Purification, Conformational Analysis and Cytotoxic Activities of Host-Defense Peptides from the Giant Gladiator Treefrog Boana boans (Hylidae: Hylinae)
Abstract
1. Introduction
2. Results
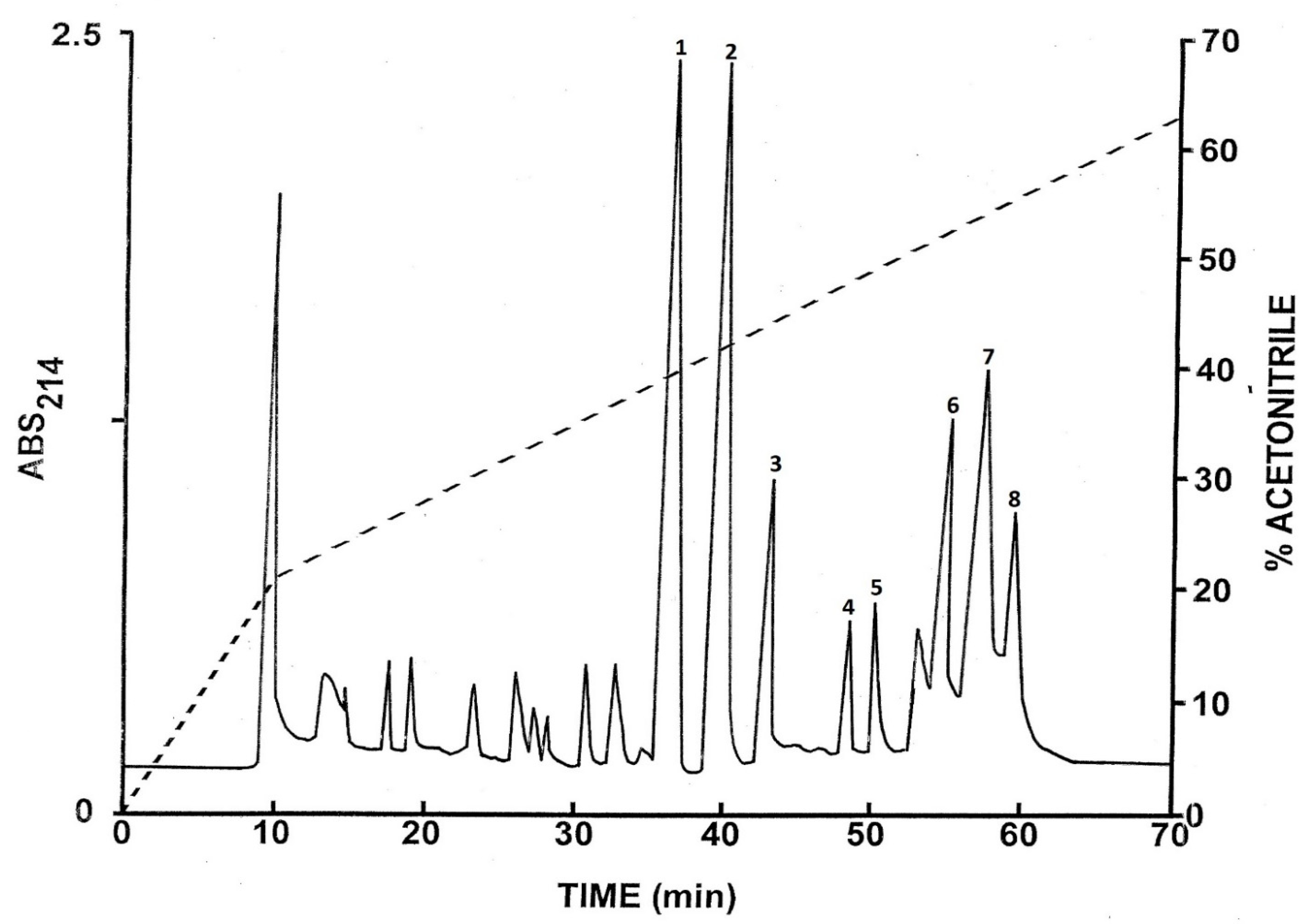
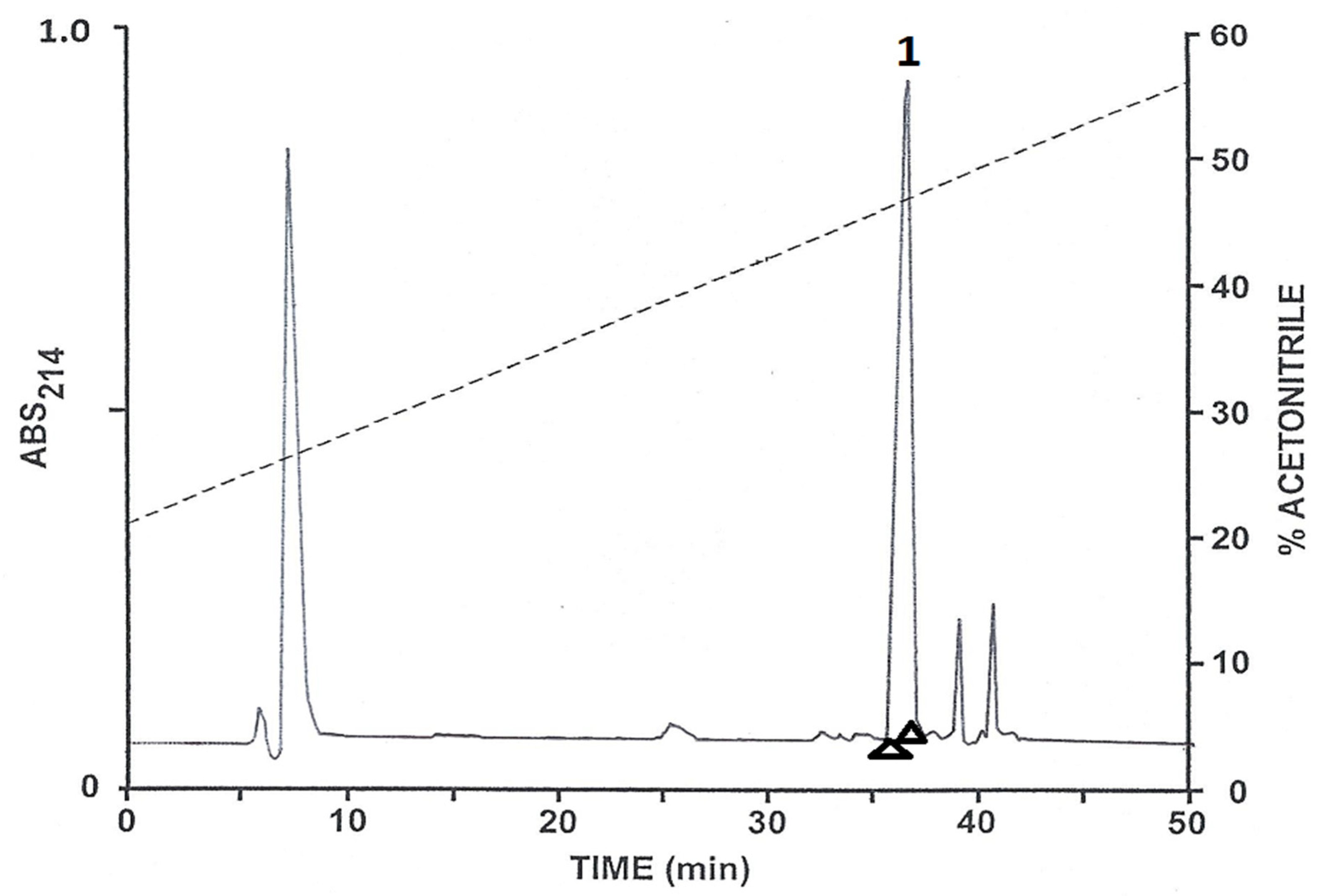
2.1. Purification of the Peptides
2.2. Structural Characterization
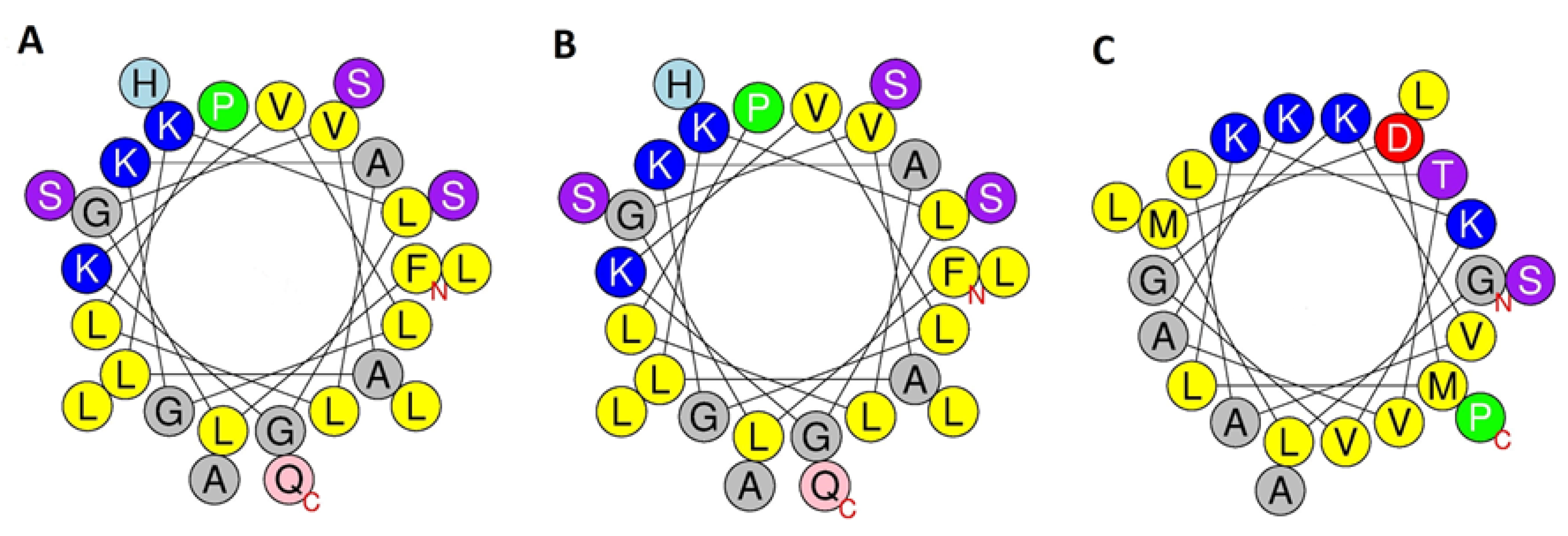
2.3. Conformational Analysis
2.4. Antimicrobial Activities
2.5. Cytotoxic Activities
3. Discussion
4. Materials and Methods
4.1. Collection of Skin Secretions
4.2. Purification of the Peptides
4.3. Structural Characterization
4.4. Peptide Synthesis
4.5. Conformational Analysis
4.6. Antimicrobial Assays
4.7. Cytotoxicity Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frost, D.R. Amphibian Species of the World; An Online Reference; Version 6.1.; American Museum of Natural History: New York, NY, USA, 2023; Available online: https://amphibiansoftheworld.amnh.org/index.php (accessed on 9 May 2023).
- Jackway, R.J.; Pukala, T.L.; Donnellan, S.C.; Sherman, P.J.; Tyler, M.J.; Bowie, J.H. Skin peptide and cDNA profiling of Australian anurans: Genus and species identification and evolutionary trends. Peptides 2011, 32, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Bartels, E.J.H.; Dekker, D.; Amiche, M. Dermaseptins, multifunctional antimicrobial peptides: A review of their pharmacology, effectivity, mechanism of action, and possible future directions. Front. Pharmacol. 2019, 10, 1421. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.S.; Ferreira, T.C.; Cilli, E.M.; Crusca, E., Jr.; Mendes-Giannini, M.J.; Sebben, A.; Ricart, C.A.; Sousa, M.V.; Fontes, W. Hylin a1, the first cytolytic peptide isolated from the arboreal South American frog Hypsiboas albopunctatus (“spotted treefrog”). Peptides 2009, 30, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.S.; Matsushita, R.H.; Sebben, A.; Sousa, M.V.; Fontes, W. Hylins: Bombinins H structurally related peptides from the skin secretion of the Brazilian tree-frog Hyla biobeba. Protein Pept. Lett. 2005, 12, 89–93. [Google Scholar] [CrossRef]
- Brunetti, A.E.; Bunk, B.; Lyra, M.L.; Fuzo, C.A.; Marani, M.M.; Spröer, C.; Haddad, C.F.B.; Lopes, N.P.; Overmann, J. Molecular basis of a bacterial-amphibian symbiosis revealed by comparative genomics, modelling, and functional testing. ISME J. 2022, 16, 788–800. [Google Scholar] [CrossRef]
- Aguilar, S.; Brunetti, A.E.; Garay, A.V.; Santos, L.C.; Perez, L.O.; Moreira, D.; Cancelarich, N.L.; Barbosa, E.A.; Basso, N.G.; de Freitas, S.M.; et al. Structure and function of cationic hylin bioactive peptides from the tree frog Boana pulchella in interaction with lipid membranes. Peptides 2023, 159, 170900. [Google Scholar] [CrossRef]
- Magalhães, B.S.; Melo, J.A.; Leite, J.R.; Silva, L.P.; Prates, M.V.; Vinecky, F.; Barbosa, E.A.; Verly, R.M.; Mehta, A.; Nicoli, J.R.; et al. Post-secretory events alter the peptide content of the skin secretion of Hypsiboas raniceps. Biochem. Biophys. Res. Commun. 2008, 377, 1057–1061. [Google Scholar] [CrossRef]
- Santana, C.J.C.; Magalhães, A.C.M.; Dos Santos Júnior, A.C.M.; Ricart, C.A.O.; Lima, B.D.; Álvares, A.D.C.M.; Freitas, S.M.; Luz, I.S.; Pires, O.R., Jr.; Fontes, W.; et al. Figainin 1, a novel amphibian skin peptide with antimicrobial and antiproliferative properties. Antibiotics 2020, 9, 625. [Google Scholar] [CrossRef]
- Santana, C.J.C.; Magalhães, A.C.M.; Prías-Márquez, C.A.; Falico, D.A.; Dos Santos Júnior, A.C.M.; Lima, B.D.; Ricart, C.A.O.; de Pilger, D.R.B.; Bonotto, R.M.; Moraes, C.B.; et al. Biological properties of a novel multifunctional host defense peptide from the skin secretion of the Chaco tree frog, Boana raniceps. Biomolecules 2020, 10, 790. [Google Scholar] [CrossRef]
- Morán-Marcillo, G.; Sánchez Hinojosa, V.; de Los Monteros-Silva, N.E.; Blasco-Zúñiga, A.; Rivera, M.; Naranjo, R.E.; Almeida, J.R.; Wang, L.; Zhou, M.; Chen, T.; et al. Picturins and Pictuseptins, two novel antimicrobial peptide families from the skin secretions of the Chachi treefrog, Boana picturata. J. Proteom. 2022, 264, 104633. [Google Scholar] [CrossRef]
- Hedges, S.B.; Powell, R.; Henderson, R.W.; Hanson, S.; Murphy, J.C. Definition of the Caribbean Islands biogeogaphic region, with checklist and recommendations for standardized common names of amphibians and reptiles. Caribb. Herpetol. 2019, 67, 1–53. [Google Scholar] [CrossRef]
- Murphy, J.C.; Downie, J.R.; Smith, J.M.; Livingstone, S.R.; Mohammed, R.S.; Lehtinen, R.M.; Eyre, M.; Sewlal, J.; Noriega, N.; Caspar, G.S.; et al. A Field Guide to the Amphibians and Reptiles of Trinidad and Tobago; Trinidad and Tobago Field Naturalists’ Club: Port of Spain, Trinidad and Tobago, 2018; p. 336. ISBN 978-976-8255-47-1. [Google Scholar]
- La Marca, E.; Azevedo-Ramos, C.; Coloma, L.A.; Solís, F.; Ibáñez, R.; Jaramillo, C.; Fuenmayor, Q.; Ron, S.; Hardy, J. Hypsiboas boans. The IUCN Red List of Threatened Species. Version 2022-2. Available online: https://iucnredlist.org (accessed on 12 May 2023).
- Montecucchi, P.C. Isolation and primary structure determination of amphibian skin tryptophyllins. Peptides 1985, 6 (Suppl. 3), 187–195. [Google Scholar] [CrossRef]
- Fauchère, J.L.; Charton, M.; Kier, L.B.; Verloop, A.; Pliska, V. Amino acid side chain parameters for correlation studies in biology and pharmacology. Int. J. Pept. Protein Res. 1988, 32, 269–278. [Google Scholar] [CrossRef]
- Eisenberg, D.; Weiss, R.M.; Terwilliger, T.C. The helical hydrophobic moment: A measure of the amphiphilicity of a helix. Nature 1982, 299, 371–374. [Google Scholar] [CrossRef]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A web server to screen sequences with specific α-helical properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef]
- Singh, H.; Singh, S.; Raghava, G.P.S. Peptide secondary structure prediction using evolutionary information. bioRxiv 2019, 558791. [Google Scholar] [CrossRef]
- Xu, X.; Lai, R. The chemistry and biological activities of peptides from amphibian skin secretions. Chem. Rev. 2015, 115, 1760–1846. [Google Scholar] [CrossRef]
- Conlon, J.M.; Mechkarska, M.; Leprince, J. Peptidomic analysis in the discovery of therapeutically valuable peptides in amphibian skin secretions. Expert Rev. Proteom. 2019, 16, 897–908. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B.A. DICHROWEB: An online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004, 32, W668–W673. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B.A. Protein secondary structure analyses from circular dichroism spectroscopy: Methods and reference databases. Biopolymers 2008, 89, 392–400. [Google Scholar] [CrossRef]
- Miles, A.J.; Ramalli, S.G.; Wallace, B.A. DichroWeb, a website for calculating protein secondary structure from circular dichroism spectroscopic data. Protein Sci. 2022, 31, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Forood, B.; Filiciano, E.J.; Niambiar, K.P. Stabilization of α-helical structures in short peptides via end capping. Proc. Natl. Acad. Sci. USA 1993, 90, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Ladram, A.; Nicolas, P. Antimicrobial peptides from frog skin: Biodiversity and therapeutic promises. Front. Biosci. 2016, 21, 1341–1371. [Google Scholar] [CrossRef] [PubMed]
- Vineeth Kumar, T.V.; Sanil, G. A review of the mechanism of action of amphibian antimicrobial peptides focusing on peptide-membrane interaction and membrane curvature. Curr. Protein Pept. Sci. 2017, 18, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Zan, B.; Ulmschneider, J.P.; Wimley, W.C.; Lu, T.K.; Ulmschneider, M.B.; Zhou, L. Development of membrane-active peptide therapeutics in oncology. J. Pept. Sci. 2023, 29, e3482. [Google Scholar] [CrossRef] [PubMed]
- Attoub, S.; Arafat, H.; Mechkarska, M.; Conlon, J.M. Anti-tumor activities of the host-defense peptide hymenochirin-1B. Regul. Pept. 2013, 187, 51–56. [Google Scholar] [CrossRef]
- Bowie, J.H.; Separovic, F.; Tyler, M.J. Host-defense peptides of Australian anurans. Part 2. Structure, activity, mechanism of action, and evolutionary significance. Peptides 2012, 37, 174–188. [Google Scholar] [CrossRef]
- Conlon, J.M.; Jouenne, T.; Cosette, P.; Cosquer, D.; Vaudry, H.; Taylor, C.K.; Abel, P.W. Bradykinin-related peptides and tryptophyllins in the skin secretions of the most primitive extant frog, Ascaphus truei. Gen. Comp. Endocrinol. 2005, 143, 193–199. [Google Scholar] [CrossRef]
- Dematei, A.; Costa, S.R.; Moreira, D.C.; Barbosa, E.A.; Friaça Albuquerque, L.F.; Vasconcelos, A.G.; Nascimento, T.; Silva, P.C.; Silva-Carvalho, A.É.; Saldanha-Araújo, F.; et al. Antioxidant and neuroprotective effects of the first tryptophyllin found in snake venom (Bothrops moojeni). J. Nat. Prod. 2022, 85, 2695–2705. [Google Scholar] [CrossRef]
- Jackway, R.J.; Maselli, V.M.; Musgrave, I.F.; Maclean, M.J.; Tyler, M.J.; Bowie, J.H. Skin peptides from anurans of the Litoria rubella group: Sequence determination using electrospray mass spectrometry. Opioid activity of two major peptides. Rapid Commun. Mass Spectrom. 2009, 23, 1189–1195. [Google Scholar] [CrossRef]
- Wang, R.; Lin, Y.; Chen, T.; Zhou, M.; Wang, L.; Shaw, C. Molecular cloning of a novel tryptophyllin peptide from the skin of the orange-legged monkey frog, Phyllomedusa hypochondrialis. Chem. Biol. Drug Des. 2014, 83, 731–740. [Google Scholar] [CrossRef]
- Tran, T.T.N.; Tran, D.P.; Nguyen, V.C.; Tran, T.D.T.; Bui, T.T.T.; Bowie, J.H. Antioxidant activities of major tryptophyllin L peptides: A joint investigation of Gaussian-based 3D-QSAR and radical scavenging experiments. J. Pept. Sci. 2021, 27, e3295. [Google Scholar] [CrossRef]
- Barran, G.; Kolodziejek, J.; Coquet, L.; Leprince, J.; Jouenne, T.; Nowotny, N.; Conlon, J.M.; Mechkarska, M. Peptidomic analysis of skin secretions of the Caribbean frogs Leptodactylus insularum and Leptodactylus nesiotus (Leptodactylidae) identifies an ocellatin with broad spectrum antimicrobial activity. Antibiotics 2020, 9, 718. [Google Scholar] [CrossRef]
- Conlon, J.M.; Moffett, R.C.; Leprince, J.; Flatt, P.R. Identification of components in frog skin secretions with therapeutic potential as antidiabetic agents. Methods Mol. Biol. 2018, 1719, 319–333. [Google Scholar] [CrossRef]
- Pantic, J.; Guilhaudis, L.; Musale, V.; Attoub, S.; Lukic, M.L.; Mechkarska, M.; Conlon, J.M. Immunomodulatory, insulinotropic, and cytotoxic activities of phylloseptins and plasticin-TR from the Trinidadian leaf frog Phyllomedusa trinitatis. J. Pept. Sci. 2019, 25, e3153. [Google Scholar] [CrossRef]
- Provencher, S.W.; Glockner, J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry 1981, 20, 33–37. [Google Scholar] [CrossRef]
- Van Stokkum, I.H.M.; Spoelder, H.J.W.; Bloemendal, M.; Van Grondelle, R.; Groen, F.C.A. Estimation of protein secondary structure and error analysis from CD spectra. Anal. Biochem. 1990, 191, 110–118. [Google Scholar] [CrossRef]
- Compton, L.A.; Johnson, W.C., Jr. Analysis of protein circular dichroism spectra for secondary structure using a simple matrix multiplication. Anal. Biochem. 1986, 155, 155–167. [Google Scholar] [CrossRef]
- Sreerema, N.; Woody, R.W. A self-consistent method for the analysis of protein secondary structure from circular dichroism. Anal. Biochem. 1993, 209, 32–44. [Google Scholar] [CrossRef]
- Sreerama, N.; Woody, R.W. Estimation of protein secondary structure from CD spectra: Comparison of CONTIN, SELCON and CDSSTR methods with an expanded reference set. Anal. Biochem. 2000, 287, 252–260. [Google Scholar] [CrossRef]
- Sreerama, N.; Venyaminov, S.Y.; Woody, R.W. Estimation of the number of helical and strand segments in proteins using CD spectroscopy. Protein Sci. 1999, 8, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Clinical Laboratory and Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Approved Standard M07; CLSI: Wayne, PA, USA, 2019. [Google Scholar]
- Manzo, G.; Scorciapino, M.A.; Srinivasan, D.; Attoub, S.; Mangoni, M.L.; Rinaldi, A.C.; Casu, M.; Flatt, P.R.; Conlon, J.M. Conformational analysis of the host-defense peptides Pseudhymenochirin-1Pb and -2Pa and design of analogues with insulin-releasing activities and reduced toxicities. J. Nat. Prod. 2015, 78, 3041–30488. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Al-Ghaferi, N.; Abraham, B.; Leprince, J. Strategies for transformation of naturally-occurring amphibian antimicrobial peptides into therapeutically valuable anti-infective agents. Methods 2007, 35, 349–357. [Google Scholar] [CrossRef] [PubMed]



| Peptide | Primary Structure | [MH+]exp | [MH+]calc |
|---|---|---|---|
| Tryptophyllin BN | WRPFPFL.NH2 | 961.5 | 961.5 |
| Tryptophyllin BN non-amidated | WRPFPFL | 962.5 | 962.5 |
| Figainin 2BN | FLGVALKLGKVLGKALLPLASSLLHSQ | 2773.8 | 2773.7 |
| [Q27E]figainin 2BN | FLGVALKLGKVLGKALLPLASSLLHSE | 2774.8 | 2774.7 |
| Picturin 1BN | GIFKDTLKKVVAAVLTTVADNIHPK | 2678.7 | 2678.6 |
| [N21D]picturin 1BN | GIFKDTLKKVVAAVLTTVADDIHPK | 2679.6 | 2679.6 |
| Picturin 2BN | GLMDMLKKVGKVALTVAKSALLP | 2383.4 | 2383.4 |
| Peptide | Charge at pH 7.0 | Hydrophobicity | Hydrophobic Moment | Predicted Helical Domain |
|---|---|---|---|---|
| Figainin 2BN | +3 | 0.667 | 0.348 | 4–25 |
| [Q27E]figainin 2BN | +2 | 0.651 | 0.337 | 4–25 |
| Picturin 1BN | +2 | 0.405 | 0.250 | 5–19 |
| [N21D]picturin 1BN | +1 | 0.398 | 0.244 | 5–19 |
| Picturin 2BN | +3 | 0.511 | 0.342 | 3–19 |
| Peptide | Medium | Method | Helix | β Sheet | Turns | Random |
|---|---|---|---|---|---|---|
| Figainin 2BN | water | Dichroweb | 2 | 21 | 10 | 33 |
| [θ]222 | 1 | - | - | - | ||
| 25% TFE | Dichroweb | 35 | 18 | 20 | 28 | |
| [θ]222 | 27 | - | - | - | ||
| 50% TFE | Dichroweb | 40 | 17 | 17 | 27 | |
| [θ]222 | 33 | - | - | - | ||
| 20 mM DPC | Dichroweb | 41 | 9 | 15 | 22 | |
| [θ]222 | 42 | - | - | - | ||
| 10 mM SDS | Dichroweb | 49 | 9 | 14 | 26 | |
| [θ]222 | 38 | |||||
| Picturin 1BN | water | Dichroweb | 2 | 4 | 2 | 89 |
| [θ]222 | 6 | - | - | - | ||
| 25% TFE | Dichroweb | 61 | 7 | 12 | 22 | |
| [θ]222 | 58 | - | - | - | ||
| 50% TFE | Dichroweb | 64 | 3 | 11 | 24 | |
| [θ]222 | 59 | - | - | - | ||
| 20 mM DPC | Dichroweb | 69 | 0 | 10 | 23 | |
| [θ]222 | 61 | - | - | - | ||
| 10 mM SDS | Dichroweb | 65 | 3 | 9 | 22 | |
| [θ]222 | 57 | - | - | - |
| Microrganism | Figainin 2BN | Picturin 1BN | Picturin 2BN |
|---|---|---|---|
| Gram-positive | |||
| S. aureus (ATCC 12600) | 7.8 | 62.5 | 31.3 |
| E. faecium (ATCC 19439) | 31.3 | ND | ND |
| E. faecalis (ATCC 51299) | 125 | ND | ND |
| Gram-negative | |||
| E. coli (ATCC 35218) | 15.6 | 7.8 | 7.8 |
| K. pneumoniae (ATCC 49472) | 31.3 | 15.6 | 15.6 |
| P. aeruginosa (ATCC 9072) | 31.3 | 15.6 | 15.6 |
| Cells | Figainin 2BN | Picturin 1BN | Picturin 2BN |
|---|---|---|---|
| A549 | 6.7 ± 0.4 | 30.7 ± 0.6 | 23.0 ± 0.7 |
| MDA-MB-231 | 8.1 ± 0.5 | 64.2 ± 0.9 | 26.3 ± 1.9 |
| HT-29 | 14.1 ± 0.8 | 84.9 ± 4.3 | 52.2 ± 3.1 |
| HUVEC | 15.1 ± 0.5 | 54.0 ± 1.2 | 53.3 ± 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conlon, J.M.; Guilhaudis, L.; Attoub, S.; Coquet, L.; Leprince, J.; Jouenne, T.; Mechkarska, M. Purification, Conformational Analysis and Cytotoxic Activities of Host-Defense Peptides from the Giant Gladiator Treefrog Boana boans (Hylidae: Hylinae). Antibiotics 2023, 12, 1102. https://doi.org/10.3390/antibiotics12071102
Conlon JM, Guilhaudis L, Attoub S, Coquet L, Leprince J, Jouenne T, Mechkarska M. Purification, Conformational Analysis and Cytotoxic Activities of Host-Defense Peptides from the Giant Gladiator Treefrog Boana boans (Hylidae: Hylinae). Antibiotics. 2023; 12(7):1102. https://doi.org/10.3390/antibiotics12071102
Chicago/Turabian StyleConlon, J. Michael, Laure Guilhaudis, Samir Attoub, Laurent Coquet, Jérôme Leprince, Thierry Jouenne, and Milena Mechkarska. 2023. "Purification, Conformational Analysis and Cytotoxic Activities of Host-Defense Peptides from the Giant Gladiator Treefrog Boana boans (Hylidae: Hylinae)" Antibiotics 12, no. 7: 1102. https://doi.org/10.3390/antibiotics12071102
APA StyleConlon, J. M., Guilhaudis, L., Attoub, S., Coquet, L., Leprince, J., Jouenne, T., & Mechkarska, M. (2023). Purification, Conformational Analysis and Cytotoxic Activities of Host-Defense Peptides from the Giant Gladiator Treefrog Boana boans (Hylidae: Hylinae). Antibiotics, 12(7), 1102. https://doi.org/10.3390/antibiotics12071102








