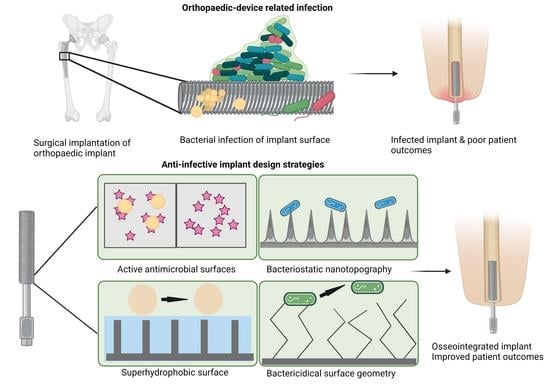
Strategies to Mitigate and Treat Orthopaedic Device-Associated Infections
Abstract
1. Introduction
2. Orthopaedic Device-Related Infection (ODI)
2.1. Origin and Causative Organisms
2.2. Biofilm-Related Infections
2.2.1. Biofilm Formation
2.2.2. Biofilms—The Clinical Consequences
3. Infection Control and Orthopaedic Implant Design
3.1. Orthopaedic Device Materials
3.2. Infection Control
4. Antimicrobials and Anti-Biofilm Strategies
4.1. Antimicrobial Cargoes and Materials
4.2. Combatting Bacterial Biofilms
5. Antimicrobial Surface Coatings
5.1. Direct Antimicrobial Application to the Implant Surface
5.2. Antimicrobial Delivery from Polymer Coatings
| Polymer | Antimicrobial | Implant | Model | Release Kinetics | Outcome | Reference |
|---|---|---|---|---|---|---|
| PDLLA | Norvancomycin | Stainless steel plate | Rabbit tibia fractures, inoculated with S. aureus | Sustained release above minimum inhibitory concentration for up to 28 days | Significant reduction in infection rate (32% compared to 92% for uncoated plates) | [152] |
| PLGA | Vancomycin Cefuroxime | Ti alloy discs | S. aureus culture in vitro | Effective antibiotic release duration from 5 to 17 days depending on antibiotic and concentration | Up to 17 days antibiotic release from optimal double layer formulation | [154] |
| PLLA | Rifampicin and fusidic acid; octenidin and triclosan (antiseptics) | Ti plates | Rabbit tibia model, inoculated with S. aureus | Release of 60–62% within 1 h, then sustained release for at least 42 days | Significant reduction in infection rate (17% for antibiotic and antiseptic groups compared to 83% for control groups) | [153] |
| PDLLA | Gentamicin and/or teicoplanin | Stainless steel and Ti alloy K-wires | S. epidermidis culture in vitro | Initial burst release within 6 h, then sustained release for at least 96 h | Reduction in adhesion of viable bacteria to undetectable levels with either or both antibiotics | [172] |
| PLGA | Gentamicin | Stainless steel fracture plates | S. aureus culture in vitro | Initial burst release, with sustained high levels for 3 weeks (for the 20% gentamicin coating) | Significant reduction in bacterial growth compared to uncoated implants) | [173] |
| PDLLA | Gentamicin | Ti K-wires | S. aureus induced intra-medullary infection | 60% release within 24 h, then sustained release over 6 weeks (from previous study) | Significant reduction in histological and radiological signs of infection in treated groups compared to control groups | [156] |
| PDLLA | Gentamicin | Ti K-wires | S. aureus induced intra-medullary infection in rats | 80% gentamicin release within 48 h (demonstrated in previous study) | Significant reduction in radiological signs of infection compared to control group | [174] |
| PDLLA | Gentamicin | Ti K-wires | S. aureus induced intra-medullary infection in rats | 60% release within 24 h, up to 90% released in the following 6 weeks (demonstrated in previous study) | Significantly lower histological infection score (with or without systemic gentamicin) compared to other groups | [175] |
| PLA | Chlorhexidine | Ti plates | S. aureus culture in vitro | Rapid release during first day, followed by slower release up to 14 days | Greatest antibacterial effects with lowest % PLA coating | [176] |
| Copolymer of glycolide, caprolactone, trimethyl carbonate, lactide | gentamicin, triclosan or combination | Stainless steel plates, covered with polymer sleeve | Adult sheep | Gentamicin: 50% release within 24 h, then sustained release over 2–3 weeks Triclosan: slow release over 2–3 weeks | Tissue biocompatibility and normal bone healing demonstrated | [177] |
| PDLLA | Gentamicin | Ti K-wires | Rat with intramedullary implant | Burst release within 1 h, then gradually reducing levels over 7 days | Significantly reduced bacterial adhesion compared to uncoated wires | [178] |
| PLGA | Gentamicin | Ti coupons | Staphylococcal cultures in vitro | Release of 90% within 24 h, then short, sustained release over 4 days | Greater antibacterial activity compared to uncoated coupons | [179] |
5.3. Antimicrobial Delivery from Inorganic and Ceramic Coatings
| Carrier | Antimicrobial | Implant | Loading and Release Kinetics | Model | Outcome | Ref. |
|---|---|---|---|---|---|---|
| Silica sol–gel films | Triclosan | Percutaneous external fixator pins—stainless steel 316L rods | Continuous release in vitro with 33% of original load by 8 weeks. | Percutaneous tibial implant rabbit model, inoculated with S. aureus (ATCC™ 25923) | No infection in animals with pin implants coated with sol–gel 20% triclosan in contrast to uncoated implants. Normal bone tissue ingrowth observed at 4 weeks in coated implant model. | [192] |
| Silica sol–gel films | Vancomycin + farnesol (adjuvant) | Ti alloy rods and K-wires | Drug release from 5-layer thin films is concomitant with film degradation over 6 days. | In vitro bacterial challenge (i) MSSA (ATCC 25923) 5 mL 1 × 106 CFU/mL (rods), (ii) MRSA (ATCC 33591) 5 mL 1 × 104 CFU/mL (wires) | Bactericidal effect impacted by drug loading and farnesol on K-wires. Bacterial (MRSA) counts 1.11 × 103 and 1.56 × 102 CFU for Vancomycin 10% w/w and 20% w/w loading, respectively. Further reduction to 2.44 × 102 CFU when farnesol added to 10% vancomycin. | [193] |
| TNT | Vancomycin | Ti rods | Drug loading by lyophilization, with rapid release −58% release within 15 min. | Femur Sprague–Dawley rat model—intramedullary implantation of rod contaminated with 0.1 mL of 1 × 108 S. aureus (29213 ATCC) | Agar plate and clinical assessment at 30 days showed all animals receiving vancomycin-containing nanotubes were infection-free, while 11 out of 12 drug free-TNT controls were infected. | [194] |
| Hybrid coating—TiO2 and PDMS | Ag | PEEK discs | Release rate depends on Ag doping and ratio of coating constituents. | S. aureus (ATCC 25923) and S. epidermidis (ATCC 35894) 5 × 106 CFU/mL used for Kirby–Bauer testing and biofilm growth studies. | Kirby–Bauer testing showed greater zones of inhibition for higher Ag loading, with similar results for both bacterium types. SEM analysis revealed small colonies of S. aureus for the lower Ag loading compared to controls. Colonies were absent in higher Ag loadings. | [195] |
| HA | Ag | PEEK | Ionic Ag is immobilized via inositol hexaphosphate chelation | A non-coated PEEK or PEEK-Ag+ plate was placed into the superficial gluteus muscle of mouse, followed by inoculation of bioluminescent S. aureus (1 × 1011 CFU/mL) | Mean bacterial photon intensity decreased after 8 days and reduced to background level at day 10 in the PEEK-Ag+ model, compared to non-coated PEEK where strong photon intensity was still observed at day 10. | [190] |
| HA | Zn, Ag and Sr | Ti (Grade 5, Ti-6Al-4V) | At 30 days <10% of Sr and Zn but > 90% of the Ag released | Antimicrobial activity of the released ions and anti-colonizing potential of the surface using modified ASTM E2149-01 and S. aureus ATCC 1448 | MG-63 osteoblast cells cultured on the Sr apatite surfaces displayed the highest metabolic activity using the MTT assay. Ion release and direct surface contact important for antibacterial effects. Ag-substituted apatite produced superior biofilm inhibition compared to Sr and Zn substituted apatite surfaces. | [107] |
| Ti | Zn | Ti | For 1 cm2 coatings, total Zn loading ranged from 1.2 to 60.2 μg, depending on time of hydrothermal treatment in Zn solution and voltage used during NT fabrication | Incubation of implants in S. aureus and viable bacteria in suspensions collected from samples were evaluated by spread plate method. | Inhibition of adherent and planktonic bacteria was greatest for the coatings with the highest Zn content. Inhibition was greatest at day 1 and decreased at days 4 and 7. | [196] |
| Three-layer. Outer and inner vanco-mycin loaded in vaterite. Middle layer IL-12 containing liposomes embedded in alginate. | Vancomycin | Ti | Vaterite coating released 100% of vancomycin within ~2 days. | In vitro: soaking of different layers of the coating with 1 mL of sterile LB broth and inoculated with 200 μL of LB broth with a concentration of 1 × 106 CFU/mL ATCC 25923 (MSSA) and ATCC 43300 (MSSA) bacterial strains. In vivo: rats were inoculated with MSRA in the tibial platform and Ti alloy screw was implanted in the tibial channel, re-injection of MSRA after two weeks and injection of 0.1 mL of LB broth containing 1 × 106 CFU/mL bacteria in the bone marrow cavity. | In vitro: complete three-layer sandwich yielded a bacterial death ratio of ~100% of ATCC 25923 by day 7; complete three-layer sandwich yielded a bacterial death ratio of ~100% of ATCC 43300 by day 7. In vivo: bacterial colonization in the bone tissue reduced in the three-layer sandwich compared to control. | [197] |
| Biphasic calcium phosphate | Vancomycin Tobramycin | Drug-loaded biphasic calcium phosphate granule complex with additive antibiotic powder in gypsum binder, coated with PLGA | Human trial composing of 43 patients with previously diagnosed chronic osteomyelitis subjected to prosthesis removal (if present), debridement of necrotic tissue, and 20–40 g of cements containing beads were implanted into bone defects before primary wound closure. | Serum concentrations of vancomycin and tobramycin decreased gradually from ~ 20 μg/mL to 0 μg/mL over 14 days. | Higher success rate, faster sepsis control and bone regeneration achieved compared to PMMA cement and parenteral antibiotic therapy. | [198] |
6. Nanotechnology and Infection Control
6.1. Antimicrobial Nanomaterials
6.2. Nanotechnology Approaches for the Delivery of Antimicrobials
7. Controlling Bacterial Infection by Preventing/Minimizing Bacterial Adhesion
7.1. Surface Topography
7.2. Surface Charge
7.3. Controlling the Hydrophilic/Hydrophobic Properties at the Biological Interface
| Material | Surface Features | Fabrication Method | Bacteria Studied | Antimicrobial Outcome | Reference |
|---|---|---|---|---|---|
| Aluminium | Micro- and nano-roughed | Wet etching | E. coli, K. pneumoniae, P. aeruginosa | Decreased cell attachment compared to non-etched controls | [221] |
| Black silicon | High aspect ratio nanoprotrusions 500 nm height; contact angle 80° | Ion etching | P. aeruginosa, S. aureus, B. subtilis | Bactericidal; reduces cell viability compared to non-etched controls | [232] |
| Ti | Nano-roughened; contact angle 59.3 ± 1.13° | Electron beam evaporation | S. aureus, S. epidermidis, P. aeruginosa | Decreased adhesion of bacterial colonies compare to conventional, nanotubular and nanotextured Ti | [220] |
| Ti | Functionalization with PMMA and silk sericin | Atom transfer radical polymerization | S. aureus, S. epidermidis | Threefold decrease in number of viable S. aureus cells compared to pristine Ti | [260] |
| Ti | Coating with PEG-polylysine | Polymer surface adsorption | S. aureus | Decreased the adhesion of S. aureus to the surfaces by 89–93% compared to bare TiO2 surface | [243] |
| Ti | Two-tier micro- and nanoscale surface structures: First tier, large grain-like convex features 10–20 µm in size. Second, ≤200 nm wide irregular undulations on the surface of these grains; superhydrophobic, contact angle 166 ± 4° | Femtosecond laser ablation | P. aeruginosa, S. aureus | S. aureus colonized the surface. No P. aeruginosa cells were able to attach to the surface (i.e., any attached bacterial cells were below the estimated lower detection limit) | [252] |
| Ti | Micro/nanoscale surface roughness | Etching and adding perfluoropolyether lubricants | P. aeruginosa, MRSA | Reduction in log CFU count of P. aeruginosa and MRSA to non-measurable | [259] |
| Ti | Nanopatterned arrays | Hydrothermal etching | P. aeruginosa, S. aureus | Killed 50% of P. aeruginosa cells and about 20% of the S. aureus cells contacting the surface | [261] |
| Ti | Nanocolumnar thin Ti films | Glancing angle sputter deposition | E. coli, S. aureus | E. coli viability significantly decreased; S. aureus viability relatively unchanged | [262] |
| Ti | Titania nanowire arrays of 100 nm diameter | Alkaline hydrothermal processing | P. aeruginosa, S. aureus | Selectively bactericidal against P. aeruginosa (highly mobile), but not against S. aureus | [263] |
| Ti and CoCrMo alloys | Surface roughened, “spiky” protrusions produced | Continuous wave fibre laser with near-infrared wavelength | S. aureus biofilm | Laser treatment of Ti surfaces decreased viable bacteria and biofilm area but effects not evident in laser treated CoCrMo. | [236] |
| Ti plasma spray implant | Ti nano-spikes | Glancing angle magnetron sputter deposition | E. coli, S. aureus | Partial destruction of E. coli adherent to the nano-spikes via a physico-mechanical mechanism, not useful against Gram-positive bacteria | [264] |
| Ti | Nanostructures with peaks and valleys on surface | Etching | P. aeruginosa, S. aureus | Decrease in viability of P. aeruginosa and S. aureus to ~4% and ~40% on nanostructured surfaces, respectively, while viability did not drop below 90% for control surface | [265] |
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef]
- McMillan, D.J.; Lutton, C.; Rosenzweig, N.; Sriprakash, K.S.; Goss, B.; Stemberger, M.; Schuetz, M.A.; Steck, R. Prevention of staphylococcus aures biofilm formation on metallic surgical implants via controlled release of gentamicin. J. Biomed. Sci. Eng. 2011, 4, 535–542. [Google Scholar] [CrossRef]
- Evans, N.T.; Torstrick, F.B.; Lee, C.S.D.; Dupont, K.M.; Safranski, D.L.; Chang, W.A.; Macedo, A.E.; Lin, A.S.P.; Boothby, J.M.; Whittingslow, D.C.; et al. High-strength, surface-porous polyether-ether-ketone for load-bearing orthopedic implants. Acta Biomater. 2015, 13, 159–167. [Google Scholar] [CrossRef]
- O’ Sullivan, C.; Kennedy, G.; O’ Neill, L.; Crean, A.M.; Ryan, K.B. Chapter 5 Inorganic Biomaterials to Support the Formation and Repair of Bone Tissue. In Biomedical Applications of Inorganic Materials; The Royal Society of Chemistry: Cambridge, UK, 2022; pp. 242–304. [Google Scholar]
- Stigter, M.; Bezemer, J.; de Groot, K.; Layrolle, P. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J. Control. Release 2004, 99, 127–137. [Google Scholar] [CrossRef]
- Trampuz, A.; Widmer, A.F. Infections associated with orthopeadic implants. Curr. Opin. Infect. Dis. 2006, 19, 349–356. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Lau, E.; Schmier, J.; Ong, K.L.; Zhao, K.; Parvizi, J. Infection Burden for Hip and Knee Arthroplasty in the United States. J. Arthroplast. 2008, 23, 984–991. [Google Scholar] [CrossRef]
- Kamath, A.F.; Ong, K.L.; Lau, E.; Chan, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J.; Bozic, K.J. Quantifying the Burden of Revision Total Joint Arthroplasty for Periprosthetic Infection. J. Arthroplast. 2015, 30, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Gulcu, A.; Akman, A.; Demirkan, A.F.; Yorukoglu, A.C.; Kaleli, I.; Bir, F. Fosfomycin Addition to Poly(D,L-Lactide) Coating Does Not Affect Prophylaxis Efficacy in Rat Implant-Related Infection Model, But That of Gentamicin Does. PLoS ONE 2016, 11, e0165544. [Google Scholar] [CrossRef] [PubMed]
- Rezapoor, M.; Parvizi, J. Prevention of Periprosthetic Joint Infection. J. Arthroplast. 2015, 30, 902–907. [Google Scholar] [CrossRef]
- Jiranek, W.A.; Waligora, A.C.; Hess, S.R.; Golladay, G.L. Surgical Treatment of Prosthetic Joint Infections of the Hip and Knee: Changing Paradigms? J. Arthroplast. 2015, 30, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.L.; Patel, R. Infection Associated with Prosthetic Joints. N. Engl. J. Med. 2009, 361, 787–794. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, T.A.G.; Walraven, J.M.B.; Geurts, J.A.P.; Arts, J.J.C. Antibiotic-Loaded Collagen Sponges in Clinical Treatment of Chronic Osteomyelitis: A Systematic Review. JBJS 2018, 100, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Citak, M.; Masri, B.A.; Springer, B.; Argenson, J.-N.; Kendoff, D.O. Are Preformed Articulating Spacers Superior To Surgeon-Made Articulating Spacers in the Treatment Of PJI in THA? A Literature Review. Open Orthop. J. 2015, 9, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Montali, A. Antibacterial coating systems. Injury 2006, 37, S81–S86. [Google Scholar] [CrossRef]
- Webb, J.C.; Spencer, R.F. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J. Bone Jt. Surg. Br. 2007, 89, 851–857. [Google Scholar] [CrossRef]
- Allen, B.; Moore, C.; Seyler, T.; Gall, K. Modulating antibiotic release from reservoirs in 3D-printed orthopedic devices to treat periprosthetic joint infection. J. Orthop. Res. 2020, 38, 2239–2249. [Google Scholar] [CrossRef] [PubMed]
- An, Y.H.; Friedman, R.J. Prevention of sepsis in total joint arthroplasty. J. Hosp. Infect. 1996, 33, 93–108. [Google Scholar] [CrossRef]
- Trampuz, A.; Zimmerli, W. Prosthetic joint infections: Update in diagnosis and treatment. Swiss Med. Wkly. 2005, 135, 243–251. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef]
- Sanderson, P.J. Infection in orthopaedic implants. J. Hosp. Infect. 1991, 18 (Suppl. A), 367–375. [Google Scholar] [CrossRef] [PubMed]
- Getzlaf, M.A.; Lewallen, E.A.; Kremers, H.M.; Jones, D.L.; Bonin, C.A.; Dudakovic, A.; Thaler, R.; Cohen, R.C.; Lewallen, D.G.; van Wijnen, A.J. Multi-disciplinary antimicrobial strategies for improving orthopaedic implants to prevent prosthetic joint infections in hip and knee. J. Orthop. Res. 2016, 34, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Peel, T.N.; Cheng, A.C.; Buising, K.L.; Choong, P.F.M. Microbiological Aetiology, Epidemiology, and Clinical Profile of Prosthetic Joint Infections: Are Current Antibiotic Prophylaxis Guidelines Effective? Antimicrob. Agents Chemother. 2012, 56, 2386–2391. [Google Scholar] [CrossRef] [PubMed]
- Sendi, P.; Zimmerli, W. Antimicrobial treatment concepts for orthopaedic device-related infection. Clin. Microbiol. Infect. 2012, 18, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Trampuz, A.; Piper, K.E.; Jacobson, M.J.; Hanssen, A.D.; Unni, K.K.; Osmon, D.R.; Mandrekar, J.N.; Cockerill, F.R.; Steckelberg, J.M.; Greenleaf, J.F.; et al. Sonication of Removed Hip and Knee Prostheses for Diagnosis of Infection. N. Engl. J. Med. 2007, 357, 654–663. [Google Scholar] [CrossRef]
- Stefánsdóttir, A.; Johansson, D.; Knutson, K.; Lidgren, L.; Robertsson, O. Microbiology of the infected knee arthroplasty: Report from the Swedish Knee Arthroplasty Register on 426 surgically revised cases. Scand. J. Infect. Dis. 2009, 41, 831–840. [Google Scholar] [CrossRef]
- Marculescu, C.E.; Cantey, J.R. Polymicrobial Prosthetic Joint Infections: Risk Factors and Outcome. Clin. Orthop. Relat. Res. 2008, 466, 1397. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Ehrlich, G.D.; Montanaro, L. Biofilm-Based Implant Infections in Orthopaedics. In Biofilm-Based Healthcare-Associated Infections: Volume I; Donelli, G., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 29–46. [Google Scholar]
- Ogawa, S.; Chikumi, H.; Tanishima, S.; Hayashi, I.; Mihara, T.; Nagashima, H. Evaluation of infections in orthopedic patients using next-generation sequencing. J. Infect. Chemother. 2021, 27, 1626–1633. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Micro. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef]
- Ter Boo, G.-J.A.; Grijpma, D.W.; Moriarty, T.F.; Richards, R.G.; Eglin, D. Antimicrobial delivery systems for local infection prophylaxis in orthopedic- and trauma surgery. Biomaterials 2015, 52, 113–125. [Google Scholar] [CrossRef]
- Zimmerli, W.; Waldvogel, F.A.; Vaudaux, P.; Nydegger, U.E. Pathogenesis of Foreign Body Infection: Description and Characteristics of an Animal Model. J. Infect. Dis. 1982, 146, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Daghighi, S.; Sjollema, J.; van der Mei, H.C.; Busscher, H.J.; Rochford, E.T.J. Infection resistance of degradable versus non-degradable biomaterials: An assessment of the potential mechanisms. Biomaterials 2013, 34, 8013–8017. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-Joint Infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Lovati, A.B.; Drago, L.; Monti, L.; De Vecchi, E.; Previdi, S.; Banfi, G.; Romano, C.L. Diabetic mouse model of orthopaedic implant-related Staphylococcus aureus infection. PLoS ONE 2013, 8, e67628. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Ravaioli, S.; Montanaro, L. Polysaccharide intercellular adhesin in biofilm: Structural and regulatory aspects. Front. Cell. Infect. Microbiol. 2015, 5, 7. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Laverty, G.; Gorman, S.P.; Gilmore, B.F. Biomolecular mechanisms of staphylococcal biofilm formation. Future Microbiol. 2013, 8, 509–524. [Google Scholar] [CrossRef]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef]
- Montanaro, L.; Speziale, P.; Campoccia, D.; Ravaioli, S.; Cangini, I.; Pietrocola, G.; Giannini, S.; Arciola, C.R. Scenery of Staphylococcus implant infections in orthopedics. Future Microbiol. 2011, 6, 1329–1349. [Google Scholar] [CrossRef]
- Monds, R.D.; O’Toole, G.A. The developmental model of microbial biofilms: Ten years of a paradigm up for review. Trends Microbiol. 2009, 17, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O. Device-Associated Infections: A Macroproblem that Starts with Microadherence. Clin. Infect. Dis. 2001, 33, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Rohde, H.; Burandt, E.C.; Siemssen, N.; Frommelt, L.; Burdelski, C.; Wurster, S.; Scherpe, S.; Davies, A.P.; Harris, L.G.; Horstkotte, M.A.; et al. Polysaccharide intercellular adhesin or protein factors in biofilm accumulation of Staphylococcus epidermidis and Staphylococcus aureus isolated from prosthetic hip and knee joint infections. Biomaterials 2007, 28, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Montanaro, L.; Arciola, C.R. Biofilm in implant infections: Its production and regulation. Int. J. Artif. Organs 2005, 28, 1062–1068. [Google Scholar] [CrossRef]
- Odekerken, J.C.E.; Welting, T.J.M.; Arts, J.J.C.; Walenkamp, G.H.I.M.; Emans, P.J. Modern Orthopaedic Implant Coatings—Their Pro’s, Con’s and Evaluation Methods. In Modern Surface Engineering Treatments; InTech Open: London, UK, 2013; p. 44875. [Google Scholar]
- Wilkins, M.; Hall-Stoodley, L.; Allan, R.N.; Faust, S.N. New approaches to the treatment of biofilm-related infections. J. Infect. 2014, 69 (Suppl. S1), S47–S52. [Google Scholar] [CrossRef]
- Rosenthal, M.E.; Dever, L.L.; Moucha, C.S.; Chavda, K.D.; Otto, M.; Kreiswirth, B.N. Molecular Characterization of an Early Invasive Staphylococcus epidermidis Prosthetic Joint Infection. Microb. Drug Resist. 2011, 17, 345–350. [Google Scholar] [CrossRef]
- Yao, Y.; Sturdevant, D.E.; Otto, M. Genomewide Analysis of Gene Expression in Staphylococcus epidermidis Biofilms: Insights into the Pathophysiology of S. epidermidis Biofilms and the Role of Phenol-Soluble Modulins in Formation of Biofilms. J. Infect. Dis. 2005, 191, 289–298. [Google Scholar] [CrossRef]
- de la Fuente-Núñez, C.; Reffuveille, F.; Haney, E.F.; Straus, S.K.; Hancock, R.E.W. Broad-Spectrum Anti-biofilm Peptide That Targets a Cellular Stress Response. PLoS Pathog. 2014, 10, e1004152. [Google Scholar] [CrossRef]
- Hoyle, B.D.; Alcantara, J.; Costerton, J.W. Pseudomonas aeruginosa biofilm as a diffusion barrier to piperacillin. Antimicrob. Agents Chemother. 1992, 36, 2054–2056. [Google Scholar] [CrossRef]
- Nichols, W.W.; Dorrington, S.M.; Slack, M.P.; Walmsley, H.L. Inhibition of tobramycin diffusion by binding to alginate. Antimicrob. Agents Chemother. 1988, 32, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.R.W.; Allison, D.G.; Gilbert, P. Resistance of bacterial biofilms to antibiotics a growth-rate related effect? J. Antimicrob. Chemother. 1988, 22, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID∗ guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21 (Suppl. S1), S1–S25. [Google Scholar] [CrossRef]
- Parsek, M.R.; Singh, P.K. Bacterial biofilms: An emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003, 57, 677–701. [Google Scholar] [CrossRef]
- Reffuveille, F.; de la Fuente-Núñez, C.; Mansour, S.; Hancock, R.E.W. A Broad-Spectrum Antibiofilm Peptide Enhances Antibiotic Action against Bacterial Biofilms. Antimicrob. Agents Chemother. 2014, 58, 5363–5371. [Google Scholar] [CrossRef]
- Arruebo, M.; Vilaboa, N.; Santamaria, J. Drug delivery from internally implanted biomedical devices used in traumatology and in orthopedic surgery. Expert Opin. Drug Deliv. 2010, 7, 589–603. [Google Scholar] [CrossRef]
- Gbejuade, H.O.; Lovering, A.M.; Webb, J.C. The role of microbial biofilms in prosthetic joint infections. Acta Orthop. 2015, 86, 147–158. [Google Scholar] [CrossRef]
- Francolini, I.; Hall-Stoodley, L.; Stoodley, P. 2.2.8—Biofilms, Biomaterials, and Device-Related Infections. In Biomaterials Science, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 823–840. [Google Scholar]
- Liu, L.; Webster, T.J. Nanotechnology for Reducing Orthopedic Implant Infections: Synthesis, Characterization, and Properties. In Orthopedic Biomaterials: Advances and Applications; Li, B., Webster, T., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 31–62. [Google Scholar]
- Hench, L.L.; Polak, J.M. Third-Generation Biomedical Materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef]
- Agarwal, R.; García, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef]
- de Guzman, R.C. Materials for Orthopedic Applications. In Orthopedic Biomaterials: Advances and Applications; Li, B., Webster, T., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 367–398. [Google Scholar]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Tan, L.; Wan, P.; Yu, X.; Ma, Z. Biodegradable Metals for Orthopedic Applications. In Orthopedic Biomaterials: Advances and Applications; Li, B., Webster, T., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 275–309. [Google Scholar]
- Heimbach, B.; Wei, M. Composite Orthopedic Fixation Devices. In Orthopedic Biomaterials: Advances and Applications; Li, B., Webster, T., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 399–425. [Google Scholar]
- O’Sullivan, C.; O’Hare, P.; Byrne, G.; O’Neill, L.; Ryan, K.B.; Crean, A.M. A Modified Surface on Titanium Deposited by a Blasting Process. Coatings 2011, 1, 53–71. [Google Scholar] [CrossRef]
- Ahern, E.; Doody, T.; Ryan, K.B. Bioinspired nanomaterials for bone tissue engineering. In Bioengineered Nanomaterials; Tiwari, A., Tiwari, A., Press, C.R.C., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2014; pp. 369–412. [Google Scholar]
- Moroni, A.; Larsson, S.; Hoang Kim, A.; Gelsomini, L.; Giannoudis, P.V. Can We Improve Fixation and Outcomes? Use of Bone Substitutes. J. Orthop. Trauma 2009, 23, 422–425. [Google Scholar] [CrossRef]
- Forde, P.F.; Ryan, K.B. Biomaterial-Mediated Drug Delivery in Primary and Metastatic Cancers of the Bone. In Orthopedic Biomaterials: Advances and Applications; Li, B., Webster, T., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 569–604. [Google Scholar]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Boyan, B.D.; Sylvia, V.L.; Liu, Y.; Sagun, R.; Cochran, D.L.; Lohmann, C.H.; Dean, D.D.; Schwartz, Z. Surface roughness mediates its effects on osteoblasts via protein kinase A and phospholipase A2. Biomaterials 1999, 20, 2305–2310. [Google Scholar] [CrossRef]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. Part A 2005, 74A, 49–58. [Google Scholar] [CrossRef]
- Freemont, A. The pathology of joint replacement and tissue engineering. Diagn. Histopathol. 2012, 18, 169–176. [Google Scholar] [CrossRef]
- Khanna, R. Advances in Bearing Materials for Total Artificial Hip Arthroplasty. In Orthopedic Biomaterials: Advances and Applications; Li, B., Webster, T., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 467–494. [Google Scholar]
- Zhu, Y.; Liu, K.; Deng, J.; Ye, J.; Ai, F.; Ouyang, H.; Wu, T.; Jia, J.; Cheng, X.; Wang, X. 3D printed zirconia ceramic hip joint with precise structure and broad-spectrum antibacterial properties. Int. J. Nanomed. 2019, 14, 5977–5987. [Google Scholar] [CrossRef]
- Eyerer, P.; Jin, R. Influence of mixing technique on some properties of PMMA bone cement. J. Biomed. Mater. Res. 1986, 20, 1057–1094. [Google Scholar] [CrossRef]
- Liu, J.Z.; Crist, B.D. Coated nails: Is their use supported by the literature? OTA Int. 2021, 4, e110. [Google Scholar] [CrossRef]
- Koo, K.H.; Yang, J.W.; Cho, S.H.; Song, H.R.; Park, H.B.; Ha, Y.C.; Chang, J.D.; Kim, S.Y.; Kim, Y.H. Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J. Arthroplast. 2001, 16, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Liu, P.; Zhang, X.; Jingguo, x.; Yongjie, w.; Zou, X.; Mei, X.; Zhang, S.; Zhang, S. Strategies to improve bioactive and antibacterial properties of polyetheretherketone (PEEK) for use as orthopedic implants. Mater. Today Bio. 2022, 16, 100402. [Google Scholar] [CrossRef]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef]
- Gristina, A. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Su, B.; Dalby, M.J. Multifunctional Coatings and Nanotopographies: Toward Cell Instructive and Antibacterial Implants. Adv. Healthc. Mater. 2019, 8, e1801103. [Google Scholar] [CrossRef]
- Busscher, H.J.; van der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; van den Dungen, J.J.A.M.; Zaat, S.A.J.; Schultz, M.J.; Grainger, D.W. Biomaterial-Associated Infection: Locating the Finish Line in the Race for the Surface. Sci. Transl. Med. 2012, 4, 153rv110. [Google Scholar] [CrossRef]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef]
- Neoh, K.G.; Hu, X.; Zheng, D.; Kang, E.T. Balancing osteoblast functions and bacterial adhesion on functionalized titanium surfaces. Biomaterials 2012, 33, 2813–2822. [Google Scholar] [CrossRef]
- Whitaker, R.; Hernaez-Estrada, B.; Hernandez, R.M.; Santos-Vizcaino, E.; Spiller, K.L. Immunomodulatory Biomaterials for Tissue Repair. Chem. Rev. 2021, 121, 11305–11335. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Boot, W.; Dimas, K.; Malizos, K.; Hänsch, G.M.; Stuyck, J.; Gawlitta, D.; Romanò, C.L. Does implant coating with antibacterial-loaded hydrogel reduce bacterial colonization and biofilm formation in vitro? Clin. Orthop. Relat. Res. 2014, 472, 3311–3323. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.W. Biofilms and antibiotic therapy: Is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv. Drug Deliv. Rev. 2005, 57, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Saleh, K.J.; Ragland, P.S.; Pour, A.E.; Mont, M.A. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop. 2008, 79, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Barnett, A.G.; Merollini, K.; Sutton, A.; Cooper, N.; Berendt, T.; Wilson, J.; Graves, N. Control strategies to prevent total hip replacement-related infections: A systematic review and mixed treatment comparison. BMJ Open 2014, 4, e003978. [Google Scholar] [CrossRef] [PubMed]
- Tsikopoulos, K.; Sidiropoulos, K.; Kitridis, D.; Hassan, A.; Drago, L.; Mavrogenis, A.; McBride, D. Is coating of titanium implants effective at preventing Staphylococcus aureus infections? A meta-analysis of animal model studies. Int. Orthop. 2021, 45, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Rossolini, G.M.; Arena, F.; Pecile, P.; Pollini, S. Update on the antibiotic resistance crisis. Curr. Opin. Pharmacol. 2014, 18, 56–60. [Google Scholar] [CrossRef]
- Reardon, S. Bacterial arms race revs up. Nature 2015, 521, 402–403. [Google Scholar] [CrossRef]
- Gilles, B.; Tom, C. Quorum Sensing Inhibitors as Anti-Biofilm Agents. Curr. Pharm. Des. 2015, 21, 5–11. [Google Scholar] [CrossRef]
- Kamble, S.; Valtchev, P.; Dao, A.; Pelras, T.; Rogers, M.J.; Savage, P.B.; Dehghani, F.; Schindeler, A. Synthesis and Characterization of Bone Binding Antibiotic-1 (BBA-1), a Novel Antimicrobial for Orthopedic Applications. Molecules 2021, 26, 1541. [Google Scholar] [CrossRef]
- Haktaniyan, M.; Bradley, M. Polymers showing intrinsic antimicrobial activity. Chem. Soc. Rev. 2022, 51, 8584–8611. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-Based Nanomaterials Advance Biomedicine: A Review. Int. J. Mol. Sci. 2022, 23, 5405. [Google Scholar] [CrossRef]
- Maillard, J.-Y.; Hartemann, P. Silver as an antimicrobial: Facts and gaps in knowledge. Crit. Rev. Microbiol. 2013, 39, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, M.; Massè, A.; Tobin, E.; Cannas, M. Silver coated materials for external fixation devices: In vitro biocompatibility and genotoxicity. Biomaterials 2002, 23, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Courtney, H.S.; Bettenga, M.; Agrawal, C.M.; Bumgardner, J.D.; Ong, J.L. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5517. [Google Scholar] [CrossRef] [PubMed]
- Fielding, G.A.; Roy, M.; Bandyopadhyay, A.; Bose, S. Antibacterial and biological characteristics of silver containing and strontium doped plasma sprayed hydroxyapatite coatings. Acta Biomater. 2012, 8, 3144–3152. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.; O’Hare, P.; O’Leary, N.D.; Crean, A.M.; Ryan, K.; Dobson, A.D.W.; O’Neill, L. Deposition of substituted apatites with anticolonizing properties onto titanium surfaces using a novel blasting process. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95B, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Fielding, G.A.; Beyenal, H.; Bandyopadhyay, A.; Bose, S. Mechanical, in vitro antimicrobial, and biological properties of plasma-sprayed silver-doped hydroxyapatite coating. ACS Appl. Mater. Interfaces 2012, 4, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Jacquart, S.; Girod-Fullana, S.; Brouillet, F.; Pigasse, C.; Siadous, R.; Fatnassi, M.; Grimoud, J.; Rey, C.; Roques, C.; Combes, C. Injectable bone cement containing carboxymethyl cellulose microparticles as a silver delivery system able to reduce implant-associated infection risk. Acta Biomater. 2022, 145, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Wang, H.; Wang, W.; Tong, L.; Pan, H.; Ruan, C.; Ma, Q.; Liu, M.; Yang, H.; Zhang, L.; et al. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials 2014, 35, 4255–4265. [Google Scholar] [CrossRef]
- Ryan, E.J.; Ryan, A.J.; González-Vázquez, A.; Philippart, A.; Ciraldo, F.E.; Hobbs, C.; Nicolosi, V.; Boccaccini, A.R.; Kearney, C.J.; O’Brien, F.J. Collagen scaffolds functionalised with copper-eluting bioactive glass reduce infection and enhance osteogenesis and angiogenesis both in vitro and in vivo. Biomaterials 2019, 197, 405–416. [Google Scholar] [CrossRef]
- Onaizi, S.A.; Leong, S.S.J. Tethering antimicrobial peptides: Current status and potential challenges. Biotechnol. Adv. 2011, 29, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Cai, J.; Zhang, B.; Wang, Y.; Wong, D.F.; Siu, S.W.I. Recent Progress in the Discovery and Design of Antimicrobial Peptides Using Traditional Machine Learning and Deep Learning. Antibiotics 2022, 11, 1451. [Google Scholar] [CrossRef]
- Brooks, B.D.; Brooks, A.E. Therapeutic strategies to combat antibiotic resistance. Adv. Drug Deliv. Rev. 2014, 78, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Parducho, K.R.; Beadell, B.; Ybarra, T.K.; Bush, M.; Escalera, E.; Trejos, A.T.; Chieng, A.; Mendez, M.; Anderson, C.; Park, H.; et al. The Antimicrobial Peptide Human Beta-Defensin 2 Inhibits Biofilm Production of Pseudomonas aeruginosa Without Compromising Metabolic Activity. Front Immunol. 2020, 11, 805. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh-Narbat, M.; Kindrachuk, J.; Duan, K.; Jenssen, H.; Hancock, R.E.W.; Wang, R. Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials 2010, 31, 9519–9526. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Carvalho, I.F.; Montelaro, R.C.; Gomes, P.; Martins, M.C.L. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011, 7, 1431–1440. [Google Scholar] [CrossRef]
- Brogden, N.K.; Brogden, K.A. Will new generations of modified antimicrobial peptides improve their potential as pharmaceuticals? Int. J. Antimicrob. Agents 2011, 38, 217–225. [Google Scholar] [CrossRef]
- Hook, A.L.; Chang, C.-Y.; Yang, J.; Luckett, J.; Cockayne, A.; Atkinson, S.; Mei, Y.; Bayston, R.; Irvine, D.J.; Langer, R.; et al. Combinatorial discovery of polymers resistant to bacterial attachment. Nat. Biotechnol. 2012, 30, 868–875. [Google Scholar] [CrossRef]
- Kiedrowski, M.R.; Horswill, A.R. New approaches for treating staphylococcal biofilm infections. Ann. N. Y. Acad. Sci. 2011, 1241, 104–121. [Google Scholar] [CrossRef]
- Beloin, C.; Renard, S.; Ghigo, J.-M.; Lebeaux, D. Novel approaches to combat bacterial biofilms. Curr. Opin. Pharmacol. 2014, 18, 61–68. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Hook, A.L.; Chang, C.-Y.; Yang, J.; Atkinson, S.; Langer, R.; Anderson, D.G.; Davies, M.C.; Williams, P.; Alexander, M.R. Discovery of Novel Materials with Broad Resistance to Bacterial Attachment Using Combinatorial Polymer Microarrays. Adv. Mater. 2013, 25, 2542–2547. [Google Scholar] [CrossRef] [PubMed]
- Thoendel, M.; Kavanaugh, J.S.; Flack, C.E.; Horswill, A.R. Peptide signaling in the Staphylococci. Chem. Rev. 2011, 111, 117–151. [Google Scholar] [CrossRef]
- Dapunt, U.; Prior, B.; Oelkrug, C.; Kretzer, J.P. IgY Targeting Bacterial Quorum-Sensing Molecules in Implant-Associated Infections. Molecules 2020, 25, 4027. [Google Scholar] [CrossRef] [PubMed]
- Anguita-Alonso, P.; Giacometti, A.; Cirioni, O.; Ghiselli, R.; Orlando, F.; Saba, V.; Scalise, G.; Sevo, M.; Tuzova, M.; Patel, R.; et al. RNAIII-Inhibiting-Peptide-Loaded Polymethylmethacrylate Prevents In Vivo Staphylococcus aureus Biofilm Formation. Antimicrob. Agents Chemother. 2007, 51, 2594–2596. [Google Scholar] [CrossRef]
- He, Y.; Luckett, J.; Begines, B.; Dubern, J.-F.; Hook, A.L.; Prina, E.; Rose, F.R.A.J.; Tuck, C.J.; Hague, R.J.M.; Irvine, D.J.; et al. Ink-jet 3D printing as a strategy for developing bespoke non-eluting biofilm resistant medical devices. Biomaterials 2022, 281, 121350. [Google Scholar] [CrossRef] [PubMed]
- Ommen, P.; Hansen, L.; Hansen, B.K.; Vu-Quang, H.; Kjems, J.; Meyer, R.L. Aptamer-Targeted Drug Delivery for Staphylococcus aureus Biofilm. Front. Cell. Infect. Microbiol. 2022, 12, 814340. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ruan, X.; Lv, X.; Xu, Y.; Wang, W.; Cai, Y.; Ding, M.; Dong, H.; Shao, J.; Yang, D.; et al. Biofilm microenvironment-responsive nanoparticles for the treatment of bacterial infection. Nano Today 2022, 46, 101602. [Google Scholar] [CrossRef]
- Xiu, W.; Shan, J.; Yang, K.; Xiao, H.; Yuwen, L.; Wang, L. Recent development of nanomedicine for the treatment of bacterial biofilm infections. View 2021, 2, 20200065. [Google Scholar] [CrossRef]
- Obuobi, S.; Ngoc Phung, A.; Julin, K.; Johannessen, M.; Škalko-Basnet, N. Biofilm Responsive Zwitterionic Antimicrobial Nanoparticles to Treat Cutaneous Infection. Biomacromolecules 2022, 23, 303–315. [Google Scholar] [CrossRef]
- Cui, S.; Qiao, J.; Xiong, M.P. Antibacterial and Biofilm-Eradicating Activities of pH-Responsive Vesicles against Pseudomonas aeruginosa. Mol. Pharm. 2022, 19, 2406–2417. [Google Scholar] [CrossRef] [PubMed]
- Lauderdale, K.J.; Malone, C.L.; Boles, B.R.; Morcuende, J.; Horswill, A.R. Biofilm dispersal of community-associated methicillin-resistant Staphylococcus aureus on orthopedic implant material. J. Orthop. Res. 2010, 28, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Mlynek, K.D.; Hettiarachchi, H.; Alamneh, Y.A.; Biggemann, L.; Zurawski, D.V.; Black, C.C.; Bane, C.E.; Kim, R.K.; Granick, M.S. Extracellular polymeric substance (EPS)-degrading enzymes reduce staphylococcal surface attachment and biocide resistance on pig skin in vivo. PLoS ONE 2018, 13, e0205526. [Google Scholar] [CrossRef] [PubMed]
- Dieltjens, L.; Appermans, K.; Lissens, M.; Lories, B.; Kim, W.; Van der Eycken, E.V.; Foster, K.R.; Steenackers, H.P. Inhibiting bacterial cooperation is an evolutionarily robust anti-biofilm strategy. Nat. Commun. 2020, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Diefenbeck, M.; Mückley, T.; Hofmann, G.O. Prophylaxis and treatment of implant-related infections by local application of antibiotics. Injury 2006, 37, S95–S104. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef]
- Wu, P.; Grainger, D.W. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials 2006, 27, 2450–2467. [Google Scholar] [CrossRef]
- Darouiche, R.O.; Farmer, J.; Chaput, C.; Mansouri, M.; Saleh, G.; Landon, G.C. Anti-Infective Efficacy of Antiseptic-Coated Intramedullary Nails*†. J. Bone Jt. Surg. 1998, 80, 1336–1340. [Google Scholar] [CrossRef]
- Darouiche, R.O.; Mansouri, M.D.; Zakarevicz, D.; Alsharif, A.; Landon, G.C. In vivo efficacy of antimicrobial-coated devices. J. Bone Jt. Surg. Am. 2007, 89, 792–797. [Google Scholar] [CrossRef]
- Aykut, S.; Öztürk, A.; Özkan, Y.; Yanik, K.; İlman, A.A.; Özdemir, R.M. Evaluation and comparison of the antimicrobial efficacy of teicoplanin- and clindamycin-coated titanium implants: An experimental study. J. Bone Jt. Surg. Br. Vol. 2010, 92-B, 159–163. [Google Scholar] [CrossRef]
- Hickok, N.J.; Shapiro, I.M. Immobilized antibiotics to prevent orthopaedic implant infections. Adv. Drug Deliv. Rev. 2012, 64, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Antoci Jr, V.; Adams, C.S.; Parvizi, J.; Davidson, H.M.; Composto, R.J.; Freeman, T.A.; Wickstrom, E.; Ducheyne, P.; Jungkind, D.; Shapiro, I.M. The inhibition of Staphylococcus epidermidis biofilm formation by vancomycin-modified titanium alloy and implications for the treatment of periprosthetic infection. Biomaterials 2008, 29, 4684–4690. [Google Scholar] [CrossRef]
- Jose, B.; Antoci Jr, V.; Zeiger, A.R.; Wickstrom, E.; Hickok, N.J. Vancomycin Covalently Bonded to Titanium Beads Kills Staphylococcus aureus. Chem. Biol. 2005, 12, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.L.; Hammond, A.A.; Mosley, T.; Cortez, J.; Gray, T.; Colmer-Hamood, J.A.; Shashtri, M.; Spallholz, J.E.; Hamood, A.N.; Reid, T.W. Organoselenium Coating on Cellulose Inhibits the Formation of Biofilms by Pseudomonas aeruginosa and Staphylococcus aureus. Appl. Environ. Microbiol. 2009, 75, 3586–3592. [Google Scholar] [CrossRef] [PubMed]
- Simplex Bone Cement. Available online: https://www.strykermeded.com/medical-devices/surgical-solutions/surgical-equipment/simplex-bone-cement/# (accessed on 1 November 2022).
- 510(k) Summary SmartSet GHV Gentamicin Bone Cement. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf3/K033563.pdf (accessed on 1 November 2022).
- Global Unique Device Identification Database (GUDID). SMARTSET (10603295174288). Available online: https://accessgudid.nlm.nih.gov/devices/10603295174288 (accessed on 1 November 2022).
- Hake, M.E.; Young, H.; Hak, D.J.; Stahel, P.F.; Hammerberg, E.M.; Mauffrey, C. Local antibiotic therapy strategies in orthopaedic trauma: Practical tips and tricks and review of the literature. Injury 2015, 46, 1447–1456. [Google Scholar] [CrossRef]
- Pichavant, L.; Amador, G.; Jacqueline, C.; Brouillaud, B.; Heroguez, V.; Durrieu, M.C. pH-controlled delivery of gentamicin sulfate from orthopedic devices preventing nosocomial infections. J. Control Release 2012, 162, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Aviv, M.; Berdicevsky, I.; Zilberman, M. Gentamicin-loaded bioresorbable films for prevention of bacterial infections associated with orthopedic implants. J. Biomed. Mater. Res. Part A 2007, 83A, 10–19. [Google Scholar] [CrossRef]
- Fei, J.; Yu, H.J.; Pan, C.J.; Zhao, C.H.; Zhou, Y.G.; Wang, Y. Efficacy of a norvancomycin-loaded, PDLLA-coated plate in preventing early infection of rabbit tibia fracture. Orthopedics 2010, 33, 310. [Google Scholar]
- Kaelicke, T.; Schierholz, J.; Schlegel, U.; Frangen, T.M.; Koeller, M.; Printzen, G.; Seybold, D.; Kloeckner, S.; Muhr, G.; Arens, S. Effect on infection resistance of a local antiseptic and antibiotic coating on osteosynthesis implants: An in vitro and in vivo study. J. Orthop. Res. 2006, 24, 1622–1640. [Google Scholar] [CrossRef]
- Yeh, M.-L.; Chen, K.-H.; Chang, N.-J.; Chen, H.-D.; Lai, K.-A. Prolonged Antibiotic Release by PLGA Encapsulation on Titanium Alloy. J. Med. Biol. Eng. 2013, 33, 17–22. [Google Scholar] [CrossRef]
- Ryan, K.B.; Maher, S.; Brayden, D.; O’Driscoll, C. Nanostructures Overcoming the Intestinal Barrier: Drug Delivery Strategies In Nanostructured Biomaterials for Overcoming Biological Barriers; Alonso, M.J., Csaba, N.S., Eds.; RSC Publishing: Cambridge, UK, 2012. [Google Scholar]
- Schmidmaier, G.; Lucke, M.; Wildemann, B.; Haas, N.P.; Raschke, M. Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: A review. Injury 2006, 37, S105–S112. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, C.M.; Athanasiou, K.A. Technique to control pH in vicinity of biodegrading PLA-PGA implants. J. Biomed. Mater. Res. 1997, 38, 105–114. [Google Scholar] [CrossRef]
- Harris, L.G.; Mead, L.; Müller-Oberländer, E.; Richards, R.G. Bacteria and cell cytocompatibility studies on coated medical grade titanium surfaces. J. Biomed. Mater. Res. Part A 2006, 78A, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V.; Desai, T.A. Nanoparticulate drug delivery platforms for advancing bone infection therapies. Expert Opin. Drug Deliv. 2014, 11, 1899–1912. [Google Scholar] [CrossRef]
- SYNTHES. Expert Tibial Nail PROtect. Why Risk an Infection? Available online: http://synthes.vo.llnwd.net/o16/LLNWMB8/INT%20Mobile/Synthes%20International/Product%20Support%20Material/legacy_Synthes_PDF/036.001.264.pdf (accessed on 1 December 2015).
- Kluin, O.S.; van der Mei, H.C.; Busscher, H.J.; Neut, D. A surface-eroding antibiotic delivery system based on poly-(trimethylene carbonate). Biomaterials 2009, 30, 4738–4742. [Google Scholar] [CrossRef]
- Huang, Z.M.; He, C.L.; Yang, A.; Zhang, Y.; Han, X.J.; Yin, J.; Wu, Q. Encapsulating drugs in biodegradable ultrafine fibers through co-axial electrospinning. J. Biomed. Mater. Res. A 2006, 77, 169–179. [Google Scholar] [CrossRef]
- Schierholz, J.M.; Steinhauser, H.; Rump, A.F.; Berkels, R.; Pulverer, G. Controlled release of antibiotics from biomedical polyurethanes: Morphological and structural features. Biomaterials 1997, 18, 839–844. [Google Scholar] [CrossRef]
- Moskowitz, J.S.; Blaisse, M.R.; Samuel, R.E.; Hsu, H.-P.; Harris, M.B.; Martin, S.D.; Lee, J.C.; Spector, M.; Hammond, P.T. The effectiveness of the controlled release of gentamicin from polyelectrolyte multilayers in the treatment of Staphylococcus aureus infection in a rabbit bone model. Biomaterials 2010, 31, 6019–6030. [Google Scholar] [CrossRef]
- Shukla, A.; Fleming, K.E.; Chuang, H.F.; Chau, T.M.; Loose, C.R.; Stephanopoulos, G.N.; Hammond, P.T. Controlling the release of peptide antimicrobial agents from surfaces. Biomaterials 2010, 31, 2348–2357. [Google Scholar] [CrossRef]
- Shukla, A.; Fuller, R.C.; Hammond, P.T. Design of multi-drug release coatings targeting infection and inflammation. J. Control Release 2011, 155, 159–166. [Google Scholar] [CrossRef]
- Ter Boo, G.-J.A.; Arens, D.; Metsemakers, W.-J.; Zeiter, S.; Richards, R.G.; Grijpma, D.W.; Eglin, D.; Moriarty, T.F. Injectable gentamicin-loaded thermo-responsive hyaluronic acid derivative prevents infection in a rabbit model. Acta Biomater. 2016, 43, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Ter Boo, G.J.; Schmid, T.; Zderic, I.; Nehrbass, D.; Camenisch, K.; Richards, R.G.; Grijpma, D.W.; Moriarty, T.F.; Eglin, D. Local application of a gentamicin-loaded thermo-responsive hydrogel allows for fracture healing upon clearance of a high Staphylococcus aureus load in a rabbit model. Eur. Cell Mater. 2018, 35, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Vallejo Diaz, A.; Deimling, C.; Morgenstern, M.; D’Este, M.; Puetzler, J.; Zeiter, S.; Arens, D.; Metsemakers, W.J.; Richards, R.G.; Eglin, D.; et al. Local Application of a Gentamicin-Loaded Hydrogel Early After Injury Is Superior to Perioperative Systemic Prophylaxis in a Rabbit Open Fracture Model. J. Orthop. Trauma 2020, 34, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Franz, D.; Raschke, M.; Giannoudis, P.V.; Leliveld, M.; Metsemakers, W.J.; Verhofstad, M.H.J.; Craig, J.A.; Shore, J.; Smith, A.; Muehlendyck, C.; et al. Use of antibiotic coated intramedullary nails in open tibia fractures: A European medical resource use and cost-effectiveness analysis. Injury 2021, 52, 1951–1958. [Google Scholar] [CrossRef]
- Rodham, P.; Giannoudis, P.V. Innovations in orthopaedic trauma: Top advancements of the past two decades and predictions for the next two. Injury 2022, 53, S2–S7. [Google Scholar] [CrossRef]
- Gollwitzer, H.; Ibrahim, K.; Meyer, H.; Mittelmeier, W.; Busch, R.; Stemberger, A. Antibacterial poly(d,l-lactic acid) coating of medical implants using a biodegradable drug delivery technology. J. Antimicrob. Chemother. 2003, 51, 585–591. [Google Scholar] [CrossRef]
- Price, J.S.; Tencer, A.F.; Arm, D.M.; Bohach, G.A. Controlled release of antibiotics from coated orthopedic implants. J. Biomed. Mater. Res. 1996, 30, 281–286. [Google Scholar] [CrossRef]
- Lucke, M.; Schmidmaier, G.; Sadoni, S.; Wildemann, B.; Schiller, R.; Haas, N.P.; Raschke, M. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone 2003, 32, 521–531. [Google Scholar] [CrossRef]
- Lucke, M.; Wildemann, B.; Sadoni, S.; Surke, C.; Schiller, R.; Stemberger, A.; Raschke, M.; Haas, N.P.; Schmidmaier, G. Systemic versus local application of gentamicin in prophylaxis of implant-related osteomyelitis in a rat model. Bone 2005, 36, 770–778. [Google Scholar] [CrossRef]
- Kim, W.-H.; Lee, S.-B.; Oh, K.-T.; Moon, S.-K.; Kim, K.-M.; Kim, K.-N. The release behavior of CHX from polymer-coated titanium surfaces. Surf. Interface Anal. 2008, 40, 202–204. [Google Scholar] [CrossRef]
- Von Plocki, S.C.; Armbruster, D.; Klein, K.; Kaempf, K.; Zlinszky, K.; Hilbe, M.; Kronen, P.; Gruskin, E.; von Rechenberg, B. Biodegradable Sleeves for Metal Implants to Prevent Implant-Associated Infection: An Experimental In Vivo Study in Sheep. Vet. Surg. 2012, 41, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Vester, H.; Wildemann, B.; Schmidmaier, G.; Stöckle, U.; Lucke, M. Gentamycin delivered from a PDLLA coating of metallic implants. Injury 2010, 41, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Neut, D.; Dijkstra, R.J.B.; Thompson, J.I.; van der Mei, H.C.; Busscher, H.J. Antibacterial efficacy of a new gentamicin-coating for cementless prostheses compared to gentamicin-loaded bone cement. J. Orthop. Res. 2011, 29, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Alt, V.; Bitschnau, A.; Österling, J.; Sewing, A.; Meyer, C.; Kraus, R.; Meissner, S.A.; Wenisch, S.; Domann, E.; Schnettler, R. The effects of combined gentamicin–hydroxyapatite coating for cementless joint prostheses on the reduction of infection rates in a rabbit infection prophylaxis model. Biomaterials 2006, 27, 4627–4634. [Google Scholar] [CrossRef]
- Radin, S.; Campbell, J.T.; Ducheyne, P.; Cuckler, J.M. Calcium phosphate ceramic coatings as carriers of vancomycin. Biomaterials 1997, 18, 777–782. [Google Scholar] [CrossRef]
- Vu, A.A.; Robertson, S.F.; Ke, D.; Bandyopadhyay, A.; Bose, S. Mechanical and biological properties of ZnO, SiO2, and Ag2O doped plasma sprayed hydroxyapatite coating for orthopaedic and dental applications. Acta Biomater. 2019, 92, 325–335. [Google Scholar] [CrossRef]
- Brohede, U.; Forsgren, J.; Roos, S.; Mihranyan, A.; Engqvist, H.; Strømme, M. Multifunctional implant coatings providing possibilities for fast antibiotics loading with subsequent slow release. J. Mater. Sci. Mater. Med. 2009, 20, 1859–1867. [Google Scholar] [CrossRef]
- Teller, M.; Gopp, U.; Neumann, H.G.; Kuhn, K.D. Release of gentamicin from bone regenerative materials: An in vitro study. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 23–29. [Google Scholar] [CrossRef]
- Moojen, D.J.; Vogely, H.C.; Fleer, A.; Nikkels, P.G.; Higham, P.A.; Verbout, A.J.; Castelein, R.M.; Dhert, W.J. Prophylaxis of infection and effects on osseointegration using a tobramycin-periapatite coating on titanium implants--an experimental study in the rabbit. J. Orthop. Res. 2009, 27, 710–716. [Google Scholar] [CrossRef]
- Thompson, K.; Petkov, S.; Zeiter, S.; Sprecher, C.M.; Richards, R.G.; Moriarty, T.F.; Eijer, H. Intraoperative loading of calcium phosphate-coated implants with gentamicin prevents experimental Staphylococcus aureus infection in vivo. PLoS ONE 2019, 14, e0210402. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, A. Bioactive materials for biomedical applications using sol-gel technology. Biomed. Mater. 2008, 3, 034005. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.S.; Antoci, V.; Harrison, G.; Patal, P.; Freeman, T.A.; Shapiro, I.M.; Parvizi, J.; Hickok, N.J.; Radin, S.; Ducheyne, P. Controlled release of vancomycin from thin sol-gel films on implant surfaces successfully controls osteomyelitis. J. Orthop. Res. 2009, 27, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Nablo, B.J.; Rothrock, A.R.; Schoenfisch, M.H. Nitric oxide-releasing sol-gels as antibacterial coatings for orthopedic implants. Biomaterials 2005, 26, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Ishihama, H.; Ishii, K.; Nagai, S.; Kakinuma, H.; Sasaki, A.; Yoshioka, K.; Kuramoto, T.; Shiono, Y.; Funao, H.; Isogai, N.; et al. An antibacterial coated polymer prevents biofilm formation and implant-associated infection. Sci. Rep. 2021, 11, 3602. [Google Scholar] [CrossRef]
- O’ Sullivan, C.; O’ Neill, L.; O’ Leary, N.D.; O’ Gara, J.P.; Crean, A.M.; Ryan, K.B. Osteointegration, antimicrobial and antibiofilm activity of orthopaedic titanium surfaces coated with silver and strontium-doped hydroxyapatite using a novel blasting process. Drug Deliv. Transl. Res. 2021, 11, 702–716. [Google Scholar] [CrossRef]
- Qu, H.; Knabe, C.; Radin, S.; Garino, J.; Ducheyne, P. Percutaneous external fixator pins with bactericidal micron-thin sol–gel films for the prevention of pin tract infection. Biomaterials 2015, 62, 95–105. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Agrawal, A.; Knabe, C.; Ducheyne, P. Sol–gel silica controlled release thin films for the inhibition of methicillin-resistant Staphylococcus aureus. Biomaterials 2014, 35, 509–517. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Tian, A.; Xue, X.X.; Wang, L.; Alquhali, A.; Bai, X. Improved antibacterial activity and biocompatibility on vancomycin-loaded TiO(2) nanotubes: In vivo and in vitro studies. Int. J. Nanomed. 2013, 8, 4379–4389. [Google Scholar] [CrossRef]
- Tran, N.; Kelley, M.N.; Tran, P.A.; Garcia, D.R.; Jarrell, J.D.; Hayda, R.A.; Born, C.T. Silver doped titanium oxide–PDMS hybrid coating inhibits Staphylococcus aureus and Staphylococcus epidermidis growth on PEEK. Mater. Sci. Eng. C 2015, 49, 201–209. [Google Scholar] [CrossRef]
- Huo, K.; Zhang, X.; Wang, H.; Zhao, L.; Liu, X.; Chu, P.K. Osteogenic activity and antibacterial effects on titanium surfaces modified with Zn-incorporated nanotube arrays. Biomaterials 2013, 34, 3467–3478. [Google Scholar] [CrossRef]
- Lian, Q.; Zheng, S.; Shi, Z.; Li, K.; Chen, R.; Wang, P.; Liu, H.; Chen, Y.; Zhong, Q.; Liu, Q.; et al. Using a degradable three-layer sandwich-type coating to prevent titanium implant infection with the combined efficient bactericidal ability and fast immune remodeling property. Acta Biomater. 2022. Online ahead of Print. [Google Scholar] [CrossRef]
- Mistry, S.; Roy, R.; Jha, A.K.; Pandit, N.; Das, S.; Burman, S.; Joy, M. Treatment of long bone infection by a biodegradable bone cement releasing antibiotics in human. J. Control. Release 2022, 346, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Galvin, P.; Thompson, D.; Ryan, K.B.; McCarthy, A.; Moore, A.; Burke, C.; Dyson, M.; MacCraith, B.; Gun’ko, Y.; Byrne, M.; et al. Nanoparticle-based drug delivery: Case studies for cancer and cardiovascular applications. Cell. Mol. Life Sci. 2012, 69, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Ndayishimiye, J.; Kumeria, T.; Popat, A.; Falconer, J.R.; Blaskovich, M.A.T. Nanomaterials: The New Antimicrobial Magic Bullet. ACS Infect. Dis. 2022, 8, 693–712. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Fromm, K.M.; Akbar Ashkarran, A.; Jimenez de Aberasturi, D.; Larramendi, I.R.d.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Klasen, H.J. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns 2000, 26, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef]
- van Hengel, I.A.J.; Putra, N.E.; Tierolf, M.; Minneboo, M.; Fluit, A.C.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Biofunctionalization of selective laser melted porous titanium using silver and zinc nanoparticles to prevent infections by antibiotic-resistant bacteria. Acta Biomater. 2020, 107, 325–337. [Google Scholar] [CrossRef]
- Ye, Z.; Sang, T.; Li, K.; Fischer, N.G.; Mutreja, I.; Echeverría, C.; Kumar, D.; Tang, Z.; Aparicio, C. Hybrid nanocoatings of self-assembled organic-inorganic amphiphiles for prevention of implant infections. Acta Biomater. 2022, 140, 338–349. [Google Scholar] [CrossRef]
- Visai, L.; De Nardo, L.; Punta, C.; Melone, L.; Cigada, A.; Imbriani, M.; Arciola, C.R. Titanium oxide antibacterial surfaces in biomedical devices Int. J. Artif. Organs 2011, 34, 929–946. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Aw, M.S.; Santos, A.; Gulati, K.; Bariana, M. Titania nanotube arrays for local drug delivery: Recent advances and perspectives. Expert Opin. Drug Deliv. 2015, 12, 103–127. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Kuijer, R.; Kaper, H.J.; van der Mei, H.C.; Busscher, H.J. Simultaneous interaction of bacteria and tissue cells with photocatalytically activated, anodized titanium surfaces. Biomaterials 2014, 35, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, K.; Poh, Z.; Kant, K.; Chan, J.; Michelmore, A.; Losic, D. Tailoring the surface functionalities of titania nanotube arrays. Biomaterials 2010, 31, 532–540. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar Arya, D.; Dua, M.; Chhatwal, G.S.; Johri, A.K. Nano-technology for targeted drug delivery to combat antibiotic resistance. Expert Opin. Drug Deliv. 2012, 9, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.-T.; Chen, C.-F.; Chu, I.M.; Li, Y.-M.; Hsu, W.-H.; Hsu, R.W.-W.; Chang, P.-J. Treatment of osteomyelitis with teicoplanin-encapsulated biodegradable thermosensitive hydrogel nanoparticles. Biomaterials 2010, 31, 5227–5236. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.J.; Gisbert-Garzarán, M.; Mediero, A.; Fernández-Aceñero, M.J.; de-Pablo-Velasco, D.; Lozano, D.; Esteban, J.; Vallet-Regí, M. Antibiotic delivery from bone-targeted mesoporous silica nanoparticles for the treatment of osteomyelitis caused by methicillin-resistant Staphylococcus aureus. Acta Biomater. 2022. Online ahead of Print. [Google Scholar] [CrossRef]
- Popat, K.C.; Eltgroth, M.; LaTempa, T.J.; Grimes, C.A.; Desai, T.A. Decreased Staphylococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loaded titania nanotubes. Biomaterials 2007, 28, 4880–4888. [Google Scholar] [CrossRef]
- Gulati, K.; Aw, M.S.; Losic, D. Drug-eluting Ti wires with titania nanotube arrays for bone fixation and reduced bone infection. Nanoscale Res. Lett. 2011, 6, 571. [Google Scholar] [CrossRef]
- Chennell, P.; Feschet-Chassot, E.; Devers, T.; Awitor, K.O.; Descamps, S.; Sautou, V. In vitro evaluation of TiO2 nanotubes as cefuroxime carriers on orthopaedic implants for the prevention of periprosthetic joint infections. Int. J. Pharm. 2013, 455, 298–305. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Anselme, K.; Davidson, P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef] [PubMed]
- Puckett, S.D.; Taylor, E.; Raimondo, T.; Webster, T.J. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 2010, 31, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Jain, S.; Padmarajan, R.; Purighalla, S.; Sambandamurthy, V.K.; Chatterjee, K. Multi-scale surface topography to minimize adherence and viability of nosocomial drug-resistant bacteria. Mater. Des. 2018, 140, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xi, Y.; Bai, J.; Jiang, Z.; Wang, S.; Zhang, H.; Dai, W.; Chen, C.; Gou, Z.; Yang, G.; et al. Covalent grafting of hyperbranched poly-L-lysine on Ti-based implants achieves dual functions of antibacteria and promoted osteointegration in vivo. Biomaterials 2021, 269, 120534. [Google Scholar] [CrossRef]
- Ammar, Y.; Swailes, D.; Bridgens, B.; Chen, J. Influence of surface roughness on the initial formation of biofilm. Surf. Coat. Technol. 2015, 284, 410–416. [Google Scholar] [CrossRef]
- Glinel, K.; Thebault, P.; Humblot, V.; Pradier, C.M.; Jouenne, T. Antibacterial surfaces developed from bio-inspired approaches. Acta Biomater. 2012, 8, 1670–1684. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural bactericidal surfaces: Mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef]
- Kelleher, S.M.; Habimana, O.; Lawler, J.; Reilly, B.O.; Daniels, S.; Casey, E.; Cowley, A. Cicada Wing Surface Topography: An Investigation into the Bactericidal Properties of Nanostructural Features. ACS Appl. Mater. Interfaces 2016, 8, 14966–14974. [Google Scholar] [CrossRef]
- Linklater, D.P.; Baulin, V.A.; Juodkazis, S.; Crawford, R.J.; Stoodley, P.; Ivanova, E.P. Mechano-bactericidal actions of nanostructured surfaces. Nat. Rev. Microbiol. 2021, 19, 8–22. [Google Scholar] [CrossRef]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Phong Nguyen, T.H.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Watson, J.A.; et al. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys J. 2013, 104, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Modaresifar, K.; Azizian, S.; Ganjian, M.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Bactericidal effects of nanopatterns: A systematic review. Acta Biomater. 2019, 83, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Berton, P.; Moraes, C.; Rogers, R.D.; Tufenkji, N. Nanodarts, nanoblades, and nanospikes: Mechano-bactericidal nanostructures and where to find them. Adv. Colloid Interface Sci. 2018, 252, 55–68. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef] [PubMed]
- Tsimbouri, P.M.; Fisher, L.; Holloway, N.; Sjostrom, T.; Nobbs, A.H.; Meek, R.M.D.; Su, B.; Dalby, M.J. Osteogenic and bactericidal surfaces from hydrothermal titania nanowires on titanium substrates. Sci. Rep. 2016, 6, 36857. [Google Scholar] [CrossRef] [PubMed]
- Damiati, L.A.; Tsimbouri, M.P.; Hernandez, V.-L.; Jayawarna, V.; Ginty, M.; Childs, P.; Xiao, Y.; Burgess, K.; Wells, J.; Sprott, M.R.; et al. Materials-driven fibronectin assembly on nanoscale topography enhances mesenchymal stem cell adhesion, protecting cells from bacterial virulence factors and preventing biofilm formation. Biomaterials 2022, 280, 121263. [Google Scholar] [CrossRef] [PubMed]
- Preedy, E.; Perni, S.; Nipiĉ, D.; Bohinc, K.; Prokopovich, P. Surface Roughness Mediated Adhesion Forces between Borosilicate Glass and Gram-Positive Bacteria. Langmuir 2014, 30, 9466–9476. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-W.; Carson, L.; Smith, G.C.; Morelli, A.; Lee, S. Enhancing the antibacterial performance of orthopaedic implant materials by fibre laser surface engineering. Appl. Surf. Sci. 2017, 404, 67–81. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Hao, L.; Fang, N.; Chang, M.W.; Xu, R.; Yang, Y.; Chen, Y. Sharper and faster "nano darts" kill more bacteria: A study of antibacterial activity of individually dispersed pristine single-walled carbon nanotube. ACS Nano 2009, 3, 3891–3902. [Google Scholar] [CrossRef]
- Berlot, S.; Aissaoui, Z.; Pavon-Djavid, G.; Belleney, J.; Jozefowicz, M.; Hélary, G.; Migonney, V. Biomimetic Poly(methyl methacrylate)-Based Terpolymers: Modulation of Bacterial Adhesion Effect. Biomacromolecules 2002, 3, 63–68. [Google Scholar] [CrossRef]
- Anagnostou, F.; Debet, A.; Pavon-Djavid, G.; Goudaby, Z.; Helary, G.; Migonney, V. Osteoblast functions on functionalized PMMA-based polymers exhibiting Staphylococcus aureus adhesion inhibition. Biomaterials 2006, 27, 3912–3919. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Razatos, A.; Ong, Y.-L.; Boulay, F.; Elbert, D.L.; Hubbell, J.A.; Sharma, M.M.; Georgiou, G. Force Measurements between Bacteria and Poly(ethylene glycol)-Coated Surfaces. Langmuir 2000, 16, 9155–9158. [Google Scholar] [CrossRef]
- Park, K.D.; Kim, Y.S.; Han, D.K.; Kim, Y.H.; Lee, E.H.B.; Suh, H.; Choi, K.S. Bacterial adhesion on PEG modified polyurethane surfaces. Biomaterials 1998, 19, 851–859. [Google Scholar] [CrossRef]
- Harris, L.G.; Tosatti, S.; Wieland, M.; Textor, M.; Richards, R.G. Staphylococcus aureus adhesion to titanium oxide surfaces coated with non-functionalized and peptide-functionalized poly(l-lysine)-grafted-poly(ethylene glycol) copolymers. Biomaterials 2004, 25, 4135–4148. [Google Scholar] [CrossRef]
- Maddikeri, R.R.; Tosatti, S.; Schuler, M.; Chessari, S.; Textor, M.; Richards, R.G.; Harris, L.G. Reduced medical infection related bacterial strains adhesion on bioactive RGD modified titanium surfaces: A first step toward cell selective surfaces. J. Biomed. Mater. Res. Part A 2008, 84A, 425–435. [Google Scholar] [CrossRef]
- Arciola, C.R.; Bustanji, Y.; Conti, M.; Campoccia, D.; Baldassarri, L.; Samori, B.; Montanaro, L. Staphylococcus epidermidis—Fibronectin binding and its inhibition by heparin. Biomaterials 2003, 24, 3013–3019. [Google Scholar] [CrossRef]
- Roosjen, A.; Kaper, H.J.; van der Mei, H.C.; Norde, W.; Busscher, H.J. Inhibition of adhesion of yeasts and bacteria by poly(ethylene oxide)-brushes on glass in a parallel plate flow chamber. Microbiology 2003, 149, 3239–3246. [Google Scholar] [CrossRef]
- Nejadnik, M.R.; van der Mei, H.C.; Norde, W.; Busscher, H.J. Bacterial adhesion and growth on a polymer brush-coating. Biomaterials 2008, 29, 4117–4121. [Google Scholar] [CrossRef]
- Cringus-Fundeanu, I.; Luijten, J.; van der Mei, H.C.; Busscher, H.J.; Schouten, A.J. Synthesis and characterization of surface-grafted polyacrylamide brushes and their inhibition of microbial adhesion. Langmuir 2007, 23, 5120–5126. [Google Scholar] [CrossRef]
- Gao, G.; Lange, D.; Hilpert, K.; Kindrachuk, J.; Zou, Y.; Cheng, J.T.J.; Kazemzadeh-Narbat, M.; Yu, K.; Wang, R.; Straus, S.K.; et al. The biocompatibility and biofilm resistance of implant coatings based on hydrophilic polymer brushes conjugated with antimicrobial peptides. Biomaterials 2011, 32, 3899–3909. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-Hydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Privett, B.J.; Youn, J.; Hong, S.A.; Lee, J.; Han, J.; Shin, J.H.; Schoenfisch, M.H. Antibacterial fluorinated silica colloid superhydrophobic surfaces. Langmuir 2011, 27, 9597–9601. [Google Scholar] [CrossRef] [PubMed]
- Fadeeva, E.; Truong, V.K.; Stiesch, M.; Chichkov, B.N.; Crawford, R.J.; Wang, J.; Ivanova, E.P. Bacterial Retention on Superhydrophobic Titanium Surfaces Fabricated by Femtosecond Laser Ablation. Langmuir 2011, 27, 3012–3019. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.-S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. Available online: http://www.nature.com/nature/journal/v477/n7365/abs/nature10447.html#supplementary-information (accessed on 1 December 2015). [CrossRef]
- Leslie, D.C.; Waterhouse, A.; Berthet, J.B.; Valentin, T.M.; Watters, A.L.; Jain, A.; Kim, P.; Hatton, B.D.; Nedder, A.; Donovan, K.; et al. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat. Biotech. 2014. Advance online Publication. [Google Scholar] [CrossRef]
- Epstein, A.K.; Wong, T.-S.; Belisle, R.A.; Boggs, E.M.; Aizenberg, J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc. Natl. Acad. Sci. USA 2012, 109, 13182–13187. [Google Scholar] [CrossRef]
- Li, J.; Kleintschek, T.; Rieder, A.; Cheng, Y.; Baumbach, T.; Obst, U.; Schwartz, T.; Levkin, P.A. Hydrophobic Liquid-Infused Porous Polymer Surfaces for Antibacterial Applications. ACS Appl. Mater. Interfaces 2013, 5, 6704–6711. [Google Scholar] [CrossRef]
- Bruchmann, J.; Pini, I.; Gill, T.S.; Schwartz, T.; Levkin, P.A. Patterned SLIPS for the Formation of Arrays of Biofilm Microclusters with Defined Geometries. Adv. Healthc. Mater. 2017, 6, 1601082. [Google Scholar] [CrossRef]
- Keller, N.; Bruchmann, J.; Sollich, T.; Richter, C.; Thelen, R.; Kotz, F.; Schwartz, T.; Helmer, D.; Rapp, B.E. Study of Biofilm Growth on Slippery Liquid-Infused Porous Surfaces Made from Fluoropor. ACS Appl. Mater. Interfaces 2019, 11, 4480–4487. [Google Scholar] [CrossRef]
- Chae, K.; Jang, W.Y.; Park, K.; Lee, J.; Kim, H.; Lee, K.; Lee, C.K.; Lee, Y.; Lee, S.H.; Seo, J. Antibacterial infection and immune-evasive coating for orthopedic implants. Sci. Adv. 2020, 6, eabb0025. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Z.; Zhu, X.; Kang, E.-T.; Neoh, K.-G. Silk-functionalized titanium surfaces for enhancing osteoblast functions and reducing bacterial adhesion. Biomaterials 2008, 29, 4751–4759. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, C.M.; Khanh Truong, V.; Pham, V.T.H.; Al Kobaisi, M.; Seniutinas, G.; Wang, J.Y.; Juodkazis, S.; Crawford, R.J.; Ivanova, E.P. Antibacterial titanium nano-patterned arrays inspired by dragonfly wings. Sci. Rep. 2015, 5, 16817. [Google Scholar] [CrossRef] [PubMed]
- Sengstock, C.; Lopian, M.; Motemani, Y.; Borgmann, A.; Khare, C.; Buenconsejo, P.J.S.; Schildhauer, T.A.; Ludwig, A.; Köller, M. Structure-related antibacterial activity of a titanium nanostructured surface fabricated by glancing angle sputter deposition. Nanotechnology 2014, 25, 195101. [Google Scholar] [CrossRef] [PubMed]
- Diu, T.; Faruqui, N.; Sjöström, T.; Lamarre, B.; Jenkinson, H.F.; Su, B.; Ryadnov, M.G. Cicada-inspired cell-instructive nanopatterned arrays. Sci. Rep. 2014, 4, 7122. [Google Scholar] [CrossRef]
- Ziegler, N.; Sengstock, C.; Mai, V.; Schildhauer, T.A.; Köller, M.; Ludwig, A. Glancing-Angle Deposition of Nanostructures on an Implant Material Surface. Nanomaterials 2019, 9, 60. [Google Scholar] [CrossRef]
- Bright, R.; Fernandes, D.; Wood, J.; Palms, D.; Burzava, A.; Ninan, N.; Brown, T.; Barker, D.; Vasilev, K. Long-term antibacterial properties of a nanostructured titanium alloy surface: An in vitro study. Mater. Today Bio. 2022, 13, 100176. [Google Scholar] [CrossRef]
- Moriarty, T.F.; Metsemakers, W.J.; Morgenstern, M.; Hofstee, M.I.; Vallejo Diaz, A.; Cassat, J.E.; Wildemann, B.; Depypere, M.; Schwarz, E.M.; Richards, R.G. Fracture-related infection. Nat. Rev. Dis. Prim. 2022, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Lex, J.R.; Koucheki, R.; Stavropoulos, N.A.; Michele, J.D.; Toor, J.S.; Tsoi, K.; Ferguson, P.C.; Turcotte, R.E.; Papagelopoulos, P.J. Megaprosthesis anti-bacterial coatings: A comprehensive translational review. Acta Biomater. 2022, 140, 136–148. [Google Scholar] [CrossRef]
- Wu, Z.; Chan, B.; Low, J.; Chu, J.J.H.; Hey, H.W.D.; Tay, A. Microbial resistance to nanotechnologies: An important but understudied consideration using antimicrobial nanotechnologies in orthopaedic implants. Bioact. Mater. 2022, 16, 249–270. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Ramakrishna, S. Surface engineering of biomaterials in orthopedic and dental implants: Strategies to improve osteointegration, bacteriostatic and bactericidal activities. Biotechnol. J. 2021, 16, e2000116. [Google Scholar] [CrossRef]
- Moriarty, T.F.; Harris, L.G.; Mooney, R.A.; Wenke, J.C.; Riool, M.; Zaat, S.A.J.; Moter, A.; Schaer, T.P.; Khanna, N.; Kuehl, R.; et al. Recommendations for design and conduct of preclinical in vivo studies of orthopedic device-related infection. J. Orthop. Res. 2019, 37, 271–287. [Google Scholar] [CrossRef] [PubMed]
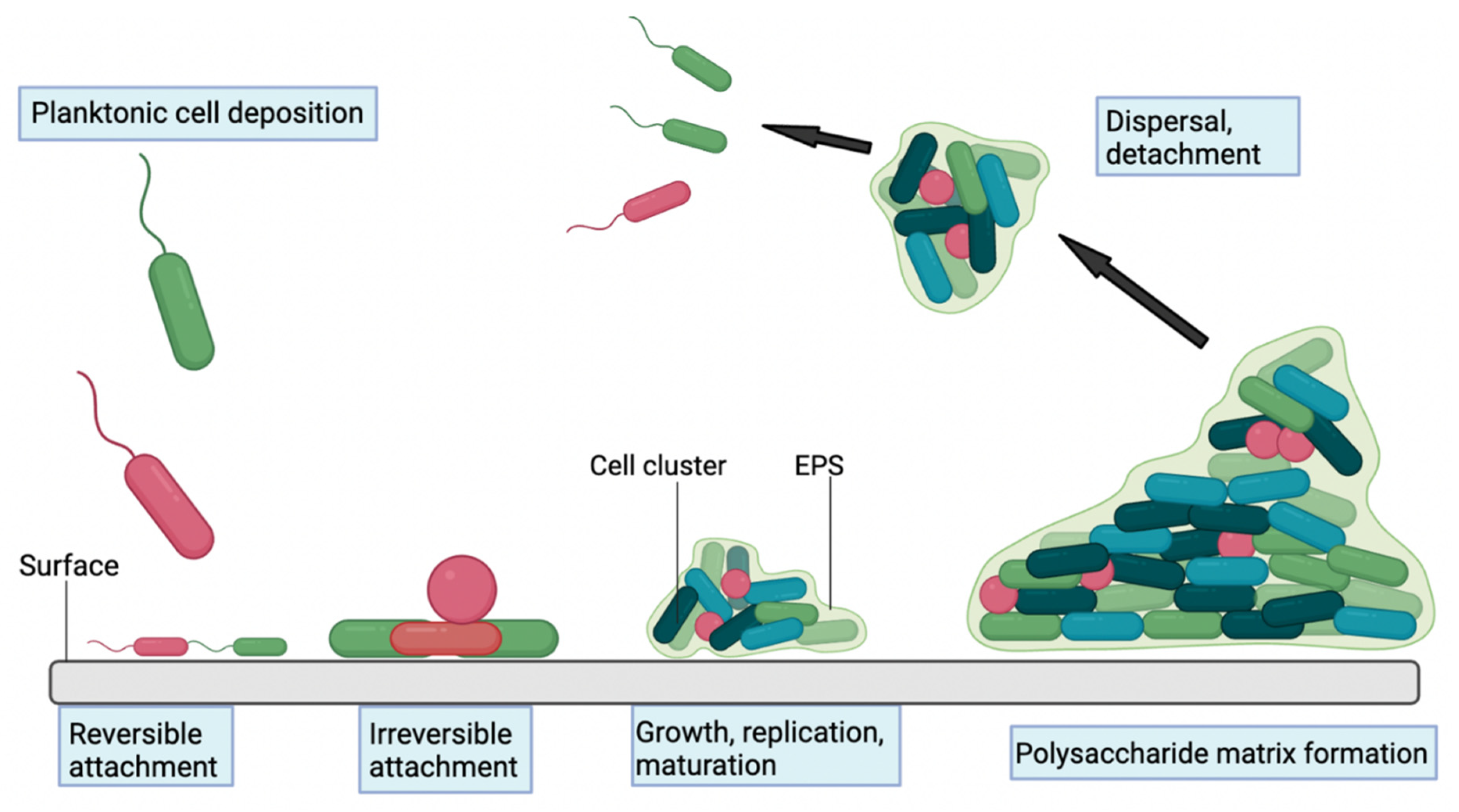
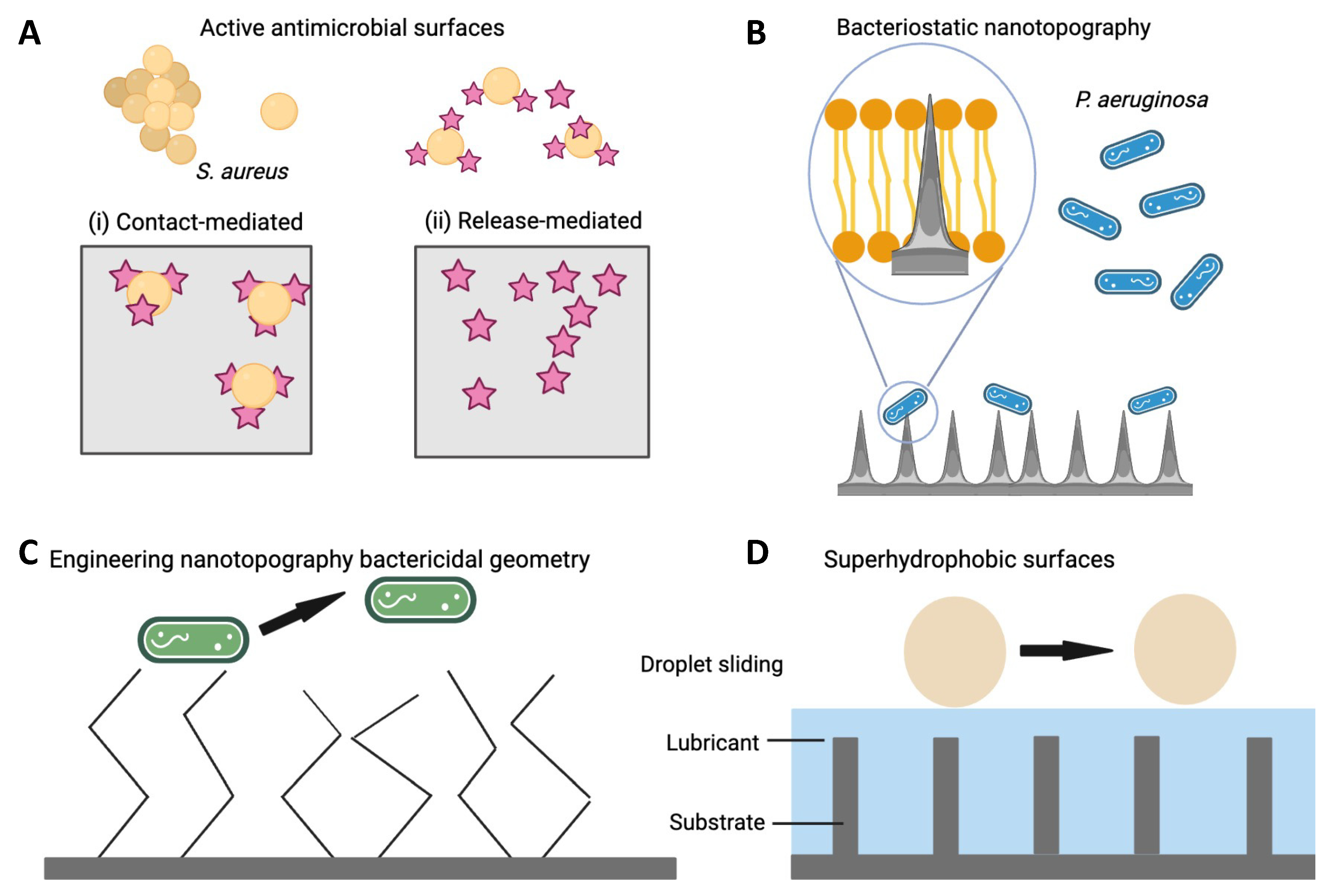
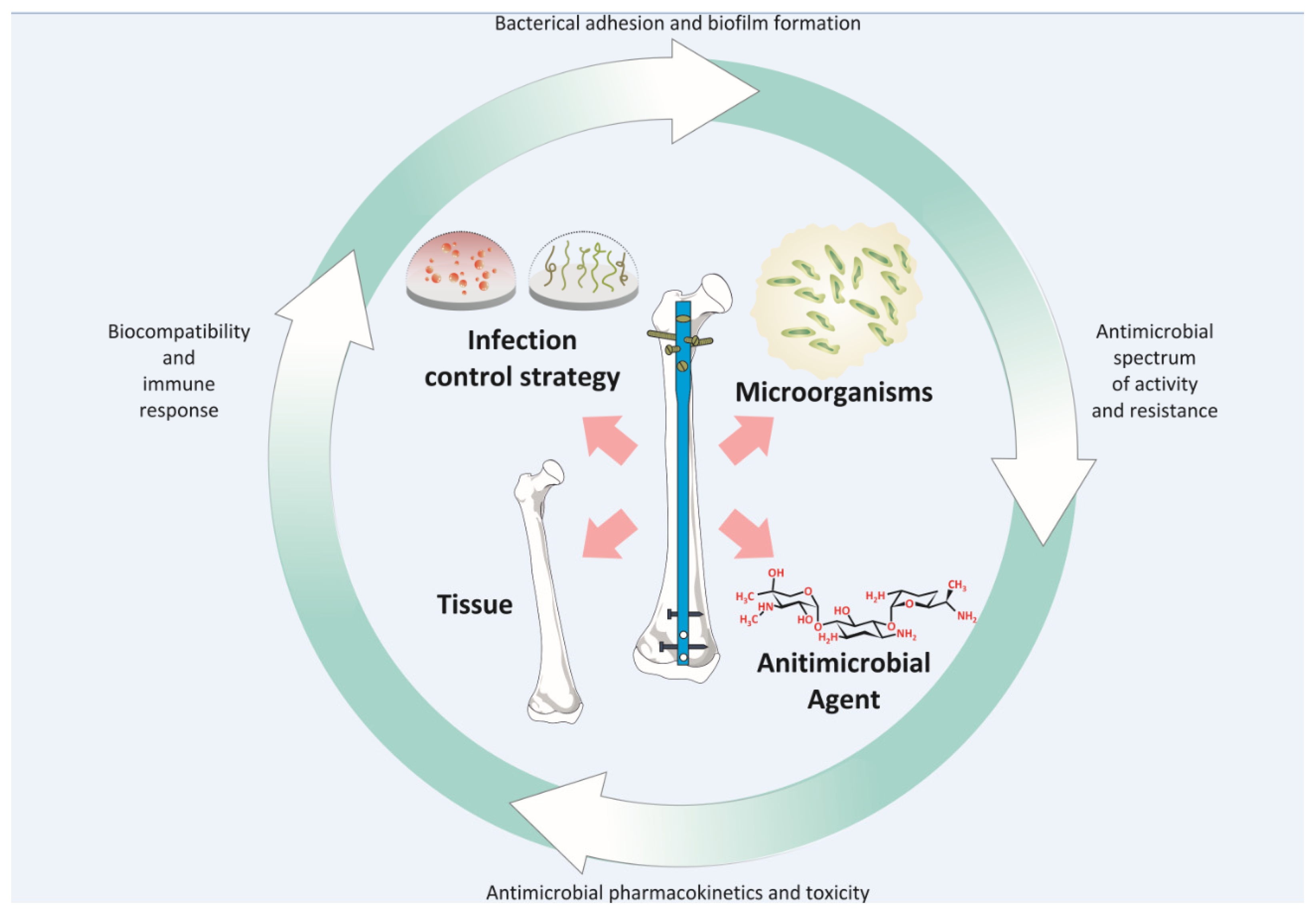
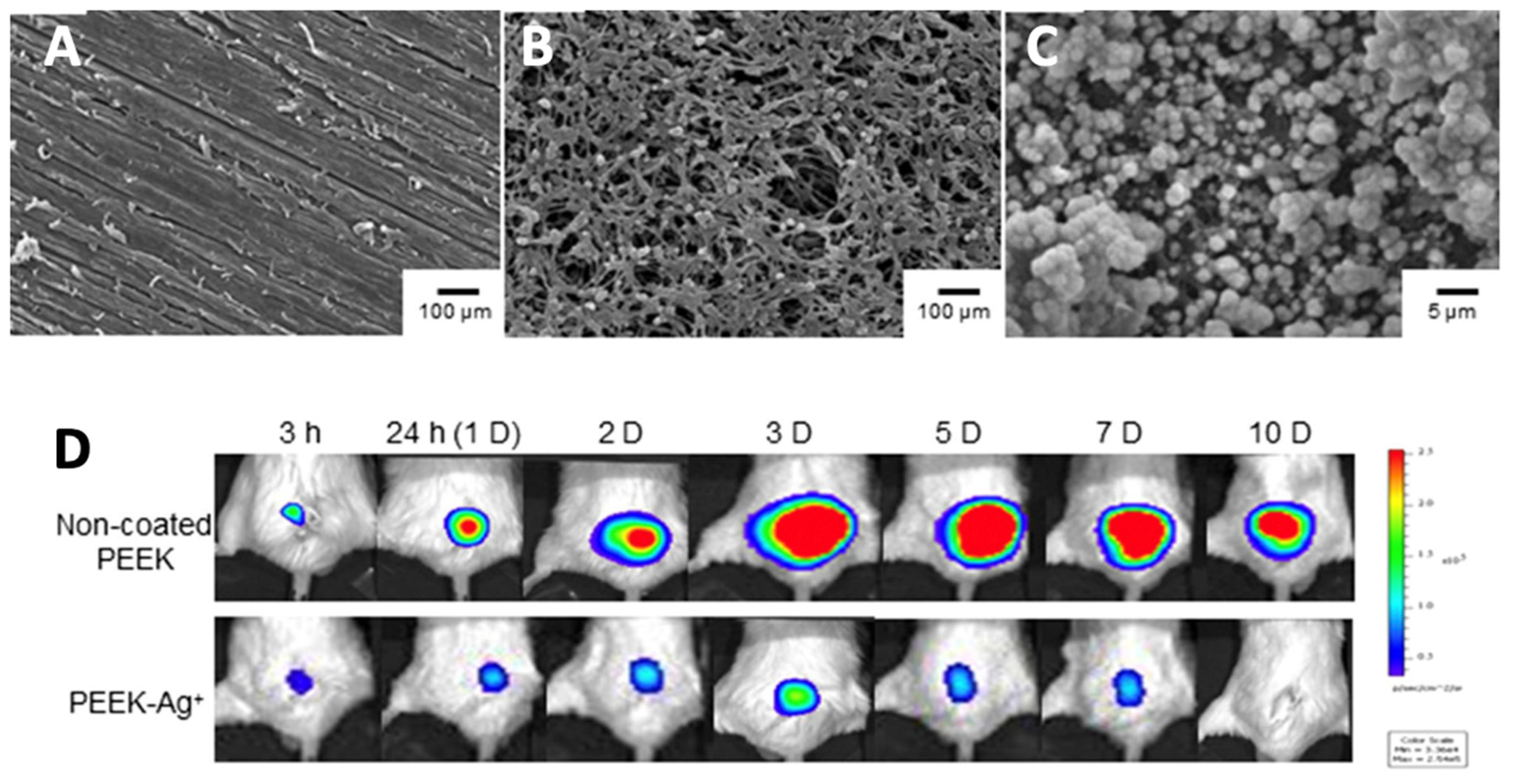
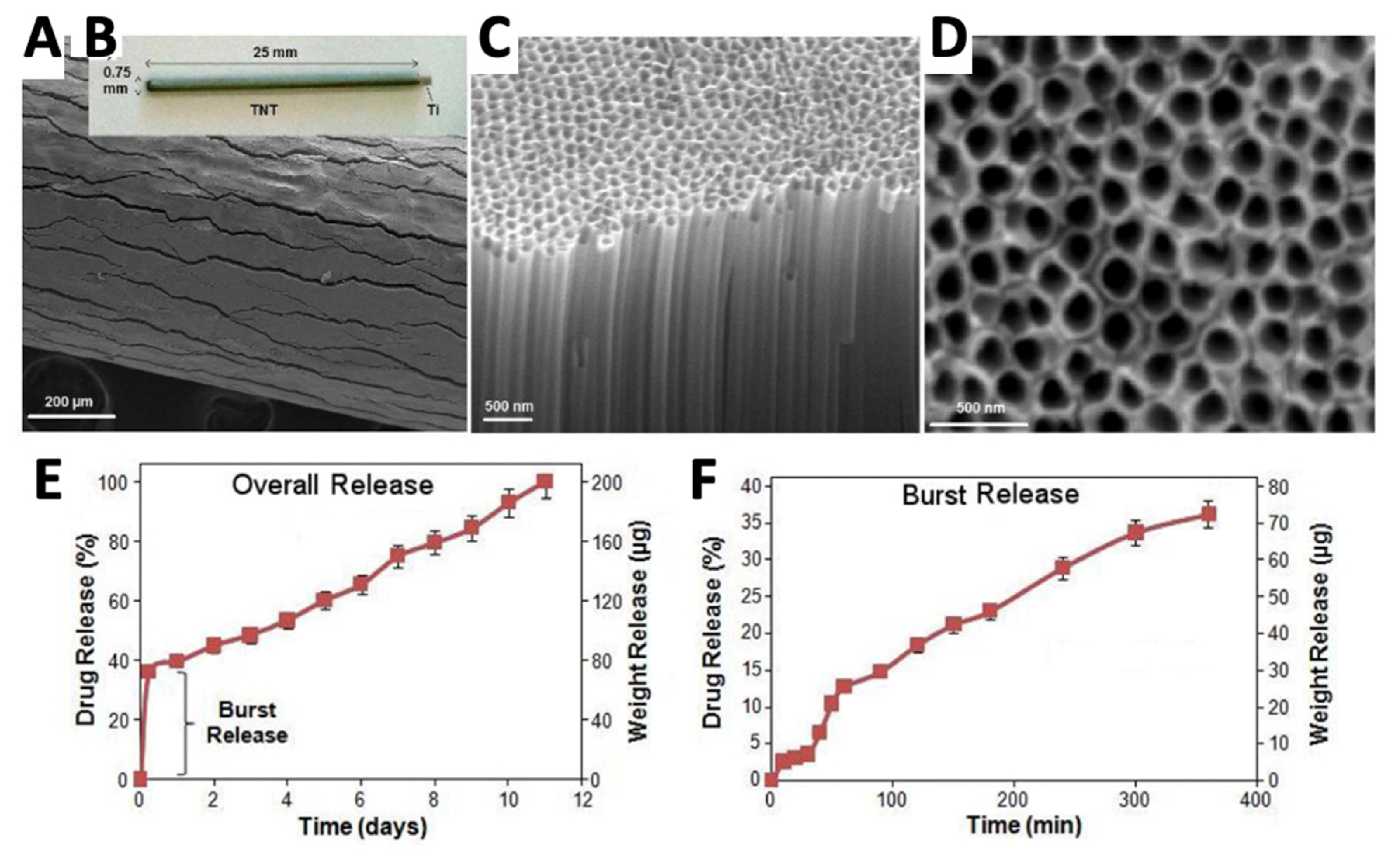
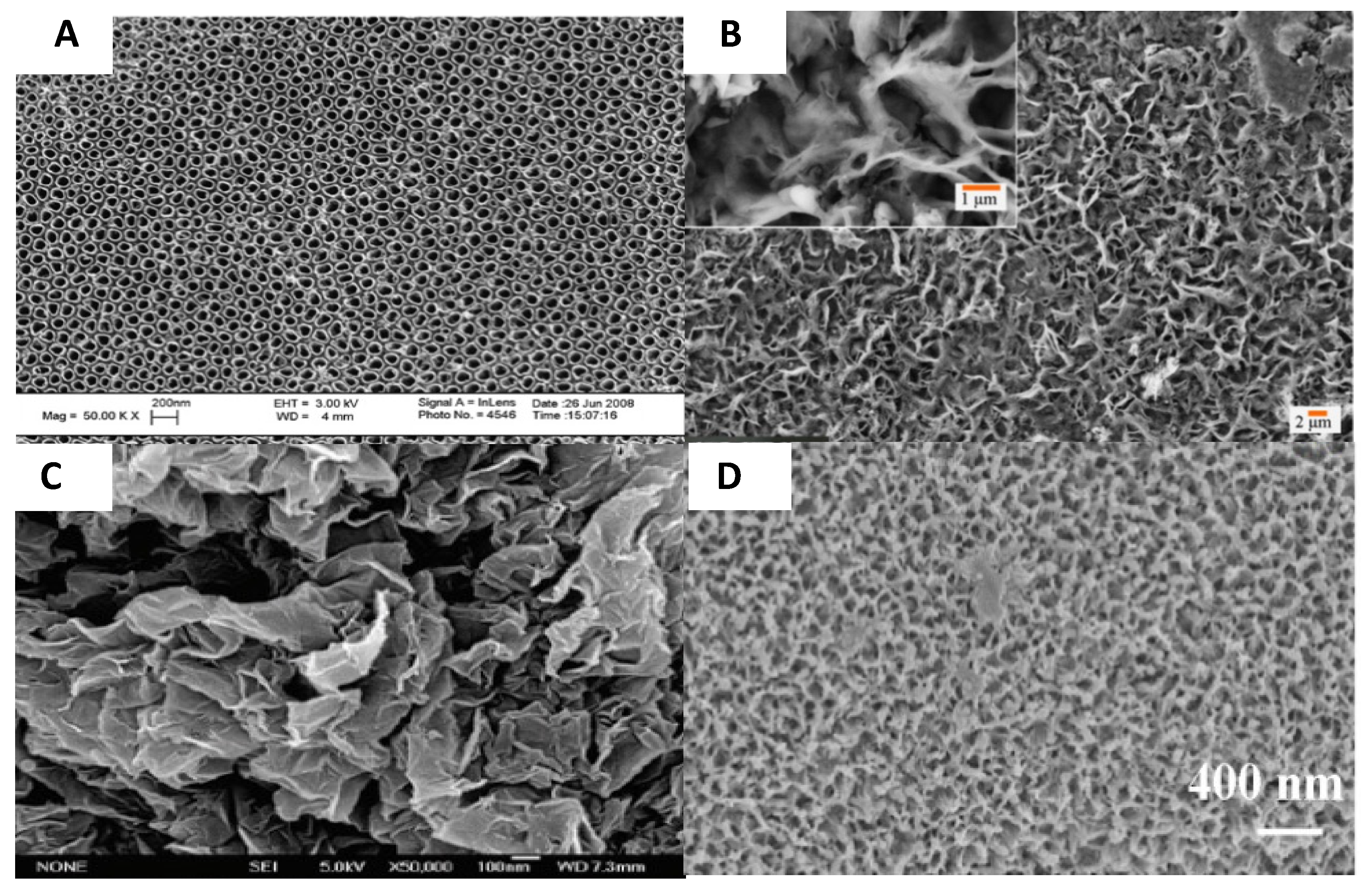
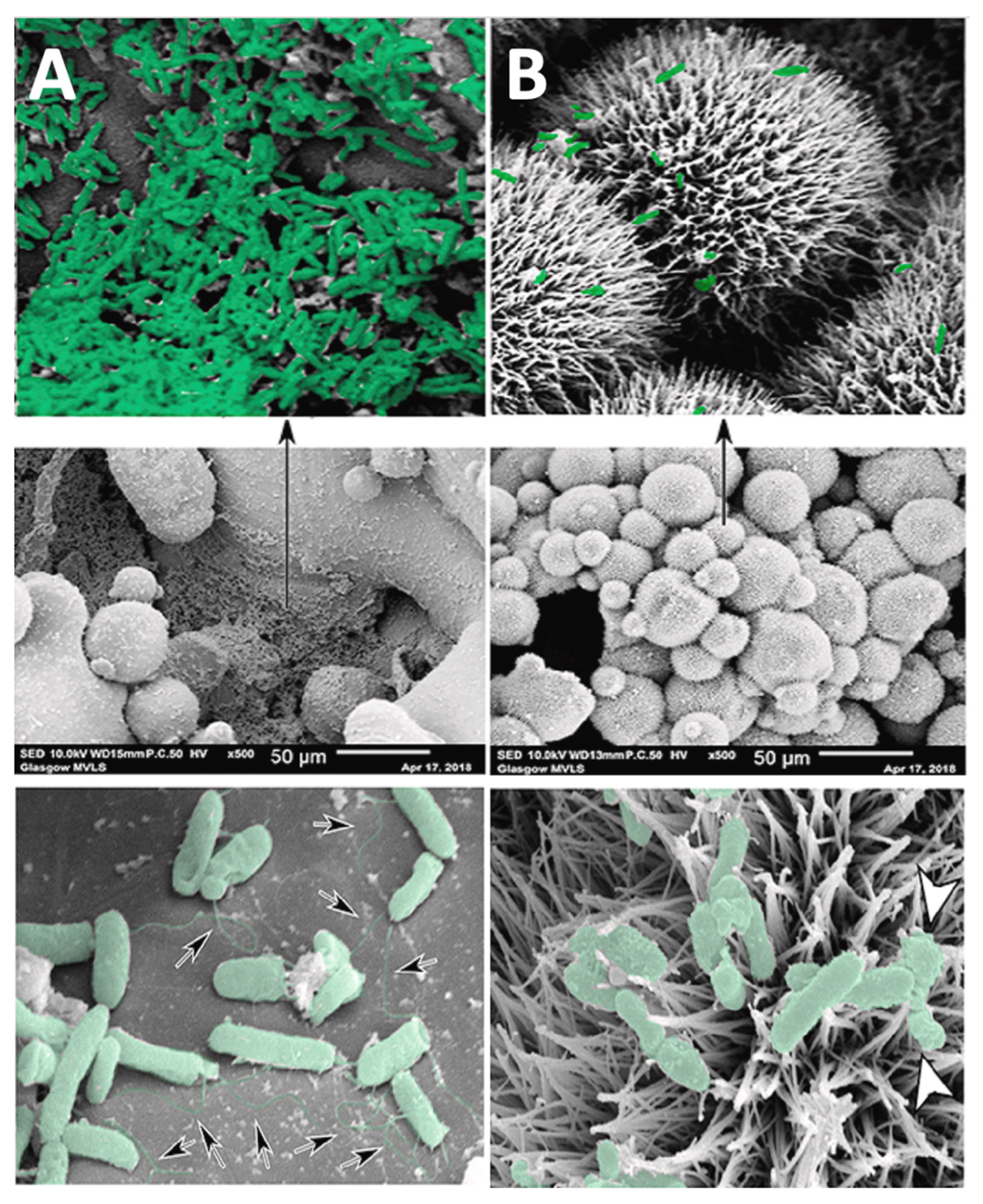

| Property | Features |
|---|---|
| Infection | Bacteria species, strain, properties (charge), susceptibility to treatment, presence of biofilm |
| Device | Material (metal–titanium alloy, stainless steel; polymer—PEEK), Shape Application—arthroplasty, fracture fixation, trauma Permanent or temporary implantation |
| Infection control strategy | Antimicrobial surfaces (antimicrobial release or contact inhibition) Anti-adhesive surfaces, bacteria repellent. Interference with biofilm formation (e.g., quorum-sensing inhibitors, quorum quenchers, enzymes, small molecules, immunotherapy) |
| Antimicrobial cargo | Controlled release profile—spatiotemporal control over presentation of antimicrobial agents, reproducible PK/PD parameters Properties—MIC, pathogen selectivity, spectrum of activity, species selectivity, mode of action, toxicity to host cells and tissue, resistance |
| Surface properties | Surface roughness, chemistry, energy, and wettability Physical architecture—nanotopography Interaction with host proteins, host cells and bacteria Influence on cell proliferation and differentiation |
| Mechanical features | Sufficient to facilitate handling and surgical implantation. Replicate those of the target tissue to provide important cues that instruct tissue development where integration is desirable while simultaneously limiting stress shielding |
| Biomaterial properties | Biocompatibility—material and breakdown products should be biocompatible to avoid foreign body responses that might otherwise lead to rejection or interfere with the healing cascade. Host responses Where integration is desirable, biomaterials should ideally be osteoconductive, osteoinductive and support osseointegration. |
| Manufacture/ Production | Technology should facilitate translation from bench scale to the clinic. Production according to GMP standards. Cost-effective. |
| Development and Regulation | Preclinical (in vitro, in vivo) and clinical models. Regulatory requirements—e.g., testing the efficacy of combination medical devices. Antimicrobial efficacy according to ASTM standards Approval pathway and regulatory requirements in different jurisdictions. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kennedy, D.G.; O’Mahony, A.M.; Culligan, E.P.; O’Driscoll, C.M.; Ryan, K.B. Strategies to Mitigate and Treat Orthopaedic Device-Associated Infections. Antibiotics 2022, 11, 1822. https://doi.org/10.3390/antibiotics11121822
Kennedy DG, O’Mahony AM, Culligan EP, O’Driscoll CM, Ryan KB. Strategies to Mitigate and Treat Orthopaedic Device-Associated Infections. Antibiotics. 2022; 11(12):1822. https://doi.org/10.3390/antibiotics11121822
Chicago/Turabian StyleKennedy, Darragh G., Aoife M. O’Mahony, Eamonn P. Culligan, Caitriona M. O’Driscoll, and Katie B. Ryan. 2022. "Strategies to Mitigate and Treat Orthopaedic Device-Associated Infections" Antibiotics 11, no. 12: 1822. https://doi.org/10.3390/antibiotics11121822
APA StyleKennedy, D. G., O’Mahony, A. M., Culligan, E. P., O’Driscoll, C. M., & Ryan, K. B. (2022). Strategies to Mitigate and Treat Orthopaedic Device-Associated Infections. Antibiotics, 11(12), 1822. https://doi.org/10.3390/antibiotics11121822