Structure-–antimicrobial activity of some natural isocoumarins and their analogues. Phytomedicine 2005, 12, 378–381. [Google Scholar] [CrossRef]
- Koppula, P.; Purohit, N. Synthesis of new biologically active triazolo, tetrazolo and coumarinoyl derivatives of isocoumarins. Org. Commun. 2013, 6, 148–161. [Google Scholar]
- Lama, A.; Pané-Farré, J.; Chon, T.; Wiersma, A.M.; Sit, C.S.; Vederas, J.C.; Hecker, M.; Nakano, M.M. Response of methicillin-resistant Staphylococcus aureus to amicoumacin A. PLoS ONE 2012, 7, e34037. [Google Scholar] [CrossRef] [PubMed]
- Polikanov, Y.S.; Osterman, I.A.; Szal, T.; Tashlitsky, V.N.; Serebryakova, M.V.; Kusochek, P.; Bulkley, D.; Malanicheva, I.A.; Efimenko, T.A.; Efremenkova, O.V.; et al. Amicoumacin A inhibits translation by stabilizing mRNA interaction with the ribosome. Mol. Cell 2014, 56, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.R.; Kumar, M.; Gupta, V. Heterocyclic Chemistry: Volume II: Five-Membered Heterocycles; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Ovsyannikova, M.N.; Vol’Eva, V.B.; Belostotskaya, I.S.; Komissarova, N.L.; Malkova, A.V.; Kurkovskaya, L.N. Antibacterial activity of substituted 1, 3-dioxolanes. Pharm. Chem. J. 2013, 47, 142–145. [Google Scholar] [CrossRef]
- Küçük, H.B.; Yusufoğlu, A.; Mataracı, E.; Döşler, S. Synthesis and biological activity of new 1, 3-dioxolanes as potential antibacterial and antifungal compounds. Molecules 2011, 16, 6806–6815. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Clinical and Laboratory Standards Institute. Clinical and Laboratory Standards Institute CLSI Document M100. In Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023. [Google Scholar]
- Wright, M.H.; Shalom, J.; Matthews, B.; Greene, A.C.; Cock, I.E. Terminalia ferdinandiana Exell: Extracts inhibit Shewanella spp. growth and prevent fish spoilage. Food Microbiol. 2019, 78, 114–122. [Google Scholar] [CrossRef]
- Tiwana, G.; Cock, I.E.; White, A.; Cheesman, M.J. Use of specific combinations of the triphala plant component extracts to potentiate the inhibition of gastrointestinal bacterial growth. J. Ethnopharmacol. 2020, 260, 112937. [Google Scholar] [CrossRef]
- Hübsch, Z.; Van Zyl, R.L.; Cock, I.E.; Van Vuuren, S.F. Interactive antimicrobial and toxicity profiles of conventional antimicrobials with Southern African medicinal plants. S. Afr. J. Bot. 2014, 93, 185–197. [Google Scholar] [CrossRef]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]
- Eloff, J.N. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement. Altern. Med. 2019, 19, 106. [Google Scholar] [CrossRef]
- Eloff, J.N. Quantification the bioactivity of plant extracts during screening and bioassay guided fractionation. Phytomedicine 2004, 11, 370–371. [Google Scholar] [CrossRef] [PubMed]
- Mogana, R.; Adhikari, A.; Tzar, M.N.; Ramliza, R.; Wiart, C. Antibacterial activities of the extracts, fractions and isolated compounds from Canarium patentinervium Miq. against bacterial clinical isolates. BMC Complement. Med. Ther. 2020, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Santana, E.F.; Saraiva, A.M.; Coutinho, F.N.; Castro, R.H.; Pisciottano, M.N.; Amorim, E.L.; Albuquerque, U.P. Which approach is more effective in the selection of plants with antimicrobial activity? Evid. Based Complementary Altern. Med. 2013, 2013, 308980. [Google Scholar] [CrossRef] [PubMed]
- Doern, C.D. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar] [CrossRef]
- Shalom, J.; Cock, I.E. Terminalia ferdinandiana Exell. fruit and leaf extracts inhibit proliferation and induce apoptosis in selected human cancer cell lines. Nutr. Cancer 2018, 70, 579–593. [Google Scholar] [CrossRef]
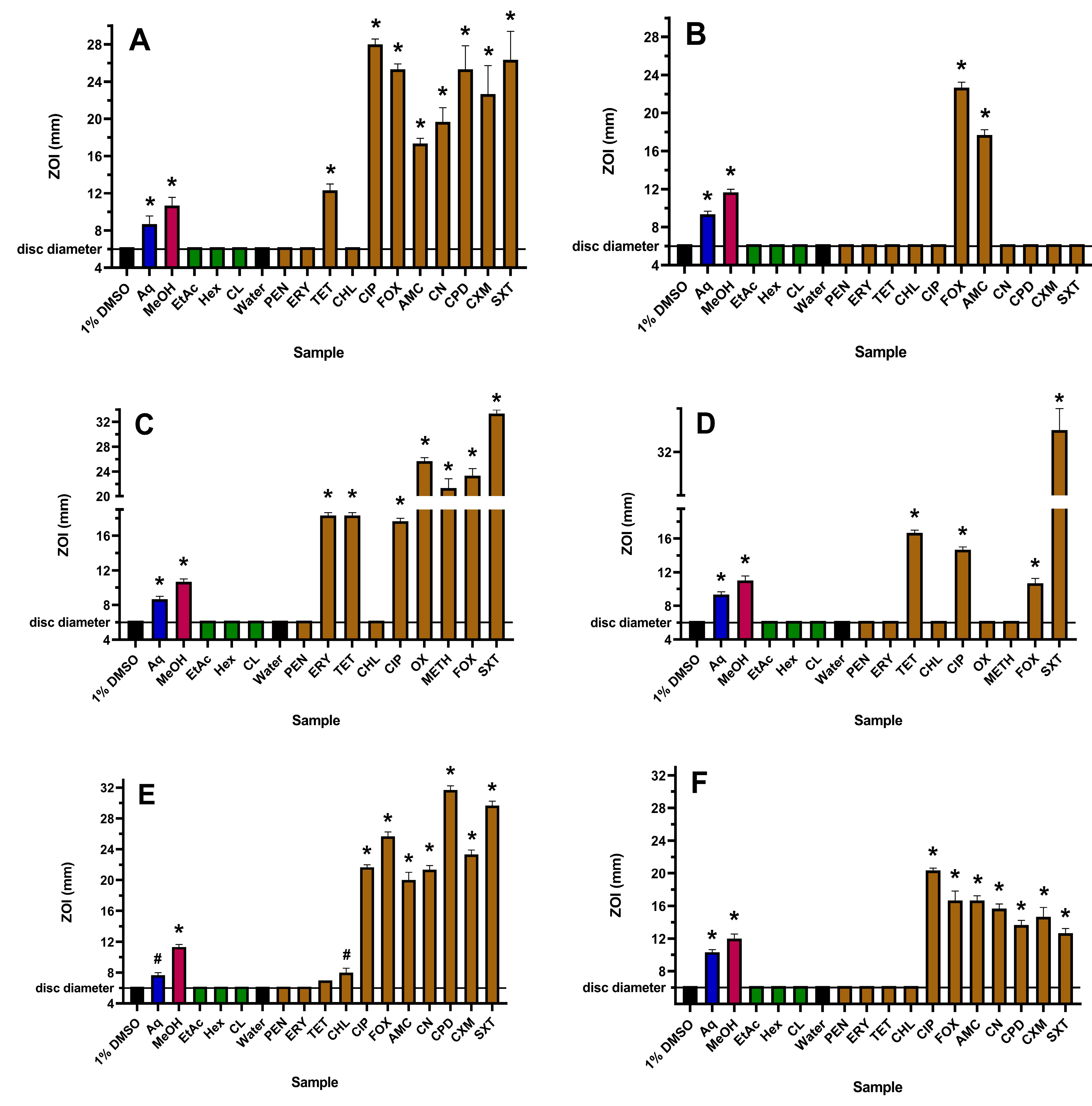

| Extract, Antibiotic or Tannin | MIC (µg/mL) | |||||
|---|---|---|---|---|---|---|
| E. coli | ESBL E. coli | S. aureus a | MRSA | K. pneumoniae | ESBL K. pneumonia | |
| WH-Aq | 3448 | 1724 | 493 | 431 | 1724 | 2463 |
| WH-MeOH | 1173 | 670 | 251 | 168 | 1341 | 1257 |
| WH-EtAc | >10,000 * | >10,000 * | >10,000 * | >10,000 * | >10,000 * | >10,000 * |
| WH-Hex | >10,000 * | >10,000 * | >10,000 * | >10,000 * | >10,000 * | >10,000 * |
| WH-CL | >10,000 * | >10,000 * | >10,000 * | >10,000 * | >10,000 * | >10,000 * |
| PEN | >2.5 * | >2.5 * | >2.5 * | >2.5 * | >2.5 * | >2.5 * |
| ERY | >2.5 * | >2.5 * | 0.313 | >2.5 * | >2.5 * | >2.5 * |
| TET | 0.625 | >2.5 * | 0.156 | 0.156 | 0.625 | >2.5 * |
| CHL | 2.5 | >2.5 * | >2.5 * | >2.5 * | >2.5 * | >2.5 * |
| CIP | CND | >2.5 * | 0.625 | 0.625 | CND | 0.625 |
| OX | NT | NT | 0.039 | >2.5 * | NT | NT |
| METH | NT | NT | 0.313 | >2.5 * | NT | NT |
| EA | >625 * | >625 * | >625 * | >625 * | >625 * | >625 * |
| GA | >1000 * | >1000 * | >1000 * | >1000 * | >1000 * | >1000 * |
| TA | 313 | 625 | 156 | 156 | >625 * | 625 |
| Extract Solvent | Antibiotic | FIC or ∑FIC Values | Bacterial Strain | Extract Solvent | Antibiotic | FIC or ∑FIC Values | Bacterial Strain | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | ESBL E. coli | S. aureusa | MRSA | K. pneumoniae | ESBL K. pneumoniae | E. coli | ESBL E. coli | S. aureusa | MRSA | K. pneumoniae | ESBL K. pneumoniae | ||||||
| Aqueous | PEN | FICEXT | Methanol | PEN | FICEXT | ||||||||||||
| FICPEN | FICPEN | ||||||||||||||||
| ∑FIC | ∑FIC | ||||||||||||||||
| ERY | FICEXT | 0.25 | ERY | FICEXT | 0.50 | ||||||||||||
| FICERY | 1.00 | FICERY | 2.00 | ||||||||||||||
| ∑FIC | 1.25 | ∑FIC | 2.50 | ||||||||||||||
| TET | FICEXT | 0.25 | 0.13 | 0.50 | 1.00 | TET | FICEXT | 1.00 | 0.50 | 0.50 | 1.00 | ||||||
| FICTET | 1.00 | 1.00 | 0.50 | 1.00 | FICTET | 1.00 | 2.00 | 0.50 | 0.50 | ||||||||
| ∑FIC | 1.25 | 1.13 | 1.00 | 2.00 | ∑FIC | 2.00 | 2.50 | 1.00 | 1.50 | ||||||||
| CHL | FICEXT | 1.00 | 2.00 | CHL | FICEXT | 0.5 | 1.00 | ||||||||||
| FICCHL | 1.00 | 0.50 | FICCHL | 0.25 | 0.50 | ||||||||||||
| ∑FIC | 2.00 | 2.50 | ∑FIC | 0.75 | 1.50 | ||||||||||||
| CIP | FICEXT | 0.25 | 1.0 | 2.00 | CIP | FICEXT | 0.50 | 1.0 | 1.96 | ||||||||
| FICCIP | 2.00 | 0.50 | 0.10 | FICCIP | 0.25 | 0.25 | 2.0 | ||||||||||
| ∑FIC | 2.25 | 1.50 | 2.10 | ∑FIC | 0.75 | 1.25 | 2.96 | ||||||||||
| Retention Time (Min) | Empirical Formula | Molecular Mass (Da) | Putative Identification | Relative Abundance (% Total Area) | ||||
|---|---|---|---|---|---|---|---|---|
| M | W | E | C | H | ||||
| 5.23 | C2H6OS | 78 | Dimethylsulfoxide | 96.15 | 89.53 | 85.08 | ||
| 5.825 | Could not be determined | 0.05 | ||||||
| 7.015 | Could not be determined | 17.88 | ||||||
| 7.047 | Could not be determined | 29.9 | ||||||
| 7.265 | Could not be determined | 4.18 | ||||||
| 8.785 | Could not be determined | 0.28 | ||||||
| 8.824 | Could not be determined | 0.32 | ||||||
| 8.92 | Could not be determined | 0.15 | ||||||
| 9.779 | C8H18O | 130 | Isobutyl ether | 0.29 | 1.05 | |||
| 10.034 | C9H20O | 144 | 3,5,5-Trimethylhexanol | 0.64 | 0.55 | |||
| 10.505 | C8H18O | 130 | 2-Ethyl-1-hexanol | 2.15 | 2.01 | 6.5 | 5.21 | 79.94 |
| 11.138 | C6H14O2 | 118 | 3-Methoxy-3-methylbutanol | 0.3 | ||||
| 12.074 | C8H8O | 120 | Phthalane | 0.07 | 0.08 | |||
| 12.737 | C9H18O | 142 | Nonanal | 0.24 | ||||
| 14.129 | C10H20O2 | 172 | 2-Ethyl-1-hexyl acetate | 0.06 | ||||
| 14.271 | C10H20O2 | 172 | 2-Heptyl-1,3-dioxolane | 11.85 | 0.32 | 0.98 | 0.42 | 7.04 |
| 14.707 | C9H20O | 144 | 1-Nonanol | 0.02 | ||||
| 14.817 | C10H20O | 156 | Menthol | 0.05 | ||||
| 15.635 | C11H16O2 | 164 | 5,6,7,8,9-octahydro-2H-benzo[a]cyclohepten-2-one | 0.13 | ||||
| 15.718 | C10H20O | 156 | Decanal | 0.06 | 0.13 | |||
| 15.837 | Could not be determined | 0.1 | 0.07 | 0.48 | 4.98 | 5.46 | ||
| 16.041 | C9H10O | 134 | Epoxy-cumene | 0.04 | 0.77 | 0.5 | ||
| 17.155 | C14H22 | 190 | 1,3-Di-tert-butylbenzene | 0.04 | 0.08 | |||
| 17.308 | C10H18O | 154 | trans-2-Decenal | 0.03 | ||||
| 17.93 | C10H16 | 136 | Camphene | 0.1 | ||||
| 19.068 | Could not be determined | 0.08 | 0.04 | 1.11 | ||||
| 19.144 | C4H8O3 | 104 | 1,3-Dioxolane-2-methanol | 1.91 | 0.08 | 0.19 | 0.07 | 2.22 |
| 19.849 | C16H30O4 | 286 | 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 0.02 | 0.08 | 0.22 | 0.1 | |
| 20.094 | C12H22O | 182 | (E)-2-Dodecen-1-al | 0.05 | ||||
| 20.42 | C12H24O3 | 216 | 1,3-Pentanediol, 2,2,4-trimethyl-, 1-isobutyrate | 0.06 | 0.16 | 0.46 | 0.21 | 0.58 |
| 25.386 | Could not be determined | 0.16 | 0.15 | 0.22 | 2.23 | |||
| 26.127 | C11H16O2 | 180 | 2,6,6-Trimethyl-2-hydroxycyclohexylidene) acetic acid lactone | 0.04 | ||||
| 26.135 | C11H16O2 | 180 | 2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl- | 0.05 | ||||
| 28.034 | C16H30O4 | 286 | 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 0.1 | 0.15 | 0.26 | 0.17 | 1.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheesman, M.J.; Alcorn, S.R.; White, A.; Cock, I.E. Hamamelis virginiana L. Leaf Extracts Inhibit the Growth of Antibiotic-Resistant Gram-Positive and Gram-Negative Bacteria. Antibiotics 2023, 12, 1195. https://doi.org/10.3390/antibiotics12071195
Cheesman MJ, Alcorn SR, White A, Cock IE. Hamamelis virginiana L. Leaf Extracts Inhibit the Growth of Antibiotic-Resistant Gram-Positive and Gram-Negative Bacteria. Antibiotics. 2023; 12(7):1195. https://doi.org/10.3390/antibiotics12071195
Chicago/Turabian StyleCheesman, Matthew J., Sean R. Alcorn, Alan White, and Ian E. Cock. 2023. "Hamamelis virginiana L. Leaf Extracts Inhibit the Growth of Antibiotic-Resistant Gram-Positive and Gram-Negative Bacteria" Antibiotics 12, no. 7: 1195. https://doi.org/10.3390/antibiotics12071195
APA StyleCheesman, M. J., Alcorn, S. R., White, A., & Cock, I. E. (2023). Hamamelis virginiana L. Leaf Extracts Inhibit the Growth of Antibiotic-Resistant Gram-Positive and Gram-Negative Bacteria. Antibiotics, 12(7), 1195. https://doi.org/10.3390/antibiotics12071195







