Effect of the COVID-19 Pandemic on Rates and Epidemiology of Clostridioides difficile Infection in One VA Hospital
Abstract
1. Introduction
2. Results
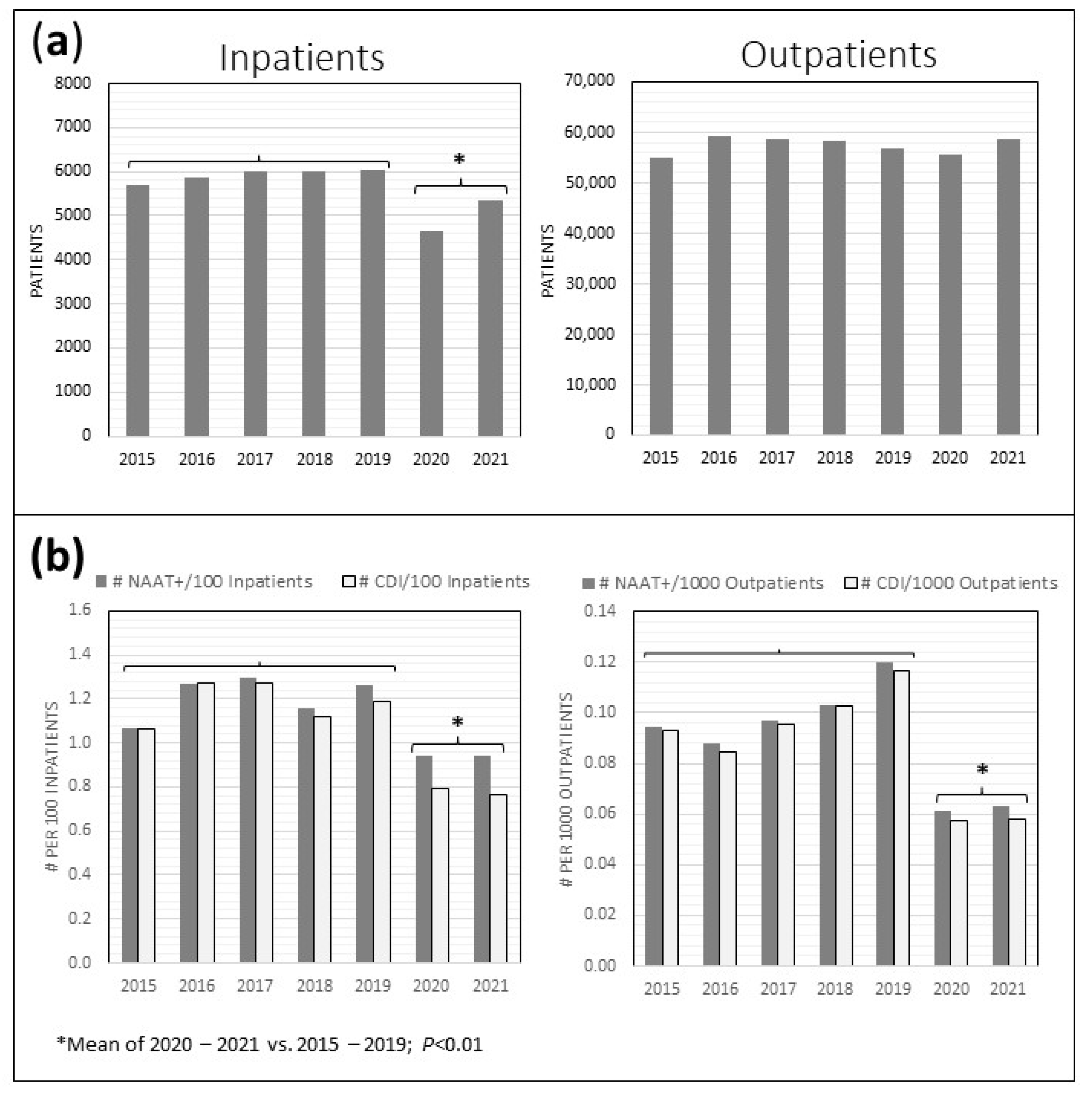
2.1. CD Stool Testing
2.2. REA Typing and Patient Characteristics
3. Discussion
4. Materials and Methods
4.1. Data Collection
4.2. REA Typing
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J.; Wilson, L.E.; Kainer, M.A.; Lynfield, R.; Greissman, S.; et al. Changes in Prevalence of Health Care–Associated Infections in U.S. Hospitals. N. Engl. J. Med. 2018, 379, 1732–1744. [Google Scholar] [CrossRef] [PubMed]
- Guh, A.Y.; Mu, Y.; Winston, L.G.; Johnston, H.; Olson, D.; Farley, M.M.; Wilson, L.E.; Holzbauer, S.M.; Phipps, E.C.; Dumyati, G.K.; et al. Trends in U.S. Burden of Clostridioides difficile Infection and Outcomes. N. Engl. J. Med. 2020, 382, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- CDC. Antibiotic/Antimicrobial Resistance (AR/AMR); Biggest Threats & Data; Clostridioides difficile (C. difficile); Centers for Disesase Control and Prevention: Atlanta, GA, USA, 2021. [Google Scholar]
- Kelly, C.P. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin. Microbiol. Infect. 2012, 18, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Shields, K.; Araujo-Castillo, R.V.; Theethira, T.G.; Alonso, C.D.; Kelly, C.P. Recurrent Clostridium difficile Infection: From Colonization to Cure. Anaerobe 2015, 34, 59–73. [Google Scholar] [CrossRef]
- Sipos, S.; Vlad, C.; Prejbeanu, R.; Haragus, H.; Vlad, D.; Cristian, H.; Dumitrascu, C.; Popescu, R.; Dumitrascu, V.; Predescu, V. Impact of COVID-19 prevention measures on Clostridioides difficile infections in a regional acute care hospital. Exp. Ther. Med. 2021, 22, 1215. [Google Scholar] [CrossRef]
- Weiner-Lastinger, L.M.; Pattabiraman, V.; Konnor, R.Y.; Patel, P.R.; Wong, E.; Xu, S.Y.; Smith, B.; Edwards, J.R.; Dudeck, M.A. The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections in 2020: A summary of data reported to the National Healthcare Safety Network. Infect. Control Hosp. Epidemiol. 2022, 43, 12–25. [Google Scholar] [CrossRef]
- Evans, M.E.; Simbartl, L.A.; Kralovic, S.M.; Clifton, M.; DeRoos, K.; McCauley, B.P.; Gauldin, N.; Flarida, L.K.; Gamage, S.D.; Jones, M.M.; et al. Healthcare-associated infections in Veterans Affairs acute-care and long-term healthcare facilities during the coronavirus disease 2019 (COVID-19) pandemic. Infect. Control Hosp. Epidemiol. 2023, 44, 420–426. [Google Scholar] [CrossRef]
- Rose, A.N.; Baggs, J.; Kazakova, S.V.; Guh, A.Y.; Yi, S.H.; McCarthy, N.L.; Jernigan, J.A.; Reddy, S.C. Trends in facility-level rates of Clostridioides difficile infections in US hospitals, 2019–2020. Infect. Control Hosp. Epidemiol. 2023, 44, 238–245. [Google Scholar] [CrossRef]
- Nielsen, R.T.; Dalby, T.; Emborg, H.D.; Larsen, A.R.; Petersen, A.; Torpdahl, M.; Hoffmann, S.; Vestergaard, L.S.; Valentiner-Branth, P. COVID-19 preventive measures coincided with a marked decline in other infectious diseases in Denmark, spring 2020. Epidemiol. Infect. 2022, 150, e138. [Google Scholar] [CrossRef]
- Advani, S.D.; Sickbert-Bennett, E.; Moehring, R.; Cromer, A.; Lokhnygina, Y.; Dodds-Ashley, E.; Kalu, I.C.; DiBiase, L.; Weber, D.J.; Anderson, D.J. The Disproportionate Impact of Coronavirus Disease 2019 (COVID-19) Pandemic on Healthcare-Associated Infections in Community Hospitals: Need for Expanding the Infectious Disease Workforce. Clin. Infect. Dis. 2023, 76, e34–e41. [Google Scholar] [CrossRef]
- Hawes, A.M.; Desai, A.; Patel, P.K. Did Clostridioides difficile testing and infection rates change during the COVID-19 pandemic? Anaerobe 2021, 70, 102384. [Google Scholar] [CrossRef] [PubMed]
- Vendrik, K.E.W.; Baktash, A.; Goeman, J.J.; Harmanus, C.; Notermans, D.W.; de Greeff, S.C.; Kuijper, E.J. Comparison of trends in Clostridioides difficile infections in hospitalised patients during the first and second waves of the COVID-19 pandemic: A retrospective sentinel surveillance study. Lancet Reg. Health Eur. 2022, 19, 100424. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Granata, G.; Petrosillo, N.; Al Moghazi, S.; Caraffa, E.; Puro, V.; Tillotson, G.; Cataldo, M.A. The burden of Clostridioides difficile infection in COVID-19 patients: A systematic review and meta-analysis. Anaerobe 2022, 74, 102484. [Google Scholar] [CrossRef] [PubMed]
- Kullar, R.; Johnson, S.; McFarland, L.V.; Goldstein, E.J.C. Potential Roles for Probiotics in the Treatment of COVID-19 Patients wnad Prevention of Complications Associated with Increawed Antibiotic Use. Antibiotics 2021, 10, 408. [Google Scholar] [CrossRef]
- Ochoa-Hein, E.; Rajme-López, S.; Rodríguez-Aldama, J.C.; Huertas-Jiménez, M.A.; Chávez-Ríos, A.R.; de Paz-García, R.; Haro-Osnaya, A.; González-Colín, K.K.; González-González, R.; González-Lara, M.F.; et al. Substantial reduction of healthcare facility-onset Clostridioides difficile infection (HO-CDI) rates after conversion of a hospital for exclusive treatment of COVID-19 patients. Am. J. Infect. Control 2021, 49, 966–968. [Google Scholar] [CrossRef]
- CDC. FluView Interactive: ILI and Viral Surveillance; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2018. [Google Scholar]
- Wiese, A.D.; Everson, J.; Grijalva, C.G. Social Distancing Measures: Evidence of Interruption of Seasonal Influenza Activity and Early Lessons of the SARS-CoV-2 Pandemic. Clin. Infect. Dis. 2021, 73, e141–e143. [Google Scholar] [CrossRef]
- Wee, L.E.I.; Conceicao, E.P.; Tan, J.Y.; Magesparan, K.D.; Amin, I.B.M.; Ismail, B.B.S.; Toh, H.X.; Jin, P.; Zhang, J.; Wee, E.G.L.; et al. Unintended consequences of infection prevention and control measures during COVID-19 pandemic. Am. J. Infect. Control 2021, 49, 469–477. [Google Scholar] [CrossRef]
- Hartnett, K.P.; Kite-Powell, A.; DeVies, J.; Coletta, M.A.; Boehmer, T.K.; Adjemian, J.; Gundlapalli, A.V. Impact of the COVID-19 Pandemic on Emergency Department Visits—United States, 1 January 2019–30 May 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 699–704. [Google Scholar] [CrossRef]
- McCracken, C.E.; Gander, J.C.; McDonald, B.; Goodrich, G.K.; Tavel, H.M.; Basra, S.; Weinfield, N.S.; Ritzwoller, D.P.; Roblin, D.W.; Davis, T.L. Impact of COVID-19 on Trends in Outpatient Clinic Utilization: A Tale of 2 Departments. Med. Care 2023, 61, S4–S11. [Google Scholar] [CrossRef]
- O’Reilly-Shah, V.N.; Van Cleve, W.; Long, D.R.; Moll, V.; Evans, F.M.; Sunshine, J.E.; Kassebaum, N.J.; Harrison, E.M.; Jabaley, C.S. Impact of COVID-19 response on global surgical volumes: An ongoing observational study. Bull. World Health Organ. 2020, 98, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, T.J.; Wilson, M.A.; Chiu, S.T.; Penny, B.W.; Chepuri, V.B.; Waggoner, J.W.; Spinelli, K.J. Case Rates, Treatment Approaches, and Outcomes in Acute Myocardial Infarction During the Coronavirus Disease 2019 Pandemic. JAMA Cardiol. 2020, 5, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A. Antibiotic Resistance (AR), Antibiotic Use (AU), and COVID-19; Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria: Rockville, MD, USA, 2021. [Google Scholar]
- Lee, H.S.; Plechot, K.; Gohil, S.; Le, J. Clostridium difficile: Diagnosis and the Consequence of Over Diagnosis. Infect. Dis. Ther. 2021, 10, 687–697. [Google Scholar] [CrossRef]
- Gentry, C.A.; Williams, R.J., 2nd; Campbell, D. Continued decline in the prevalence of the Clostridioides difficile BI/NAP1/027 strain across the United States Veterans Health Administration. Diagn. Microbiol. Infect. Dis. 2021, 100, 115308. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, I.; Johnson, S.; Sambol, S.P.; Goldstein, E.J.; Citron, D.M.; Gerding, D.N. Relapse versus reinfection: Recurrent Clostridium difficile infection following treatment with fidaxomicin or vancomycin. Clin. Infect. Dis. 2012, 55 (Suppl. S2), S104–S109. [Google Scholar] [CrossRef] [PubMed]
- Petrella, L.A.; Sambol, S.P.; Cheknis, A.; Nagaro, K.; Kean, Y.; Sears, P.S.; Babakhani, F.; Johnson, S.; Gerding, D.N. Decreased cure and increased recurrence rates for Clostridium difficile infection caused by the epidemic C. difficile BI strain. Clin. Infect. Dis. 2012, 55, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Kociolek, L.K.; Perdue, E.R.; Fawley, W.N.; Wilcox, M.H.; Gerding, D.N.; Johnson, S. Correlation between restriction endonuclease analysis and PCR ribotyping for the identification of Clostridioides (Clostridium) difficile clinical strains. Anaerobe 2018, 54, 1–7. [Google Scholar] [CrossRef]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, 987–994. [Google Scholar] [CrossRef]
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin. Infect. Dis. 2021, 73, 755–757. [Google Scholar] [CrossRef]
- CDC. Multidrug-Resistant Organisms and Clostridioides difficile Infection (MDRO/CDI) Module; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2023. [Google Scholar]
- Kociolek, L.K.; Patel, S.J.; Shulman, S.T.; Gerding, D.N. Molecular epidemiology of Clostridium difficile infections in children: A retrospective cohort study. Infect. Control Hosp. Epidemiol. 2015, 36, 445–451. [Google Scholar] [CrossRef]
- Clabots, C.R.; Johnson, S.; Bettin, K.M.; Mathie, P.A.; Mulligan, M.E.; Schaberg, D.R.; Peterson, L.R.; Gerding, D.N. Development of a rapid and efficient restriction endonuclease analysis typing system for Clostridium difficile and correlation with other typing systems. J. Clin. Microbiol. 1993, 31, 1870–1875. [Google Scholar] [CrossRef] [PubMed]
- Manzo, C.E.; Merrigan, M.M.; Johnson, S.; Gerding, D.N.; Riley, T.V.; Silva, J., Jr.; Brazier, J.S. International typing study of Clostridium difficile. Anaerobe 2014, 28, 4–7. [Google Scholar] [CrossRef] [PubMed]

| Pre-Pandemic (2015–2019) | First Pandemic Year (2020) | p-Value * | Second Pandemic Year (2021) | p-Value † | Pandemic Years (2020–2021) | p-Value ‡ | |
|---|---|---|---|---|---|---|---|
| Mean Monthly NAAT 1+ Tests (95% CI; n) | 15.45 (14.53–16.37; n = 927) | 9.33 (7.79–10.87; n = 112) | <0.01 | 10.67 (9.06–12.28; n = 128) | <0.01 | 10.00 (8.94–11.06; n = 240) | <0.01 |
| Mean Monthly Total CDI 2 Cases (95% CI; n) | 15.12 (14.20–16.03; n = 907) | 8.17 (6.90–9.43; n = 98) | <0.01 | 9.00 (7.54–10.46; n = 108) | <0.01 | 8.58 (7.68–9.49; n = 206) | <0.01 |
| Mean Monthly rCDI 3 Cases (95% CI; n) | 3.38 (2.89–3.87; n = 203) | 1.25 (0.53–1.97; n = 15) | <0.01 | 2.58 (1.55–3.61; n = 31) | 0.18 | 1.92 (1.27–2.56; n = 46) | <0.01 |
| No. Total NAAT+ CD 4 Stool Tests in 2018–2021 (Total Stool Tests Run; %NAAT+) | 393 (2287; 17.2%) | 112 (789; 14.2%) | 0.05 | 128 (892; 14.4%) | 0.05 | 240 (1681; 14.3%) | 0.01 |
| Mean Monthly CD Colonization (95% CI; n) | 0.33 (0.15–0.52; n = 20) | 1.17 (0.51–1.82; n = 14) | <0.01 | 1.67 (0.98–2.35; n = 20) | <0.01 | 1.42 (0.97–1.86; n = 34) | <0.01 |
| All Encounters | Pre-Pandemic (1 January 2019 – 7 March 2020) | Initial Pandemic Wave (8 March 2020 – 30 June 2020) | Post-Initial Wave (1 July 2020 – 31 March 2022 | p-Value | |
|---|---|---|---|---|---|
| Case Characteristics | (n = 327) | (n = 82) | (n = 32) | (n = 213) | |
| Median Age (IQR 1) | 73 (67–78) | 73.0 (67–82) | 73.0 (68–82) | 73.0 (67–77) | 0.96 * |
| No. Male (%) | 316 (96.6) | 77 (93.9) | 31 (96.9) | 208 (97.7) | 0.28 † |
| No. Immunocompromised (%) | 63 (19.3) | 9 (11.0) | 7 (21.9) | 47 (22.2) | 0.09 † |
| No. PPI 2 (%) | 192 (58.9) | 45 (55.0) | 19 (59.4) | 128 (60.4) | 0.69 † |
| Median No. Days Prior Antibiotics Exposure (IQR) | 14 (3–29) | 15 (3–35) | 19 (6–36) | 14 (2–27) | 0.24 * |
| Mean Temperature, C (95% CI) | 36.8 (36.7–36.8) | 36.8 (36.6–37.0) | 37.3 (36.9–37.7) | 36.7 (36.6–36.7) | <0.01 ‡ |
| Mean WBC 3 (95% CI) | 10.5 (9.8–11.2) | 11.3 (9.7–12.8) | 8.7 (7.3–10.0) | 10.5 (9.6–11.4) | 0.17 ‡ |
| Mean Creatinine (95% CI) | 2.0 (1.8–2.3) | 1.7 (1.4–2.0) | 1.4 (1.1–1.8) | 2.3 (1.9–2.6) | 0.05 ‡ |
| Mean Albumin (95% CI) | 2.6 (2.5–2.7) | 2.4 (2.2–2.6) | 2.7 (2.5–2.9) | 2.6 (2.5–2.8) | 0.14 ‡ |
| REA 4 Strain Typing | (n = 159) | (n = 44) | (n = 15) | (n = 100) | |
| No. REA Group Y (RT 5 014/020) (%) | 32 (20.1) | 12 (27.3) | 1 (6.7) | 19 (19.0) | 0.21 † |
| No. REA Group BI (RT 027) (%) | 19 (12.0) | 4 (9.1) | 4 (26.7) | 11 (11.0) | 0.17 † |
| No. REA Group DH (RT 106) (%) | 20 (12.6) | 3 (6.8) | 2 (13.3) | 15 (15.0) | 0.39 † |
| Other REA Groups (%) | 88 (55.4) | 25 (56.8) | 8 (53.3) | 55 (55.0) | 0.97 † |
| First Pandemic Wave Compared to Pre-Pandemic Period * | |
|---|---|
| Adjusted Odds Ratio (95% CI 1) | |
| REA 2 Group BI (RT 027) | 6.41 (1.03–39.91) |
| REA Group DH (RT 106) | 2.87 (0.35–23.27) |
| REA Group Y (RT 014/020) | 0.13 (0.01–1.26) |
| Post First Pandemic Wave Compared to First Pandemic Wave † | |
| REA Group BI (RT 027) | 0.20 (0.04–0.99) |
| REA Group DH (RT 106) | 0.35 (0.04–2.82) |
| REA Group Y (RT 014/020) | 3.56 (0.41–31.43) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wright, L.M.; Skinner, A.M.; Cheknis, A.; McBurney, C.; Ge, L.; Pacheco, S.M.; Leehey, D.; Gerding, D.N.; Johnson, S. Effect of the COVID-19 Pandemic on Rates and Epidemiology of Clostridioides difficile Infection in One VA Hospital. Antibiotics 2023, 12, 1159. https://doi.org/10.3390/antibiotics12071159
Wright LM, Skinner AM, Cheknis A, McBurney C, Ge L, Pacheco SM, Leehey D, Gerding DN, Johnson S. Effect of the COVID-19 Pandemic on Rates and Epidemiology of Clostridioides difficile Infection in One VA Hospital. Antibiotics. 2023; 12(7):1159. https://doi.org/10.3390/antibiotics12071159
Chicago/Turabian StyleWright, Lorinda M., Andrew M. Skinner, Adam Cheknis, Conor McBurney, Ling Ge, Susan M. Pacheco, David Leehey, Dale N. Gerding, and Stuart Johnson. 2023. "Effect of the COVID-19 Pandemic on Rates and Epidemiology of Clostridioides difficile Infection in One VA Hospital" Antibiotics 12, no. 7: 1159. https://doi.org/10.3390/antibiotics12071159
APA StyleWright, L. M., Skinner, A. M., Cheknis, A., McBurney, C., Ge, L., Pacheco, S. M., Leehey, D., Gerding, D. N., & Johnson, S. (2023). Effect of the COVID-19 Pandemic on Rates and Epidemiology of Clostridioides difficile Infection in One VA Hospital. Antibiotics, 12(7), 1159. https://doi.org/10.3390/antibiotics12071159






