Daptomycin Pharmacokinetics in Blood and Wound Fluid in Critical Ill Patients with Left Ventricle Assist Devices
Abstract
1. Introduction
2. Results
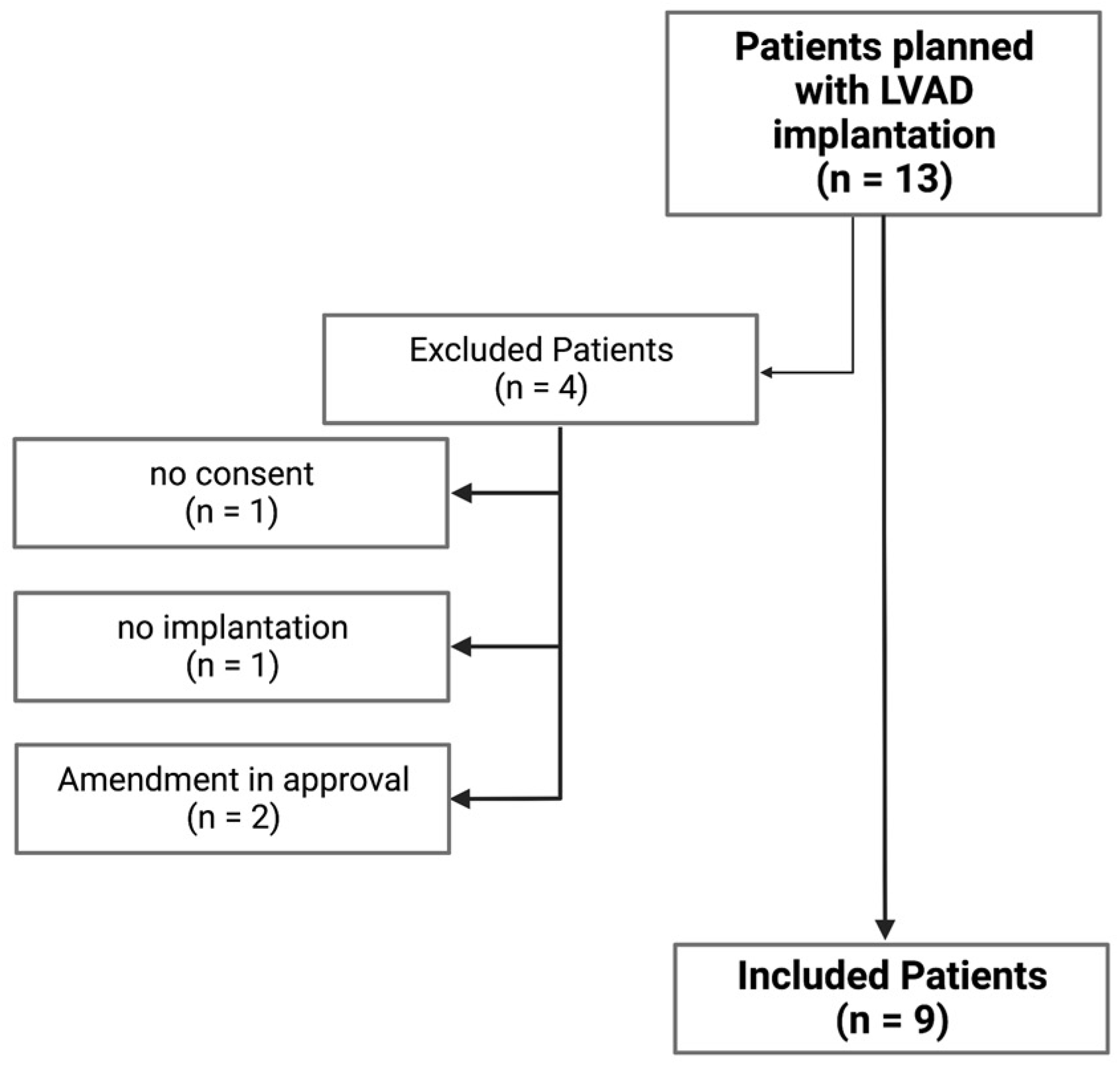
2.1. Cohort Baseline Epidemiological Data and Clinical Characteristics
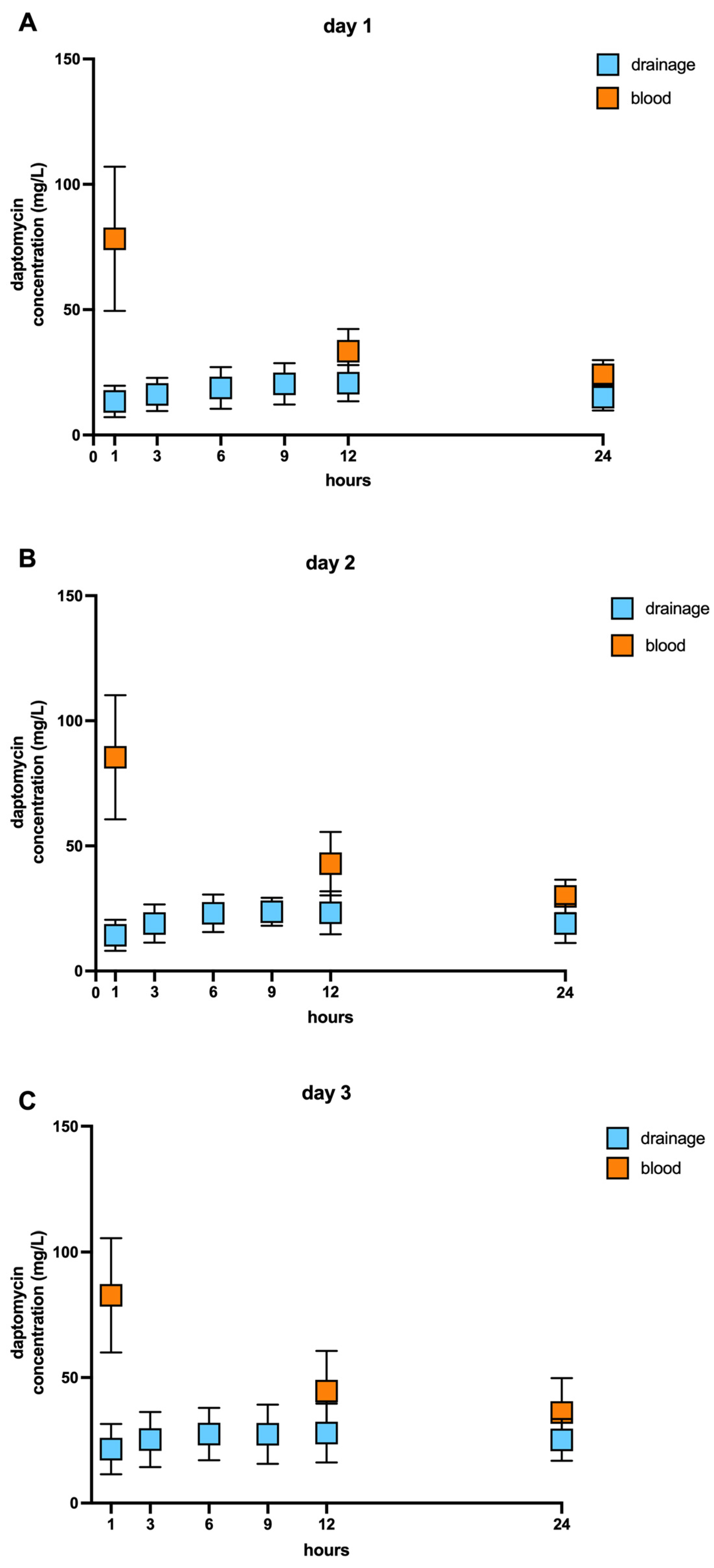
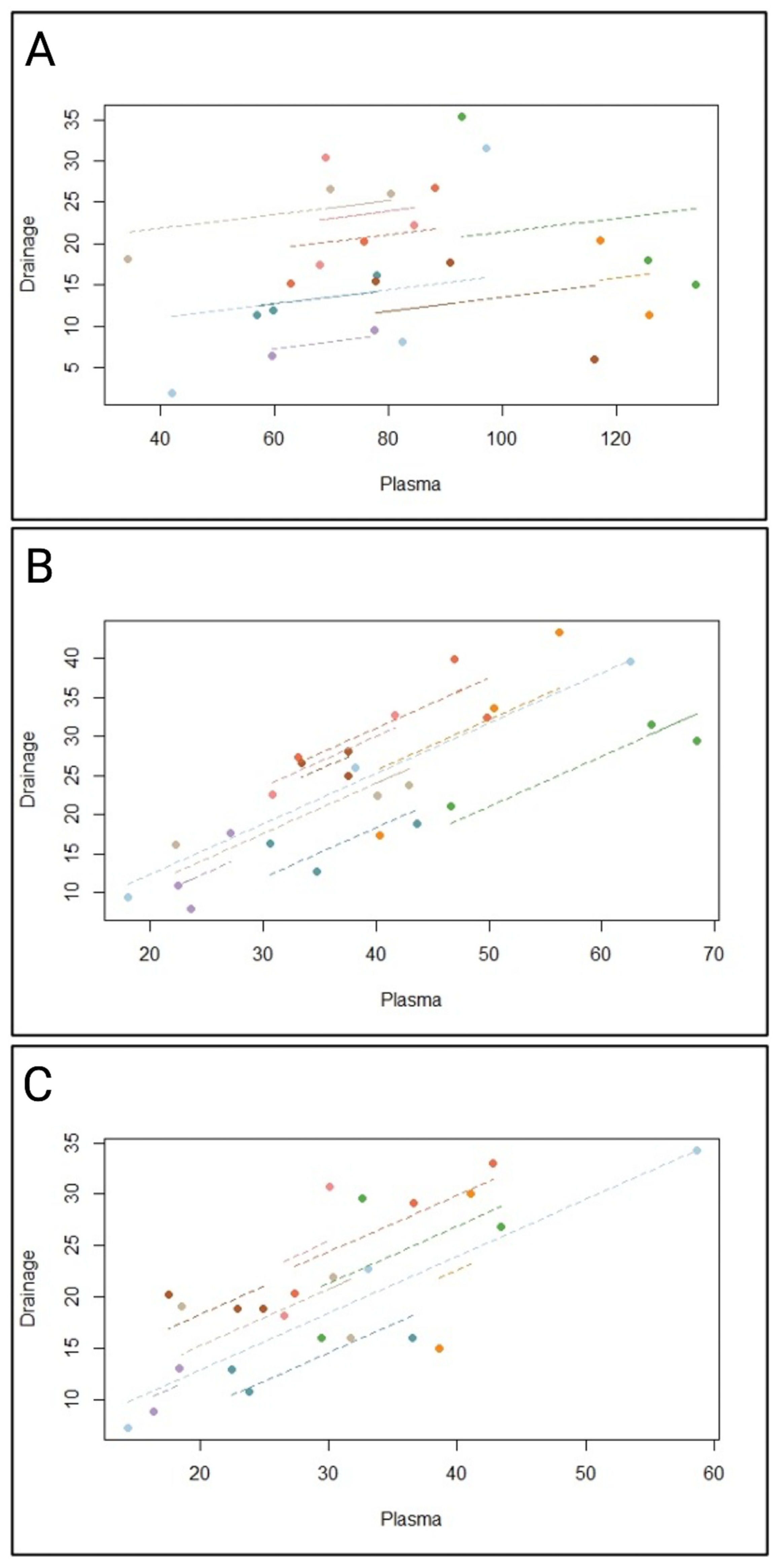
2.2. Pharmacokinetic of Daptomycin
2.3. Safety Results
3. Discussion
3.1. Limitations
3.2. Conclusions
4. Materials and Methods
4.1. Study Design
4.2. Daptomycin Concentration in Blood Serum and Wound Fluid
4.3. High-Performance Liquid Chromatography with UV Detection
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bassetti, M.; Righi, E.; Astilean, A.; Corcione, S.; Petrolo, A.; Farina, E.C.; De Rosa, F.G. Antimicrobial prophylaxis in minor and major surgery. Minerva Anestesiol. 2015, 81, 76–91. [Google Scholar]
- Abdul-Aziz, M.H.; Alffenaar, J.W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Wilson, R.C.; O’hare, D.; Herrero, P.; Kambugu, A.; Lamorde, M.; Ellington, M.; Georgiou, P.; Cass, A.; Hope, W.W.; et al. Optimizing antimicrobial use: Challenges, advances and opportunities. Nat. Rev. Microbiol. 2021, 19, 747–758. [Google Scholar] [CrossRef]
- Kumar, A.; Ellis, P.; Arabi, Y.; Roberts, D.; Light, B.; Parrillo, J.E.; Dodek, P.; Wood, G.; Kumar, A.; Simon, D.; et al. Initiation of Inappropriate Antimicrobial Therapy Results in a Fivefold Reduction of Survival in Human Septic Shock. Chest 2009, 136, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Liebchen, U.; Paal, M.; Scharf, C.; Schroeder, I.; Grabein, B.; Zander, J.; Siebers, C.; Zoller, M. The ONTAI study—A survey on antimicrobial dosing and the practice of therapeutic drug monitoring in German intensive care units. J. Crit. Care 2020, 60, 260–266. [Google Scholar] [CrossRef]
- Mabilat, C.; Gros, M.F.; Nicolau, D.; Mouton, J.W.; Textoris, J.; Roberts, J.A.; Cotta, M.O.; van Belkum, A.; Caniaux, I. Diagnostic and medical needs for therapeutic drug monitoring of antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 791–797. [Google Scholar] [CrossRef]
- Rahal, A.; Ruch, Y.; Meyer, N.; Perrier, S.; Minh, T.H.; Schneider, C.; Lavigne, T.; Marguerite, S.; Ajob, G.; Cristinar, M.; et al. Left ventricular assist device-associated infections: Incidence and risk factors. J. Thorac. Dis. 2020, 12, 2654–2662. [Google Scholar] [CrossRef]
- Okoh, A.K.; Fugar, S.; Dodoo, S.; Selevany, M.; Al-Obaidi, N.; Ozturk, E.; Singh, S.; Tayal, R.; Lee, L.Y.; Russo, M.J.; et al. Derivation and validation of the bridge to transplantation with left ventricular assist device score for 1 year mortality after heart transplantation. The BTT-LVAD score. Int. J. Artif. Organs 2022, 45, 470–477. [Google Scholar] [CrossRef]
- Casida, J.M.; Pavol, M.; Budhathoki, C.; Craddock, H.; Schroeder, S.E.; Hoff, D.; Tiburcio, M.; Ewald, G. A pilot clinical trial of a self-management intervention in patients with a left ventricular assist device. J. Artif. Organs 2022, 25, 91–104. [Google Scholar] [CrossRef]
- Patel, C.B.; Blue, L.; Cagliostro, B.; Bailey, S.H.; Entwistle, J.W.; John, R.; Thohan, V.; Cleveland, J.C., Jr.; Goldstein, D.J.; Uriel, N.; et al. Left ventricular assist systems and infection-related outcomes: A comprehensive analysis of the MOMENTUM 3 trial. J. Heart Lung Transplant. 2020, 39, 774–781. [Google Scholar] [CrossRef]
- Moayedi, Y.; Multani, A.; Bunce, P.E.; Henricksen, E.; Lee, R.; Yang, W.; Gomez, C.A.; Garvert, D.W.; Tremblay-Gravel, M.; Duclos, S.; et al. Outcomes of patients with infection related to a ventricular assist device after heart transplantation. Clin. Transplant. 2019, 33, e13692. [Google Scholar] [CrossRef] [PubMed]
- Siméon, S.; Flécher, E.; Revest, M.; Niculescu, M.; Roussel, J.C.; Michel, M.; Leprince, P.; Tattevin, P. Left ventricular assist device-related infections: A multicentric study. Clin. Microbiol. Infect. 2017, 23, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Kusne, S.; Mooney, M.; Danziger-Isakov, L.; Kaan, A.; Lund, L.H.; Lyster, H.; Wieselthaler, G.; Aslam, S.; Cagliostro, B.; Chen, J.; et al. An ISHLT consensus document for prevention and management strategies for mechanical circulatory support infection. J. Heart Lung Transplant. 2017, 36, 1137–1153. [Google Scholar] [CrossRef]
- Juraszek, A.; Smolski, M.; Kolsut, P.; Szymanski, J.; Litwinski, P.; Kusmierski, K.; Zakrzewska-Koperska, J.; Sterlinski, M.; Dziodzio, T.; Kusmierczyk, M. Prevalence and management of driveline infections in mechanical circulatory support—A single center analysis. J. Cardiothorac. Surg. 2021, 16, 216. [Google Scholar] [CrossRef]
- Fenton, C.; Keating, G.M.; Curran, M.P. Daptomycin. Drugs 2004, 64, 445–455; discussion 457–458. [Google Scholar] [CrossRef]
- Karas, J.A.; Carter, G.P.; Howden, B.P.; Turner, A.M.; Paulin, O.K.A.; Swarbrick, J.D.; Baker, M.A.; Li, J.; Velkov, T. Structure–Activity Relationships of Daptomycin Lipopeptides. J. Med. Chem. 2020, 63, 13266–13290. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Wang, J.T.; Lin, H.Y.; Chang, S.C. Daptomycin versus linezolid for treatment of vancomycin-resistant enterococcal bacteremia: Systematic review and meta-analysis. BMC Infect. Dis. 2014, 14, 687. [Google Scholar] [CrossRef]
- Khan, A.; Wilson, B.; Gould, I.M. Current and future treatment options for community-associated MRSA infection. Expert Opin. Pharmacother. 2018, 19, 457–470. [Google Scholar] [CrossRef]
- Fowler, V.G., Jr.; Boucher, H.W.; Corey, G.R.; Abrutyn, E.; Karchmer, A.W.; Rupp, M.E.; Levine, D.P.; Chambers, H.F.; Tally, F.P.; Vigliani, G.A.; et al. Daptomycin versus Standard Therapy for Bacteremia and Endocarditis Caused by Staphylococcus aureus. N. Engl. J. Med. 2006, 355, 653–665. [Google Scholar] [CrossRef]
- Walter, V.; Stock, U.A.; Soriano-Romero, M.; Schnitzbauer, A.; Moritz, A.; Beiras-Fernandez, A. Eradication of a chronic wound and driveline infection after redo-LVAD implantation. J. Cardiothorac. Surg. 2014, 9, 63. [Google Scholar] [CrossRef]
- Levy, D.T.; Steed, M.E.; Rybak, M.J.; Guo, Y.; Gialanella, P.; Hanau, L.; Muggia, V.; Ostrowsky, B. Successful treatment of a left ventricular assist device infection with daptomycin non-susceptible methicillin-resistant Staphylococcus aureus: Case report and review of the literature. Transpl. Infect. Dis. 2012, 14, E89–E96. [Google Scholar] [CrossRef]
- Moser, C.; Lerche, C.J.; Thomsen, K.; Hartvig, T.; Schierbeck, J.; Jensen, P.; Ciofu, O.; Høiby, N. Antibiotic therapy as personalized medicine—General considerations and complicating factors. Apmis 2019, 127, 361–371. [Google Scholar] [CrossRef]
- Cusumano, J.A.; Klinker, K.P.; Huttner, A.; Luther, M.K.; Roberts, J.A.; LaPlante, K.L. Towards precision medicine: Therapeutic drug monitoring-guided dosing of vancomycin and beta-lactam antibiotics to maximize effectiveness and minimize toxicity. Am. J. Health Syst. Pharm. 2020, 77, 1104–1112. [Google Scholar] [CrossRef]
- Hites, M.; Dell’Anna, A.M.; Scolletta, S.; Taccone, F.S. The challenges of multiple organ dysfunction syndrome and extra-corporeal circuits for drug delivery in critically ill patients. Adv. Drug Deliv. Rev. 2014, 77, 12–21. [Google Scholar] [CrossRef]
- Galar, A.; Muñoz, P.; Valerio, M.; Cercenado, E.; García-González, X.; Burillo, A.; Sánchez-Somolinos, M.; Juárez, M.; Verde, E.; Bouza, E. Current use of daptomycin and systematic therapeutic drug monitoring: Clinical experience in a tertiary care institution. Int. J. Antimicrob. Agents 2019, 53, 40–48. [Google Scholar] [CrossRef]
- Matsumoto, K.; Samura, M.; Tashiro, S.; Shishido, S.; Saiki, R.; Takemura, W.; Misawa, K.; Liu, X.; Enoki, Y.; Taguchi, K. Target Therapeutic Ranges of Anti-MRSA Drugs, Linezolid, Tedizolid and Daptomycin, and the Necessity of TDM. Biol. Pharm. Bull. 2022, 45, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Jentzer, J.C.; Kashyap, R.; Keegan, M.T.; Dunlay, S.M.; Passe, M.A.; Loftsgard, T.; Murphree, D.H.; Stulak, J.M. Sequential organ failure assessment score improves survival prediction for left ventricular assist device recipients in intensive care. Artif. Organs 2022, 46, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- D’avolio, A.; Pensi, D.; Baietto, L.; Pacini, G.; Di Perri, G.; De Rosa, F.G. Daptomycin Pharmacokinetics and Pharmacodynamics in Septic and Critically Ill Patients. Drugs 2016, 76, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, F.; Rogers, J.G. Left ventricular assist device therapy in advanced heart failure: Patient selection and outcomes. Eur. J. Heart Fail. 2017, 19, 595–602. [Google Scholar] [CrossRef]
- Frigerio, M. Left Ventricular Assist Device: Indication, Timing, and Management. Heart Fail. Clin. 2021, 17, 619–634. [Google Scholar] [CrossRef]
- O’Horo, J.C.; Abu Saleh, O.M.; Stulak, J.M.; Wilhelm, M.P.; Baddour, L.M.; Rizwan Sohail, M. Left Ventricular Assist Device Infections: A Systematic Review. ASAIO J. 2018, 64, 287–294. [Google Scholar] [CrossRef]
- Papathanasiou, M.; Pohl, J.; Jánosi, R.A.; Pizanis, N.; Kamler, M.; Rassaf, T.; Luedike, P. Colonization With Multiresistant Bacteria: Impact on Ventricular Assist Device Patients. Ann. Thorac. Surg. 2018, 105, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, A.L.; Hart, A.; Brouse, S.D.; Charnigo, R.J.; Branam, S.; Guglin, M.E. Left ventricular assist device-related infections: Does the time of onset matter? J. Artif. Organs 2019, 22, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Nienaber, J.J.C.; Kusne, S.; Riaz, T.; Walker, R.C.; Baddour, L.M.; Wright, A.J.; Park, S.J.; Vikram, H.R.; Keating, M.R.; Arabia, F.A.; et al. Clinical Manifestations and Management of Left Ventricular Assist Device-Associated Infections. Clin. Infect. Dis. 2013, 57, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Hamad, Y.; Blanco-Guzman, M.O.; Olsen, M.A.; Wang, X.; Vader, J.; Verma, A.; Dubberke, E.R. The role of chronic suppressive antibiotics therapy in superficial drive line infection relapse of left ventricular assist devices: A retrospective cohort from a tertiary care center. Transpl. Infect. Dis. 2021, 23, e13686. [Google Scholar] [CrossRef]
- Spano, G.; Buffle, E.; Walti, L.N.; Mihalj, M.; Cameron, D.R.; Martinelli, M.; Fürholz, M.; Que, Y.A.; Hayward, C.; Reineke, D.; et al. Ten-year retrospective cohort analysis of ventricular assist device infections. Artif. Organs 2022, 47, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Pogliano, J.; Pogliano, N.; Silverman, J.A. Daptomycin-Mediated Reorganization of Membrane Architecture Causes Mislocalization of Essential Cell Division Proteins. J. Bacteriol. 2012, 194, 4494–4504. [Google Scholar] [CrossRef] [PubMed]
- Howden, B.P.; McEvoy, C.R.E.; Allen, D.L.; Chua, K.; Gao, W.; Harrison, P.F.; Bell, J.; Coombs, G.; Bennett-Wood, V.; Porter, J.L.; et al. Evolution of Multidrug Resistance during Staphylococcus aureus Infection Involves Mutation of the Essential Two Component Regulator WalKR. PLoS Pathog. 2011, 7, e1002359. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.A.; Panesso, D.; McGrath, D.M.; Qin, X.; Mojica, M.F.; Miller, C.; Diaz, L.; Tran, T.T.; Rincon, S.; Barbu, E.M.; et al. Genetic Basis for In Vivo Daptomycin Resistance in Enterococci. N. Engl. J. Med. 2011, 365, 892–900. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Miyakis, S.; Ward, D.V.; Earl, A.M.; Rubio, A.; Cameron, D.R.; Pillai, S.; Moellering, R.C., Jr.; Eliopoulos, G.M. Whole Genome Characterization of the Mechanisms of Daptomycin Resistance in Clinical and Laboratory Derived Isolates of Staphylococcus aureus. PLoS ONE 2012, 7, e28316. [Google Scholar] [CrossRef] [PubMed]
- Sakoulas, G.; Moise, P.A.; Casapao, A.M.; Nonejuie, P.; Olson, J.; Okumura, C.Y.; Rybak, M.J.; Kullar, R.; Dhand, A.; Rose, W.E.; et al. Antimicrobial Salvage Therapy for Persistent Staphylococcal Bacteremia Using Daptomycin Plus Ceftaroline. Clin. Ther. 2014, 36, 1317–1333. [Google Scholar] [CrossRef]
- Benvenuto, M.; Benziger, D.P.; Yankelev, S.; Vigliani, G. Pharmacokinetics and Tolerability of Daptomycin at Doses up to 12 Milligrams per Kilogram of Body Weight Once Daily in Healthy Volunteers. Antimicrob. Agents Chemother. 2006, 50, 3245–3249. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J. The efficacy and safety of daptomycin: First in a new class of antibiotics for Gram-positive bacteria. Clin. Microbiol. Infect. 2006, 12 (Suppl. S1), 24–32. [Google Scholar] [CrossRef]
- Dvorchik, B.; Arbeit, R.D.; Chung, J.; Liu, S.; Knebel, W.; Kastrissios, H. Population Pharmacokinetics of Daptomycin. Antimicrob. Agents Chemother. 2004, 48, 2799–2807. [Google Scholar] [CrossRef]
- Kanzler, I.; Weis, F.; Beiras-Fernandez, A. Current use of daptomycin in cardiac surgery and postoperative intensive care. Expert Rev. Anti-Infect. Ther. 2013, 11, 309–320. [Google Scholar] [CrossRef]
- Huang, Y.; Lv, G.; Hu, L.; Wu, Y.; Guo, N.; Zhu, Y.; Ding, L.; Li, Q.; Liu, S.; Yang, Y.; et al. Efficacy and Safety of High Vs Standard Daptomycin Doses Examined in Chinese Patients With Severe Burn Injuries by Pharmacokinetic Evaluation. J. Burn. Care Res. 2020, 41, 705–713. [Google Scholar] [CrossRef]
- Xu, X.; Khadzhynov, D.; Peters, H.; Chaves, R.L.; Hamed, K.; Levi, M.; Corti, N. Population pharmacokinetics of daptomycin in adult patients undergoing continuous renal replacement therapy. Br. J. Clin. Pharmacol. 2017, 83, 498–509. [Google Scholar] [CrossRef]
- Chakraborty, A.; Roy, S.; Loeffler, J.; Chaves, R.L. Comparison of the pharmacokinetics, safety and tolerability of daptomycin in healthy adult volunteers following intravenous administration by 30 min infusion or 2 min injection. J. Antimicrob. Chemother. 2009, 64, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.; Gee, T.; Andrews, J.M.; Dvorchik, B.; Marshall, G. Pharmacokinetics and Inflammatory Fluid Penetration of Intravenous Daptomycin in Volunteers. Antimicrob. Agents Chemother. 2002, 46, 31–33. [Google Scholar] [CrossRef][Green Version]
- Kim, A.; Suecof, L.A.; Sutherland, C.A.; Gao, L.; Kuti, J.L.; Nicolau, D.P. In Vivo Microdialysis Study of the Penetration of Daptomycin into Soft Tissues in Diabetic versus Healthy Volunteers. Antimicrob. Agents Chemother. 2008, 52, 3941–3946. [Google Scholar] [CrossRef]
- Traunmüller, F.; Schintler, M.V.; Metzler, J.; Spendel, S.; Mauric, O.; Popovic, M.; Konz, K.H.; Scharnagl, E.; Joukhadar, C. Soft tissue and bone penetration abilities of daptomycin in diabetic patients with bacterial foot infections. J. Antimicrob. Chemother. 2010, 65, 1252–1257. [Google Scholar] [CrossRef]
- Copeland, H.; Baran, D. A persistent problem—The dreaded LVAD driveline infection. J. Card. Surg. 2021, 37, 105–106. [Google Scholar] [CrossRef]
- Lambadaris, M.; Vishram-Nielsen, J.K.K.; Amadio, J.M.; Husain, S.; Rao, V.; Billia, F.; Alba, A.C. Association between continuous-flow left ventricular assist device infections requiring long-term antibiotic use and post-heart transplant morbidity and mortality. J. Card. Surg. 2021, 37, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Piérard, D.; Stone, G.G. In vitro activity of ceftaroline and comparators against bacterial isolates collected globally from patients with skin infections. J. Glob. Antimicrob. Resist. 2021, 26, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Satlin, M.J.; Nicolau, D.P.; Humphries, R.M.; Kuti, J.L.; Campeau, S.A.; Ii, J.S.L.; Weinstein, M.P.; Jorgensen, J.H. Development of Daptomycin Susceptibility Breakpoints for Enterococcus faecium and Revision of the Breakpoints for Other Enterococcal Species by the Clinical and Laboratory Standards Institute. Clin. Infect. Dis. 2020, 70, 1240–1246. [Google Scholar] [CrossRef]
- Kullar, R.; Davis, S.L.; Levine, D.P.; Zhao, J.J.; Crank, C.W.; Segreti, J.; Sakoulas, G.; Cosgrove, S.E.; Rybak, M.J. High-Dose Daptomycin for Treatment of Complicated Gram-Positive Infections: A Large, Multicenter, Retrospective Study. Pharmacotherapy 2011, 31, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Kullar, R.; Sakoulas, G.; Deresinski, S.; van Hal, S.J. When sepsis persists: A review of MRSA bacteraemia salvage therapy. J. Antimicrob. Chemother. 2016, 71, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Bhavnani, S.M.; Rubino, C.M.; Ambrose, P.G.; Drusano, G.L. Daptomycin Exposure and the Probability of Elevations in the Creatine Phosphokinase Level: Data from a Randomized Trial of Patients with Bacteremia and Endocarditis. Clin. Infect. Dis. 2010, 50, 1568–1574. [Google Scholar] [CrossRef]
- Missault, S.; Causenbroeck, J.V.; Vandewiele, K.; Czapla, J.; Philipsen, T.; François, K.; Bové, T. Analysis of clinical outcome and postoperative organ function effects in a propensity-matched comparison between conventional and minimally invasive mitral valve surgery. J. Card. Surg. 2020, 35, 3276–3285. [Google Scholar] [CrossRef]
- Abdul–Aziz, M.H.; Brady, K.M.P.; Cotta, M.O.; Roberts, J.A. Therapeutic Drug Monitoring of Antibiotics: Defining the Therapeutic Range. Ther. Drug Monit. 2022, 44, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Bakdash, J.Z.; Marusich, L.R. Repeated Measures Correlation. Front. Psychol. 2017, 8, 456. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Vlasblom, A.A.; Verstappen, K.M.; Zomer, A.L.; Fluit, A.C.; Rogers, M.R.C.; Wagenaar, J.A.; Claesson, M.J.; Duim, B. Differential Analysis of Longitudinal Methicillin-Resistant Staphylococcus aureus Colonization in Relation to Microbial Shifts in the Nasal Microbiome of Neonatal Piglets. Msystems 2021, 6, e0015221. [Google Scholar] [CrossRef] [PubMed]
- Ogami, C.; Tsuji, Y.; Kasai, H.; Hiraki, Y.; Yamamoto, Y.; Matsunaga, K.; Karube, Y.; To, H. Evaluation of pharmacokinetics and the stability of daptomycin in serum at various temperatures. Int. J. Infect. Dis. 2017, 57, 38–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Characteristics of Participants | |
|---|---|
| Age (years/median/min–max) | 61.1 (54.0–68.0) |
| Sex (female/male) | 1/8 |
| Daptomycin mg/kg body weight | |
| Dose adjustment d2 | 2 out of 9 |
| Clinical Scores (mean ± SD) | |
| APACHEII Score | 22.4 ± 1.3 |
| SOFA Score | 11.2 ± 0.8 |
| SAPS Score | 44.6 ± 4.4 |
| Administration of vasoactive and inotropic medication | |
| epinephrine | 3 out of 9 |
| norepinephrine | 9 out of 9 |
| dobutamin | 3 out of 9 |
| vasopressin | 4 out of 9 |
| milrinon | 9 out of 9 |
| levosimendan | 1 out of 9 |
| Laboratory values | |
| creatinine kinase (U/L) | |
| postoperative | 542.9 ± 170.7 |
| day 1 | 631.8 ± 209.2 |
| day 2 | 409.8 ± 193.8 |
| day 3 | 362.6 ± 168.3 |
| Myoglobin (U/L) | |
| day 1 | 407 ± 201 |
| day 2 | 305 ± 256 |
| day 3 | 410 ± 359 |
| Lactatdehydrogenase (U/L) | |
| postoperative | 366.3 ± 29.7 |
| day 1 | 343.9 ± 23.1 |
| day 2 | 299.8 ± 15.9 |
| day 3 | 286. 8 ± 23.6 |
| Day 1 (Mean ± SD) | Day 2 (Mean ± SD) | Day 3 (Mean ± SD) | |
|---|---|---|---|
| AUCblood (µg*h/mL) | 1025 ± 334 | 1298 ± 478 | 1372 ± 464 |
| AUCdrain (µg*h/mL) | 383 ± 140 | 512 ± 173 | 695 ± 291 |
| PR | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 |
| Cminblood (µg/mL) | 24.1 ± 5.9 | 29.8 ± 6.7 | 29.9 ± 10.2 |
| Cmaxblood (µg/mL) | 78.3 ± 28.8 | 85.4 ± 24.8 | 82.8 ± 22.8 |
| t1/2blood (hours) | 14.1 ± 4.0 | 15.8 ± 3.2 | 20.5 ± 6.7 |
| Day 1 | Day 2 | Day 3 | |||||||||||||||
| Time to sampling (blood) after daptomycin infusion (h) | |||||||||||||||||
| 1 | 12 | 24 | 1 | 12 | 24 | 1 | 12 | 24 | |||||||||
| Day 1 | Day 2 | Day 3 | |||||||||||||||
| Time to sampling (drains) after daptomycin infusion (h) | |||||||||||||||||
| 1 | 3 | 6 | 9 | 12 | 24 | 1 | 3 | 6 | 9 | 12 | 24 | 1 | 3 | 6 | 9 | 12 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calov, S.; Munzel, F.; Roehr, A.C.; Frey, O.; Higuita, L.M.S.; Wied, P.; Rosenberger, P.; Haeberle, H.A.; Ngamsri, K.-C. Daptomycin Pharmacokinetics in Blood and Wound Fluid in Critical Ill Patients with Left Ventricle Assist Devices. Antibiotics 2023, 12, 904. https://doi.org/10.3390/antibiotics12050904
Calov S, Munzel F, Roehr AC, Frey O, Higuita LMS, Wied P, Rosenberger P, Haeberle HA, Ngamsri K-C. Daptomycin Pharmacokinetics in Blood and Wound Fluid in Critical Ill Patients with Left Ventricle Assist Devices. Antibiotics. 2023; 12(5):904. https://doi.org/10.3390/antibiotics12050904
Chicago/Turabian StyleCalov, Stefanie, Frederik Munzel, Anka C. Roehr, Otto Frey, Lina Maria Serna Higuita, Petra Wied, Peter Rosenberger, Helene A. Haeberle, and Kristian-Christos Ngamsri. 2023. "Daptomycin Pharmacokinetics in Blood and Wound Fluid in Critical Ill Patients with Left Ventricle Assist Devices" Antibiotics 12, no. 5: 904. https://doi.org/10.3390/antibiotics12050904
APA StyleCalov, S., Munzel, F., Roehr, A. C., Frey, O., Higuita, L. M. S., Wied, P., Rosenberger, P., Haeberle, H. A., & Ngamsri, K.-C. (2023). Daptomycin Pharmacokinetics in Blood and Wound Fluid in Critical Ill Patients with Left Ventricle Assist Devices. Antibiotics, 12(5), 904. https://doi.org/10.3390/antibiotics12050904







