Activity of Delafloxacin and Comparator Fluoroquinolones against Multidrug-Resistant Pseudomonas aeruginosa in an In Vitro Cystic Fibrosis Sputum Model
Abstract
1. Introduction
2. Results
2.1. Susceptibility Testing
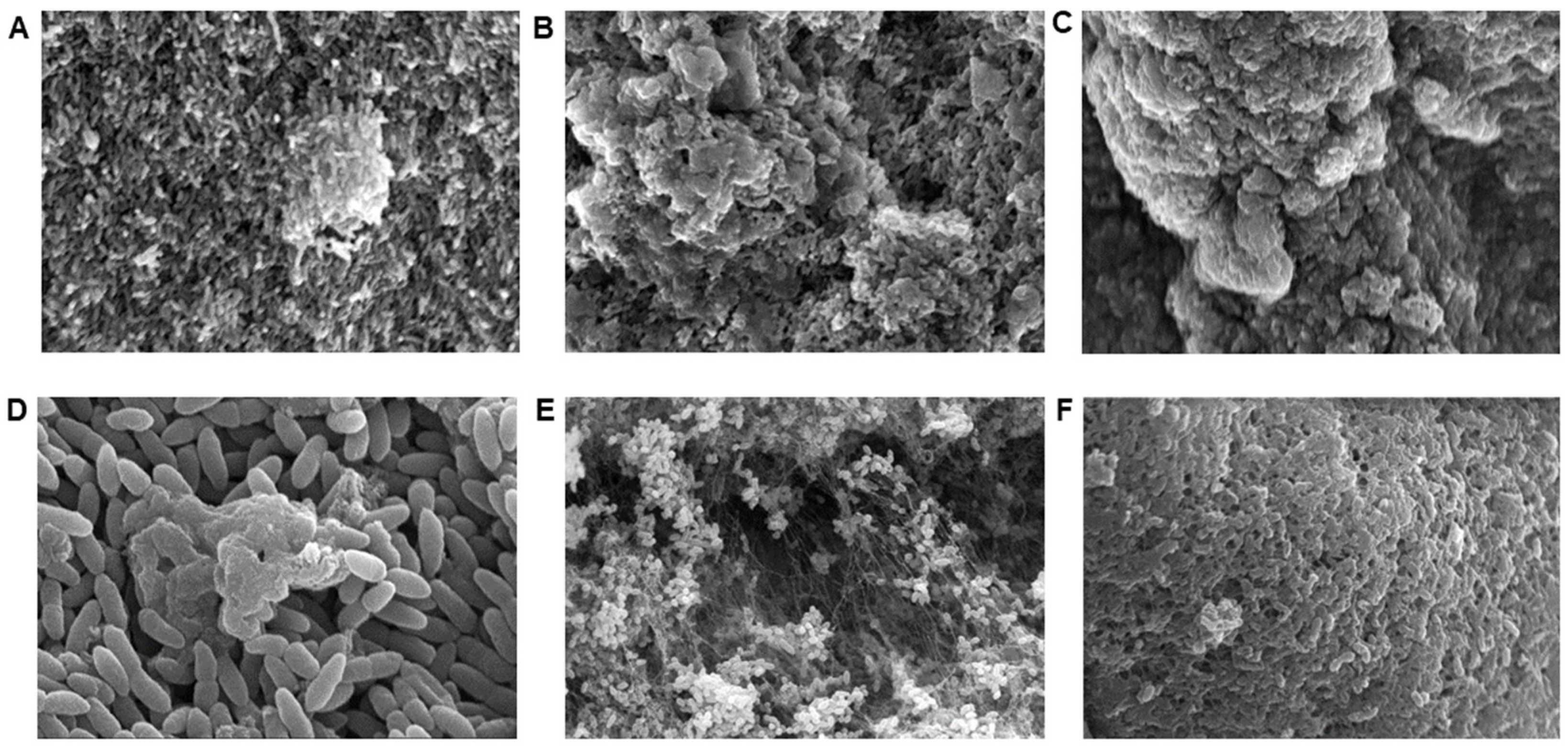
2.2. Scanning Electron Microscopy (SEM)
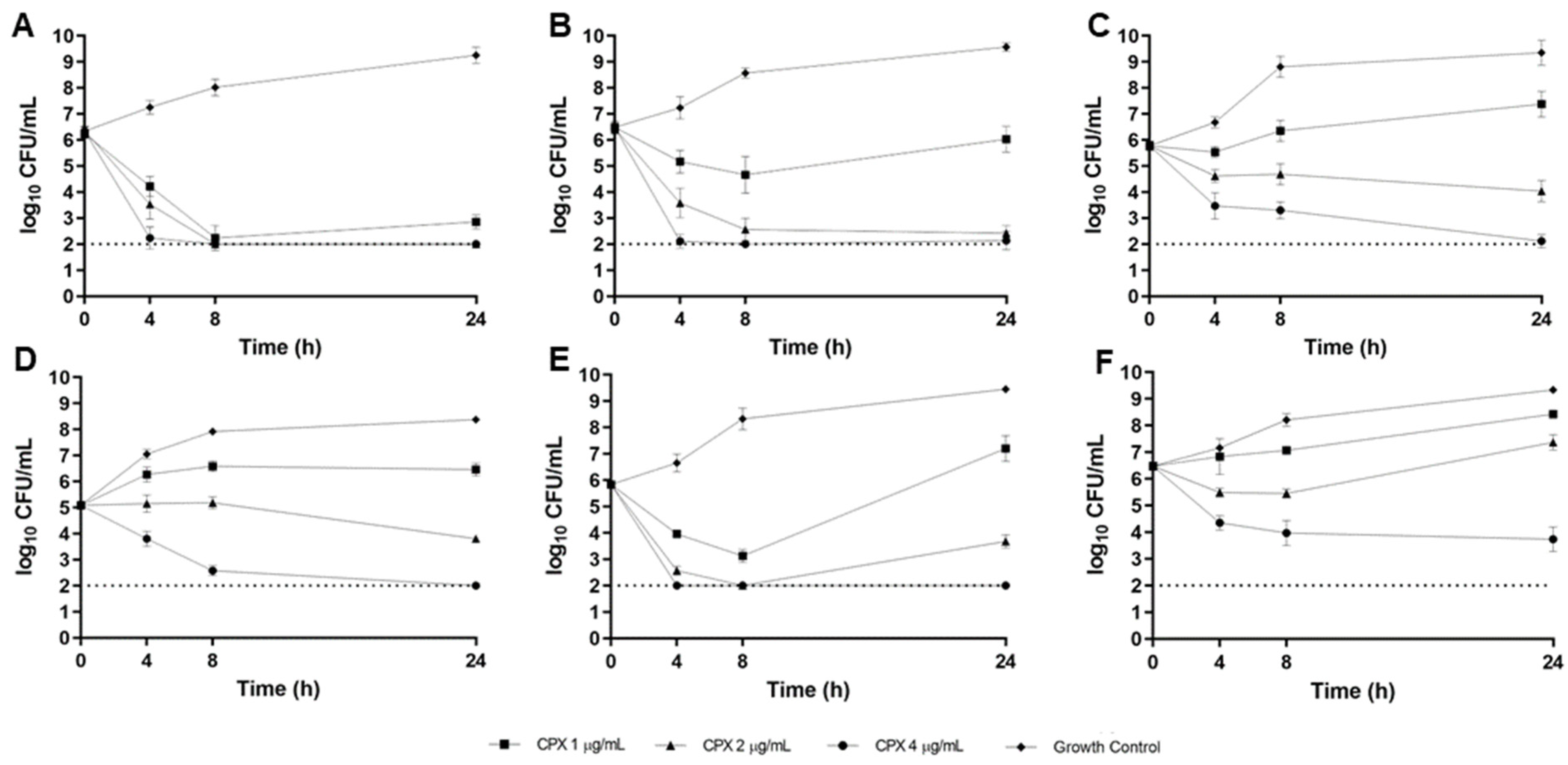
2.3. CF Sputum Biofim Time-Kill Model
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Antimicrobials
4.3. Media
4.4. Susceptibility Testing
4.5. Scanning Electron Microscopy
4.6. CF Sputum Time-Kill Model
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibson, R.L.; Burns, J.L.; Ramsey, B.W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 918–951. [Google Scholar] [CrossRef] [PubMed]
- Bhagirath, A.Y.; Li, Y.; Somayajula, D.; Dadashi, M.; Badr, S.; Duan, K. Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulm. Med. 2016, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Emerson, J.; Rosenfeld, M.; McNamara, S.; Ramsey, B.; Gibson, R.L. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 2002, 34, 91–100. [Google Scholar] [CrossRef]
- Cystic Fibrosis Foundation. Annual Data Report; Cystic Fibrosis Foundation: Bethesda, MD, USA, 2017. [Google Scholar]
- Cho, D.Y.; Lim, D.; Mackey, C.; Skinner, D.; Zhan, S.; McCormick, J.; Woodworth, B.A. Ivacaftor, a Cystic Fibrosis Transmembrane Conductance Regulator Potentiator, Enhances Ciprofloxacin Activity against Pseuodomonas aeeurginosa. Am. J. Rhinol. Allergy 2019, 33, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Millar, B.C.; McCaughan, J.; Rendall, J.C.; Moore, J.E. Delafloxacin—A novel fluoroquinolone for the treatment of ciprofloxacin-resistant Pseudomonas aeruginosa in patients with cystic fibrosis. Clin. Respir. J. 2021, 15, 116–120. [Google Scholar] [CrossRef]
- Lemaire, S.; Tulkens, P.M.; Van Bambeke, F. Contrasting effects of acidic pH on the extracellular and intracellular activities of the anti-gram-positive fluoroquinolones moxifloxacin and delafloxacin against Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 649–658. [Google Scholar] [CrossRef]
- Pezzulo, A.A.; Tang, X.X.; Hoegger, M.J.; Abou Alaiwa, M.H.; Ramachandran, S.; Moninger, T.O.; Karp, P.H.; Wohlford-Lenane, C.L.; Haagsman, H.P.; van Eijk, M.; et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 2012, 487, 109–113. [Google Scholar] [CrossRef]
- Thabit, A.K.; Crandon, J.L.; Nicolau, D.P. Pharmacodynamic and pharmacokinetic profiling of delafloxacin in a murine lung model against community-acquired respiratory tract pathogens. Int. J. Antimicrob. Agents 2016, 48, 535–541. [Google Scholar] [CrossRef]
- Siala, W.; Mingeot-Leclercq, M.P.; Tulkens, P.M.; Hallin, M.; Denis, O.; Van Bambeke, F. Comparison of the antibiotic activities of Daptomycin, Vancomycin, and the investigational Fluoroquinolone Delafloxacin against biofilms from Staphylococcus aureus clinical isolates. Antimicrob. Agents Chemother. 2014, 58, 6385–6397. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Mercuro, N.J.; Davis, S.L.; Rybak, M.J. Delafloxacin: Place in Therapy and Review of Microbiologic, Clinical and Pharmacologic Properties. Infect. Dis. Ther. 2018, 7, 197–217. [Google Scholar] [CrossRef]
- Yoon, S.S.; Coakley, R.; Lau, G.W.; Lymar, S.V.; Gaston, B.; Karabulut, A.C.; Hennigan, R.F.; Hwang, S.H.; Buettner, G.; Schurr, M.J.; et al. Anaerobic killing of mucoid Pseudomonas aeruginosa by acidified nitrite derivatives under cystic fibrosis airway conditions. J. Clin. Investig. 2006, 116, 436–446. [Google Scholar] [CrossRef]
- Massip-Copiz, M.M.; Santa-Coloma, T.A. Extracellular pH and lung infections in cystic fibrosis. Eur. J. Cell Biol. 2018, 97, 402–410. [Google Scholar] [CrossRef]
- Coakley, R.D.; Grubb, B.R.; Paradiso, A.M.; Gatzy, J.T.; Johnson, L.G.; Kreda, S.M.; O’Neal, W.K.; Boucher, R.C. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc. Natl. Acad. Sci. USA 2003, 100, 16083–16088. [Google Scholar] [CrossRef]
- McShane, D.; Davies, J.C.; Davies, M.G.; Bush, A.; Geddes, D.M.; Alton, E.W. Airway surface pH in subjects with cystic fibrosis. Eur. Respir. J. 2003, 21, 37–42. [Google Scholar] [CrossRef]
- Hunter, R.C.; Beveridge, T.J. Application of a pH-sensitive fluoroprobe (C-SNARF-4) for pH microenvironment analysis in Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 2005, 71, 2501–2510. [Google Scholar] [CrossRef] [PubMed]
- Baxdela (Delafloxacin) [Package Insert]; Melinta Therapeutics, Inc.: Lincolnshire, IL, USA, 2017.
- CLSI M100-Ed32; Performance Standards for Antimicrobial Susceptibility Testing—32nd Edition. Clinical Laboratory Standards Institute: Wayne, PA, USA, 2022.
- Somayaji, R.; Parkins, M.D.; Shah, A.; Martiniano, S.L.; Tunney, M.M.; Kahle, J.S.; Waters, V.J.; Elborn, J.S.; Bell, S.C.; Flume, P.A.; et al. Antimicrobial susceptibility testing (AST) and associated clinical outcomes in individuals with cystic fibrosis: A systematic review. J. Cyst. Fibros. 2019, 18, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Hengzhuang, W.; Song, Z.; Ciofu, O.; Onsoyen, E.; Rye, P.D.; Hoiby, N. OligoG CF-5/20 Disruption of Mucoid Pseudomonas aeruginosa Biofilm in a Murine Lung Infection Model. Antimicrob. Agents Chemother. 2016, 60, 2620–2626. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Lebeaux, D.; Chauhan, A.; Rendueles, O.; Beloin, C. From in vitro to in vivo Models of Bacterial Biofilm-Related Infections. Pathogens 2013, 2, 288–356. [Google Scholar] [CrossRef]
- McBain, A.J. Chapter 4: In vitro biofilm models: An overview. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2009; Volume 69, pp. 99–132. [Google Scholar]
- Kirchner, S.; Fothergill, J.L.; Wright, E.A.; James, C.E.; Mowat, E.; Winstanley, C. Use of artificial sputum medium to test antibiotic efficacy against Pseudomonas aeruginosa in conditions more relevant to the cystic fibrosis lung. J. Vis. Exp. 2012, 64, e3857. [Google Scholar] [CrossRef]
- Haley, C.L.; Colmer-Hamood, J.A.; Hamood, A.N. Characterization of biofilm-like structures formed by Pseudomonas aeruginosa in a synthetic mucus medium. BMC Microbiol. 2012, 12, 181. [Google Scholar] [CrossRef]
- Jayaraman, S.; Joo, N.S.; Reitz, B.; Wine, J.J.; Verkman, A.S. Submucosal gland secretions in airways from cystic fibrosis patients have normal [Na(+)] and pH but elevated viscosity. Proc. Natl. Acad. Sci. USA 2001, 98, 8119–8123. [Google Scholar] [CrossRef] [PubMed]
- Tate, S.; MacGregor, G.; Davis, M.; Innes, J.A.; Greening, A.P. Airways in cystic fibrosis are acidified: Detection by exhaled breath condensate. Thorax 2002, 57, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.L.; Aye, L.M.; Whiteley, M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 2007, 189, 8079–8087. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.C.; Fatima, M.A.; Santos, V.V.; Brandao, C.M.; Alves, I.A.; Azeredo, F.J. Pharmacokinetic/Pharmacodynamic Modeling and Application in Antibacterial and Antifungal Pharmacotherapy: A Narrative Review. Antibiotics 2022, 11, 986. [Google Scholar] [CrossRef]
- CLSI M100-Ed29; Performance Standards for Antimicrobial Susceptibility Testing—29th Edition. Clinical Laboratory Standards Institute: Wayne, PA, USA, 2019.
- Sriramulu, D.D.; Lunsdorf, H.; Lam, J.S.; Romling, U. Microcolony formation: A novel biofilm model of Pseudomonas aeruginosa for the cystic fibrosis lung. J. Med. Microbiol. 2005, 54 Pt 7, 667–676. [Google Scholar] [CrossRef]
- Cipro (Ciprofloxacin) [Package Insert]; Bayer HealthCare Pharmaceuticals: Whippany, NJ, USA, 2016.
- Levaquin (Levofloxacin) [Package Insert]; Ortho-McNeil-Janssen Pharmaceuticals: Raritan, NJ, USA, 2008.


| CAMHB (pH 7.3) | ASM (pH 6.9) | Adjusted CAMHB (pH 6.9) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Isolate and Morphotype | DLX | CPX | LVX | DLX | CPX | LVX | DLX | CPX | LVX |
| B727 (flat) | 0.0156 | 0.25 | 0.5 | 0.0156 | 0.5 | 0.5 | 0.0156 | 0.25 | 0.5 |
| B660 (mucoid) | 0.25 | 0.25 | 1 | 0.0625 | 1 | 4 | 0.125 | 1 | 4 |
| B661 (flat) | 1 | 1 | 8 | 1 | 2 | 8 | 0.5 | 1 | 8 |
| B677 (flat) | 2 | 1 | 4 | 1 | 2 | 4 | 1 | 1 | 4 |
| B310 (flat) | 0.25 | 0.25 | 1 | 0.25 | 1 | 2 | 0.25 | 0.5 | 1 |
| ATCC® BAA-2108 (flat) | 0.5 | 0.5 | 2 | 0.25 | 2 | 4 | 0.5 | 1 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craddock, V.D.; Steere, E.L.; Harman, H.; Britt, N.S. Activity of Delafloxacin and Comparator Fluoroquinolones against Multidrug-Resistant Pseudomonas aeruginosa in an In Vitro Cystic Fibrosis Sputum Model. Antibiotics 2023, 12, 1078. https://doi.org/10.3390/antibiotics12061078
Craddock VD, Steere EL, Harman H, Britt NS. Activity of Delafloxacin and Comparator Fluoroquinolones against Multidrug-Resistant Pseudomonas aeruginosa in an In Vitro Cystic Fibrosis Sputum Model. Antibiotics. 2023; 12(6):1078. https://doi.org/10.3390/antibiotics12061078
Chicago/Turabian StyleCraddock, Vaughn D., Evan L. Steere, Hannah Harman, and Nicholas S. Britt. 2023. "Activity of Delafloxacin and Comparator Fluoroquinolones against Multidrug-Resistant Pseudomonas aeruginosa in an In Vitro Cystic Fibrosis Sputum Model" Antibiotics 12, no. 6: 1078. https://doi.org/10.3390/antibiotics12061078
APA StyleCraddock, V. D., Steere, E. L., Harman, H., & Britt, N. S. (2023). Activity of Delafloxacin and Comparator Fluoroquinolones against Multidrug-Resistant Pseudomonas aeruginosa in an In Vitro Cystic Fibrosis Sputum Model. Antibiotics, 12(6), 1078. https://doi.org/10.3390/antibiotics12061078




