An Evaluation of the Impact of Increasing the Awareness of the WHO Access, Watch, and Reserve (AWaRe) Antibiotics Classification on Knowledge, Attitudes, and Hospital Antibiotic Prescribing Practices
Abstract
1. Introduction
2. Results
2.1. Demographic and Medical Characteristics of the Study Sample
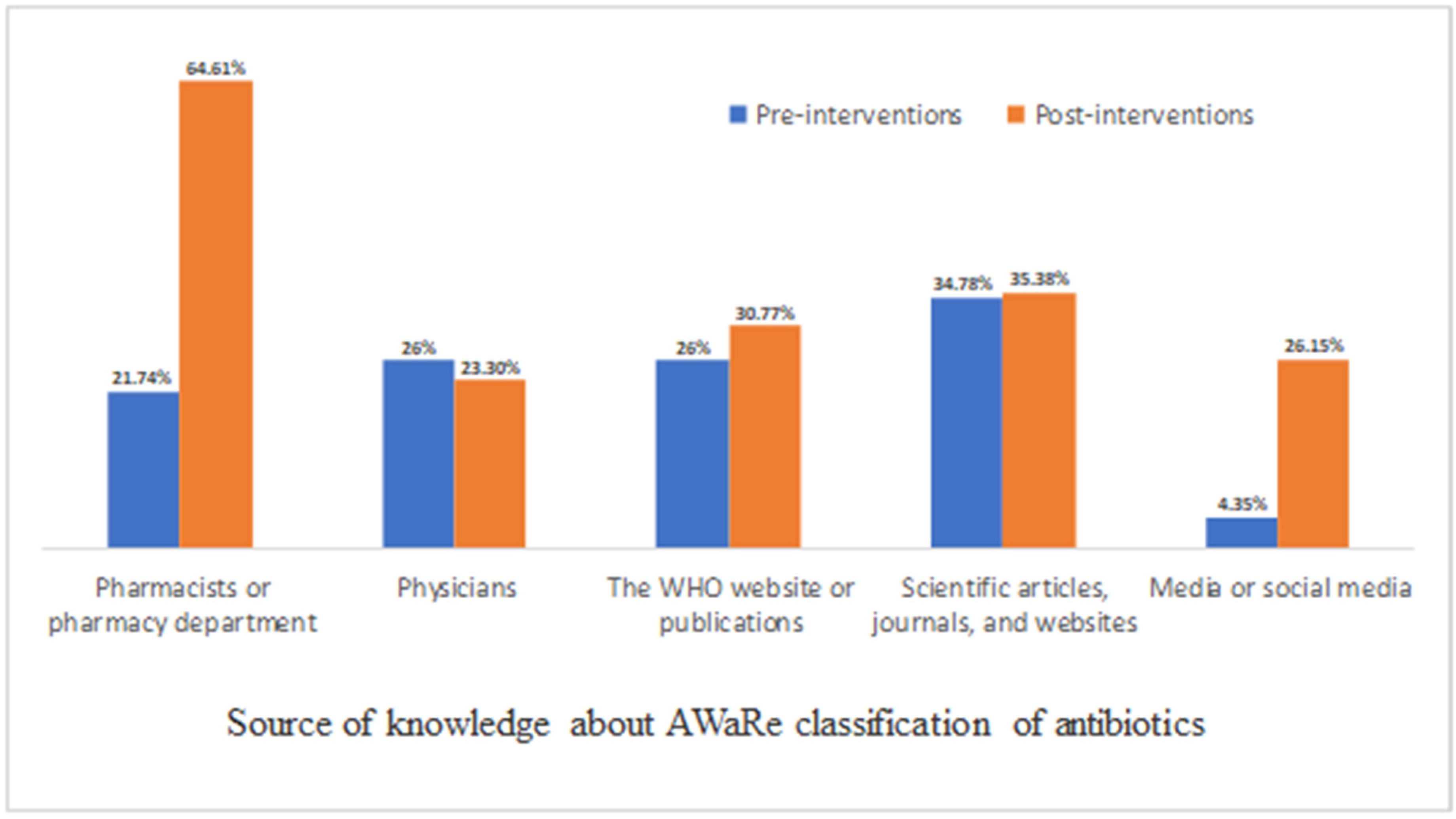
2.2. Knowledge, Perceptions, and Attitude about (AWaRe) Antibiotics Classification
2.3. Prescribed Antibiotics Measured by Global-PPS Method
2.4. Pharmacy Antibiotics Dispensing
3. Discussion
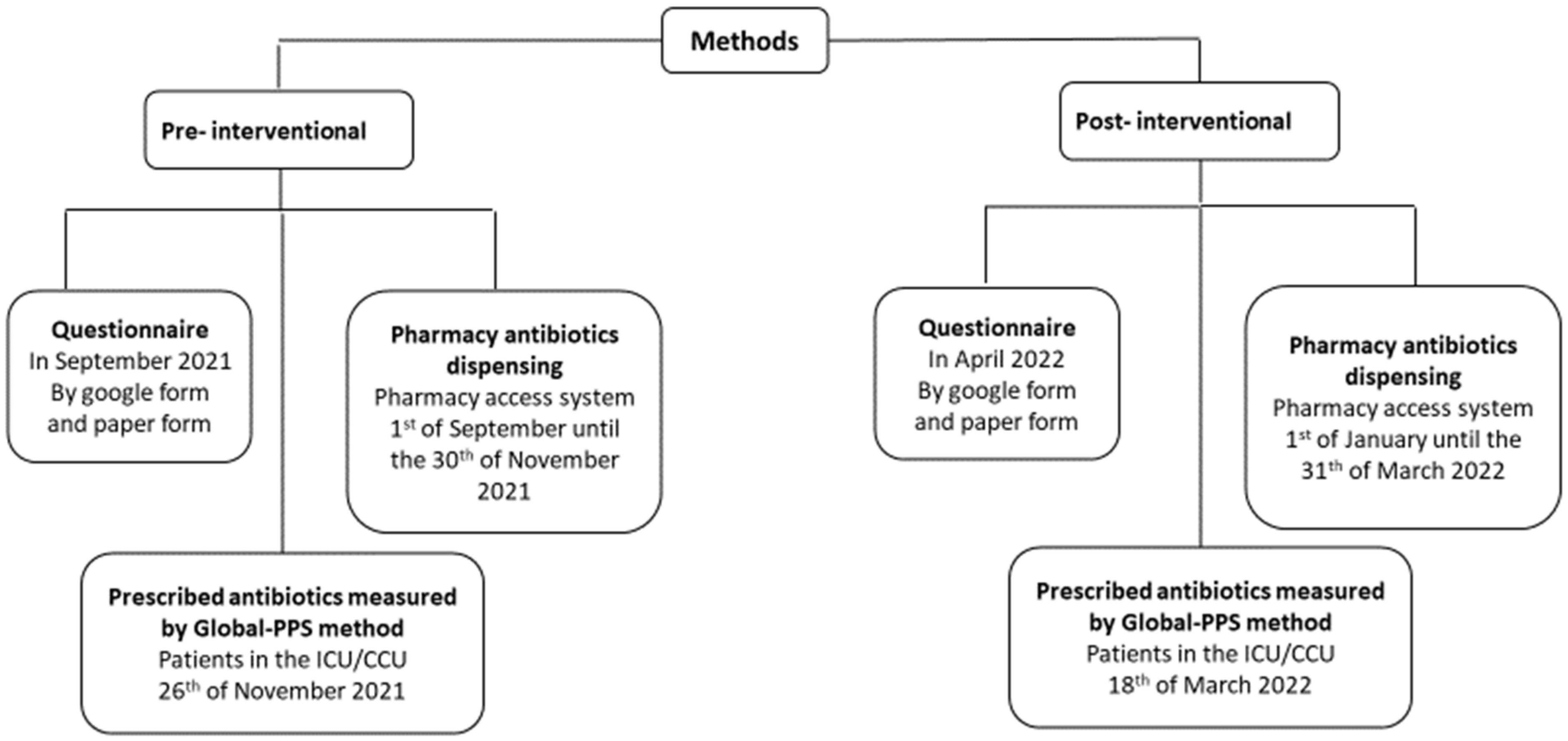
4. Methods
4.1. Study Design and Sample Size
4.2. Pre-Intervention Phase
4.3. Educational Interventions
4.4. Post-Intervention Phase
4.5. Data Collection
4.6. Defined Daily Dose (DDD)
4.7. Global-PPS
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Collignon, P.J.; Conly, J.M.; Andremont, A.; McEwen, S.A.; Aidara-Kane, A.; Agerso, Y.; Andremont, A.; Collignon, P.; Conly, J.; Dang Ninh, T.; et al. World Health Organization Ranking of Antimicrobials According to Their Importance in Human Medicine: A Critical Step for Developing Risk Management Strategies to Control Antimicrobial Resistance From Food Animal Production. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 63, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine; Forum on Emerging Infections. The National Academies Collection: Reports funded by National Institutes of Health. In The Resistance Phenomenon in Microbes and Infectious Disease Vectors: Implications for Human Health and Strategies for Containment: Workshop Summary; National Academies Press: Washington, DC, USA, 2003. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Resistance: Global Report on Surveillance. World Health Organization. 2014. Available online: https://apps.who.int/iris/handle/10665/112642 (accessed on 1 January 2023).
- WHO. WHO Report on Surveillance of Antibiotic Consumption: 2016–2018 Early Implementation. World Health Organization. 2018. Available online: https://apps.who.int/iris/handle/10665/277359 (accessed on 1 January 2023).
- Jirjees, F.; Al-Obaidi, H.; Sartaj, M.; Conlon-Bingham, G.; Farren, D.; Scott, M.; Gould, I.M.; Lopez-Lozano, J.M.; Aldeyab, M.A. Antibiotic Use and Resistance in Hospitals: Time-Series Analysis Strategy for Determining and Prioritising Interventions. Hospital Pharmacy Europe. 2020. Available online: https://hospitalpharmacyeurope.com/news/reviews-research/antibiotic-use-and-resistance-in-hospitals-time-series-analysis-strategy-for-determining-and-prioritising-interventions/ (accessed on 1 January 2023).
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Gandra, S.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; Levin, S.A.; Laxminarayan, R. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef]
- Lewis, K. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 2013, 12, 371–387. [Google Scholar] [CrossRef]
- Fishman, N. Antimicrobial stewardship. Am. J. Infect. Control 2006, 34, S55–S63; discussion S64–S73. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Cho, I.H.; Jeong, B.C.; Lee, S.H. Strategies to minimize antibiotic resistance. Int. J. Environ. Res. Public Health 2013, 10, 4274–4305. [Google Scholar] [CrossRef]
- Dyar, O.J.; Huttner, B.; Schouten, J.; Pulcini, C. What is antimicrobial stewardship? Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2017, 23, 793–798. [Google Scholar] [CrossRef]
- WHO. Anti-Infective Drug Resistance Surveillance and Containment Team. WHO Global Strategy for Containment of Antimicrobial Resistance. World Health Organization. 2001. Available online: https://apps.who.int/iris/handle/10665/66860 (accessed on 1 January 2023).
- Mendelson, M.; Matsoso, M.P. The World Health Organization Global Action Plan for antimicrobial resistance. S. Afr. Med. J. Suid-Afrik. Tydskr. Vir Geneeskd. 2015, 105, 325. [Google Scholar] [CrossRef]
- Mugada, V.; Mahato, V.; Andhavaram, D.; Vajhala, S.M. Evaluation of Prescribing Patterns of Antibiotics Using Selected Indicators for Antimicrobial Use in Hospitals and the Access, Watch, Reserve (AWaRe) Classification by the World Health Organization. Turk. J. Pharm. Sci. 2021, 18, 282–288. [Google Scholar] [CrossRef]
- WHO. The 2019 WHO AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use. Geneva: World Health Organ. 2019. Available online: https://www.who.int/publications/i/item/WHOEMPIAU2019.11 (accessed on 1 January 2023).
- Alzoubi, K.; Al-Azzam, S.; Alhusban, A.; Mukattash, T.; Al-Zubaidy, S.; Alomari, N.; Khader, Y. An audit on the knowledge, beliefs and attitudes about the uses and side-effects of antibiotics among outpatients attending 2 teaching hospitals in Jordan. East. Mediterr. Health J. 2013, 19, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Scaioli, G.; Gualano, M.R.; Gili, R.; Masucci, S.; Bert, F.; Siliquini, R. Antibiotic use: A cross-sectional survey assessing the knowledge, attitudes and practices amongst students of a school of medicine in Italy. PLoS ONE 2015, 10, e0122476. [Google Scholar] [CrossRef] [PubMed]
- WHO. More Countries Committing to Tackling Antimicrobial Resistance. World Health Organ. (WHO). 2021. Available online: https://www.who.int/news/item/11-11-2021-more-countries-committing-to-tackling-antimicrobial-resistance (accessed on 1 January 2023).
- Morehead, M.S.; Scarbrough, C. Emergence of global antibiotic resistance. Prim. Care Clin. Off. Pract. 2018, 45, 467–484. [Google Scholar] [CrossRef]
- Kalungia, A.; Godman, B. Implications of non-prescription antibiotic sales in China. Lancet Infect. Dis. 2019, 19, 1272–1273. [Google Scholar] [CrossRef]
- Sawair, F.A.; Baqain, Z.H.; Abu Karaky, A.; Abu Eid, R. Assessment of self-medication of antibiotics in a Jordanian population. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2009, 18, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, H.; Sun, Q. Analysis of Antibiotic Consumption by AWaRe Classification in Shandong Province, China, 2012–2019: A Panel Data Analysis. Front. Pharmacol. 2021, 12, 790817. [Google Scholar] [CrossRef]
- Chae, J.; Kim, B.; Kim, D.S. Changes in antibiotic consumption patterns after the implementation of the National Action Plan according to the Access, Watch, Reserve (AWaRe) classification system. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2022, 122, 345–351. [Google Scholar] [CrossRef]
- Apisarnthanarak, A.; Danchaivijitr, S.; Khawcharoenporn, T.; Limsrivilai, J.; Warachan, B.; Bailey, T.C.; Fraser, V.J. Effectiveness of education and an antibiotic-control program in a tertiary care hospital in Thailand. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2006, 42, 768–775. [Google Scholar] [CrossRef]
- Mustafa, Z.U.; Salman, M.; Yasir, M.; Godman, B.; Majeed, H.A.; Kanwal, M.; Iqbal, M.; Riaz, M.B.; Hayat, K.; Hasan, S.S. Antibiotic consumption among hospitalized neonates and children in Punjab province, Pakistan. Expert Rev. Anti-Infect. Ther. 2022, 20, 931–939. [Google Scholar] [CrossRef]
- Nguyen, N.V.; Do, N.T.T.; Nguyen, C.T.K.; Tran, T.K.; Ho, P.D.; Nguyen, H.H.; Vu, H.T.L.; Wertheim, H.F.L.; van Doorn, H.R.; Lewycka, S. Community-level consumption of antibiotics according to the AWaRe (Access, Watch, Reserve) classification in rural Vietnam. JAC-Antimicrob. Resist. 2020, 2, dlaa048. [Google Scholar] [CrossRef]
- Elhajji, F.D.; Al-Taani, G.M.; Anani, L.; Al-Masri, S.; Abdalaziz, H.; Qabba’h, S.H.; Al Bawab, A.Q.; Scott, M.; Farren, D.; Gilmore, F.; et al. Comparative point prevalence survey of antimicrobial consumption between a hospital in Northern Ireland and a hospital in Jordan. BMC Health Serv. Res. 2018, 18, 849. [Google Scholar] [CrossRef] [PubMed]
- Al-Taani, G.M.; Scott, M.; Farren, D.; Gilmore, F.; McCullagh, B.; Hibberd, C.; McCorry, A.; Versporten, A.; Goossens, H.; Zarb, P.; et al. Longitudinal point prevalence survey of antibacterial use in Northern Ireland using the European Surveillance of Antimicrobial Consumption (ESAC) PPS and Global-PPS tool. Epidemiol. Infect. 2018, 146, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Definition and General Considerations. WHO Collaborating Centre for Drug Statistics Methodology. Available online: https://www.whocc.no/ddd/definition_and_general_considera/ (accessed on 1 January 2023).
- WHO. WHO Access, Watch, Reserve (AWaRe) Classification of Antibiotics for Evaluation and Monitoring of Use; World Health Organ: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/2021-aware-classification (accessed on 1 January 2023).
| Pre-Interventional | Post-Interventional | |
|---|---|---|
| Parameter | n (%) | n (%) |
| Age (years) | ||
| ○ <30 | 48 (44.9) | 53 (45.3) |
| ○ 30–39 | 19 (17.8) | 19 (16.2) |
| ○ 40–49 | 16 (15.0) | 17 (14.5) |
| ○ ≥50 | 24 (22.4) | 28 (23.9) |
| Gender | ||
| ○ Male | 68 (63.6) | 75 (64.1) |
| ○ Female | 39 (36.4) | 42 (35.9) |
| Profession | ||
| ○ Specialist or consultant doctors | 48 (44.9) | 50 (42.7) |
| ○ Resident doctors | 49 (45.8) | 42 (35.9) |
| ○ Clinical pharmacists | 0 (0) | 5 (4.3) |
| ○ Pharmacists | 10 (9.3) | 20 (17.1) |
| Years of practice in prescribing or dispensing medicines | ||
| ○ 1–2 years | 32 (29.9) | 29 (24.8) |
| ○ 3–4 years | 13 (12.1) | 22 (18.8) |
| ○ 5–7 years | 20 (18.7) | 17 (14.5) |
| ○ More than 8 years | 42 (39.3) | 49 (41.9) |
| Pre-Interventional | Post-Interventional | |
|---|---|---|
| Parameter | n (%) | n (%) |
| Have you heard about (AWaRe) Classification of antibiotics? | ||
| ○ Yes | 23 (21.5) | 65 (55.6) |
| ○ No | 84 (78.5) | 52 (44.4) |
| I have a good knowledge regarding the meaning and purpose of AWaRe classification of antibiotics | ||
| ○ Agree/Strongly agree | 9 (39.13) | 49 (75.38) |
| ○ Disagree/Strongly disagree | 5 (21.74) | 5 (7.69) |
| ○ Neutral | 9 (39.13) | 11 (16.92) |
| Pre-Interventional | Post-Interventional | |
|---|---|---|
| Parameter | n (%) | n (%) |
| I follow the AWaRe classification of antibiotics in my practice. | ||
| ○ Agree/Strongly agree | 5 (21.74) | 38 (58.46) |
| ○ Disagree/Strongly disagree | 9 (39.14) | 6 (9.23) |
| ○ Neutral | 9 (39.14) | 21 (32.31) |
| The hospital’s regulations and guidelines encourage considering AWaRe classification of antibiotics in my practice | ||
| ○ Agree/Strongly agree | 9 (39.13) | 46 (70.77) |
| ○ Disagree/Strongly disagree | 8 (34.78) | 2 (3.08) |
| ○ Neutral | 6 (26.09) | 17 (26.15) |
| I believe that following the AWaRe classification of antibiotics helps in reduction the rate of antibiotics resistance | ||
| ○ Agree/Strongly agree | 19 (82.16) | 57 (87.69) |
| ○ Disagree/Strongly disagree | 2 (8.70) | 4 (6.15) |
| ○ Neutral | 2 (8.70) | 4 (6.15) |
| The AWaRe classification of antibiotics is compatible with the scientific knowledge I have gain | ||
| ○ Agree/Strongly agree | 16 (69.57) | 56 (86.15) |
| ○ Disagree/Strongly disagree | 2 (8.70) | 3 (4.62) |
| ○ Neutral | 5 (21.74) | 6 (9.23) |
| More insight should be excreted on promoting AWaRe classification of antibiotics | ||
| ○ Agree/Strongly agree | 19 (82.61) | 56 (86.15) |
| ○ Disagree/Strongly disagree | 2 (8.7) | 3 (4.62) |
| ○ Neutral | 2 (8.7) | 6 (9.23) |
| I believe that AWaRe classification of antibiotics can suggest safe choices of antibiotics | ||
| ○ Agree/Strongly agree | 13 (56.52) | 59 (90.77) |
| ○ Disagree/Strongly disagree | 2 (8.7) | 2 (3.08) |
| ○ Neutral | 8 (34.78) | 4 (6.15) |
| I believe that AWaRe classification of antibiotics can suggest cost- effective choices of antibiotics | ||
| ○ Agree/Strongly agree | 15 (65.22) | 55 (84.62) |
| ○ Disagree/Strongly disagree | 1 (4.34) | 3 (4.62) |
| ○ Neutral | 7 (30.43) | 7 (10.77) |
| Training is needed on antibiotics resistance, antimicrobial stewardship, and AWaRe classification of antibiotics | ||
| ○ Agree/Strongly agree | 12 (11.2) | 62 (95.38) |
| ○ Disagree/Strongly disagree | 4 (3.7) | 1 (1.54) |
| ○ Neutral | 7 (6.5) | 2 (3.08) |
| Pre-Interventional | Post-Interventional | |
|---|---|---|
| Parameter | (n = 18) | (n = 14) |
| Mean age (SD) | 57.5 (±6.3) | 52.9 (±6.5) |
| Gender | ||
| ○ Male | 14 (77.8%) | 10 (71.4%) |
| ○ Female | 4 (22.2%) | 4 (28.6%) |
| Prescribed Access antibiotics (DDDs/patient) | 0.33 (7.6%) | 0.43 (20.2%) |
| Prescribed Watch antibiotics (DDDs/patient) | 3.02 (69.3%) | 1.05 (49.5%) |
| Prescribed Reserve antibiotics (DDDs/patient) | 1.01 (23.1%) | 0.64 (30.3%) |
| Prescribed total antibiotics (DDDs/patient) | 4.36 | 2.12 |
| Pre-Interventional | Post-Interventional | |||
|---|---|---|---|---|
| DDDs/100 Patient Days | % of Total Antibiotic Use | DDDs/100 Patient Days | % of Total Antibiotic Use | |
| Access | ||||
| Amikacin | 1.326 | 0.684 | 1.219 | 0.569 |
| Amoxicillin and Co-amoxiclav | 27.357 | 14.105 | 33.215 | 15.507 |
| Ampicillin | 0.290 | 0.150 | 0.333 | 0.155 |
| Cefalexin | 0.116 | 0.060 | 0.222 | 0.104 |
| Cefazolin | 2.428 | 1.252 | 3.362 | 1.570 |
| Clindamycin | 2.932 | 1.512 | 3.003 | 1.402 |
| Doxycycline | 0.853 | 0.440 | 3.736 | 1.744 |
| Gentamicin | 0.753 | 0.388 | 0.712 | 0.332 |
| Metronidazole | 7.633 | 3.936 | 5.636 | 2.631 |
| Watch | ||||
| Azithromycin | 5.693 | 2.935 | 7.320 | 3.417 |
| Cefaclor | 1.314 | 0.677 | 2.320 | 1.083 |
| Cefdinir | 21.858 | 11.270 | 20.061 | 9.366 |
| Cefditoren | 1.629 | 0.840 | 1.245 | 0.581 |
| Cefixime | 25.022 | 12.901 | 30.664 | 14.316 |
| Cefotaxime | 0.878 | 0.453 | 0.818 | 0.382 |
| Cefpodoxime | 5.013 | 2.585 | 3.269 | 1.526 |
| Cefprozil | 1.745 | 0.900 | 1.290 | 0.602 |
| Ceftazidime | 0.950 | 0.490 | 0.845 | 0.394 |
| Ceftizoxime | 4.064 | 2.095 | 3.965 | 1.851 |
| Ceftriaxone | 5.893 | 3.038 | 6.117 | 2.856 |
| Cefuroxime | 15.749 | 8.120 | 16.417 | 7.664 |
| Ciprofloxacin | 19.703 | 10.159 | 17.545 | 8.191 |
| Clarithromycin | 1.249 | 0.644 | 1.548 | 0.723 |
| Erythromycin | 0.322 | 0.166 | 0.365 | 0.170 |
| Ertapenem | 1.226 | 0.632 | 1.245 | 0.581 |
| Imipenem/Cilastatin | 0.812 | 0.419 | 0.938 | 0.438 |
| Levofloxacin | 24.501 | 12.633 | 31.902 | 14.894 |
| Lincomycin | 0.092 | 0.047 | 0.097 | 0.045 |
| Meropenem | 1.834 | 0.946 | 2.855 | 1.333 |
| Moxifloxacin | 4.997 | 2.576 | 6.288 | 2.936 |
| Piperacillin/Tazobactam | 1.290 | 0.665 | 1.344 | 0.627 |
| Teicoplanin | 1.881 | 0.970 | 1.810 | 0.845 |
| Vancomycin | 1.687 | 0.870 | 1.883 | 0.879 |
| Cefepime | 0.043 | 0.022 | 0.018 | 0.008 |
| Reserve | ||||
| Ceftazidime/Avibactam | 0.084 | 0.043 | 0.044 | 0.021 |
| Ceftobiprole | 0.013 | 0.007 | 0.006 | 0.003 |
| Colistimethate | 0.121 | 0.062 | 0.050 | 0.023 |
| Linezolid | 0.019 | 0.010 | 0.0 | 0.000 |
| Tigecycline | 0.582 | 0.300 | 0.418 | 0.195 |
| Total antibiotics | 193.96 | 100 | 214.20 | 100 |
| Access | Watch | Reserve | |
|---|---|---|---|
| DDD/100 patient days (%) | |||
| Pre-intervention | 43.69 (22.53) | 149.45 (77.05) | 0.82 (0.42) |
| Post-intervention | 51.44 (24.01) | 162.22 (75.73) | 0.54 (0.25) |
| Relative rate of change * | 1.066 | 0.983 | 0.569 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Ajaleh, S.; Darwish Elhajji, F.; Al-Bsoul, S.; Abu Farha, R.; Al-Hammouri, F.; Amer, A.; Al Rusasi, A.; Al-Azzam, S.; Araydah, M.; Aldeyab, M.A. An Evaluation of the Impact of Increasing the Awareness of the WHO Access, Watch, and Reserve (AWaRe) Antibiotics Classification on Knowledge, Attitudes, and Hospital Antibiotic Prescribing Practices. Antibiotics 2023, 12, 951. https://doi.org/10.3390/antibiotics12060951
Abu-Ajaleh S, Darwish Elhajji F, Al-Bsoul S, Abu Farha R, Al-Hammouri F, Amer A, Al Rusasi A, Al-Azzam S, Araydah M, Aldeyab MA. An Evaluation of the Impact of Increasing the Awareness of the WHO Access, Watch, and Reserve (AWaRe) Antibiotics Classification on Knowledge, Attitudes, and Hospital Antibiotic Prescribing Practices. Antibiotics. 2023; 12(6):951. https://doi.org/10.3390/antibiotics12060951
Chicago/Turabian StyleAbu-Ajaleh, Salam, Feras Darwish Elhajji, Shatha Al-Bsoul, Rana Abu Farha, Fawzi Al-Hammouri, Amer Amer, Ahmed Al Rusasi, Sayer Al-Azzam, Mohammad Araydah, and Mamoon A. Aldeyab. 2023. "An Evaluation of the Impact of Increasing the Awareness of the WHO Access, Watch, and Reserve (AWaRe) Antibiotics Classification on Knowledge, Attitudes, and Hospital Antibiotic Prescribing Practices" Antibiotics 12, no. 6: 951. https://doi.org/10.3390/antibiotics12060951
APA StyleAbu-Ajaleh, S., Darwish Elhajji, F., Al-Bsoul, S., Abu Farha, R., Al-Hammouri, F., Amer, A., Al Rusasi, A., Al-Azzam, S., Araydah, M., & Aldeyab, M. A. (2023). An Evaluation of the Impact of Increasing the Awareness of the WHO Access, Watch, and Reserve (AWaRe) Antibiotics Classification on Knowledge, Attitudes, and Hospital Antibiotic Prescribing Practices. Antibiotics, 12(6), 951. https://doi.org/10.3390/antibiotics12060951






