Antibiotic Resistance in the Apennine Wolf (Canis lupus italicus): Implications for Wildlife and Human Health
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Samples Collection
4.2. Laboratory Investigations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mech, L.D.; Boitani, L. Wolves: Behavior, Ecology, and Conservation; Chicago University Press: Chicago, IL, USA, 2003. [Google Scholar]
- Breintenmoser, U. Large predators in the Alps: The fall and rise of man’s competitors. Biol. Conserv. 1998, 83, 279–289. [Google Scholar] [CrossRef]
- Nowak, R.M. Walker’s Mammals of the World, 5th ed.; Johns Hopkins University Press: Baltimore, MD, USA, 1991. [Google Scholar]
- Apollonio, M. Wolves in the Casentinesi Forests: Insights for wolf conservation in Italy from a protected area with a rich wild prey community. Biol. Conserv. 2004, 120, 249–260. [Google Scholar] [CrossRef]
- Zimen, E.; Boitani, L. Number and distribution of wolf in Italy. Z. Säugetierkd. 1975, 40, 102–112. [Google Scholar] [CrossRef]
- Macdonald, D.W.; Boitani, L.; Barrasso, P. Foxes, Wolves and Conservation in the Abruzzo Mountains. In The Red Fox; Zimen, E.B., Ed.; Springer: Dordrecht, The Netherlands, 1980. [Google Scholar] [CrossRef]
- Di Marco, M.; Boitani, L.; Mallon, D.; Hoffmann, M.; Iacucci, A.; Meijaard, E.; Visconti, P.; Schipper, J.; Rondinini, C. A retrospective evaluation of the global decline of carnivores and ungulates. Conserv. Biol. 2014, 28, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. Official Journal of the European Communities. 1992. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31992L0043 (accessed on 22 April 2023).
- Council of Europe. Convention on the Conservation of European Wildlife and Natural Habitats. Council of Europe. 1979. Available online: https://rm.coe.int/1680078aff (accessed on 22 April 2023).
- Fabbri, E.; Miquel, C.; Lucchini, V.; Santini, A.; Caniglia, R.; Duchamp, C.; Weber, J.M.; Lequette, B.; Marucco, F.; Boitani, L.; et al. From the Apennines to the Alps: Colonization genetics of the naturally expanding Italian wolf (Canis lupus) population. Mol. Ecol. 2007, 16, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- La Morgia, V.; Marucco, F.; Aragno, P.; Salvatori, V.; Gervasi, V.; De Angelis, D.; Fabbri, E.; Caniglia, R.; Velli, E.; Avanzinelli, E.; et al. Stima Della Distribuzione e Consistenza del Lupo a Scala Nazionale 2020/2021. 2022. Available online: https://www.isprambiente.gov.it/it/attivita/biodiversita/monitoraggio-nazionale-del-lupo/file-monitoraggio/report-nazionale-lupo-20_21.pdf (accessed on 22 April 2023).
- Salafsky, N.; Margoluis, R.; Redford, K.H.; Robinson, J.G. Improving the practice of conservation: A conceptual framework and research agenda for conservation science. Conserv. Biol. 2002, 16, 1469–1479. [Google Scholar] [CrossRef]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging infectious diseases of wildlife—Threats to biodiversity and human health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef]
- Di Sabatino, D.; Lorusso, A.; Di Francesco, C.E.; Gentile, L.; Di Pirro, V.; Bellacicco, A.L.; Giovannini, A.; Di Francesco, G.; Marruchella, G.; Marsilio, F.; et al. Arctic lineage-canine distemper virus as a cause of death in Apennine wolves (Canis lupus) in Italy. PLoS ONE 2014, 9, e82356. [Google Scholar] [CrossRef]
- Di Francesco, C.E.; Smoglica, C.; Paoletti, B.; Angelucci, S.; Innocenti, M.; Antonucci, A.; Di Domenico, G.; Marsilio, F. Detection of selected pathogens in Apennine wolf (Canis lupus italicus) by a non-invasive GPS-based telemetry sampling of two packs from Majella National Park, Italy. Eur. J. Wildl. Res. 2019, 65, 84. [Google Scholar] [CrossRef]
- Musto, C.; Cerri, J.; Galaverni, M.; Caniglia, R.; Fabbri, E.; Apollonio, M.; Mucci, N.; Bonilauri, P.; Maioli, G.; Fontana, M.C.; et al. Men and wolves: Anthropogenic causes are an important driver of wolf mortality in human-dominated landscapes in Italy. Glob. Ecol. Conserv. 2021, 32, e01892. [Google Scholar] [CrossRef]
- Vittecoq, M.; Godreuil, S.; Prugnolle, F.; Durand, P.; Brazier, L.; Renaud, N.; Arnal, A.; Aberkane, S.; Jean-Pierre, H.; Gauthier-Clerc, M.; et al. Antimicrobial resistance in wildlife. J. Appl. Ecol. 2016, 53, 519–529. [Google Scholar] [CrossRef]
- Ramey, A.M.; Ahlstrom, C.A. Antibiotic resistant bacteria in wildlife: Perspectives on trends, acquisition and dissemination, data gaps, and future directions. J. Wildl. Dis. 2020, 56, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Laborda, P.; Sanz-García, F.; Ochoa-Sánchez, L.E.; Gil-Gil, T.; Hernando-Amado, S.; Martínez, J.L. Wildlife and Antibiotic Resistance. Front. Cell. Infect. Microbiol. 2022, 12, 873989. [Google Scholar] [CrossRef] [PubMed]
- Smoglica, C.; Vergara, A.; Angelucci, S.; Festino, A.R.; Antonucci, A.; Marsilio, F.; Di Francesco, C.E. Evidence of Linezolid Resistance and Virulence Factors in Enterococcus spp. Isolates from Wild and Domestic Ruminants, Italy. Antibiotics 2022, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Smoglica, C.; Vergara, A.; Angelucci, S.; Festino, A.R.; Antonucci, A.; Moschetti, L.; Farooq, M.; Marsilio, F.; Di Francesco, C.E. Resistance Patterns, mcr-4 and OXA-48 Genes, and Virulence Factors of Escherichia coli from Apennine Chamois Living in Sym- patry with Domestic Species, Italy. Animals 2022, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Smoglica, C.; Vergara, A.; Angelucci, S.; Festino, A.R.; Antonucci, A.; Marsilio, F.; Di Francesco, C.E. Antibiotic Resistant Bacteria Dissemination in Wildlife, Livestock and Water of Maiella National Park, Italy. Animals 2023, 13, 432. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, D.P. Development and Validation of a Body Condition Score System for Dogs 15-10:22;1997 Pract Canine. Available online: https://wsava.org/wp-content/uploads/2020/01/Body-Condition-Score-Dog.pdf (accessed on 22 April 2023).
- Santos, N.; Rio Maior, H.; Nakamura, M.; Roque, S.; Brandão, R.; Petrucci-Fonseca, F.; Palacios, V.; Garcia, E.; López-Bao, J.V.; Llaneza, L.; et al. Hematology and serum biochemistry values of free-ranging Iberian wolves (Canis lupus) trapped by leg-hold snares. Eur. J. Wildl. Res. 2014, 61, 135–141. [Google Scholar] [CrossRef]
- Thoresen, S.I.; Arnemo, J.M.; Liberg, O. Hematology and Serum Clinical Chemistry Reference Intervals for Free-Ranging Scandinavian Gray Wolves (Canis Lupus). Vet. Clin. Pathol. 2009, 38, 224–229. [Google Scholar] [CrossRef]
- Constable, P.; Hinchcliff, K.; Demma, N.; Callahan, M.; Dale, B.; Fox, K.; Adams, L.; Wack, R.; Kramer, L. Serum Biochemistry of Captive and Free-Ranging Gray Wolves (Canis Lupus). J. Zoo Wildl. Med. 1998, 29, 435–440. [Google Scholar]
- Turchi, B.; Dec, M.; Bertelloni, F.; Winiarczyk, S.; Gnat, S.; Bresciani, F.; Viviani, F.; Cerri, D.; Fratini, F. Antibiotic Susceptibility and Virulence Factors in Escherichia coli from Sympatric Wildlife of the Apuan Alps Regional Park (Tuscany, Italy). Microb. Drug Resist. 2019, 25, 772–780. [Google Scholar] [CrossRef]
- Dec, M.; Stępień-Pyśniak, D.; Gnat, S.; Fratini, F.; Urban-Chmiel, R.; Cerri, D.; Winiarczyk, S.; Turchi, B. Antibiotic Susceptibility and Virulence Genes in Enterococcus Isolates from Wild Mammals Living in Tuscany, Italy. Microb. Drug Resist. 2020, 26, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Igrejas, G.; Radhouani, H.; Correia, S.; Pacheco, R.; Santos, T.; Monteiro, R.; Guerra, A.; Petrucci-Fonseca, F.; Brito, F.; et al. Antimicrobial resistance in faecal enterococci and Escherichia coli isolates recovered from Iberian wolf. Lett. Appl. Microbiol. 2013, 56, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Igrejas, G.; Radhouani, H.; López, M.; Guerra, A.; Petrucci-Fonseca, F.; Alcaide, E.; Zorrilla, I.; Serra, R.; Torres, C.; et al. Detection of vancomycin-resistant enterococci from faecal samples of Iberian wolf and Iberian lynx, including Enterococcus faecium strains of CC17 and the new singleton ST573. Sci. Total Environ. 2011, 410–411, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.A.; Helbig, K.J. The Complex Diseases of Staphylococcus pseudintermedius in Canines: Where to Next? Vet. Sci. 2021, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Smoglica, C.; Evangelisti, G.; Fani, C.; Marsilio, F.; Trotta, M.; Messina, F.; Di Francesco, C.E. Antimicrobial Resistance Profile of Bacterial Isolates from Urinary Tract Infections in Companion Animals in Central Italy. Antibiotics 2022, 11, 1363. [Google Scholar] [CrossRef]
- Bhooshan, S.; Negi, V.; Khatri, P.K. Staphylococcus pseudintermedius: An undocumented, emerging pathogen in humans. GMS Hyg. Infect. Control 2020, 15, Doc32. [Google Scholar] [CrossRef]
- Oh, S.I.; Kim, J.W.; Jung, J.Y.; Chae, M.; Lee, Y.R.; Kim, J.H.; So, B.; Kim, H.Y. Pathologic and molecular characterization of Streptococcus dysgalactiae subsp. equisimilis infection in neonatal piglets. J. Vet. Sci. 2018, 19, 313–317. [Google Scholar] [CrossRef]
- Massé, J.; Dufour, S.; Archambault, M. Characterization of Klebsiella isolates obtained from clinical mastitis cases in dairy cattle. J. Dairy Sci. 2020, 103, 3392–3400. [Google Scholar] [CrossRef]
- Lee, D.; Oh, J.Y.; Sum, S.; Park, H.M. Prevalence and antimicrobial resistance of Klebsiella species isolated from clinically ill companion animals. J. Vet. Sci. 2021, 22, e17. [Google Scholar] [CrossRef]
- Neog, N.; Phukan, U.; Puzari, M.; Sharma, M.; Chetia, P. Klebsiella oxytoca and Emerging Nosocomial Infections. Curr. Microbiol. 2021, 78, 1115–1123. [Google Scholar] [CrossRef]
- Pagnossin, D.; Smith, A.; Oravcová, K.; Weir, W. Streptococcus canis, the underdog of the genus. Vet. Microbiol. 2022, 273, 109524. [Google Scholar] [CrossRef] [PubMed]
- Pottier, M.; Castagnet, S.; Gravey, F.; Leduc, G.; Sévin, C.; Petry, S.; Giard, J.C.; Le Hello, S.; Léon, A. Antimicrobial Resistance and Genetic Diversity of Pseudomonas aeruginosa Strains Isolated from Equine and Other Veterinary Samples. Pathogens 2022, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.; Choudhary, B.K.; Bhoyar, S.; Kale, S.B.; Chaudhari, S.P.; Bera, B.C.; Jain, A.; Barbuddhe, S.B. Isolation and characterization of multidrug-resistant Leclercia species from animal clinical case. Lett. Appl. Microbiol. 2018, 66, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Zayet, S.; Lang, S.; Garnier, P.; Pierron, A.; Plantin, J.; Toko, L.; Royer, P.Y.; Villemain, M.; Klopfenstein, T.; Gendrin, V. Leclercia adecarboxylata as Emerging Pathogen in Human Infections: Clinical Features and Antimicrobial Susceptibility Testing. Pathogens 2021, 10, 1399. [Google Scholar] [CrossRef]
- World Health Organization. WHO List of Critically Important Antimicrobials for Human Medicine (WHO CIA List). Available online: https://www.who.int/publications/i/item/9789241515528 (accessed on 22 April 2023).
- Smoglica, C.; Di Francesco, C.E.; Angelucci, S.; Antonucci, A.; Innocenti, M.; Marsilio, F. Occurrence of the tetracycline resistance gene tetA(P) in Apennine wolves (Canis lupus italicus) from different human-wildlife interfaces. J. Glob. Antimicrob. Resist. 2020, 23, 184–185. [Google Scholar] [CrossRef]
- Antonucci, A.; Valerio, A.; Petrizzelli, L.; Verschuur, L.; Gandolfi, M.; Manchi, S.; Di Domenico, G.; Innocenti, M.; Smoglica, C.; Di Tana, F.; et al. Maiella Wolves Do Not Like Livestock? 20 Years of Research and Experience on Feeding Ecology of Apennine Wolf (Canis Lupus Italicus). In Proceedings of the Wolves Across Bordes Conference, Stockholm, Sweden, 8–11 May 2023. [Google Scholar]
- Aguirre, A.A. Wild canids as sentinels of ecological health: A conservation medicine perspective. Parasites Vectors 2009, 2 (Suppl. S1), S7. [Google Scholar] [CrossRef]
- Prandi, I.; Bellato, A.; Nebbia, P.; Stella, M.C.; Ala, U.; von Degerfeld, M.M.; Quaranta, G.; Robino, P. Antibiotic resistant Escherichia coli in wild birds hospitalised in a wildlife rescue centre. Comp. Immunol. Microbiol. Infect. Dis. 2023, 93, 101945. [Google Scholar] [CrossRef]
- Casalino, G.; D’Amico, F.; Dinardo, F.R.; Bozzo, G.; Napoletano, V.; Camarda, A.; Bove, A.; Lombardi, R.; D’Onghia, F.P.; Circella, E. Prevalence and Antimicrobial Resistance of Campylobacter jejuni and Campylobacter coli in Wild Birds from a Wildlife Rescue Centre. Animals 2022, 12, 2889. [Google Scholar] [CrossRef]
- Baros Jorquera, C.; Moreno-Switt, A.I.; Sallaberry-Pincheira, N.; Munita, J.M.; Flores Navarro, C.; Tardone, R.; González-Rocha, G.; Singer, R.S.; Bueno, I. Antimicrobial resistance in wildlife and in the built environment in a wildlife rehabilitation center. One Health 2021, 13, 100298. [Google Scholar] [CrossRef]
- Marrow, J.; Whittington, J.K.; Mitchell, M.; Hoyer, L.L.; Maddox, C. Prevalence and antibiotic-resistance characteristics of Enterococcus spp. Isolated from free-living and captive raptors in Central Illinois. J. Wildl. Dis. 2009, 45, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Giacopello, C.; Foti, M.; Mascetti, A.; Grosso, F.; Ricciardi, D.; Fisichella, V.; Lo Piccolo, F. Antimicrobial resistance patterns of Enterobacteriaceae in European wild bird species admitted in a wildlife rescue centre. Vet. Ital. 2016, 52, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Darwich, L.; Vidal, A.; Seminati, C.; Albamonte, A.; Casado, A.; López, F.; Molina-López, R.A.; Migura-Garcia, L. High prevalence and diversity of extended-spectrum β- lactamase and emergence of OXA-48 producing Enterobacterales in wildlife in Catalonia. PLoS ONE 2019, 14, e0210686. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Castillo, D.; Farfán-López, M.; Esposito, F.; Moura, Q.; Fernandes, M.R.; Lopes, R.; Cardoso, B.; Muñoz, M.E.; Cerdeira, L.; Najle, I.; et al. Wild owls colonized by international clones of extended-spectrum β-lactamase (CTX-M)-producing Escherichia coli and Salmonella Infantis in the Southern Cone of America. Sci. Total Environ. 2019, 674, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Barroso, P.; Relimpio, D.; Zearra, J.A.; Cerón, J.J.; Palencia, P.; Cardoso, B.; Ferreras, E.; Escobar, M.; Cáceres, G.; López-Olvera, J.R.; et al. Using integrated wildlife monitoring to prevent future pandemics through one health approach. One Health 2022, 16, 100479. [Google Scholar] [CrossRef]
- Johnson, M.R.; Boyd, D.K.; Pletscher, D.H. Serologic investigations of canine parvovirus and canine distemper in relation to wolf (Canis lupus) pup mortalities. J. Wildl. Dis. 1994, 30, 270–273. [Google Scholar] [CrossRef]
- Molnar, B.; Duchamp, C.; Mostl, K.; Diehl, P.A.; Betschart, B. Comparative survey of canine parvovirus, canine distemper virus and canine enteric coronavirus infection in free-ranging wolves of Central Italy and South-Eastern France. Eur. Wildl. Res. 2014, 60, 613–624. [Google Scholar] [CrossRef]
- Mazzi, A. Elementi di Anestesia Degli Animali Esotici e Selvatici, 2nd ed.; Libreria Cortina Verona: Verona, Italy, 2008; pp. 59–73. [Google Scholar]
- EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs an Zone Diameters. Version 11.0. 2021. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 22 April 2023).
- World Bank. Drug-Resistant Infections: A Threat to Our Economic Future; World Bank: Washington, DC, USA, 2017. Available online: https://documents1.worldbank.org/curated/en/323311493396993758/pdf/final-report.pdf (accessed on 18 May 2023).
| ID Animals | Sex | Age | BCS and Weight | Samples |
|---|---|---|---|---|
| LU251122VA | F | 6 m | 2/9 5.3 kg | Endocardium |
| Lung | ||||
| Thoracic effusion | ||||
| LU120922 | F | 2 y | 4/9 25.4 kg | Peritoneal effusion |
| Lung | ||||
| Endocardium | ||||
| Liver | ||||
| Pleural effusion | ||||
| LU270222AB | F | 7 y | 3/9 22.9 kg | Forearm wound swab |
| Exposed fracture swab | ||||
| LU120522OUT | M | 1 y | 5/9 26.3 kg | Carpal wound swab |
| Intraarticular swab |
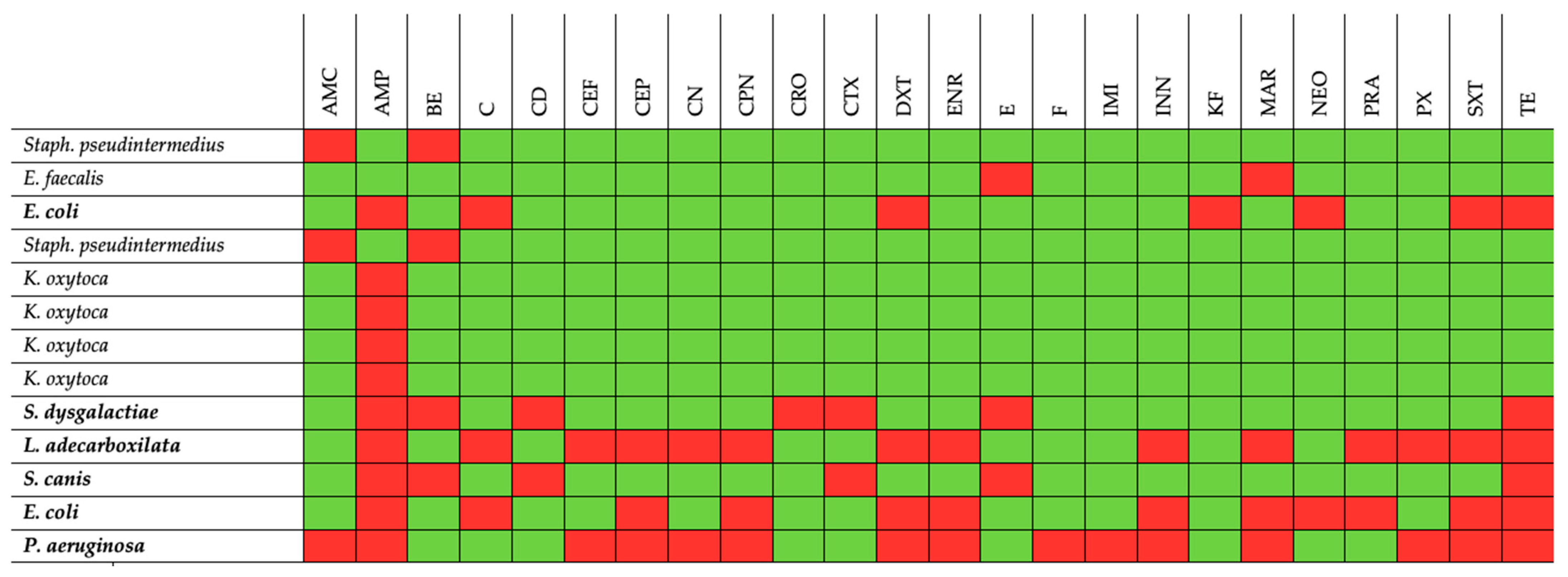
| ID Animals | Sample | Bacteria | Antibiotic Resistance Profiles |
|---|---|---|---|
| LU251122VA | Endocardial swab | Staphylococcus pseudintermedius | AMC BE |
| Enterococcus faecalis | E * ENR * MAR * | ||
| Lung | Escherichia coli | AMP DXT C KF NEO * SXT TE | |
| Thoracic effusion | Staphylococcus pseudintermedius | AMC BE | |
| LU120922 | Peritoneal effusion | Klebsiella oxytoca | AMP |
| Lung | Klebsiella oxytoca | AMP | |
| Endocardial swab | Klebsiella oxytoca | AMP | |
| Liver parenchyma | Klebsiella oxytoca | AMP | |
| Pleural effusion | Negative | - | |
| LU270222AB | Forearm wound swab | Streptococcus dysgalactiae ssp equisimilis | AMP BE CD CRO CTX E TE |
| Exposed fracture swab | Leclercia adecarboxilata | AMP C CEF CEP CN CPN DXT ENR INN MAR PRA PX SXT TE | |
| LU120522OUT | Carpal wound swab | Streptococcus canis | AMP BE CD CTX E TE |
| Escherichia coli | AMP C CPN CEP DXT ENR INN MAR NEO * PRA SXT TE | ||
| Intraarticular swab | Pseudomonas aeruginosa | AMC AMP CEF CEP CN CPN DXT ENR F IMI INN MAR * PX SXT TE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smoglica, C.; Angelucci, S.; Di Tana, F.; Antonucci, A.; Marsilio, F.; Di Francesco, C.E. Antibiotic Resistance in the Apennine Wolf (Canis lupus italicus): Implications for Wildlife and Human Health. Antibiotics 2023, 12, 950. https://doi.org/10.3390/antibiotics12060950
Smoglica C, Angelucci S, Di Tana F, Antonucci A, Marsilio F, Di Francesco CE. Antibiotic Resistance in the Apennine Wolf (Canis lupus italicus): Implications for Wildlife and Human Health. Antibiotics. 2023; 12(6):950. https://doi.org/10.3390/antibiotics12060950
Chicago/Turabian StyleSmoglica, Camilla, Simone Angelucci, Fabrizia Di Tana, Antonio Antonucci, Fulvio Marsilio, and Cristina Esmeralda Di Francesco. 2023. "Antibiotic Resistance in the Apennine Wolf (Canis lupus italicus): Implications for Wildlife and Human Health" Antibiotics 12, no. 6: 950. https://doi.org/10.3390/antibiotics12060950
APA StyleSmoglica, C., Angelucci, S., Di Tana, F., Antonucci, A., Marsilio, F., & Di Francesco, C. E. (2023). Antibiotic Resistance in the Apennine Wolf (Canis lupus italicus): Implications for Wildlife and Human Health. Antibiotics, 12(6), 950. https://doi.org/10.3390/antibiotics12060950







