Isavuconazole Exposure in Critically Ill Patients Treated with Extracorporeal Membrane Oxygenation: Two Case Reports and a Narrative Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Sample Collection and Bioanalysis
2.3. Pharmacokinetic Analysis
2.4. Narrative Literature Review
2.5. Ethics
3. Results
3.1. Case Descriptions
3.1.1. Case 1
3.1.2. Case 2
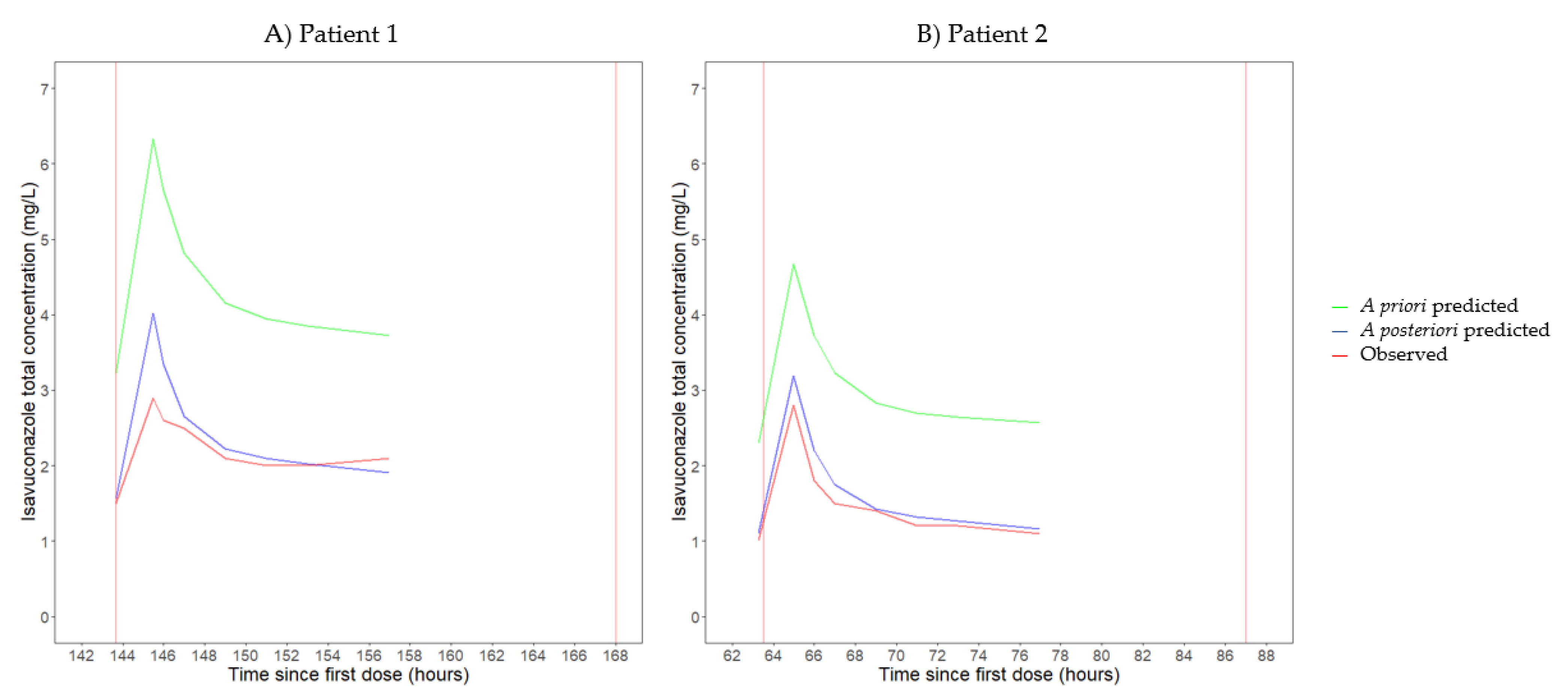
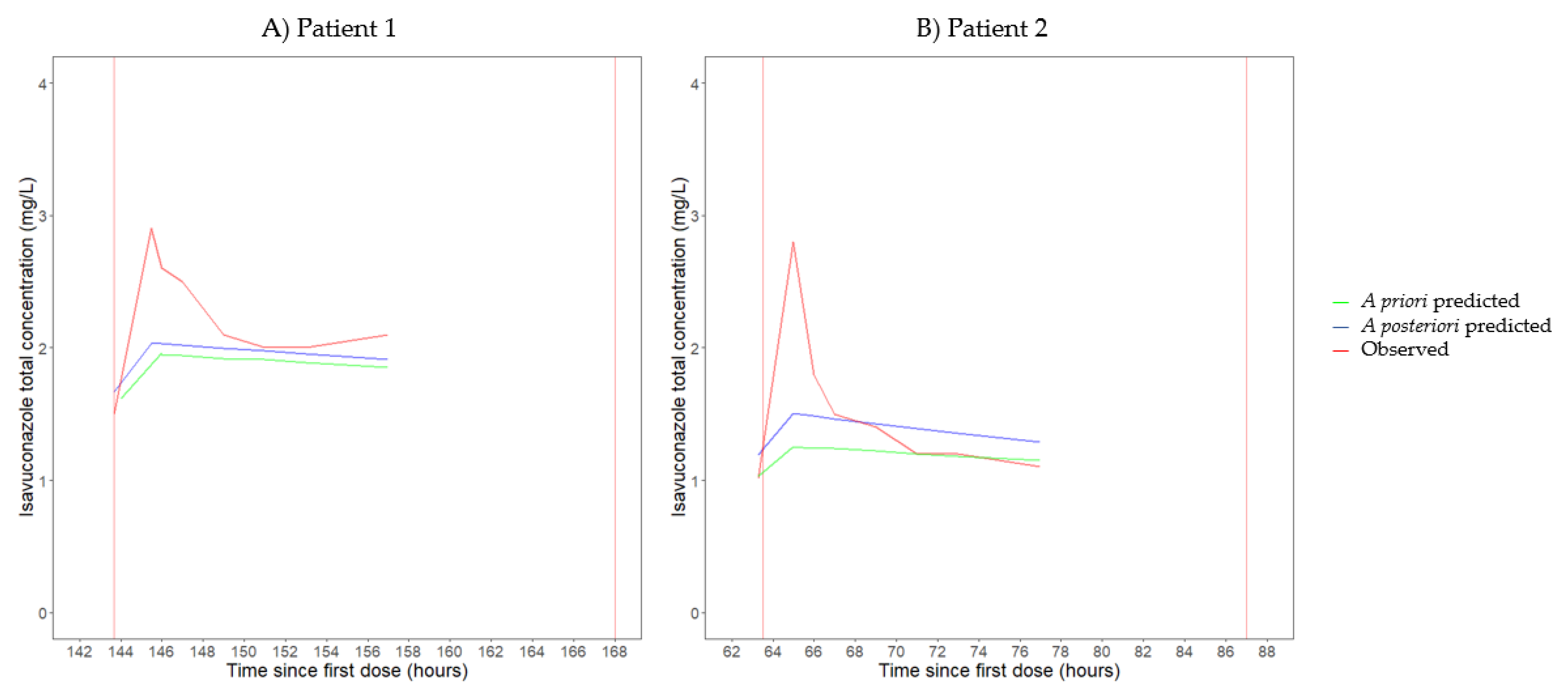
3.2. Pharmacokinetics
3.3. Literature Review
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patterson, T.F.; Thompson, G.R., III; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Executive summary: Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, 433–442. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24, e1–e38. [Google Scholar] [CrossRef]
- Verweij, P.E.; Rijnders, B.J.A.; Brüggemann, R.J.M.; Azoulay, E.; Bassetti, M.; Blot, S.; Calandra, T.; Clancy, C.J.; Cornely, O.A.; Chiller, T.; et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: An expert opinion. Intensive Care Med. 2020, 46, 1524–1535. [Google Scholar] [CrossRef]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef] [PubMed]
- Bellmann, R.; Smuszkiewicz, P. Pharmacokinetics of antifungal drugs: Practical implications for optimized treatment of patients. Infection 2017, 45, 737–779. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Vfend: European Public Assessment Report. Available online: https://www.ema.europa.eu/en/documents/product-information/vfend-epar-product-information_en.pdf (accessed on 5 May 2023).
- European Medicines Agency. Cresemba: European Public Assessment Report. Available online: https://www.ema.europa.eu/en/documents/product-information/cresemba-epar-product-information_en.pdf (accessed on 5 May 2023).
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A position paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.A.; Raad, I.I.; Marr, K.A.; Patterson, T.F.; Kontoyiannis, D.P.; Cornely, O.A.; Bow, E.J.; Rahav, G.; Neofytos, D.; Aoun, M.; et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A phase 3, randomised-controlled, non-inferiority trial. Lancet 2016, 387, 760–769. [Google Scholar] [CrossRef]
- Lewis, R.; Brüggemann, R.; Padoin, C.; Maertens, J.; Marchetti, O.; Groll, A.; Johnson, E.; Arendrup, M. Triazole antifungal therapeutic drug monitoring. In Proceedings of the 6th European Conference Infections in Leukemia Meeting, Sophia Antipolis, France, 11–12 September 2015; Available online: https://www.ebmt.org/sites/default/files/migration_legacy_files/document/ECIL%206-Triazole-TDM-07-12-2015-Lewis-R-et-al.pdf (accessed on 3 February 2023).
- Kaindl, T.; Andes, D.; Engelhardt, M.; Saulay, M.; Larger, P.; Groll, A.H. Variability and exposure-response relationships of isavuconazole plasma concentrations in the phase 3 SECURE trial of patients with invasive mould diseases. J. Antimicrob. Chemother. 2019, 74, 761–767. [Google Scholar] [CrossRef]
- Desai, A.V.; Kovanda, L.L.; Hope, W.W.; Andes, D.; Mouton, J.W.; Kowalski, D.L.; Townsend, R.W.; Mujais, S.; Bonate, P.L. Exposure-response relationships for isavuconazole in patients with invasive aspergillosis and other filamentous fungi. Antimicrob. Agents Chemother. 2017, 61, e01034-17. [Google Scholar] [CrossRef]
- Zurl, C.; Waller, M.; Schwameis, F.; Muhr, T.; Bauer, N.; Zollner-Schwetz, I.; Valentin, T.; Meinitzer, A.; Ullrich, E.; Wunsch, S.; et al. Isavuconazole treatment in a mixed patient cohort with invasive fungal infections: Outcome, tolerability and clinical implications of isavuconazole plasma concentrations. J. Fungi 2020, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Risum, M.; Vestergaard, M.B.; Weinreich, U.M.; Helleberg, M.; Vissing, N.H.; Jørgensen, R. Therapeutic drug monitoring of isavuconazole: Serum concentration variability and success rates for reaching target in comparison with voriconazole. Antibiotics 2021, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Höhl, R.; Bertram, R.; Kinzig, M.; Haarmeyer, G.S.; Baumgärtel, M.; Geise, A.; Muschner, D.; Prosch, D.; Reger, M.; Naumann, H.T.; et al. Isavuconazole therapeutic drug monitoring in critically ill ICU patients: A monocentric retrospective analysis. Mycoses 2022, 65, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.; Corne, P.; Pasquier, G.; Konecki, C.; Sadek, M.; Le Bihan, C.; Klouche, K.; Mathieu, O.; Reynes, J.; Cazaubon, Y. Population pharmacokinetics of isavuconazole in critical care patients with COVID-19-associated pulmonary aspergillosis and Monte Carlo simulations of high off-label doses. J. Fungi 2023, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing. Clinical Breakpoints of Fungi, Version 10. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/AFST_BP_v10.0_200204_updatd_links_200924.pdf (accessed on 3 February 2023).
- Furfaro, E.; Signori, A.; Di Grazia, C.; Dominietto, A.; Raiola, A.M.; Aquino, S.; Ghiggi, C.; Ghiso, A.; Ungaro, R.; Angelucci, E.; et al. Serial monitoring of isavuconazole blood levels during prolonged antifungal therapy. J. Antimicrob. Chemother. 2019, 74, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- Extracorporeal Life Support Organization. General Guidelines for All ECLS Cases, Version 1.4. Available online: https://www.elso.org/ecmo-resources/elso-ecmo-guidelines.aspx (accessed on 9 May 2023).
- Alessandri, F.; Di Nardo, M.; Ramanathan, K.; Brodie, D.; MacLaren, G. Extracorporeal membrane oxygenation for COVID-19-related acute respiratory distress syndrome: A narrative review. J. Intensive Care 2023, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Tonna, J.E.; Abrams, D.; Brodie, D.; Greenwood, J.C.; RUBIO Mateo-Sidron, J.A.; Usman, A.; Fan, E. Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J. 2021, 67, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Choi, J.H.; Chang, M.J. Pharmacokinetic changes of antibiotic, antiviral, antituberculosis and antifungal agents during extracorporeal membrane oxygenation in critically ill adult patients. J. Clin. Pharm. Ther. 2017, 42, 661–671. [Google Scholar] [CrossRef]
- Dzierba, A.L.; Abrams, D.; Brodie, D. Medicating patients during extracorporeal membrane oxygenation: The evidence is building. Crit. Care 2017, 21, 66. [Google Scholar] [CrossRef]
- Shekar, K.; Fraser, J.F.; Smith, M.T.; Roberts, J.A. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J. Crit. Care 2012, 27, 741.e749–718. [Google Scholar] [CrossRef]
- Cheng, V.; Abdul-Aziz, M.H.; Roberts, J.A.; Shekar, K. Optimising drug dosing in patients receiving extracorporeal membrane oxygenation. J. Thorac. Dis. 2018, 10, S629–S641. [Google Scholar] [CrossRef]
- Desai, A.; Kovanda, L.; Kowalski, D.; Lu, Q.; Townsend, R.; Bonate, P.L. Population pharmacokinetics of isavuconazole from phase 1 and phase 3 (SECURE) trials in adults and target attainment in patients with invasive infections due to Aspergillus and other filamentous fungi. Antimicrob. Agents Chemother. 2016, 60, 5483–5491. [Google Scholar] [CrossRef]
- Huddart, R.; Fohner, A.E.; Whirl-Carrillo, M.; Wojcik, G.L.; Gignoux, C.R.; Popejoy, A.B.; Bustamante, C.D.; Altman, R.B.; Klein, T.E. Standardized biogeographic grouping system for annotating populations in pharmacogenetic research. Clin. Pharmacol. Ther. 2019, 105, 1256–1262. [Google Scholar] [CrossRef]
- Miller, M.; Kludjian, G.; Mohrien, K.; Morita, K. Decreased isavuconazole trough concentrations in the treatment of invasive aspergillosis in an adult patient receiving extracorporeal membrane oxygenation support. Am. J. Health Syst. Pharm. 2022, 79, 1245–1249. [Google Scholar] [CrossRef]
- Zhao, Y.; Seelhammer, T.G.; Barreto, E.F.; Wilson, J.W. Altered pharmacokinetics and dosing of liposomal amphotericin B and isavuconazole during extracorporeal membrane oxygenation. Pharmacotherapy 2020, 40, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Mertens, B.; Wauters, J.; Debaveye, Y.; Van Regenmortel, N.; Degezelle, K.; Meersseman, P.; Hermans, G.; Vandenbriele, C.; Van Daele, R.; Spriet, I. The impact of extracorporeal membrane oxygenation on the exposure to isavuconazole: A plea for thorough pharmacokinetic evaluation. Crit. Care 2022, 26, 227. [Google Scholar] [CrossRef] [PubMed]
- Kriegl, L.; Hatzl, S.; Zurl, C.; Reisinger, A.C.; Schilcher, G.; Eller, P.; Gringschl, Y.; Muhr, T.; Meinitzer, A.; Prattes, J.; et al. Isavuconazole plasma concentrations in critically ill patients during extracorporeal membrane oxygenation. J. Antimicrob. Chemother. 2022, 77, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Schmitt-Hoffmann, A.; Roos, B.; Heep, M.; Schleimer, M.; Weidekamm, E.; Brown, T.; Roehrle, M.; Beglinger, C. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrob. Agents Chemother. 2006, 50, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Schmitt-Hoffmann, A.; Roos, B.; Maares, J.; Heep, M.; Spickerman, J.; Weidekamm, E.; Brown, T.; Roehrle, M. Multiple-dose pharmacokinetics and safety of the new antifungal triazole BAL4815 after intravenous infusion and oral administration of its prodrug, BAL8557, in healthy volunteers. Antimicrob. Agents Chemother. 2006, 50, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Schmitt-Hoffmann, A.; Roos, B.; Spickermann, J.; Heep, M.; Peterfaí, E.; Edwards, D.J.; Stoeckel, K. Effect of mild and moderate liver disease on the pharmacokinetics of isavuconazole after intravenous and oral administration of a single dose of the prodrug BAL8557. Antimicrob. Agents Chemother. 2009, 53, 4885–4890. [Google Scholar] [CrossRef] [PubMed]
- Townsend, R.W.; Akhtar, S.; Alcorn, H.; Berg, J.K.; Kowalski, D.L.; Mujais, S.; Desai, A.V. Phase I trial to investigate the effect of renal impairment on isavuconazole pharmacokinetics. Eur. J. Clin. Pharmacol. 2017, 73, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Kovanda, L.L.; Desai, A.V.; Lu, Q.; Townsend, R.W.; Akhtar, S.; Bonate, P.; Hope, W.W. Isavuconazole population pharmacokinetic analysis using nonparametric estimation in patients with invasive fungal disease (results from the VITAL study). Antimicrob. Agents Chemother. 2016, 60, 4568–4576. [Google Scholar] [CrossRef] [PubMed]
- Biagi, M.; Butler, D.; Tan, X.; Qasmieh, S.; Tejani, K.; Patel, S.; Rivosecchi, R.M.; Nguyen, M.H.; Clancy, C.J.; Shields, R.K.; et al. Pharmacokinetics and dialytic clearance of isavuconazole during in vitro and in vivo continuous renal replacement therapy. Antimicrob. Agents Chemother. 2019, 63, e01085-19. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.S.; Yogaratnam, D.; Levasseur-Franklin, K.E.; Forni, A.; Fong, J. Introduction to drug pharmacokinetics in the critically ill patient. Chest 2012, 141, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; van der Lee, M.; van Gelder, T.; Swen, J.J. Why we need to take a closer look at genetic contributions to CYP3A activity. Front. Pharmacol. 2022, 13, 912618. [Google Scholar] [CrossRef] [PubMed]
- PharmGKB. Gene-Specific Information Tables for CYP3A5. Available online: https://www.pharmgkb.org/page/cyp3a5RefMaterials (accessed on 14 March 2023).
- Shekar, K.; Abdul-Aziz, M.H.; Cheng, V.; Burrows, F.; Buscher, H.; Cho, Y.J.; Corley, A.; Diehl, A.; Gilder, E.; Jakob, S.M.; et al. Antimicrobial exposures in critically ill patients receiving extracorporeal membrane oxygenation. Am. J. Respir. Crit. Care Med. 2022, 207, 704–720. [Google Scholar] [CrossRef]
| Patient 1 | Patient 2 | |
|---|---|---|
| Demographics | ||
| Age, years | 61 | 52 |
| Sex | Male | Female |
| Biogeographical ancestry a | Near Eastern | Near Eastern |
| Body weight, kg | 81.3 | 67.0 |
| BMI, kg/m2 | 24.3 | 27.5 |
| Clinical characteristics | ||
| Length of ICU stay b, days | 30 | 48 |
| Host factors c | COVID-19 infection needing intensive care | COVID-19 infection needing intensive care |
| APACHE II score on admission | 18 | 28 |
| SOFA score d | 10 | 15 |
| Serum albumin d, g/L | 25.7 | 44.8 |
| ALT d, U/L | 15 | 167 |
| AST d, U/L | 14 | 152 |
| eGFR (CKD-EPI) d, mL/min/1.73 m2 | 46 | NA e |
| CRRT during isavuconazole therapy | No | Yes |
| Total duration of CRRT, days | NA | 36 |
| Duration of CRRT until extensive PK sampling, days | NA | 24 |
| Deceased during ICU admission | Yes | Yes |
| ECMO support | ||
| Indication of ECMO support | COVID-19-associated ARDS | COVID-19-associated ARDS |
| ECMO modality | Veno-venous | Veno-venous |
| ECMO oxygenator | Medos Hilite 7000LT | Medos Hilite 7000LT/Eurosets A.L.ONE |
| ECMO blood pump | Medos Deltastream DP3 | Medos Deltastream DP3 |
| Priming solution | Plasma-Lyte A | Plasma-Lyte A |
| Priming volume, mL | 670 | 670 |
| Total duration of ECMO support, days | 22 | 43 |
| Duration of ECMO support at initiation of isavuconazole, days | NA | 28 |
| Duration of ECMO support until extensive PK sampling, days | 1 | 31 |
| ECMO circuit exchanges f | No exchanges | Days 12, 17 *, 27 |
| Isavuconazole therapy | ||
| Indication | Probable CAPA g | Probable CAPA g |
| Therapy duration, days | 10 | 16 |
| Duration of isavuconazole therapy until extensive PK sampling, days | 7 | 4 |
| Maintenance dose on the extensive PK sampling day | 300 mg q24h h | 200 mg q24h |
| Trough concentrations i, mg/L | Day 4: 1.4 ** Day 7: 1.5 Day 9: 2.2 ** | Day 4: 1.0 Day 7: 0.8 ** Day 14: 1.0 ** |
| Sampling and Isavuconazole Dosing | PK Parameters a | |||||||
|---|---|---|---|---|---|---|---|---|
| Number of Samples (n) | Isavuconazole Maintenance Dose b | CL (L/h) | Q (L/h) | Vd (L) | T1/2 (h) | AUC0–24 (mg × h/L) | AUC0–24,ss (mg × h/L) | |
| Non-compartmental analysis | ||||||||
| Patient 1 | 8 | 300 mg q24h | 6.3 | NA | 310.8 | 34.2 | 47.8 | NA |
| Patient 2 | 8 | 200 mg q24h | 6.7 | NA | 212.6 | 21.9 | 29.8 | NA |
| Population PK model by Desai et al. [28] | ||||||||
| Patient 1 | 8 | 300 mg q24h | 5.4 [5.1–5.7] | 34.8 [25.0–48.3] | Vc: 49.1 Vp: 258.3 [219.9–323.4] | T1/2,α: 0.74 T1/2,β: 44.1 | 52.7 | 55.7 |
| Patient 2 | 8 | 200 mg q24h | 7.2 [6.5–8.1] | 28.3 [20.3–39.5] | Vc: 49.1 Vp: 342.1 [243.4–355.0] | T1/2,α: 0.85 T1/2,β: 35.6 | 33.4 | 27.7 |
| Typical patient c | NA | 200 mg q24h | 2.4 | 26.6 | Vc: 49.1 Vp: 260.0 | T1/2,α: 1.01 T1/2,β: 96.5 | NA | 89.3 |
| Population PK model by Perez et al. [17] | ||||||||
| Patient 1 | 8 | 300 mg q24h | 4.2 [2.9–6.2] | NA | 776.5 [616.5–978.0] | 128.5 | 46.6 | 62.5 |
| Patient 2 | 8 | 200 mg q24h | 7.5 [5.5–10.2] | NA | 588.8 [432.7–801.3] | 54.4 | 30.9 | 26.9 |
| Typical patient d | NA | 200 mg q24h | 4.0 | NA | 850.0 | 148.1 | NA | 50.2 |
| Reference (Year) | Perez et al. (2023) [17] | Kriegl et al. (2022) [33] | Mertens et al. (2022) [32] | Miller et al. (2022) [30] | Zhao et al. (2020) [31] | Zurl et al. (2020) [14] |
|---|---|---|---|---|---|---|
| Study characteristics | ||||||
| Study design | Single-center retrospective study | Single-center prospective observational PK study | Single-center retrospective study | Case report | Case report | Multicenter prospective observational cohort study |
| Number of patients | 5 a | 7 | 4 | 1 | 1 | 3 b |
| Demographics | ||||||
| Age, years | Median [IQR] in total cohort: 65 [56–70]; NS for patients with ECMO | Median [IQR]: 58 [50–62] | * Patient 1: 61 * Patient 2: 59 * Patient 3: 65 * Patient 4: 38 | 56 | 26 | Median [IQR] in total cohort: 60 [46–69]; NS for patients with ECMO |
| Sex | Total cohort: 22% female; NS for patients with ECMO | 43% female | 100% male | Male | Male | Total cohort: 28% female; NS for patients with ECMO |
| BMI, kg/m2 | Median [IQR] in total cohort: 29.2 [25.6–31.8]; NS for patients with ECMO | Median [IQR]: 29.8 [26.9–35.2] | * Patient 1: 26.7 * Patient 2: 20.0 * Patient 3: 29.2 * Patient 4: 24.7 | 20.3 | NS | Median [IQR] in total cohort: 24.6 [23.3–28.5]; NS for patients with ECMO |
| Clinical characteristics | ||||||
| Host factors c | COVID-19 infection needing intensive care (n = 5) | COVID-19 infection needing intensive care (n = 6); NS (n = 1) | Patients 1, 3, 4 COVID-19 infection needing intensive care Patient 2 Solid organ Tx | Immunosuppressive therapy | None | Patients 1, 2 Influenza virus infection needing intensive care Patient 3 NS |
| CRRT during ICU admission | NS for patients with ECMO | None of the included patients received CRRT | Patients 1, 3 Concomitant ISA-CRRT-ECMO therapy Patients 2, 4 No CRRT | No CRRT | Concomitant ISA-CRRT-ECMO therapy | Patient 1 Concomitant ISA-CRRT-ECMO therapy Patient 2 Concomitant ISA-CRRT-ECMO therapy (6 days) → ISA-CRRT therapy Patient 3 Concomitant ISA-ECMO therapy (11 days) → ISA-CRRT-ECMO therapy (3 days) → ISA-CRRT therapy |
| ECMO support | ||||||
| Indication of ECMO support | COVID-19-associated ARDS (n = 5) | COVID-19-associated ARDS (n = 6); cardiac arrest during cardiac surgery (n = 1) | Patients 1, 3, 4 COVID-19-associated ARDS Patient 2 Respiratory insufficiency due to complications after lung Tx | Respiratory insufficiency in patient with aspergilloma | ARDS and cardiogenic shock secondary to pulmonary blastomycosis (veno-arterial)/acute primary pulmonary allograft dysfunction (veno-venous) | Patients 1, 2 Influenza-associated ARDS Patient 3 ARDS after major cardiac surgery |
| ECMO modality | NS | Veno-venous (n = 6); veno-arterial (n = 1) | Veno-venous (n = 4) | Veno-venous | Veno-arterial → veno-arterial and veno-pulmoarterial → veno-venous | Veno-venous (n = 3) |
| Composition of ECMO circuit | NS | Novalung XLUNG kit 230 membrane oxygenator (Xenios AG); Deltastream DP3 blood pump (Medos Medizintechnik AG) | NS | Quadrox-iD adult membrane oxygenator (Maquet); Jostra Rotaflow pump (Maquet); PVC tubing with phosphorylcholine coating (LivaNova) | Cardiohelp v7 polymethylpentene membrane oxygenator and centrifugal pump (Maquet); PVC tubing | Patients 1, 2 Novalung iLA activve system (Xenios AG) Patient 3 NS |
| Circuit exchanges d | NS | NS | Patients 1, 4 No exchanges Patient 2 Day 8 * Patient 3 Days 31, 49, 57, 64 * | Day 23 | * Day 33: addition of veno-pulmoarterial ECMO circuit * Day 152: single veno-venous ECMO circuit | NS |
| Duration of ECMO at initiation of ISA therapy, days | NS | NS | * Patient 1: 16 * Patient 2: 6 * Patient 3: 24 * Patient 4: NA | 13 | 64 | NS |
| Antifungal therapy | ||||||
| Indication of antifungal therapy | CAPA (n = 5) | Antifungal prophylaxis in patients with COVID-19 infection (n = 6); probable IPA (n = 1) | Patients 1, 4 Probable CAPA Patient 2 Probable IPA Patient 3 Proven CAPA | Aspergilloma (A. fumigatus) | Pulmonary blastomycosis (Blastomyces dermatitidis) | Patients 1,3 IPA Patient 2 Probable intra-abdominal Candida parapsilosis infection |
| Choice of antifungal therapy | ISA IV/PO No information on previous/subsequent antifungal therapies in patients with ECMO. | ISA IV No information on previous/subsequent antifungal therapies. | Patient 1 VRC IV (8 days) → VRC IV + L-AmB IV + ABLC nebulization (10 days) → ISA IV + L-AmB IV + ABLC nebulization (9 days) Patient 2 ISA IV Patient 3 L-AmB IV (21 days) → Caspofungin IV + ISA IV (10 days) → L-AmB IV (36 days) → L-AmB IV + VRC IV (9 days) → VRC IV (82 days) → L-AmB IV (4 days) Patient 4 VRC IV (7 days) → ISA IV (14 + 25 days) | VRC IV (20 days) → ISA IV | L-AmB IV (5 mg/kg q24h → 7.5 mg/kg q24h → 10 mg/kg q24h) + L-AmB nebulization (25 mg q12h) + ISA IV (from day 67 of antifungal therapy onwards) | Patient 1 VRC IV → ISA IV Patient 2 Caspofungin IV → ISA IV Patient 3 NS |
| ISA dosing regimen | Total cohort: LD: 200 mg q8h (72h) in 6 patients/ MD (mean ± SD): 264 ± 79 mg q24h; NS for patients with ECMO | LD: 200 mg q8h (48h)/MD: 200 mg q24h | Patient 1 LD: 200 mg q8h (48h)/MD: 200 mg q12h (3 days) → 200–150 mg (1 day) → 150 mg q12h (3 days) Patient 2 LD: 200 mg q8h (48h)/MD: 200 mg q24h Patient 3 LD: 200 mg q8h (48h)/MD: 200 mg q24h (2 days) → 200 mg q8h (6 days) Patient 4 (course 1) e LD: 200 mg q8h (48h)/MD: 200 mg q24h (1 day) → 200 mg q18h (3 days) → 200 mg q12h (1 day) → 200 mg q24h (3 days) → 200 mg q12h (3 days) Patient 4 (course 2) LD: 200 mg q8h (48h)/MD: 200 mg q24h (6 days) → 400 mg q24h (17 days) | LD: 200 mg q8h (48h)/MD: 200 mg q24h (18 days) → 400 mg q24h (14 days) | LD: NS/MD: 200 mg q24h (9 days) → 200 mg q12h (115 days) | LD: 200 mg q8h (48h)/MD: 200 mg q24h |
| Duration of ISA therapy, days | NS | Median [IQR]: 11 [5–18] | * Patient 1: 9 * Patient 2: 7 * Patient 3: 10 * Patient 4: 14/25 | 34 | 124 | Patient 1 14 Patient 2 NS Patient 3 18 |
| Pharmacokinetics | ||||||
| PK sampling | Median [IQR] time to first Cmin measurement in total cohort: 5 [4.3–7.5] days; NS for patients with ECMO | * Blood samples from arterial line at 2h, 4h, 8h, 12h, 18h, 24h, 48h, 72h, 96h, 120h, 144h, 168h after first ISA dose (n = 64) * Samples from in- and outflow line of membrane oxygenator (n = 27) | Patient 1 Cmin on days 5 and 6 after ISA initiation (ECMO + CRRT) Patient 2 Cmin on day 7 (ECMO) Patient 3 Cmin on days 2, 4, 8 and 10 (ECMO + CRRT) Patient 4 (course 1) Cmin on day 7 (ECMO) Patient 4 (course 2) Cmin on day 6 after ISA re-initiation (no ECMO) | Cmin on days 5, 17 after ISA initiation and days 5, 8 after dosage adjustment | * Cmin on days 8, 23 and 105 after ISA initiation * Pre- and post-oxygenator concentrations on day 23 | Patient 1 Cmin on day 12 after ISA initiation (ECMO + CRRT) Patient 2 * Cmin on days 1 and 4 after ISA initiation (ECMO + CRRT) * Cmin after ECMO discontinuation (day of ISA therapy NS) (CRRT) Patient 3 Daily Cmin measurements during ISA-ECMO, ISA-ECMO-CRRT and ISA-CRRT therapy |
| Duration of ISA therapy/ECMO support at sampling day(s), days | * Median [IQR] time to first Cmin measurement in total cohort: 5 [4.3–7.5] days; NS for patients with ECMO * Duration of ECMO support at sampling day(s): NS | 1–7 days/NS | Patient 1 5, 6 days/20, 21 days Patient 2 7 days/13 days Patient 3 2, 4, 8, 10 days/27, 29, 33, 35 days Patient 4 (course 1) 7 days/2 days Patient 4 (course 2) 6 days/NA | 5, 17, 25, 28 days/18, 30, 38, 41 days | 8, 23, 105 days/71, 86, 168 days | Patient 1 12 days/NS Patient 2 * 1, 4 day(s)/NS * NS/NA Patient 3 Daily measurements |
| Reported ISA concentrations | * Median [IQR] Cmin in total cohort: 1.87 [1.29–2.25] mg/L; NS for patients with ECMO | * Median [IQR] Cmin,24h/Cmin,48h/Cmin,72h/Cmin,168h: 1.09 [0.92–1.30]/0.99 [0.94–1.51]/1.21 [1.03–2.07]/2.81 [2.09–3.76] mg/L * Significant correlation between pre- and post-membrane oxygenator ISA concentrations and between post-membrane oxygenator and arterial concentrations | Patient 1 * Cmin,d5: 4.3 mg/L * Cmin,d6: 5.0 mg/L Patient 2 Cmin,d7: 3.1 mg/L Patient 3 * Cmin,d2: 0.7 mg/L * Cmin,d4: 0.6 mg/L * Cmin,d8: 1.1 mg/L * Cmin,d10: 1.7 mg/L Patient 4 (course 1) Cmin,d7: 0.8 mg/L Patient 4 (course 2) Cmin,d6: 0.7 mg/L | * Cmin,d5: 1.7 mg/L * Cmin,d17: 0.7 mg/L * Cmin,d25: 3.7 mg/L * Cmin,d28: 2.9 mg/L | ISA 200 mg q24h— Double-circuit veno-arterial and veno-pulmoarterial ECMO Cmin,d8: 1.9 mg/L ISA 200 mg q12h— Double-circuit veno-arterial and veno-pulmoarterial ECMO Cmin/pre-/post-oxygenator concentration day 23: 4.1/3.7/3.4 mg/L ISA 200 mg q12h – Veno-venous ECMO Cmin,d105: 4.7 mg/L | Patient 1 Cmin,d12: 1.79 mg/L (ECMO + CRRT) Patient 2 * Cmin,d1: 0.74 mg/L (ECMO + CRRT) * Cmin,d4: 0.57 mg/L (ECMO + CRRT) * Cmin after ECMO discontinuation: 2.44 mg/L (CRRT) Patient 3 * Median Cmin: 1.7 mg/L (ECMO) * Median Cmin: 0.8 mg/L (ECMO + CRRT) * Median Cmin: <0.9 mg/L (CRRT) |
| Results of PK analysis | * No significant effect of ECMO on Vd or CL in a population PK analysis (nonlinear mixed-effects modeling) | * Median f [IQR] CL: 26.9 [18.4–35.3] L/h * Median f [IQR] Vd: 6.1 [3.9–8.3] L/kg * Median f [IQR] AUC0–24: 15.6 [12.2–18.9] mg × h/L | NA | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mertens, B.; Elkayal, O.; Dreesen, E.; Wauters, J.; Meersseman, P.; Debaveye, Y.; Degezelle, K.; Vermeersch, P.; Gijsen, M.; Spriet, I. Isavuconazole Exposure in Critically Ill Patients Treated with Extracorporeal Membrane Oxygenation: Two Case Reports and a Narrative Literature Review. Antibiotics 2023, 12, 1085. https://doi.org/10.3390/antibiotics12071085
Mertens B, Elkayal O, Dreesen E, Wauters J, Meersseman P, Debaveye Y, Degezelle K, Vermeersch P, Gijsen M, Spriet I. Isavuconazole Exposure in Critically Ill Patients Treated with Extracorporeal Membrane Oxygenation: Two Case Reports and a Narrative Literature Review. Antibiotics. 2023; 12(7):1085. https://doi.org/10.3390/antibiotics12071085
Chicago/Turabian StyleMertens, Beatrijs, Omar Elkayal, Erwin Dreesen, Joost Wauters, Philippe Meersseman, Yves Debaveye, Karlien Degezelle, Pieter Vermeersch, Matthias Gijsen, and Isabel Spriet. 2023. "Isavuconazole Exposure in Critically Ill Patients Treated with Extracorporeal Membrane Oxygenation: Two Case Reports and a Narrative Literature Review" Antibiotics 12, no. 7: 1085. https://doi.org/10.3390/antibiotics12071085
APA StyleMertens, B., Elkayal, O., Dreesen, E., Wauters, J., Meersseman, P., Debaveye, Y., Degezelle, K., Vermeersch, P., Gijsen, M., & Spriet, I. (2023). Isavuconazole Exposure in Critically Ill Patients Treated with Extracorporeal Membrane Oxygenation: Two Case Reports and a Narrative Literature Review. Antibiotics, 12(7), 1085. https://doi.org/10.3390/antibiotics12071085






