Uncovering the Important Genetic Factors for Growth during Cefotaxime-Gentamicin Combination Treatment in blaCTX-M-1 Encoding Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Determination of MIC and Interaction between CTX and GEN
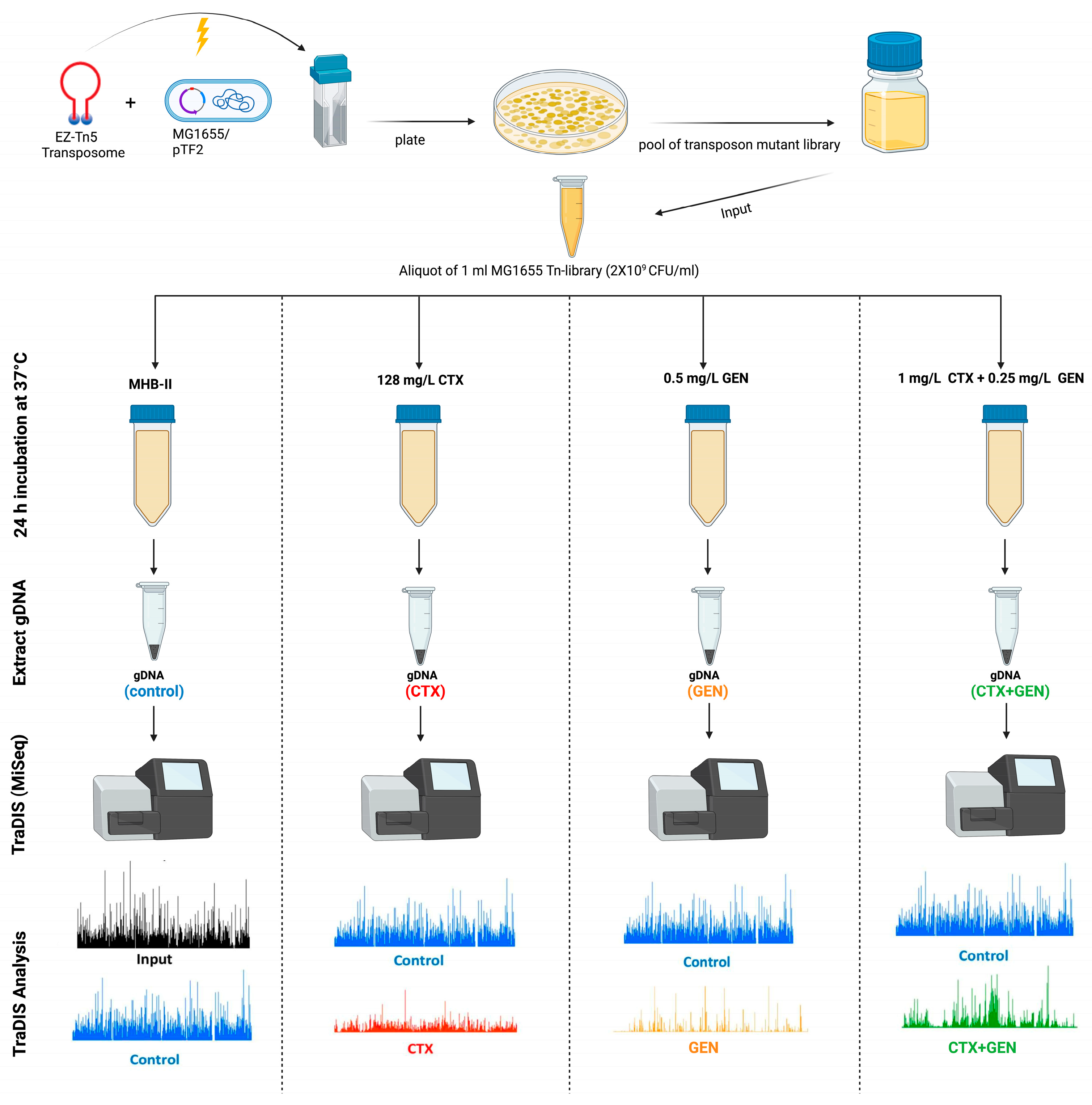
2.3. TraDIS Library Construction and Validation
2.4. TraDIS Library Antimicrobial Exposure and Sequencing
2.5. Analysis of TraDIS Data
2.6. Deletion of Selected Genes in MG1655/pTF2
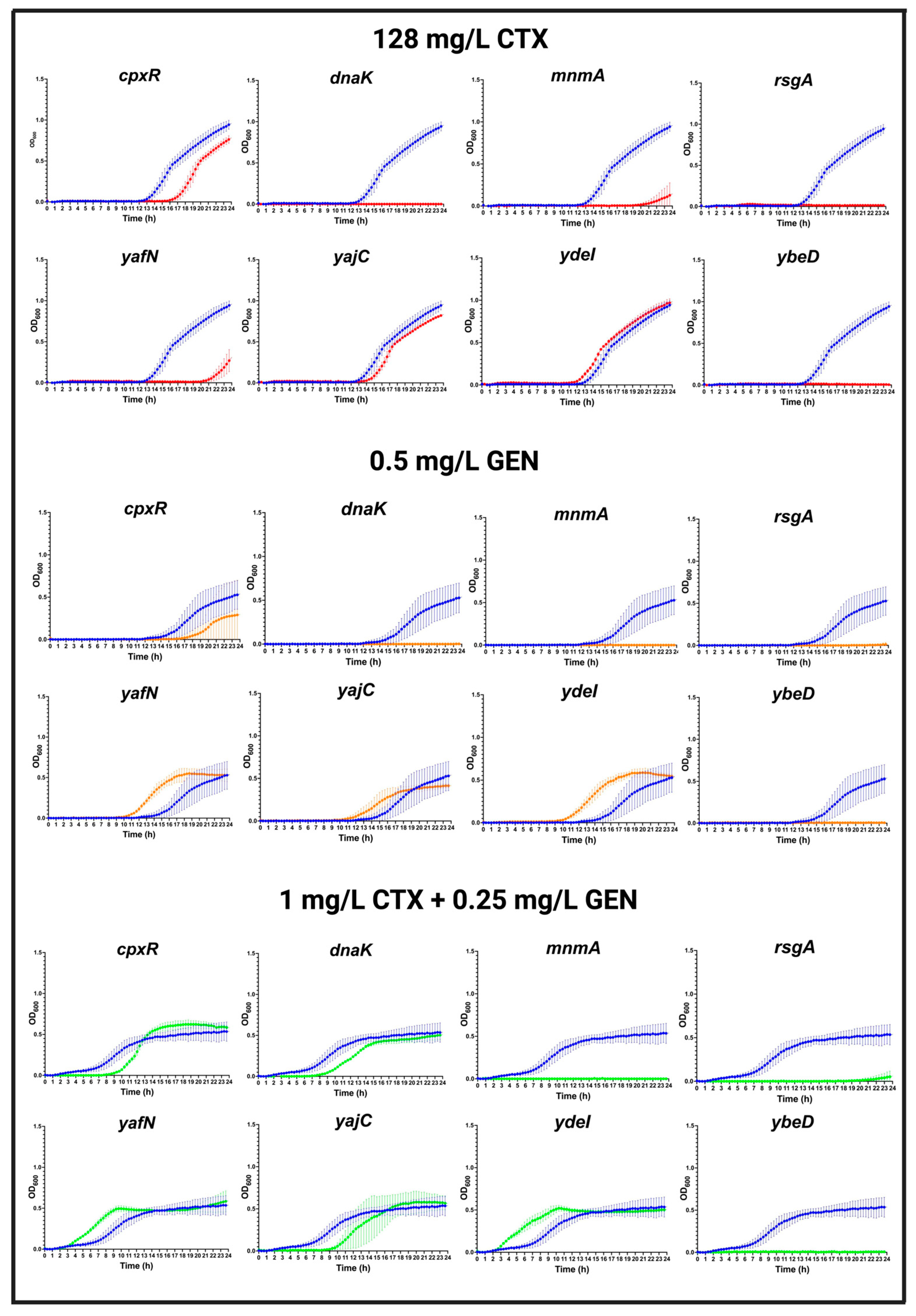
2.7. Growth Experiments
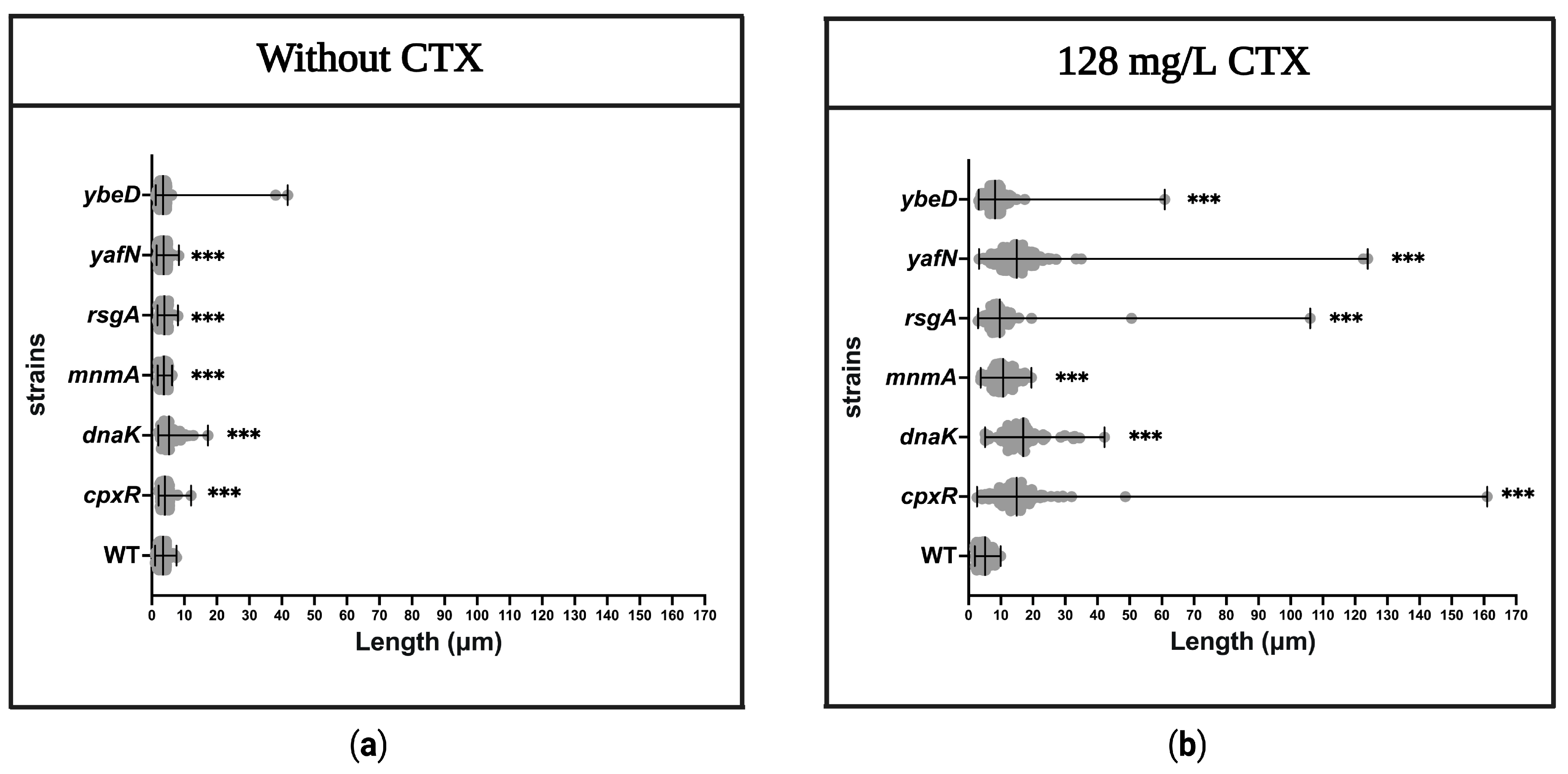
2.8. Morphology of Bacteria
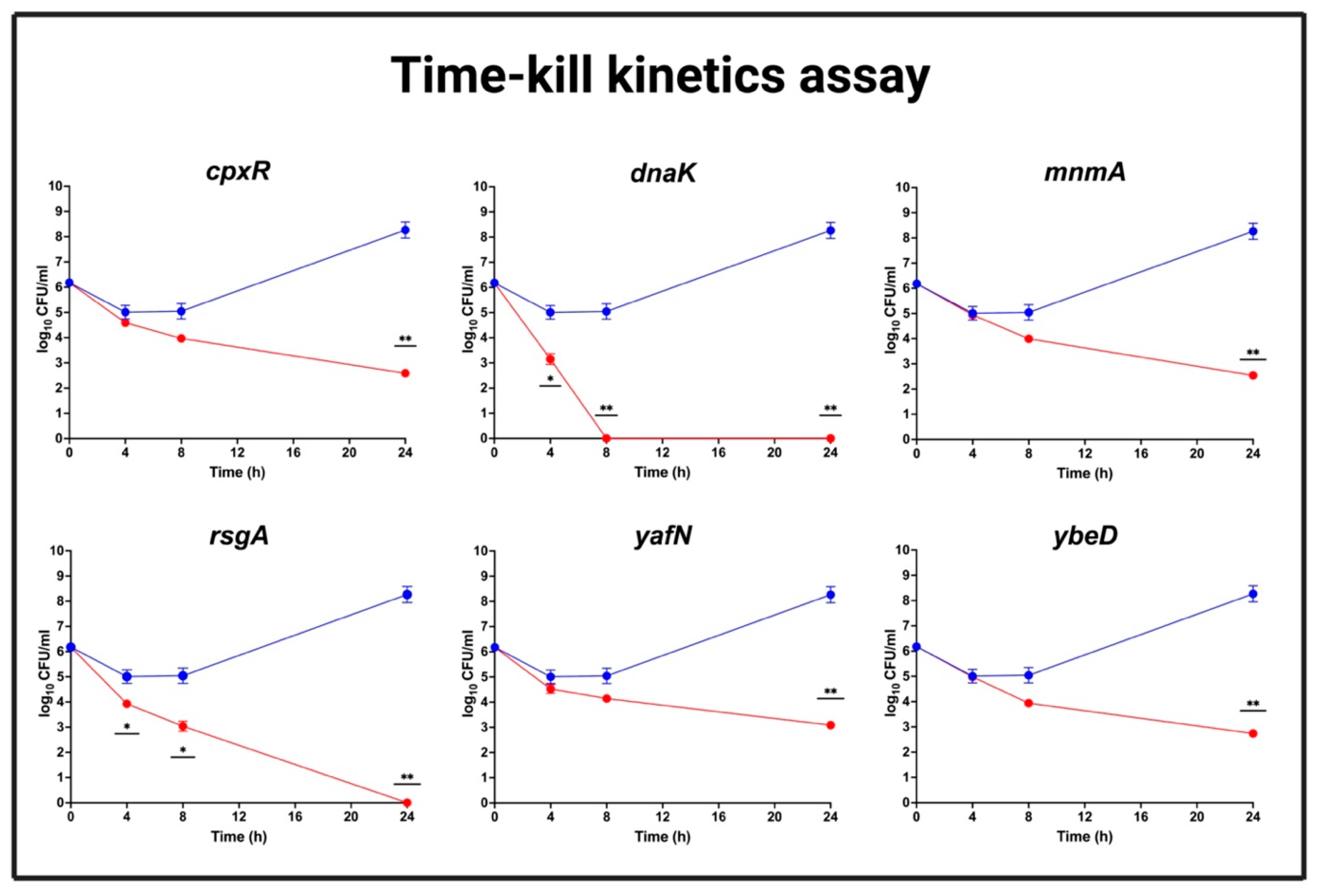
2.9. Time-Kill Assays
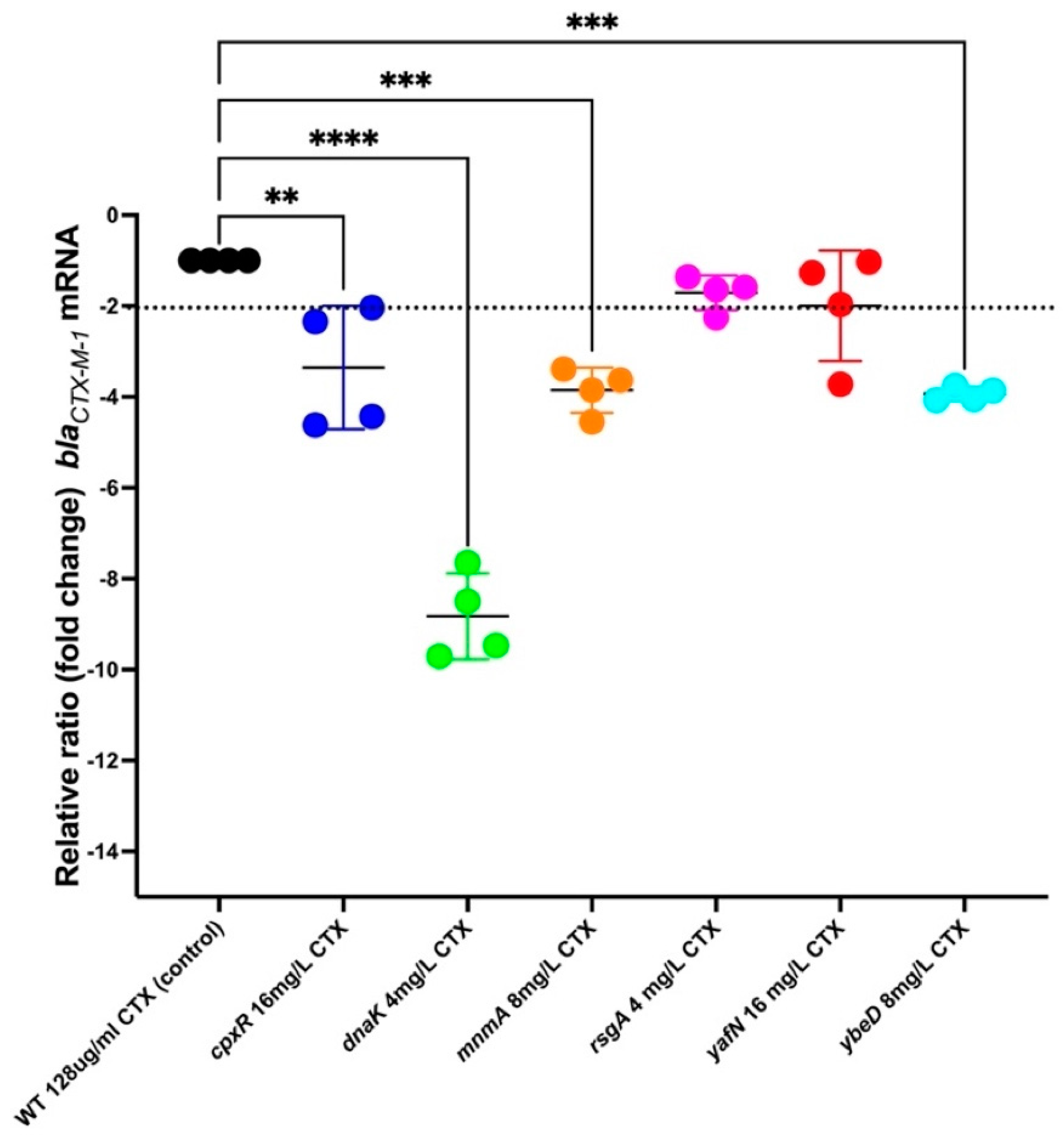
2.10. RNA Extraction and RT-qPCR
2.11. In Silico Homology Study
2.12. Statistical Analysis
3. Results
3.1. Validation of Synergy between CTX and GEN
3.2. Generation of a High-Density Mutant Library and Sequencing Using TraDIS Protocol
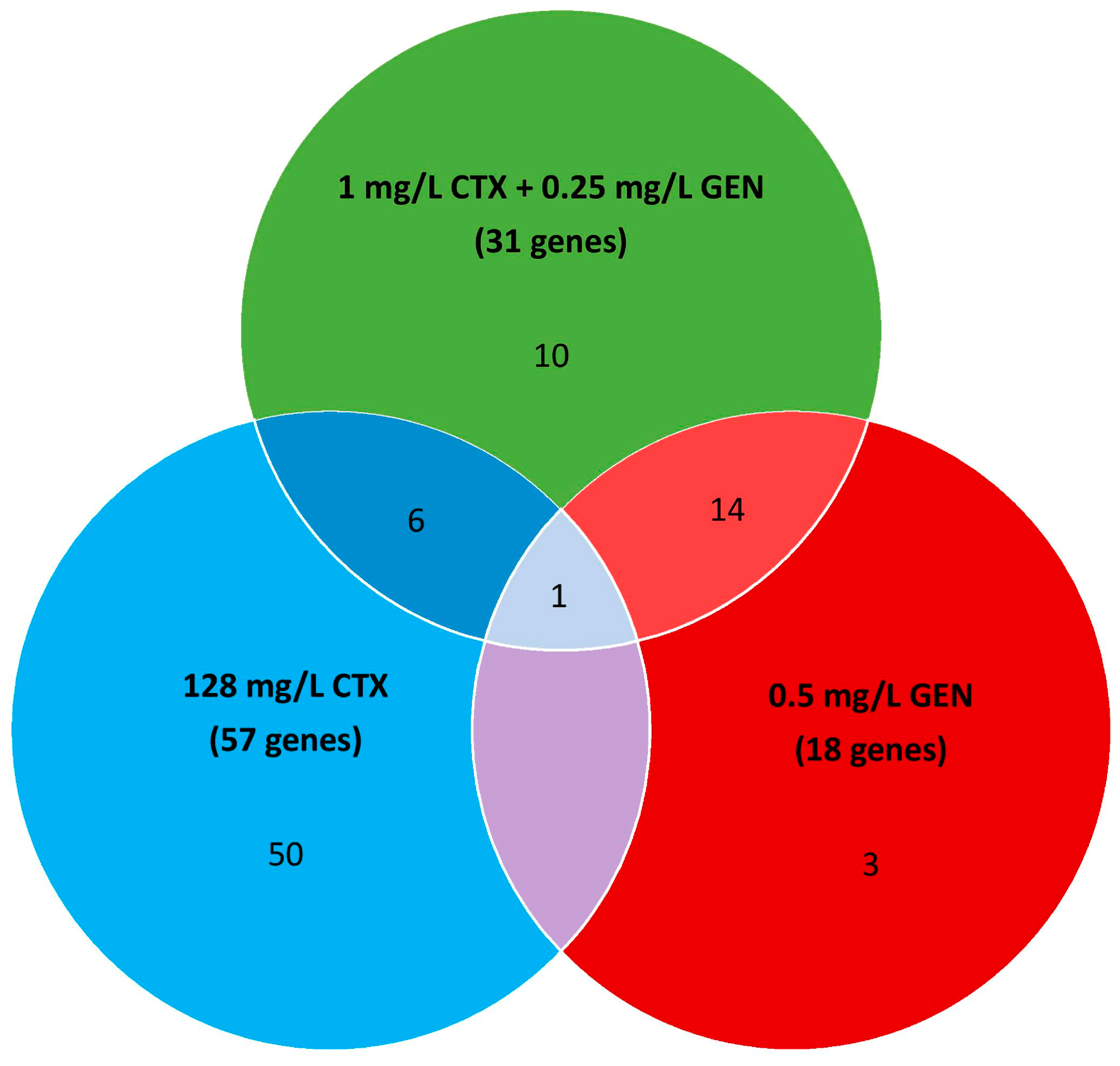
3.3. Identification of Genes Relevant for Growth with and without Antibiotics
3.4. The Secondary Resistome to CTX
3.5. The Secondary Resistome to GEN
3.6. The Secondary Resistome to CTX-GEN Combination Treatment
3.7. Validation of Genes Identified by TraDIS
3.8. Increased Susceptibility to Antimicrobials
3.9. Determination of Filament Formation in the Mutant Strains
3.10. CTX Efficacy for Mutants Showing Reduced MIC to CTX
3.11. blaCTX-M-1 mRNA Levels in the Mutants Identified to Increase Efficacy of CTX
3.12. Homology to Human Proteome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTX-M | Cefotaximase from Munich |
| ESBL | Extended-Spectrum β-Lactamase |
| CTX | Cefotaxime |
| GEN | Gentamicin |
| MIC | Minimum Inhibitory Concentration |
| TraDIS | Transposon Directed Insertion-site Sequencing |
| USD | United States dollar |
| WHO | World Health Organization |
| PBPs | Penicillin-binding proteins |
| CHL | Chloramphenicol |
| SR | Secondary resistome |
| LB | Lysogeny broth |
| Kan | Kanamycin |
| CFZ | Cefazolin |
| FOX | Cefoxitin |
| CAZ | Ceftazidime |
| FEP | Cefepime |
| CPT | Ceftaroline |
| CLSI | Clinical and Laboratory Standards Institute |
| CFU/ml | Colony-Forming Unit per millilitre |
| MHB-II | Mueller –Hinton broth II |
| FIC | Fractional Inhibitory Concentration |
| FICI | Fractional Inhibitory Concentration Index |
| SOC | Super Optimal broth with Catabolites repression |
| UISs | Unique Insertion Sites |
| ENA | European Nucleotide Archive |
| STRING | Search Tool for the Retrieval of Interacting Genes/Proteins |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GO | Gene Ontology |
| WT | Wild type |
| OD600 | Optical density at 600 nm |
| RT-qPCR | Reverse-Transcribed-quantitative Real Time Polymerase Chain Reaction |
| BLAST | Basic Local Alignment Search Tool |
| TCA | Tricarboxylic acid |
| ATP | Adenosine triphosphate |
| kDa | kilodalton |
| HSPs | Heat shock proteins |
| ST | Sequence Type |
| TAT | Twin arginine translocation |
References
- Piddock, L.J.V. Reflecting on the final report of the O’Neill Review on Antimicrobial Resistance. Lancet Infect. Dis. 2016, 16, 767–768. [Google Scholar] [CrossRef]
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 12 May 2023).
- Bonnet, R. Growing group of extended-spectrum beta-lactamases: The CTX-M enzymes. Antimicrob. Agents Chemother. 2004, 48, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Alyamani, E.J.; Khiyami, A.M.; Booq, R.Y.; Majrashi, M.A.; Bahwerth, F.S.; Rechkina, E. The occurrence of ESBL-producing Escherichia coli carrying aminoglycoside resistance genes in urinary tract infections in Saudi Arabia. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 1. [Google Scholar] [CrossRef]
- Al Naiemi, N.; Duim, B.; Bart, A. A CTX-M extended-spectrum beta-lactamase in Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J. Med. Microbiol. 2006, 55, 1607–1608. [Google Scholar] [CrossRef]
- Hu, X.; Gou, J.; Guo, X.; Cao, Z.; Li, Y.; Jiao, H.; He, X.; Ren, Y.; Tian, F. Genetic contexts related to the diffusion of plasmid-mediated CTX-M-55 extended-spectrum beta-lactamase isolated from Enterobacteriaceae in China. Ann Clin Microbiol Antimicrob 2018, 17, 12. [Google Scholar] [CrossRef]
- Ramadan, A.A.; Abdelaziz, N.A.; Amin, M.A.; Aziz, R.K. Novel blaCTX-M variants and genotype-phenotype correlations among clinical isolates of extended spectrum beta lactamase-producing Escherichia coli. Sci. Rep. 2019, 9, 4224. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.A.; Tyler, S.; Christianson, S.; McGeer, A.; Muller, M.P.; Willey, B.M.; Bryce, E.; Gardam, M.; Nordmann, P.; Mulvey, M.R. Complete nucleotide sequence of a 92-kilobase plasmid harboring the CTX-M-15 extended-spectrum beta-lactamase involved in an outbreak in long-term-care facilities in Toronto, Canada. Antimicrob. Agents Chemother. 2004, 48, 3758–3764. [Google Scholar] [CrossRef] [PubMed]
- Cottarel, G.; Wierzbowski, J. Combination drugs, an emerging option for antibacterial therapy. Trends Biotechnol. 2007, 25, 547–555. [Google Scholar] [CrossRef]
- Vilchèze, C.; Jacobs, W.R., Jr. The combination of sulfamethoxazole, trimethoprim, and isoniazid or rifampin is bactericidal and prevents the emergence of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2012, 56, 5142–5148. [Google Scholar] [CrossRef]
- Jana, B.; Cain, A.K.; Doerrler, W.T.; Boinett, C.J.; Fookes, M.C.; Parkhill, J.; Guardabassi, L. The secondary resistome of multidrug-resistant Klebsiella pneumoniae. Sci. Rep. 2017, 7, 42483. [Google Scholar] [CrossRef]
- Møller, T.S.; Rau, M.H.; Bonde, C.S.; Sommer, M.O.; Guardabassi, L.; Olsen, J.E. Adaptive responses to cefotaxime treatment in ESBL-producing Escherichia coli and the possible use of significantly regulated pathways as novel secondary targets. J. Antimicrob. Chemother. 2016, 71, 2449–2459. [Google Scholar] [CrossRef] [PubMed]
- Yunis, A.A. Chloramphenicol toxicity: 25 years of research. Am. J. Med. 1989, 87, 44n–48n. [Google Scholar]
- Fatsis-Kavalopoulos, N.; Roelofs, L.; Andersson, D.I. Potential risks of treating bacterial infections with a combination of β-lactam and aminoglycoside antibiotics: A systematic quantification of antibiotic interactions in E. coli blood stream infection isolates. EBioMedicine 2022, 78, 103979. [Google Scholar] [CrossRef]
- Martínez, J.A.; Cobos-Trigueros, N.; Soriano, A.; Almela, M.; Ortega, M.; Marco, F.; Pitart, C.; Sterzik, H.; Lopez, J.; Mensa, J. Influence of empiric therapy with a beta-lactam alone or combined with an aminoglycoside on prognosis of bacteremia due to gram-negative microorganisms. Antimicrob. Agents Chemother. 2010, 54, 3590–3596. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, T.S.; Overgaard, M.; Nielsen, S.S.; Bortolaia, V.; Jelsbak, L.; Sommer, M.; Guardabassi, L.; Olsen, J.E. CTX-M-1 β-lactamase expression in Escherichia coli is dependent on cefotaxime concentration, growth phase and gene location. J. Antimicrob. Chemother. 2015, 70, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.B.; Cockerill, F.; Bradford, P.A. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fifth Informational Supplement; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2015. [Google Scholar]
- Aparna, V.; Dineshkumar, K.; Mohanalakshmi, N.; Velmurugan, D.; Hopper, W. Identification of natural compound inhibitors for multidrug efflux pumps of Escherichia coli and Pseudomonas aeruginosa using in silico high-throughput virtual screening and in vitro validation. PLoS ONE 2014, 9, e101840. [Google Scholar] [CrossRef]
- Klitgaard, R.N.; Jana, B.; Guardabassi, L.; Nielsen, K.L.; Løbner-Olesen, A. DNA Damage Repair and Drug Efflux as Potential Targets for Reversing Low or Intermediate Ciprofloxacin Resistance in E. coli K-12. Front. Microbiol. 2018, 9, 1438. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Matsumura, S.O.; Louie, L.; Louie, M.; Simor, A.E. Synergy testing of vancomycin-resistant Enterococcus faecium against quinupristin-dalfopristin in combination with other antimicrobial agents. Antimicrob. Agents Chemother. 1999, 43, 2776–2779. [Google Scholar] [CrossRef]
- Pillai, S.K.; Moellering, R.; Eliopoulos, G.M. Antimicrobial combinations. Antibiot. Lab. Med. 2005, 5, 365–440. [Google Scholar]
- Phan, M.D.; Peters, K.M.; Sarkar, S.; Lukowski, S.W.; Allsopp, L.P.; Gomes Moriel, D.; Achard, M.E.; Totsika, M.; Marshall, V.M.; Upton, M.; et al. The serum resistome of a globally disseminated multidrug resistant uropathogenic Escherichia coli clone. PLoS Genet. 2013, 9, e1003834. [Google Scholar] [CrossRef] [PubMed]
- Andersen-Civil, A.I.S.; Ahmed, S.; Guerra, P.R.; Andersen, T.E.; Hounmanou, Y.M.G.; Olsen, J.E.; Herrero-Fresno, A. The impact of inactivation of the purine biosynthesis genes, purN and purT, on growth and virulence in uropathogenic E. coli. Mol. Biol. Rep. 2018, 45, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- García, V.; Grønnemose, R.B.; Torres-Puig, S.; Kudirkiene, E.; Piantelli, M.; Ahmed, S.; Andersen, T.E.; Møller-Jensen, J.; Olsen, J.E.; Herrero-Fresno, A. Genome-wide analysis of fitness-factors in uropathogenic Escherichia coli during growth in laboratory media and during urinary tract infections. Microb. Genom. 2021, 7, 000719. [Google Scholar] [CrossRef]
- Barquist, L.; Mayho, M.; Cummins, C.; Cain, A.K.; Boinett, C.J.; Page, A.J.; Langridge, G.C.; Quail, M.A.; Keane, J.A.; Parkhill, J. The TraDIS toolkit: Sequencing and analysis for dense transposon mutant libraries. Bioinformatics 2016, 32, 1109–1111. [Google Scholar] [CrossRef]
- Carver, T.; Harris, S.R.; Berriman, M.; Parkhill, J.; McQuillan, J.A. Artemis: An integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics 2012, 28, 464–469. [Google Scholar] [CrossRef]
- Carver, T.; Thomson, N.; Bleasby, A.; Berriman, M.; Parkhill, J. DNAPlotter: Circular and linear interactive genome visualization. Bioinformatics 2009, 25, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Doublet, B.; Douard, G.; Targant, H.; Meunier, D.; Madec, J.Y.; Cloeckaert, A. Antibiotic marker modifications of lambda Red and FLP helper plasmids, pKD46 and pCP20, for inactivation of chromosomal genes using PCR products in multidrug-resistant strains. J. Microbiol. Methods 2008, 75, 359–361. [Google Scholar] [CrossRef]
- Stringer, C.; Wang, T.; Michaelos, M.; Pachitariu, M. Cellpose: A generalist algorithm for cellular segmentation. Nat. Methods 2021, 18, 100–106. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic. Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Mondal, S.I.; Ferdous, S.; Jewel, N.A.; Akter, A.; Mahmud, Z.; Islam, M.M.; Afrin, T.; Karim, N. Identification of potential drug targets by subtractive genome analysis of Escherichia coli O157:H7: An in silico approach. Adv. Appl. Bioinform. Chem. 2015, 8, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Paradis-Bleau, C.; Markovski, M.; Uehara, T.; Lupoli, T.J.; Walker, S.; Kahne, D.E.; Bernhardt, T.G. Lipoprotein cofactors located in the outer membrane activate bacterial cell wall polymerases. Cell 2010, 143, 1110–1120. [Google Scholar] [CrossRef]
- Typas, A.; Banzhaf, M.; van den Berg van Saparoea, B.; Verheul, J.; Biboy, J.; Nichols, R.J.; Zietek, M.; Beilharz, K.; Kannenberg, K.; von Rechenberg, M.; et al. Regulation of peptidoglycan synthesis by outer-membrane proteins. Cell 2010, 143, 1097–1109. [Google Scholar] [CrossRef]
- Deckers-Hebestreit, G.; Altendorf, K. The F0F1-type ATP synthases of bacteria: Structure and function of the F0 complex. Annu. Rev. Microbiol. 1996, 50, 791–824. [Google Scholar] [CrossRef]
- Danese, P.N.; Snyder, W.B.; Cosma, C.L.; Davis, L.J.; Silhavy, T.J. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 1995, 9, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Christensen-Dalsgaard, M.; Jørgensen, M.G.; Gerdes, K. Three new RelE-homologous mRNA interferases of Escherichia coli differentially induced by environmental stresses. Mol. Microbiol. 2010, 75, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hiibel, S.R.; Reardon, K.F.; Wood, T.K. Identification of stress-related proteins in Escherichia coli using the pollutant cis-dichloroethylene. J. Appl. Microbiol. 2010, 108, 2088–2102. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Langer, T.; Hartl, F.U.; Bukau, B. DnaK, DnaJ and GrpE form a cellular chaperone machinery capable of repairing heat-induced protein damage. Embo J. 1993, 12, 4137–4144. [Google Scholar] [CrossRef]
- Tamaru, D.; Amikura, K.; Shimizu, Y.; Nierhaus, K.H.; Ueda, T. Reconstitution of 30S ribosomal subunits in vitro using ribosome biogenesis factors. Rna 2018, 24, 1512–1519. [Google Scholar] [CrossRef]
- Jomaa, A.; Stewart, G.; Martín-Benito, J.; Zielke, R.; Campbell, T.L.; Maddock, J.R.; Brown, E.D.; Ortega, J. Understanding ribosome assembly: The structure of in vivo assembled immature 30S subunits revealed by cryo-electron microscopy. Rna 2011, 17, 697–709. [Google Scholar] [CrossRef]
- Kambampati, R.; Lauhon, C.T. MnmA and IscS are required for in vitro 2-thiouridine biosynthesis in Escherichia coli. Biochemistry 2003, 42, 1109–1117. [Google Scholar] [CrossRef]
- Schulze, R.J.; Komar, J.; Botte, M.; Allen, W.J.; Whitehouse, S.; Gold, V.A.; Lycklama, A.N.J.A.; Huard, K.; Berger, I.; Schaffitzel, C.; et al. Membrane protein insertion and proton-motive-force-dependent secretion through the bacterial holo-translocon SecYEG-SecDF-YajC-YidC. Proc. Natl. Acad. Sci. USA 2014, 111, 4844–4849. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, Y.; Yoon, S.H. Overexpression of YbeD in Escherichia coli Enhances Thermotolerance. J. Microbiol. Biotechnol. 2019, 29, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Dudley, M.N.; Ambrose, P.G.; Bhavnani, S.M.; Craig, W.A.; Ferraro, M.J.; Jones, R.N. Background and rationale for revised clinical and laboratory standards institute interpretive criteria (Breakpoints) for Enterobacteriaceae and Pseudomonas aeruginosa: I. Cephalosporins and Aztreonam. Clin. Infect. Dis. 2013, 56, 1301–1309. [Google Scholar] [CrossRef]
- Gould, I.M.; MacKenzie, F.M. The response of Enterobacteriaceae to beta-lactam antibiotics—‘round forms, filaments and the root of all evil’. J. Antimicrob. Chemother. 1997, 40, 495–499. [Google Scholar] [CrossRef]
- Hanberger, H.; Nilsson, L.E.; Nilsson, M.; Maller, R. Post-antibiotic effect of beta-lactam antibiotics on gram-negative bacteria in relation to morphology, initial killing and MIC. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 927–934. [Google Scholar] [CrossRef]
- Greenwood, D. Antibiotic effects in vitro and the prediction of clinical response. J. Antimicrob. Chemother. 1997, 40, 499–501. [Google Scholar] [CrossRef]
- Murray, P.R. Activity of cefotaxime-aminoglycoside combinations against aminoglycoside-resistant Pseudomonas. Antimicrob. Agents Chemother. 1980, 17, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Klastersky, J.; Swings, G.; Daneau, D. Antimicrobial activity of the carbenicillin-gentamicin combination against gram-negative bacilli. Am. J. Med. Sci. 1970, 260, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Giamarellou, H. Aminoglycosides plus beta-lactams against gram-negative organisms. Evaluation of in vitro synergy and chemical interactions. Am. J. Med. 1986, 80, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Neu, H.C.; Fu, K.P. Synergy of azlocillin and mezlocillin combined with aminoglycoside antibiotics and cephalosporins. Antimicrob. Agents Chemother. 1978, 13, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.D.; Bottomley, A.L.; Peters, K.M.; Harry, E.J.; Schembri, M.A. Uncovering novel susceptibility targets to enhance the efficacy of third-generation cephalosporins against ESBL-producing uropathogenic Escherichia coli. J. Antimicrob. Chemother. 2020, 75, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Lobritz, M.A.; Belenky, P.; Porter, C.B.; Gutierrez, A.; Yang, J.H.; Schwarz, E.G.; Dwyer, D.J.; Khalil, A.S.; Collins, J.J. Antibiotic efficacy is linked to bacterial cellular respiration. Proc. Natl. Acad. Sci. USA 2015, 112, 8173–8180. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Leng, B.; Haaber, J.; Bojer, M.S.; Vegge, C.S.; Ingmer, H. Genome-Wide Identification of Antimicrobial Intrinsic Resistance Determinants in Staphylococcus aureus. Front. Microbiol. 2016, 7, 2018. [Google Scholar] [CrossRef]
- Tamae, C.; Liu, A.; Kim, K.; Sitz, D.; Hong, J.; Becket, E.; Bui, A.; Solaimani, P.; Tran, K.P.; Yang, H.; et al. Determination of antibiotic hypersensitivity among 4000 single-gene-knockout mutants of Escherichia coli. J. Bacteriol. 2008, 190, 5981–5988. [Google Scholar] [CrossRef]
- Numata, T.; Ikeuchi, Y.; Fukai, S.; Suzuki, T.; Nureki, O. Snapshots of tRNA sulphuration via an adenylated intermediate. Nature 2006, 442, 419–424. [Google Scholar] [CrossRef]
- Spratt, B.G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc. Natl. Acad. Sci. USA 1975, 72, 2999–3003. [Google Scholar] [CrossRef]
- Kadam, U.S.; Lossie, A.C.; Schulz, B.; Irudayaraj, J. Gene Expression Analysis Using Conventional and Imaging Methods. In DNA and RNA Nanobiotechnologies in Medicine: Diagnosis and Treatment of Diseases; Erdmann, V.A., Barciszewski, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 141–162. [Google Scholar] [CrossRef]
- Wild, J.; Altman, E.; Yura, T.; Gross, C.A. DnaK and DnaJ heat shock proteins participate in protein export in Escherichia coli. Genes Dev. 1992, 6, 1165–1172. [Google Scholar] [CrossRef]
- Wild, J.; Rossmeissl, P.; Walter, W.A.; Gross, C.A. Involvement of the DnaK-DnaJ-GrpE chaperone team in protein secretion in Escherichia coli. J. Bacteriol. 1996, 178, 3608–3613. [Google Scholar] [CrossRef]
- Deuerling, E.; Schulze-Specking, A.; Tomoyasu, T.; Mogk, A.; Bukau, B. Trigger factor and DnaK cooperate in folding of newly synthesized proteins. Nature 1999, 400, 693–696. [Google Scholar] [CrossRef]
- Teter, S.A.; Houry, W.A.; Ang, D.; Tradler, T.; Rockabrand, D.; Fischer, G.; Blum, P.; Georgopoulos, C.; Hartl, F.U. Polypeptide flux through bacterial Hsp70: DnaK cooperates with trigger factor in chaperoning nascent chains. Cell 1999, 97, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Skowyra, D.; Georgopoulos, C.; Zylicz, M. The E. coli dnaK gene product, the hsp70 homolog, can reactivate heat-inactivated RNA polymerase in an ATP hydrolysis-dependent manner. Cell 1990, 62, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Ziemienowicz, A.; Skowyra, D.; Zeilstra-Ryalls, J.; Fayet, O.; Georgopoulos, C.; Zylicz, M. Both the Escherichia coli chaperone systems, GroEL/GroES and DnaK/DnaJ/GrpE, can reactivate heat-treated RNA polymerase. Different mechanisms for the same activity. J. Biol. Chem. 1993, 268, 25425–25431. [Google Scholar] [CrossRef] [PubMed]
- Alix, J.H.; Guérin, M.F. Mutant DnaK chaperones cause ribosome assembly defects in Escherichia coli. Proc. Natl. Acad. Sci. USA 1993, 90, 9725–9729. [Google Scholar] [CrossRef]
- Singh, V.K.; Utaida, S.; Jackson, L.S.; Jayaswal, R.K.; Wilkinson, B.J.; Chamberlain, N.R. Role for dnaK locus in tolerance of multiple stresses in Staphylococcus aureus. Microbiology 2007, 153, 3162–3173. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Tomoyasu, T.; Takaya, A.; Morioka, M.; Yamamoto, T. Effects of disruption of heat shock genes on susceptibility of Escherichia coli to fluoroquinolones. BMC Microbiol. 2003, 3, 16. [Google Scholar] [CrossRef]
- Daugaard, M.; Rohde, M.; Jäättelä, M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007, 581, 3702–3710. [Google Scholar] [CrossRef]
- Boudesco, C.; Cause, S.; Jego, G.; Garrido, C. Hsp70: A Cancer Target Inside and Outside the Cell. Methods Mol. Biol. 2018, 1709, 371–396. [Google Scholar] [CrossRef]
- Arigoni, F.; Talabot, F.; Peitsch, M.; Edgerton, M.D.; Meldrum, E.; Allet, E.; Fish, R.; Jamotte, T.; Curchod, M.L.; Loferer, H. A genome-based approach for the identification of essential bacterial genes. Nat. Biotechnol. 1998, 16, 851–856. [Google Scholar] [CrossRef]
- Dassain, M.; Leroy, A.; Colosetti, L.; Carolé, S.; Bouché, J.P. A new essential gene of the ‘minimal genome’ affecting cell division. Biochimie 1999, 81, 889–895. [Google Scholar] [CrossRef]
- Nilsson, K.; Lundgren, H.K.; Hagervall, T.G.; Björk, G.R. The cysteine desulfurase IscS is required for synthesis of all five thiolated nucleosides present in tRNA from Salmonella enterica serovar typhimurium. J. Bacteriol. 2002, 184, 6830–6835. [Google Scholar] [CrossRef] [PubMed]
- Bimai, O.; Arragain, S.; Golinelli-Pimpaneau, B. Structure-based mechanistic insights into catalysis by tRNA thiolation enzymes. Curr. Opin. Struct. Biol. 2020, 65, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.L.; Brown, E.D. Genetic interaction screens with ordered overexpression and deletion clone sets implicate the Escherichia coli GTPase YjeQ in late ribosome biogenesis. J. Bacteriol. 2008, 190, 2537–2545. [Google Scholar] [CrossRef]
- Jomaa, A.; Stewart, G.; Mears, J.A.; Kireeva, I.; Brown, E.D.; Ortega, J. Cryo-electron microscopy structure of the 30S subunit in complex with the YjeQ biogenesis factor. Rna 2011, 17, 2026–2038. [Google Scholar] [CrossRef] [PubMed]
- López-Alonso, J.P.; Kaminishi, T.; Kikuchi, T.; Hirata, Y.; Iturrioz, I.; Dhimole, N.; Schedlbauer, A.; Hase, Y.; Goto, S.; Kurita, D.; et al. RsgA couples the maturation state of the 30S ribosomal decoding center to activation of its GTPase pocket. Nucleic Acids Res. 2017, 45, 6945–6959. [Google Scholar] [CrossRef]
- Himeno, H.; Hanawa-Suetsugu, K.; Kimura, T.; Takagi, K.; Sugiyama, W.; Shirata, S.; Mikami, T.; Odagiri, F.; Osanai, Y.; Watanabe, D.; et al. A novel GTPase activated by the small subunit of ribosome. Nucleic Acids Res. 2004, 32, 5303–5309. [Google Scholar] [CrossRef]
- Thurlow, B.; Davis, J.H.; Leong, V.; Moraes, T.F.; Williamson, J.R.; Ortega, J. Binding properties of YjeQ (RsgA), RbfA, RimM and Era to assembly intermediates of the 30S subunit. Nucleic Acids Res. 2016, 44, 9918–9932. [Google Scholar] [CrossRef]
- Kozlov, G.; Elias, D.; Semesi, A.; Yee, A.; Cygler, M.; Gehring, K. Structural similarity of YbeD protein from Escherichia coli to allosteric regulatory domains. J. Bacteriol. 2004, 186, 8083–8088. [Google Scholar] [CrossRef]
- Zhang, Y.; Yamaguchi, Y.; Inouye, M. Characterization of YafO, an Escherichia coli toxin. J. Biol. Chem. 2009, 284, 25522–25531. [Google Scholar] [CrossRef]
- Singletary, L.A.; Gibson, J.L.; Tanner, E.J.; McKenzie, G.J.; Lee, P.L.; Gonzalez, C.; Rosenberg, S.M. An SOS-regulated type 2 toxin-antitoxin system. J. Bacteriol. 2009, 191, 7456–7465. [Google Scholar] [CrossRef]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 2006.0008. [Google Scholar] [CrossRef]
- Raivio, T.L.; Silhavy, T.J. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J. Bacteriol. 1997, 179, 7724–7733. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Nishino, K.; Yamada, J.; Hirata, T.; Yamaguchi, A. Beta-lactam resistance modulated by the overexpression of response regulators of two-component signal transduction systems in Escherichia coli. J. Antimicrob. Chemother. 2003, 52, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef]
- Berg, D.E.; Weiss, A.; Crossland, L. Polarity of Tn5 insertion mutations in Escherichia coli. J. Bacteriol. 1980, 142, 439–446. [Google Scholar] [CrossRef]
- Meyer, C.; Hoffmann, C.; Haas, R.; Schubert, S. The role of the galU gene of uropathogenic Escherichia coli in modulating macrophage TNF-α response. Int. J. Med. Microbiol. 2015, 305, 893–901. [Google Scholar] [CrossRef]


| Library | Total Reads | Reads Mapped (%) 1 | Total UIS 2 | Total Seq Len/Total UIS |
|---|---|---|---|---|
| Input | ||||
| MG1655_pTF2_input_1 | 10,795,077 | 90.79 | 234,898 | 19.69 |
| MG1655_pTF2_input_2 | 11,001,585 | 85.73 | 279,308 | 16.56 |
| Input 1 + Input 2 (combined) | 21,796,662 | 88.23 | 315,925 | 14.64 |
| Output | ||||
| MG1655_pTF2_without_antibiotic_1 | 11,238,753 | 89.03 | 229,617 | 20.14 |
| MG1655_pTF2_without_antibiotic_2 | 12,879,322 | 89.20 | 233,968 | 19.77 |
| MG1655_pTF2_128mg_CTX_1 | 11,456,775 | 88.42 | 219,223 | 21.10 |
| MG1655_pTF2_128mg_CTX_2 | 9,862,543 | 85.08 | 227,102 | 20.37 |
| MG1655_pTF2_0.5mg_GEN_1 | 11,632,252 | 84.38 | 224,341 | 20.61 |
| MG1655_pTF2_0.5mg_GEN_2 | 9,398,550 | 89.74 | 205,101 | 22.55 |
| MG1655_pTF2_CTX+GEN_1 | 12,080,082 | 92.03 | 194,803 | 23.74 |
| MG1655_pTF2_CTX+GEN_2 | 9,614,653 | 89.80 | 194,408 | 23.79 |
| Gene | Function | 128 mg/L CTX | |
|---|---|---|---|
| Log2FC | q.Value | ||
| lpoB | penicillin-binding protein activator LpoB | −10.10 | 2.31 × 10−17 |
| gatB | PTS galactitol transporter subunit IIB | −8.85 | 1.40 × 10−11 |
| minE | cell division topological specificity factor MinE | −7.99 | 3.06 × 10−8 |
| tolQ | Tol-Pal system protein TolQ | −7.33 | 6.65 × 10−23 |
| pal | peptidoglycan-associated lipoprotein Pal | −6.96 | 1.63 × 10−22 |
| tolA | cell envelope integrity protein TolA | −6.92 | 8.42 × 10−19 |
| tolB | Tol-Pal system protein TolB | −6.62 | 1.08 × 10−23 |
| mrcB | bifunctional glycosyl transferase/transpeptidase | −6.28 | 1.40 × 10−80 |
| dicC | dicB transcriptional regulator DicC | −5.88 | 0.00303 |
| tolR | colicin uptake protein TolR | −5.38 | 1.04 × 10−27 |
| rlmH | 23S rRNA (pseudouridine(1915)-N(3))-methyltransferase RlmH | −4.89 | 7.91 × 10−05 |
| sdhC | succinate dehydrogenase cytochrome b556 subunit | −4.86 | 2.88 × 10−10 |
| ldcA | muramoyltetrapeptide carboxypeptidase | −4.83 | 9.77 × 10−14 |
| sucD | succinate--CoA ligase subunit alpha | −4.68 | 5.13 × 10−22 |
| plsX | phosphate acyltransferase PlsX | −4.48 | 4.72 × 10−50 |
| dapB | 4-hydroxy-tetrahydrodipicolinate reductase | −4.30 | 0.0005 |
| dacA | D-alanyl-D-alanine carboxypeptidase DacA | −4.14 | 2.22 × 10−23 |
| rfaD/hldD | ADP-glyceromanno-heptose 6-epimerase | −4.13 | 1.31 × 10−5 |
| rsmH | 16S rRNA (cytosine(1402)-N(4))-methyltransferase RsmH | −3.91 | 2.52 × 10−6 |
| sltY | murein transglycosylase | −3.88 | 5.43 × 10−103 |
| Gene | Function | 0.5 mg/L GEN | |
|---|---|---|---|
| Log2FC | q.Value | ||
| atpF | F0F1 ATP synthase subunit B | −8.30 | 2.65 × 10−16 |
| atpG | F0F1 ATP synthase subunit gamma | −6.49 | 1.19 × 10−23 |
| ybeD | YbeD family protein | −5.60 | 0.005 |
| atpD | F0F1 ATP synthase subunit beta | −5.55 | 6.69 × 10−41 |
| atpH | F0F1 ATP synthase subunit delta | −5.50 | 0.009 |
| plsC | 1-acylglycerol-3-phosphate O-acyltransferase | −5.48 | 0.010 |
| atpC | F0F1 ATP synthase subunit epsilon | −4.87 | 5.94 × 10−11 |
| atpA | F0F1 ATP synthase subunit alpha | −4.06 | 1.70 × 10−48 |
| atpB | F0F1 ATP synthase subunit A | −3.99 | 0.0003 |
| yajC | preprotein translocase subunit YajC | −3.84 | 5.57 × 10−8 |
| gmhB | D-glycero-beta-D-manno-heptose 1,7-bisphosphate 7-phosphatase | −3.22 | 5.11 × 10−6 |
| sdaC | HAAAP family serine/threonine permease SdaC | −3.03 | 8.89 × 10−148 |
| hflC | protease modulator HflC | −2.86 | 2.14 × 10−155 |
| ackA | acetate kinase | −2.79 | 2.53 × 10−9 |
| cpxR | envelope stress response regulator transcription factor CpxR | −2.66 | 1.40 × 10−134 |
| galU | UTP--glucose-1-phosphate uridylyltransferase GalU | −2.57 | 6.36 × 10−16 |
| hflK | FtsH protease activity modulator HflK | −2.55 | 8.42 × 10−133 |
| pta | phosphate acetyltransferase | −2.02 | 5.07 × 10−16 |
| Gene | Function | 1 + 0.25 mg/L CTX+GEN | 128 mg/L CTX | 0.5 mg/L GEN | |||
|---|---|---|---|---|---|---|---|
| Log2FC | q.Value | Log2FC | q.Value | Log2FC | q.Value | ||
| atpD | F0F1 ATP synthase subunit beta | −10.12 | 1.90 × 10−40 | 0.11 | 0.87 | −5.55 | 6.69 × 10−41 |
| atpG | F0F1 ATP synthase subunit gamma | −8.97 | 5.70 × 10−23 | −0.27 | 0.74 | −6.49 | 1.19 × 10−23 |
| atpF | F0F1 ATP synthase subunit B | −8.36 | 1.25 × 10−15 | 1.21 | 0.04 | −8.30 | 2.65 × 10−16 |
| atpC | F0F1 ATP synthase subunit epsilon | −8.21 | 1.54 × 10−12 | 0.05 | 0.98 | −4.87 | 5.94 × 10−11 |
| yajC | preprotein translocase subunit YajC | −7.71 | 1.22 × 10−10 | 1.48 | 0.01 | −3.84 | 5.57 × 10−8 |
| atpB | F0F1 ATP synthase subunit A | −6.47 | 5.39 × 10−5 | 2.58 | 7.83 × 10−5 | −3.99 | 0.0003 |
| mnmA | tRNA 2-thiouridine(34) synthase MnmA | −6.04 | 0.0009 | 1.98 | 0.012 | −0.14 | 0.94 |
| rsgA | small ribosomal subunit biogenesis GTPase RsgA | −5.86 | 0.002 | 1.23 | 0.15 | 0.24 | 0.94 |
| ybeD | YbeD family protein | −5.65 | 0.006 | 0.83 | 0.41 | −5.60 | 0.005 |
| atpH | F0F1 ATP synthase subunit delta | −5.55 | 0.006 | 2.49 | 0.01 | −5.50 | 0.009 |
| IEU92_RS07640 | Pseudogene | −5.54 | 0.010 | 3.31 | 0.0003 | −0.33 | 0.81 |
| ackA | acetate kinase | −3.98 | 3.69 × 10−11 | 5.49 | 2.02 × 10−98 | −2.79 | 2.53 × 10-9 |
| tolA | cell envelope integrity protein TolA | −3.90 | 9.54 × 10−33 | −6.92 | 8.42 × 10−19 | 0.2 | 0.48 |
| tolB | Tol-Pal system protein TolB | −3.75 | 1.30 × 10−36 | −6.62 | 1.08 × 10−23 | 0.13 | 0.59 |
| sdaC | HAAAP family serine/threonine permease SdaC | −3.73 | 2.00 × 10−138 | 0.03 | 0.88 | −3.03 | 8.89 × 10−148 |
| pal | peptidoglycan-associated lipoprotein Pal | −3.66 | 3.35 × 10−27 | −6.96 | 1.63 × 10−22 | 0.27 | 0.2 |
| atpA | F0F1 ATP synthase subunit alpha | −3.64 | 1.47 × 10−15 | 0.77 | 0.08 | −4.06 | 1.70 × 10−48 |
| IEU92_RS06395 | septation protein A | −3.49 | 0.010 | 1.91 | 0.04 | −1.05 | 0.28 |
| yafN | type I toxin-antitoxin system antitoxin YafN | −3.44 | 1.94 × 10−5 | 1.06 | 0.14 | −0.42 | 0.56 |
| hflC | protease modulator HflC | −3.43 | 1.78 × 10−102 | −0.61 | 0.0008 | −2.86 | 2.14 × 10−155 |
| tolQ | Tol-Pal system protein TolQ | −3.36 | 3.31 × 10−35 | −7.33 | 6.65 × 10−23 | 0.1 | 0.65 |
| cpxR | envelope stress response regulator transcription factor | −3.33 | 1.14 × 10−177 | −2.24 | 3.62×10−20 | −2.66 | 1.40 × 10−134 |
| ydeI | YdeI family stress tolerance OB fold protein | −3.12 | 3.90 × 10−5 | −0.86 | 0.33 | −0.58 | 0.37 |
| tusD | sulfurtransferase complex subunit TusD | −3.05 | 0.0005 | 3.75 | 3.03 × 10−10 | −1.28 | 0.06 |
| rsmH | 16S rRNA (cytosine(1402)-N(4))-methyltransferase | −3.01 | 8.78 × 10−9 | −3.91 | 2.52 × 10−6 | −1.62 | 0.0002 |
| dnaK | molecular chaperone DnaK | −2.95 | 8.72 × 10−5 | 3.41 | 1.98 × 10−9 | 0.98 | 0.02 |
| tolR | colicin uptake protein TolR | −2.89 | 2.37 × 10−53 | −5.38 | 1.04 × 10−27 | 0.29 | 0.04 |
| hflK | FtsH protease activity modulator HflK | −2.88 | 5.39 × 10−173 | −0.08 | 0.62 | −2.55 | 8.42 × 10−133 |
| pflA | pyruvate formate lyase 1-activating protein | −2.36 | 2.19 × 10−57 | 1.33 | 7.91 × 10−12 | −1.94 | 9.74 × 10−47 |
| epmA | elongation factor P--(R)-beta-lysine ligase | −2.18 | 1.88 × 10−8 | 1.17 | 0.0002 | −1.47 | 2.69 × 10−13 |
| pta | phosphate acetyltransferase | −2.17 | 1.39 × 10−12 | 4.2 | 1.70 × 10−99 | −2.02 | 5.07 × 10−16 |
| Strain | CTX (mg/L) | GEN (mg/L) | CFZ (mg/L) | FOX (mg/L) | CAZ (mg/L) | FEP (mg/L) | CPT (mg/L) |
|---|---|---|---|---|---|---|---|
| MG1655/pTF2 (WT) | 256 | 1 | 1024 | 4–8 | 2 | 2 | 1024 |
| ΔcpxR | 32 | 0.5–1 | 512 | 4 | 2 | 2 | 1024 |
| ΔdnaK | 8 | 0.5 | 32 | 0.5 | 0.25 | 1 | 256 |
| ΔmnmA | 16 | 0.5 | 256 | 2 | 0.25 | 0.25 | 512 |
| ΔrgsA | 8 | 0.5 | 128 | 0.5 | 0.125 | 0.06 | 512 |
| ΔyafN | 32 | 1–2 | 512 | 2 | 2 | 2 | 1024 |
| ΔyajC | 128–256 | 0.5–1 | - | - | - | - | - |
| ΔydeI | 256 | 1–2 | - | - | - | - | - |
| ΔybeD | 16 | 0.25 | 512 | 2 | 0.25 | 0.5 | 1024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alobaidallah, M.S.A.; García, V.; De Mets, R.; Wellner, S.M.; Thomsen, L.E.; Herrero-Fresno, A.; Olsen, J.E. Uncovering the Important Genetic Factors for Growth during Cefotaxime-Gentamicin Combination Treatment in blaCTX-M-1 Encoding Escherichia coli. Antibiotics 2023, 12, 993. https://doi.org/10.3390/antibiotics12060993
Alobaidallah MSA, García V, De Mets R, Wellner SM, Thomsen LE, Herrero-Fresno A, Olsen JE. Uncovering the Important Genetic Factors for Growth during Cefotaxime-Gentamicin Combination Treatment in blaCTX-M-1 Encoding Escherichia coli. Antibiotics. 2023; 12(6):993. https://doi.org/10.3390/antibiotics12060993
Chicago/Turabian StyleAlobaidallah, Mosaed Saleh A., Vanesa García, Richard De Mets, Sandra M. Wellner, Line E. Thomsen, Ana Herrero-Fresno, and John Elmerdahl Olsen. 2023. "Uncovering the Important Genetic Factors for Growth during Cefotaxime-Gentamicin Combination Treatment in blaCTX-M-1 Encoding Escherichia coli" Antibiotics 12, no. 6: 993. https://doi.org/10.3390/antibiotics12060993
APA StyleAlobaidallah, M. S. A., García, V., De Mets, R., Wellner, S. M., Thomsen, L. E., Herrero-Fresno, A., & Olsen, J. E. (2023). Uncovering the Important Genetic Factors for Growth during Cefotaxime-Gentamicin Combination Treatment in blaCTX-M-1 Encoding Escherichia coli. Antibiotics, 12(6), 993. https://doi.org/10.3390/antibiotics12060993






