Bacterial Adhesion Strength on Titanium Surfaces Quantified by Atomic Force Microscopy: A Systematic Review
Abstract
1. Introduction
2. Results
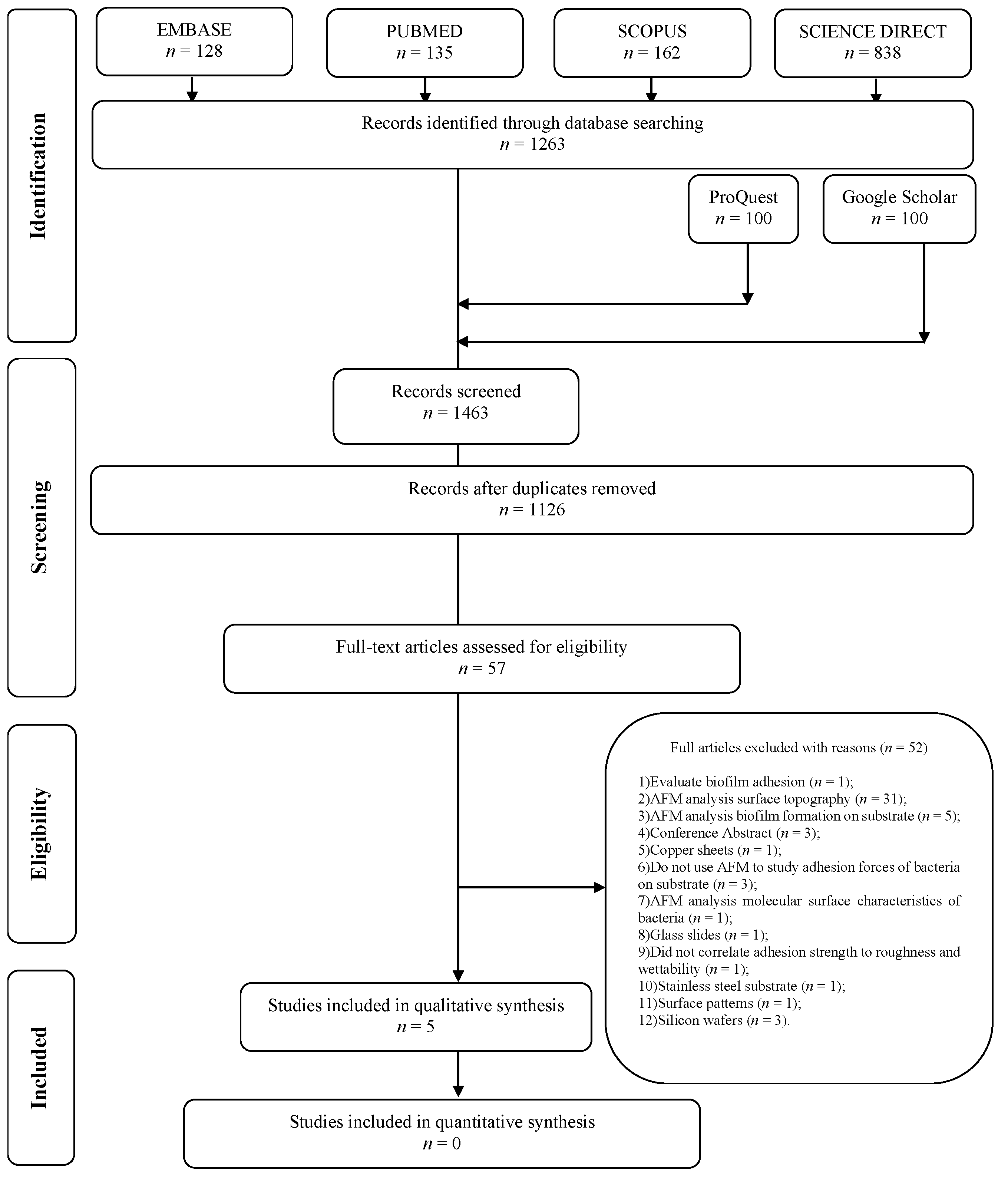
2.1. Result of the Selection Process
2.2. Qualitative Assessment of the Studies
2.3. Risk of Bias
2.4. Meta-Analysis
3. Discussion
3.1. Wettability
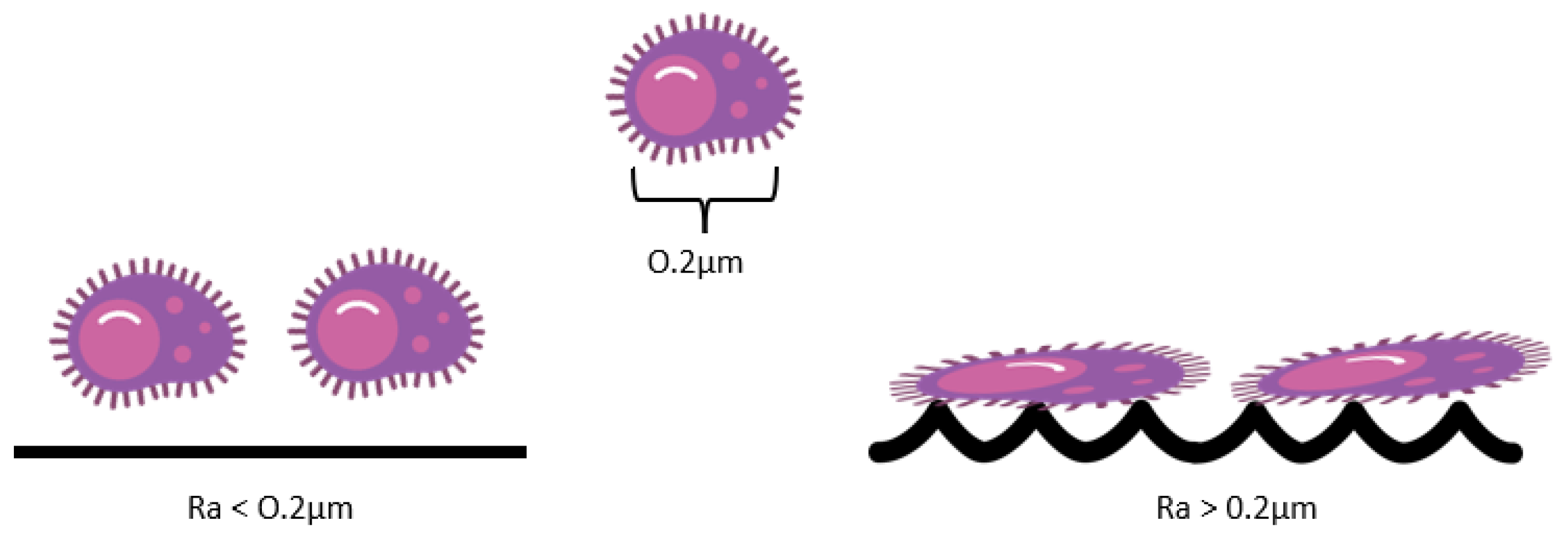
3.2. Roughness
3.3. Survival Mechanism
3.4. Clinical Implications
4. Materials and Methods
5. Conclusions
- (1)
- Current literature shows the preference of bacteria in adhering to the surfaces of the same hydrophilicity. However, this is contradicted by the present systematic review, which showed that this might not be the case, since hydrophobic bacteria developed hydrogen bonds and adhered to hydrophilic surfaces.
- (2)
- The application of surface treatments, such as potentiostat anodization and lasers, that induce the reduction of areas favorable for bacterial adhesion interferes more in the formation of biofilm than the surface roughness.
- (3)
- Bacterial colonization should be evaluated in time-dependent studies as they develop adaptation mechanisms related to time, which are not examined in this review. Furthermore, the electrostatic condition of the surface is a property that should be highlighted.
- (4)
- For clinical conditions, the literature demonstrates the following: 1. Positioning the implant at the tissue level is recommended as it allows for better hygiene; 2. There is still no consensus on the ideal roughness for the transmucosal region of the implant; 3. Printed implants allow better adhesion and osteoblastic proliferation and control of their printing parameters for mechanical biocompatibility with bone tissue; and 4. The oral cavity is a polymicrobial area so implant contamination can occur during its installation; thus, the clinician must be aware of the possibility of contamination and avoid it by using strict antisepsis methods and instruct the patient on the need for regular consultations and on how to sanitize.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguayo, S.; Donos, N.; Spratt, D.; Bozec, L. Nanoadhesion of Staphylococcus aureus onto titanium implant surfaces. J. Dent. Res. 2015, 94, 1078–1084. [Google Scholar] [CrossRef]
- Barbour, M.E.; O’Sullivan, D.J.; Jenkinson, H.F.; Jagger, D.C. The effects of polishing methods on surface morphology, roughness and bacterial colonisation of titanium abutments. J. Mater. Sci. Mater. Med. 2007, 18, 1439–1447. [Google Scholar] [CrossRef]
- Beltrán-Partida, E.; Valdez-Salas, B.; Escamilla, A.; Moreno-Ulloa, A.; Burtseva, L.; Valdez-Salas, E.; Curiel Alvarez, M.; Nedev, N. The Promotion of Antibacterial Effects of Ti6Al4V Alloy Modified with TiO2 Nanotubes Using a Superoxidized Solution. J. Nanomater. 2015, 16, 183. [Google Scholar] [CrossRef]
- Ou, K.L.; Weng, C.C.; Lin, Y.H.; Huang, M.S. A promising of alloying modified beta-type Titanium-Niobium implant for biomedical applications: Microstructural characteristics, in vitro biocompatibility and antibacterial performance. J. Alloys Compd. 2017, 697, 231–238. [Google Scholar] [CrossRef]
- Kazek-Kęsik, A.; Nosol, A.; Płonka, J.; Śmiga-Matuszowicz, M.; Gołda-Cępa, M.; Krok-Borkowicz, M.; Brzychczy-Włoch, M.; Pamuła, E.; Simka, W. PLGA-amoxicillin-loaded layer formed on anodized Ti alloy as a hybrid material for dental implant applications. Mater. Sci. Eng. C 2019, 94, 998–1008. [Google Scholar] [CrossRef]
- Walter, M.S.; Frank, M.J.; Satué, M.; Monjo, M.; Rønold, H.J.; Lyngstadaas, S.P.; Haugen, H.J. Bioactive implant surface with electrochemically bound doxycycline promotes bone formation markers in vitro and in vivo. Dent. Mater. 2014, 30, 200–214. [Google Scholar] [CrossRef]
- Kazek-Kęsik, A.; Nosol, A.; Płonka, J.; Śmiga-Matuszowicz, M.; Student, S.; Brzychczy-Włoch, M.; Krok-Borkowicz, M.; Pamuła, E.; Simka, W. Physico-chemical and biological evaluation of doxycycline loaded into hybrid oxide-polymer layer on Ti–Mo alloy. Bioact. Mater. 2020, 5, 553–563. [Google Scholar] [CrossRef]
- Rangel, A.L.R.; Falentin-Daudré, C.; da Silva Pimentel, B.N.A.; Vergani, C.E.; Migonney, V.; Alves Claro, A.P.R. Nanostructured titanium alloy surfaces for enhanced osteoblast response: A combination of morphology and chemistry. Surf. Coat. Technol. 2020, 383, 125226. [Google Scholar] [CrossRef]
- Kazek-Kęsik, A.; Jaworska, J.; Krok-Borkowicz, M.; Gołda-Cępa, M.; Pastusiak, M.; Brzychczy-Włoch, M.; Pamuła, E.; Kotarba, A.; Simka, W. Hybrid oxide-polymer layer formed on Ti-15Mo alloy surface enhancing antibacterial and osseointegration functions. Surf. Coat. Technol. 2016, 302, 158–165. [Google Scholar] [CrossRef]
- Kreve, S.; Cândido dos Reis, A. Influence of the electrostatic condition of the titanium surface on bacterial adhesion: A systematic review. J. Prosthet. Dent. 2021, 125, 416–420. [Google Scholar] [CrossRef]
- Ahimou, F.; Semmens, M.J.; Novak, P.J.; Haugstad, G. Biofilm cohesiveness measurement using a novel atomic force microscopy methodology. Appl. Environ. Microbiol. 2007, 73, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.O.; Ricomini-Filho, A.P.; Beline, T.; Ogawa, E.S.; Costa-Oliveira, B.E.; de Almeida, A.B.; Nociti Junior, F.H.; Rangel, E.C.; da Cruz, N.C.; Sukotjo, C.; et al. Three-species biofilm model onto plasma-treated titanium implant surface. Colloids Surf. B Biointerfaces 2017, 152, 354–366. [Google Scholar] [CrossRef]
- Boshkovikj, V.; Fluke, C.J.; Crawford, R.J.; Ivanova, E.P. Three-dimensional visualization of nanostructured surfaces and bacterial attachment using Autodesk® Maya®. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Aguayo, S.; Donos, N.; Spratt, D.; Bozec, L. Probing the nanoadhesion of Streptococcus sanguinis to titanium implant surfaces by atomic force microscopy. Int. J. Nanomed. 2016, 11, 1443–1450. [Google Scholar] [CrossRef]
- Alam, F.; Balani, K. Adhesion force of staphylococcus aureus on various biomaterial surfaces. J. Mech. Behav. Biomed. Mater. 2017, 65, 872–880. [Google Scholar] [CrossRef]
- Caro-Lara, L.; Ramos-Moore, E.; Vargas, I.T.; Walczak, M.; Fuentes, C.; Gómez, A.V.; Barrera, N.P.; Castillo, J.; Pizarro, G. Initial adhesion suppression of biofilm-forming and copper-tolerant bacterium Variovorax sp. on laser microtextured copper surfaces. Colloids Surf. B Biointerfaces 2021, 202, 111656. [Google Scholar] [CrossRef] [PubMed]
- Gadenne, V.; Lebrun, L.; Jouenne, T.; Thebault, P. Antiadhesive activity of ulvan polysaccharides covalently immobilized onto titanium surface. Colloids Surf. B Biointerfaces 2013, 112, 229–236. [Google Scholar] [CrossRef]
- Golda-Cepa, M.; Brzychczy-Wloch, M.; Engvall, K.; Aminlashgari, N.; Hakkarainen, M.; Kotarba, A. Microbiological investigations of oxygen plasma treated parylene C surfaces for metal implant coating. Mater. Sci. Eng. C 2015, 52, 273–281. [Google Scholar] [CrossRef]
- Na, C.; McNamara, C.J.; Konkol, N.R.; Bearce, K.A.; Mitchell, R.; Martin, S.T. The use of force-volume microscopy to examine bacterial attachment to titanium surfaces. Ann. Microbiol. 2010, 60, 495–502. [Google Scholar] [CrossRef]
- Kreve, S.; Reis, A.C.D. Bacterial adhesion to biomaterials: What regulates this attachment? A Rev. Jpn. Dent. Sci. Rev. 2021, 57, 85–96. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Bertolini, M.M.; Costa, R.C.; Nagay, B.E.; Dongari-Bagtzoglou, A.; Barão, V.A.R. Targeting implant-associated infections: Titanium surface loaded with antimicrobial. iScience 2021, 24, 102008. [Google Scholar] [CrossRef] [PubMed]
- Bürgers, R.; Morsczeck, C.; Felthaus, O.; Gosau, M.; Beck, H.C.; Reichert, T.E. Induced surface proteins of Streptococcus epidermidis adhering to titanium implant substrata. Clin. Oral Investig. 2018, 22, 2663–2668. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, D.A.; Fidai, A.B.; Natarajan, S.G.; Rodrigues, D.C. Succession of oral bacterial colonizers on dental implant materials: An in vitro biofilm model. Dent. Mater. 2022, 38, 384–396. [Google Scholar] [CrossRef]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implant. Res. 2007, 18, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Rickard, A.H.; Gilbert, P.; High, N.J.; Kolenbrander, P.E.; Handley, P.S. Bacterial coaggregation: An integral process in the development of multi-species biofilms. Trends Microbiol. 2003, 11, 94–100. [Google Scholar] [CrossRef]
- Wake, N.; Asahi, Y.; Noiri, Y.; Hayashi, M.; Motooka, D.; Nakamura, S.; Gotoh, K.; Miura, J.; Machi, H.; Iida, T.; et al. Temporal dynamics of bacterial microbiota in the human oral cavity determined using an in situ model of dental biofilms. Npj Biofilms Microbiomes 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Bavanilathamuthiah, M.; Kirubaharan, K.; Ramachandran, D.; Dharini, T.; Viswanathan, K.; Vishwakarma, V. Bio-inspired YSZ coated titanium by EB-PVD for biomedical applications. Surf. Coat. Technol. 2016, 307, 227–235. [Google Scholar] [CrossRef]
- Azelmad, K.; Hamadi, F.; Mimouni, R.; Amzil, K.; Latrache, H.; Mabrouki, M.; El Boulani, A. Adhesion of Staphylococcus aureus and Staphylococcus xylosus to materials commonly found in catering and domestic kitchens. Food Control 2017, 73, 156–163. [Google Scholar] [CrossRef]
- Yoda, I.; Koseki, H.; Tomita, M.; Shida, T.; Horiuchi, H.; Sakoda, H.; Osaki, M. Effect of surface roughness of biomaterials on Staphylococcus epidermidis adhesion. BMC Microbiol. 2014, 14, 1–7. [Google Scholar] [CrossRef]
- Yang, K.; Shi, J.; Wang, L.; Chen, Y.; Liang, C.; Yang, L.; Wang, L.N. Bacterial anti-adhesion surface design: Surface patterning, roughness and wettability: A review. J. Mater. Sci. Technol. 2022, 99, 82–100. [Google Scholar] [CrossRef]
- Vanhaecke, E.; Remon, J.P.; Moors, M.; Raes, F.; De Rudder, D.; Van Peteghem, A. Kinetics of Pseudomonas aeruginosa adhesion to 304 and 316-L stainless steel: Role of cell surface hydrophobicity. Appl. Environ. Microbiol. 1990, 56, 788–795. [Google Scholar] [CrossRef]
- Braceras, I.; Pacha-Olivenza, M.A.; Calzado-Martín, A.; Multigner, M.; Vera, C.; Broncano, L.L.; Gallardo-Moreno, A.M.; González-Carrasco, J.L.; Vilaboa, N.; González-Martín, M.L. Decrease of Staphylococcal adhesion on surgical stainless steel after Si ion implantation. Appl. Surf. Sci. 2014, 310, 36–41. [Google Scholar] [CrossRef]
- Wassmann, T.; Kreis, S.; Behr, M.; Buergers, R. The influence of surface texture and wettability on initial bacterial adhesion on titanium and zirconium oxide dental implants. Int. J. Implant. Dent. 2017, 3, 1–11. [Google Scholar] [CrossRef]
- Wu, Y.; Zitelli, J.P.; TenHuisen, K.S.; Yu, X.; Libera, M.R. Differential response of Staphylococci and osteoblasts to varying titanium surface roughness. Biomaterials 2011, 32, 951–960. [Google Scholar] [CrossRef]
- Lüdecke, C.; Roth, M.; Yu, W.; Horn, U.; Bossert, J.; Jandt, K.D. Nanorough titanium surfaces reduce adhesion of Escherichia coli and Staphylococcus aureus via nano adhesion points. Colloids Surf. B Biointerfaces 2016, 145, 617–625. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Gong, X.; Wu, B.; Zhang, G. Landing Dynamics of Swimming Bacteria on a Polymeric Surface: Effect of Surface Properties. Langmuir 2017, 33, 3525–3533. [Google Scholar] [CrossRef] [PubMed]
- Boks, N.P.; Busscher, H.J.; Van Der Mei, H.C.; Norde, W. Hydrophobic Surfaces Using Atomic Force Microscopy. Langmuir 2008, 24, 12990–12994. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Kumar, S.; Varadarajan, K.M. Quantification of Adhesion Force of Bacteria on the Surface of Biomaterials: Techniques and Assays. ACS Biomater. Sci. Eng. 2019, 5, 2093–2110. [Google Scholar] [CrossRef]
- Viljoen, A.; Mignolet, J.; Viela, F.; Mathelié-Guinlet, M.; Dufrêne, Y.F. How Microbes Use Force to Control Adhesion. J. Bacteriol. 2020, 202, e00125-20. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Pou, P.; Abe, M.; Jelinek, P.; Pérez, R.; Morita, S.; Custance, O. Chemical identification of individual surface atoms by atomic force microscopy. Nature 2007, 446, 64–67. [Google Scholar] [CrossRef]
- Giessibl, F.J. Advances in atomic force microscopy. Rev. Mod. Phys. 2003, 75, 949–983. [Google Scholar] [CrossRef]
- Berne, C.; Ellison, C.K.; Ducret, A.; Brun, Y.V. Bacterial adhesion at the single-cell level. Nat. Rev. Microbiol. 2018, 16, 616–627. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, C.; Chen, Z.; Allan, E.; van der Mei, H.C.; Busscher, H.J. Emergent heterogeneous microenvironments in biofilms: Substratum surface heterogeneity and bacterial adhesion force-sensing. FEMS Microbiol. Rev. 2018, 42, 259–272. [Google Scholar] [CrossRef]
- Milles, L.F.; Gaub, H.E. Extreme mechanical stability in protein complexes. Curr. Opin. Struct. Biol. 2020, 60, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Grzeszczuk, Z.; Rosillo, A.; Owens, Ó.; Bhattacharjee, S. Atomic Force Microscopy (AFM) As a Surface Mapping Tool in Microorganisms Resistant Toward Antimicrobials: A Mini-Review. Front. Pharmacol. 2020, 11, 517165. [Google Scholar] [CrossRef]
- James, S.A.; Hilal, N.; Wright, C.J. Atomic force microscopy studies of bioprocess engineering surfaces—Imaging, interactions and mechanical properties mediating bacterial adhesion. Biotechnol. J. 2017, 12, 1600698. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Dong, Y.; Zhu, J.; Rao, C. Adhesion and friction forces in biofouling attachments to nanotube- and PEG-patterned TiO2 surfaces. Colloids Surf. B Biointerfaces 2017, 159, 108–117. [Google Scholar] [CrossRef]
- Du, C.; Wang, C.; Zhang, T.; Zheng, L. Antibacterial Performance of Zr-BMG, Stainless Steel, and Titanium Alloy with Laser-Induced Periodic Surface Structures. ACS Appl. Bio. Mater. 2022, 5, 272–284. [Google Scholar] [CrossRef]
- Sarkis-Onofre, R.; Skupien, J.A.; Cenci, M.S.; Moraes, R.R.; Pereira-Cenci, T. The role of resin cement on bond strength of glass-fiber posts luted into root canals: A systematic review and metaanalysis of in vitro studies. Oper. Dent. 2014, 39, 31–44. [Google Scholar] [CrossRef]
- Cunha, A.; Elie, A.M.; Plawinski, L.; Serro, A.P.; Botelho Do Rego, A.M.; Almeida, A.; Urdaci, M.C.; Durrieu, M.C.; Vilar, R. Femtosecond laser surface texturing of titanium as a method to reduce the adhesion of Staphylococcus aureus and biofilm formation. Appl. Surf. Sci. 2016, 360, 485–493. [Google Scholar] [CrossRef]
- Dong, Y.; Ye, H.; Liu, Y.; Xu, L.; Wu, Z.; Hu, X.; Ma, J.; Pathak, J.L.; Liu, J.; Wu, G. pH dependent silver nanoparticles releasing titanium implant: A novel therapeutic approach to control peri-implant infection. Colloids Surf. B Biointerfaces 2017, 158, 127–136. [Google Scholar] [CrossRef]
- Bartmanski, M.; Cieslik, B.; Glodowska, J.; Kalka, P.; Pawlowski, L.; Pieper, M.; Zielinski, A. Electrophoretic deposition (EPD) of nanohydroxyapatite—Nanosilver coatings on Ti13Zr13Nb alloy. Ceram. Int. 2017, 43, 11820–11829. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Komarova, E.G.; Sharkeev, Y.P.; Ugodchikova, A.V.; Mushtovatova, L.S.; Karpova, M.R.; Sheikin, V.V.; Litvinova, L.S.; Khlusov, I.A. Zn-, Cu- or Ag-incorporated micro-arc coatings on titanium alloys: Properties and behavior in synthetic biological media. Surf. Coat. Technol. 2019, 369, 52–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, K.; He, S.; Wang, B.; Zhu, W.; Ren, F. Fabrication of high strength, antibacterial and biocompatible Ti-5Mo-5Ag alloy for medical and surgical implant applications. Mater. Sci. Eng. C 2020, 106, 110165. [Google Scholar] [CrossRef]
- Tsao, L.C. Effect of Sn addition on the corrosion behavior of Ti-7Cu-Sn cast alloys for biomedical applications. Mater. Sci. Eng. C 2015, 46, 246–252. [Google Scholar] [CrossRef]
- Santhosh Kumar, S.; Hiremath, S.S.; Ramachandran, B.; Muthuvijayan, V. Effect of Surface Finish on Wettability and Bacterial Adhesion of Micromachined Biomaterials. Biotribology 2019, 18, 100095. [Google Scholar] [CrossRef]
- Costa, R.C.; Nagay, B.E.; Bertolini, M.; Costa-Oliveira, B.E.; Sampaio, A.A.; Retamal-Valdes, B.; Shibli, J.A.; Feres, M.; Barão, V.A.R.; Souza, J.G.S. Fitting pieces into the puzzle: The impact of titanium-based dental implant surface modifications on bacterial accumulation and polymicrobial infections. Adv. Colloid Interface Sci. 2021, 298, 102551. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Truffa Giachet, F.; Miola, M.; Bertone, E.; Varesano, A.; Vineis, C.; Cochis, A.; Sorrentino, R.; Rimondini, L.; Spriano, S. Nanogrooves and keratin nanofibers on titanium surfaces aimed at driving gingival fibroblasts alignment and proliferation without increasing bacterial adhesion. Mater. Sci. Eng. C 2017, 76, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Davidson, P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef]
- Jain, S.; Williamson, R.S.; Marquart, M.; Janorkar, A.V.; Griggs, J.A.; Roach, M.D. Photofunctionalization of anodized titanium surfaces using UVA or UVC light and its effects against Streptococcus sanguinis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2284–2294. [Google Scholar] [CrossRef]
- Gittens, R.A.; Olivares-Navarrete, R.; Cheng, A.; Anderson, D.M.; McLachlan, T.; Stephan, I.; Geis-Gerstorfer, J.; Sandhage, K.H.; Fedorov, A.G.; Rupp, F.; et al. The roles of titanium surface micro/nanotopography and wettability on the differential response of human osteoblast lineage cells. Acta Biomater. 2013, 9, 6268–6277. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef]
- Verdeguer, P.; Gil, J.; Punset, M.; Manero, J.M.; Nart, J.; Vilarrasa, J.; Ruperez, E. Citric Acid in the Passivation of Titanium Dental Implants: Corrosion Resistance and Bactericide Behavior. Materials 2022, 15, 545. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Wang, Y.H.; Kuo, C.H.; Ou, S.F.; Huang, P.Z.; Song, T.Y.; Chen, Y.C.; Chen, S.T.; Wu, C.H.; Hsueh, Y.H.; et al. Hybrid ZnO/chitosan antimicrobial coatings with enhanced mechanical and bioactive properties for titanium implants. Carbohydr. Polym. 2021, 257, 117639. [Google Scholar] [CrossRef]
- Verran, J.; Packer, A.; Kelly, P.J.; Whitehead, K.A. Use of the atomic force microscope to determine the strength of bacterial attachment to grooved surface features. J. Adhes. Sci. Technol. 2010, 24, 2271–2285. [Google Scholar] [CrossRef]
- Petrini, M.; Giuliani, A.; Di Campli, E.; Di Lodovico, S.; Iezzi, G.; Piattelli, A.; D’ercole, S. The bacterial anti-adhesive activity of double-etched titanium (Dae) as a dental implant surface. Int. J. Mol. Sci. 2020, 21, 8315. [Google Scholar] [CrossRef]
- Amoroso, P.F.; Adams, R.J.; Waters, M.G.J.; Williams, D.W. Titanium surface modification and its effect on the adherence of Porphyromonas gingivalis: An in vitro study. Clin. Oral Implant. Res. 2006, 17, 633–637. [Google Scholar] [CrossRef]
- Vadillo-Rodríguez, V.; Guerra-García-Mora, A.I.; Perera-Costa, D.; Gónzalez-Martín, M.L.; Fernández-Calderón, M.C. Bacterial response to spatially organized microtopographic surface patterns with nanometer scale roughness. Colloids Surf. B Biointerfaces 2018, 169, 340–347. [Google Scholar] [CrossRef]
- Badihi Hauslich, L.; Sela, M.N.; Steinberg, D.; Rosen, G.; Kohavi, D. The adhesion of oral bacteria to modified titanium surfaces: Role of plasma proteins and electrostatic forces. Clin. Oral Implant. Res. 2013, 24, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Ren, C.; Ding, Y.; Chen, G.; Lu, X.; Wang, K.; Ren, F.; Yang, M.; Wang, Z.; Li, J.; et al. Micro/nano-structured TiO2 surface with dual-functional antibacterial effects for biomedical applications. Bioact. Mater. 2019, 4, 346–357. [Google Scholar] [CrossRef]
- Matsko, A.; França, R. Design, manufacturing and clinical outcomes for additively manufactured titanium dental implants: A systematic review. Dent. Rev. 2022, 2, 100041. [Google Scholar] [CrossRef]
- Tunchel, S.; Blay, A.; Kolerman, R.; Mijiritsky, E.; Shibli, J.A. 3D Printing/Additive Manufacturing Single Titanium Dental Implants: A Prospective Multicenter Study with 3 Years of Follow-Up. Int. J. Dent. 2016, 2016, 8590971. [Google Scholar] [CrossRef]
- Shibli, J.A.; Mangano, C.; Mangano, F.; Rodrigues, J.A.; Cassoni, A.; Bechara, K.; Ferreia, J.D.B.; Dottore, A.M.; Iezzi, G.; Piattelli, A. Bone-to-Implant Contact Around Immediately Loaded Direct Laser Metal-Forming Transitional Implants in Human Posterior Maxilla. J. Periodontol. 2013, 84, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Kloss, F.R.; Steinmüller-Nethl, D.; Stigler, R.G.; Ennemoser, T.; Rasse, M.; Hächl, O. In vivo investigation on connective tissue healing to polished surfaces with different surface wettability. Clin. Oral Implant. Res. 2011, 22, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, I.; Zitzmann, N.U.; Berglundh, T.; Linder, E.; Wennerberg, A.; Lindhe, J. The mucosal attachment to titanium implants with different surface characteristics: An experimental study in dogs. J. Clin. Periodontol. 2002, 29, 448–455. [Google Scholar] [CrossRef]
- Albrektsson, T.; Canullo, L.; Cochran, D.; De Bruyn, H. “Peri-Implantitis”: A Complication of a Foreign Body or a Man-Made “Disease”. Facts and Fiction. Clin. Implant. Dent. Relat. Res. 2016, 18, 840–849. [Google Scholar] [CrossRef]
- Schliephake, H.; Scharnweber, D.; Dard, M.; Sewing, A.; Aref, A.; Roessler, S. Functionalization of dental implant surfaces using adhesion molecules. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 73, 88–96. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.; Ladas, S.; Sotiropoulou, D.; Amedee, J.; Missirlis, Y.F. Effect of surface roughness of the titanium alloy Ti–6Al–4V on human bone marrow cell response and on protein adsorption. Biomaterials 2001, 22, 1241–1251. [Google Scholar] [CrossRef]
- Fröjd, V.; Linderbäck, P.; Wennerberg, A.; Chávez de Paz, L.; Svensäter, G.; Davies, J.R. Effect of nanoporous TiO2 coating and anodized Ca2+ modification of titanium surfaces on early microbial biofilm formation. BMC Oral Health 2011, 11, 8. [Google Scholar] [CrossRef]
- Degidi, M.; Artese, L.; Piattelli, A.; Scarano, A.; Shibli, J.A.; Piccirilli, M.; Perrotti, V.; Iezzi, G. Histological and immunohistochemical evaluation of the peri-implant soft tissues around machined and acid-etched titanium healing abutments: A prospective randomised study. Clin. Oral Investig. 2012, 16, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Rompen, E.; Domken, O.; Degidi, M.; Farias Pontes, A.E.; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral Implant. Res. 2006, 17, 55–67. [Google Scholar] [CrossRef]
- Johansson, K.; Jimbo, R.; Östlund, P.; Tranæus, S.; Becktor, J.P. Effects of Bacterial Contamination on Dental Implants during Surgery: A Systematic Review. Implant. Dent. 2017, 26, 778–789. [Google Scholar] [CrossRef]
- Mark Welch, J.L.; Dewhirst, F.E.; Borisy, G.G. Biogeography of the Oral Microbiome: The Site-Specialist Hypothesis. Annu. Rev. Microbiol. 2019, 73, 335–358. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseo integrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Gulati, K.; Arora, H.; Han, P.; Fournier, B.; Ivanovski, S. Race to invade: Understanding soft tissue integration at the transmucosal region of titanium dental implants. Dent. Mater. 2021, 37, 816–831. [Google Scholar] [CrossRef]
- Ivanovski, S.; Lee, R. Comparison of peri-implant and periodontal marginal soft tissues in health and disease. Periodontol. 2000 2018, 76, 116–130. [Google Scholar] [CrossRef]
- Branemark, P.-I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Chan, D.; Pelekos, G.; Ho, D.; Cortellini, P.; Tonetti, M.S. The depth of the implant mucosal tunnel modifies the development and resolution of experimental peri-implant mucositis: A case–control study. J. Clin. Periodontol. 2019, 46, 248–255. [Google Scholar] [CrossRef]
- Rokn, A.; Aslroosta, H.; Akbari, S.; Najafi, H.; Zayeri, F.; Hashemi, K. Prevalence of peri-implantitis in patients not participating in well-designed supportive periodontal treatments: A cross-sectional study. Clin. Oral Implant. Res. 2017, 28, 314–319. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro- and Nanotopography Sensitive Bacterial Attachment Mechanisms: A Review. Front. Microbiol. 2019, 10, 191. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Title |
|---|---|
| Aguayo et al., 2015 [1] | Nanoadhesion of Staphylococcus aureus onto Titanium Implant Surfaces |
| Aguayo et al., 2016 [14] | Probing the nanoadhesion of Streptococcus sanguinis to titanium implant surfaces by atomic force microscopy |
| Alam and Balani 2017 [15] | Adhesion force of Staphylococcus aureus on various biomaterial surfaces |
| An et al., 2017 [48] | Adhesion and friction forces in biofouling attachments to nanotubeand PEG-patterned TiO2 surfaces |
| Du et al., 2022 [49] | Antibacterial Performance of Zr-BMG, Stainless Steel, and Titanium Alloy with Laser-Induced Periodic Surface Structures |
| Author, Year | Population | Wettability Assessment Method | Wettability Results | Roughness Assessment Method | Roughness Results | Bacteria | Bacterial Adhesion Strength by AFM Result | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Aguayo et al., 2015 [1] | Ti Sample= Ti | Optical contact angle meter (CAM 200, KSV Instruments, Biolin Scientific, MD, USA) | 67.0 ± 5° | Profilometry (Proscan 1000, Scantron Ltd., Somerset, UK) | Ra 0.61 µm | S. aureus | 0 s = −0.27 ± 0.30 nN 60 s = −9.15 ± 0.78 nN | The binding strength of S. aureus on Ti increases with time. The slightly hydrophilic surface favored the formation of hydrogen bonds between the bacterium and the substrate. The roughness characterizes the surface as smooth Ra < 1 µm. |
| Aguayo et al., 2016 [14] | Ti Sample = Ti | Optical contact angle meter (KSV Instruments, CAM 200, Monroe, CT, USA) | 67.0° ± 5.0° | Conventional profilometry (Proscan 1000, Scantron, Somerset, UK) and AFM profilometry (Gwyddion 2.31 software, n = 9256 × 256 pixel scans) | Ra CP= 0.61 ± 0.01 μm and AFM = 0.17 ± 0.02 μm | S. sanguinis | 0 s = 0.32 ± 0.00 nN 1 s = 1.07 ± 0.06 nN 60 s = 4.85 ± 0.56 nN | The binding strength of S. sanguinis on Ti increases with time. The slightly hydrophilic surface that favored the formation of hydrogen bonds between the bacterium and the substrate. The roughness characterizes the surface as smooth Ra < 1 µm. |
| Alam and Balani 2017 [15] | G1 = Ti-6Al-4V; G2 = UHMWPE; G3 = SS; G4 = HA. | contact angle goniometer (Dataphysics Contact Angle System OCA) | Ti-6Al-4V = 68.8 ± 5.6 UHMWPE= 81.9 ± 2.3 SS= 48.7 ± 1.9 HA= 94.9 ± 1.2 | AFM by Nanoscope Analysis (Bruker, version 1.40) | Ra Ti-6Al-4V = 289 nm UHMWPE = 70 nm SS = 219 nm HA = 229 nm | S. aureus | 0 a 10 s Maximum adhesion strength = Ti-6Al-4V = 11.12 ± 1.07 UHMWPE = 4.10 ± 0.65 SS = 15.21 ± 1.41 HA = 7.66 ± 0.67 | Adhesion strength increased with time; the greater the roughness and wettability, the greater the adhesion force. |
| An et al., 2017 [48] | Ti Groups = G1 = DT; G2 = TN20; G3 = TN80; G4 = DT-P; G5 = TN20-P; G6 = TN80-P. | Contact angles by a DSA100 (Kruss, Germany) | DT = 96.1 ± 2.6°; TN20 = 78 ± 0.9°; TN80 = 19.5 ± 0.7°; DT-P = 68.8 ± 1.5°; TN20-P, 15.2 ± 0.1°; TN80-P, 21.2 ± 0.6°. | AFM (AFM, Dimension Icon, Bruker, USA) | RMS DT = 1.06 nm; TN20 = 19.0 nm; TN80 = 29.2 nm; DT-P = 5.13 nm; TN20-P = 28.1 nm; TN80-P = 41.2 nm. | S. aureus | Maximum adhesion strength = = DT = 172.6 ± 6.12 nN; TN20 = 31.3 ± 6.1 nN; TN80 = 32.4 ± 1.89 nN; DT-P = 149.3 ± 6.22 nN; TN20-P = 72.5 ± 3.43 nN; TN80-P = 26.4 ± 3.75 nN. | The addition of PEG increased the hydrophilicity and roughness. However, it decreased bacterial adhesion and adhesion strength. It is noteworthy that the presence of nanotubes decreased the surface area and increased the hydrophilicity. |
| Du et al., 2022 [49] | G1 = Zr-BMG; G2 = 316L; G3 = TC4; G4 = Zr-BMG-LIPSS; G5 = 316L-LIPSS; G6 = TC4-LIPSS. | Contact angle goniometer (OSA-200) | Zr-BMG= 73.6 ± 0.7°; 316L = 72.9 ± 0.5°; TC4 = 64.3 ± 0.7°; Zr-BMG-LIPSS = 28.3 ± 1.4°; 316L-LIPSS = 25.2 ± 0.9°; TC4-LIPSS = 20.6 ± 1.1°. | AFM (Bruker fast scan) | Ra Zr-BMG = NS; 316L = NS; TC4 = NS; Zr-BMG-LIPSS = 68.3 ± 9.1 nm; 316L-LIPSS = 54.7 ± 7.3 nm; TC4-LIPSS = 118 ± 10.6 nm. | E. coli and S. aureus. | E. coli Zr-BMG = 4.47 ± 0.73 nN; 316L = 4.37 ± 0.73 nN; TC4 = 4.87 ± 0.63 nN; Zr-BMG-LIPSS = 2.51 ± 0.59 nN; 316L-LIPSS = 2.61 ± 0.79 nN; TC4-LIPSS = 2.47 ± 0.73 nN. S. aureus Zr-BMG = 4.05 ± 0.45 nN; 316L = 3.98 ± 0.62 nN; TC4 = 4.74 ± 0.56 nN; Zr-BMG-LIPSS = 0.88 ± 0.52 nN; 316L-LIPSS = 0.93 ± 0.47 nN; TC4-LIPSS = 1.06 ± 0.44 nN. | The LIPSS treatment reduced the bacterial adhesion strength for E. coli and S. aureus in all samples and increased the roughness and wettability of the samples. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tardelli, J.D.C.; Bagnato, V.S.; Reis, A.C.d. Bacterial Adhesion Strength on Titanium Surfaces Quantified by Atomic Force Microscopy: A Systematic Review. Antibiotics 2023, 12, 994. https://doi.org/10.3390/antibiotics12060994
Tardelli JDC, Bagnato VS, Reis ACd. Bacterial Adhesion Strength on Titanium Surfaces Quantified by Atomic Force Microscopy: A Systematic Review. Antibiotics. 2023; 12(6):994. https://doi.org/10.3390/antibiotics12060994
Chicago/Turabian StyleTardelli, Juliana Dias Corpa, Vanderlei Salvador Bagnato, and Andréa Cândido dos Reis. 2023. "Bacterial Adhesion Strength on Titanium Surfaces Quantified by Atomic Force Microscopy: A Systematic Review" Antibiotics 12, no. 6: 994. https://doi.org/10.3390/antibiotics12060994
APA StyleTardelli, J. D. C., Bagnato, V. S., & Reis, A. C. d. (2023). Bacterial Adhesion Strength on Titanium Surfaces Quantified by Atomic Force Microscopy: A Systematic Review. Antibiotics, 12(6), 994. https://doi.org/10.3390/antibiotics12060994







