Antimicrobial Stewardship in COVID-19 Patients: Those Who Sow Will Reap Even through Hard Times
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Consumption
2.2. Microbiological and Clinical Outcomes
2.3. Qualitative Indicators
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Setting
4.2. Intervention
- Since October 2020, an ID specialist has attended daily Unit 1 clinical rounds to support antibiotic prescription and withholding and advise on the diagnostic process. Biweekly revision of ongoing antibiotic therapies was also resumed in Unit 2, already involved in the pre-COVID-19 AS intervention, to refresh physicians’ diagnostic and prescribing skills.
- Starting from the third wave, the COVID-19 guidelines as well as the hospital antibiotic guidelines were made available through the Firstline app, available for iOS and Android, and on the web (https://firstline.org/, accessed on 28 April 2023) for Units 1–3, to increase their usability; local epidemiological data and monographs of antibiotics were also accessible from the same platform.
- Prospective audits were conducted weekly in the three intervention units during the third wave. All the patients receiving antibiotic therapy on the audit day had their electronic health records reviewed; quality indicators such as compliance with empirical therapy guidelines and appropriateness of therapy were evaluated and recorded.
4.3. Outcomes
- consumption data broken down to a single agent and stratified by WHO AWaRe classification (access, watch, reserve) [25].
- prescribing appropriateness as registered by prospective audits. All the patients receiving antibiotic therapy on the audit day had their clinical charts reviewed for presumptive infective diagnosis, antimicrobial prescription, and microbiological results. Appropriateness of therapy was defined as compliance with antibiotic guidelines for empirical therapy and as adequate coverage plus de-escalation if needed for targeted, microbiological-based, therapy. The prevalence of patients receiving antibiotics on the audit day was also collected.
- Clinical outcomes, including in-hospital mortality (measured as crude rate in-hospital mortality) and length of stay (LOS).
- microbiological outcomes, including total bloodstream infection (BSI), BSI caused by MDR bacteria (i.e., methicillin-resistant S. aureus and S. epidermidis, carbapenem-resistant Enterobacterales and P. aeruginosa, ESBL-producing gram-negative, vancomycin-resistant Enterococci), and C. difficile infections (incidence per 100 admitted patients, deduplication was applied, counting only the first isolates/positive tests per patient in a 28-day interval, common contaminants were manually removed).
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calderon, M.; Gysin, G.; Gujjar, A.; McMaster, A.; King, L.; Comandé, D.; Hunter, E.; Payne, B. Bacterial co-infection and antibiotic stewardship in patients with COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2023, 23, 14. [Google Scholar] [CrossRef]
- Langford, B.J.; Soucy, J.-P.R.; Leung, V.; So, M.; Kwan, A.T.; Portnoff, J.S.; Bertagnolio, S.; Raybardhan, S.; MacFadden, D.R.; Daneman, N. Antibiotic resistance associated with the COVID-19 pandemic: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2023, 29, 302–309. [Google Scholar] [CrossRef]
- Grau, S.; Hernández, S.; Echeverría-Esnal, D.; Almendral, A.; Ferrer, R.; Limón, E.; Horcajada, J.P.; Catalan Infection Control and Antimicrobial Stewardship Program (VINCat-PROA). Antimicrobial Consumption among 66 Acute Care Hospitals in Catalonia: Impact of the COVID-19 Pandemic. Antibiotics 2021, 10, 943. [Google Scholar] [CrossRef]
- Murgadella-Sancho, A.; Coloma-Conde, A.; Oriol-Bermúdez, I. Impact of the strategies implemented by an antimicrobial stewardship program on the antibiotic consumption in the coronavirus disease 2019 (COVID-19) pandemic. Infect. Control. Hosp. Epidemiol. 2022, 43, 1292–1293. [Google Scholar] [CrossRef]
- Vaughn, V.M.; Gandhi, T.N.; Petty, L.A.; Patel, P.K.; Prescott, H.C.; Malani, A.N.; Ratz, D.; McLaughlin, E.; Chopra, V.; Flanders, S.A. Empiric Antibacterial Therapy and Community-onset Bacterial Coinfection in Patients Hospitalized With Coronavirus Disease 2019 (COVID-19): A Multi-hospital Cohort Study. Clin. Infect. Dis. 2021, 72, e533–e541. [Google Scholar] [CrossRef]
- Karami, Z.; Knoop, B.T.; Dofferhoff, A.S.M.; Blaauw, M.J.T.; Janssen, N.A.; van Apeldoorn, M.; Kerckhoffs, A.P.M.; van de Maat, J.S.; Hoogerwerf, J.J.; Oever, J.T. Few bacterial co-infections but frequent empiric antibiotic use in the early phase of hospitalized patients with COVID-19: Results from a multicentre retrospective cohort study in The Netherlands. Infect. Dis. 2021, 53, 102–110. [Google Scholar] [CrossRef]
- Van Laethem, J.; Wuyts, S.; Van Laere, S.; Koulalis, J.; Colman, M.; Moretti, M.; Seyler, L.; De Waele, E.; Pierard, D.; Lacor, P.; et al. Antibiotic prescriptions in the context of suspected bacterial respiratory tract superinfections in the COVID-19 era: A retrospective quantitative analysis of antibiotic consumption and identification of antibiotic prescription drivers. Intern. Emerg. Med. 2022, 17, 141–151. [Google Scholar] [CrossRef]
- Declercq, J.; A Van Damme, K.F.; De Leeuw, E.; Maes, B.; Bosteels, C.; Tavernier, S.J.; De Buyser, S.; Colman, R.; Hites, M.; Verschelden, G.; et al. Effect of anti-interleukin drugs in patients with COVID-19 and signs of cytokine release syndrome (COV-AID): A factorial, randomised, controlled trial. Lancet Respir. Med. 2021, 9, 1427–1438. [Google Scholar] [CrossRef]
- Meschiari, M.; Onorato, L.; Bacca, E.; Orlando, G.; Menozzi, M.; Franceschini, E.; Bedini, A.; Cervo, A.; Santoro, A.; Sarti, M.; et al. Long-Term Impact of the COVID-19 Pandemic on In-Hospital Antibiotic Consumption and Antibiotic Resistance: A Time Series Analysis (2015–2021). Antibiotics 2022, 11, 826. [Google Scholar] [CrossRef]
- Friedli, O.; Gasser, M.; Cusini, A.; Fulchini, R.; Vuichard-Gysin, D.; Tobler, R.H.; Wassilew, N.; Plüss-Suard, C.; Kronenberg, A. Impact of the COVID-19 Pandemic on Inpatient Antibiotic Consumption in Switzerland. Antibiotics 2022, 11, 792. [Google Scholar] [CrossRef]
- Bartoletti, M.; Azap, O.; Barac, A.; Bussini, L.; Ergonul, O.; Krause, R.; Paño-Pardo, J.R.; Power, N.R.; Sibani, M.; Szabo, B.G.; et al. ESCMID COVID-19 living guidelines: Drug treatment and clinical management. Clin. Microbiol. Infect. 2022, 28, 222–238. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Living Guidance for Clinical Management of COVID-19: Living Guidance; World Health Organization (WHO): Geneva, Switzerland, 2021.
- Alshaikh, F.S.; Godman, B.; Sindi, O.N.; Seaton, R.A.; Kurdi, A. Prevalence of bacterial coinfection and patterns of antibiotics prescribing in patients with COVID-19: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0272375. [Google Scholar] [CrossRef]
- Khan, S.; Bond, S.E.; Bakhit, M.; Hasan, S.S.; Sadeq, A.A.; Conway, B.R.; Aldeyab, M.A. COVID-19 Mixed Impact on Hospital Antimicrobial Stewardship Activities: A Qualitative Study in UK-Based Hospitals. Antibiotics 2022, 11, 1600. [Google Scholar] [CrossRef]
- Fukushige, M.; Ngo, N.-H.; Lukmanto, D.; Fukuda, S.; Ohneda, O. Effect of the COVID-19 pandemic on antibiotic consumption: A systematic review comparing 2019 and 2020 data. Front. Public Heal. 2022, 10. [Google Scholar] [CrossRef]
- Matteson, C.L.; Czaja, C.A.; Kronman, M.P.; Ziniel, S.; Parker, S.K.; Dodson, D.S. Impact of the coronavirus disease 2019 (COVID-19) pandemic on antimicrobial stewardship programs in Colorado hospitals. Antimicrob Steward Healthc Epidemiol 2022, 2, e172. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; I de Silva, T.; Egan, C.; et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: A multicentre, prospective cohort study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef]
- Liew, Y.; Lee, W.; Tan, L.; Kwa, A.; Thien, S.; Cherng, B.; Chung, S.J. Antimicrobial stewardship programme: A vital resource for hospitals during the global outbreak of coronavirus disease 2019 (COVID-19). Int. J. Antimicrob. Agents 2020, 56, 106145. [Google Scholar] [CrossRef]
- Huttner, B.; Catho, G.; Pao-Pardo, J.; Pulcini, C.; Schouten, J. COVID-19: Don’t neglect antimicrobial stewardship principles! Clin. Microbiol. Infect. 2020, 26, 808–810. [Google Scholar] [CrossRef]
- Staub, M.B.; Beaulieu, R.M.; Graves, J.; Nelson, G.E. Changes in antimicrobial utilization during the coronavirus disease 2019 (COVID-19) pandemic after implementation of a multispecialty clinical guidance team. Infect. Control. Hosp. Epidemiol. 2021, 42, 266–275. [Google Scholar] [CrossRef]
- Venturini, S.; Avolio, M.; Fossati, S.; Callegari, A.; De Rosa, R.; Basso, B.; Zanusso, C.; Orso, D.; Cugini, F.; Crapis, M. Antimicrobial Stewardship in the Covid-19 Pandemic. Hosp. Pharm. 2022, 57, 416–418. [Google Scholar] [CrossRef]
- Carrara, E.; Sibani, M.; Barbato, L.; Mazzaferri, F.; Salerno, N.D.; Conti, M.; Azzini, A.M.; Dalbeni, A.; Pellizzari, L.; Fontana, G.; et al. How to ‘SAVE’ antibiotics: Effectiveness and sustainability of a new model of antibiotic stewardship intervention in the internal medicine area. Int J Antimicrob Agents. 2022, 60, 106672. [Google Scholar] [CrossRef]
- The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book; World Health Organization: Geneva, Switzerland, 2022; Licence: CC BY-NC-SA 3.0 IGO.
- Ponce-Alonso, M.; de la Fuente, J.S.; Rincón-Carlavilla, A.; Moreno-Nunez, P.; Martínez-García, L.; Escudero-Sánchez, R.; Pintor, R.; García-Fernández, S.; Cobo, J. Impact of the coronavirus disease 2019 (COVID-19) pandemic on nosocomial Clostridioides difficile infection. Infect. Control. Hosp. Epidemiol. 2021, 42, 406–410. [Google Scholar] [CrossRef]
- Alberici, F.; Affatato, S.; Moratto, D.; Mescia, F.; Delbarba, E.; Guerini, A.; Tedesco, M.; Burbelo, P.; Zani, R.; Castagna, I.; et al. SARS-CoV-2 infection in dialysis and kidney transplant patients: Immunological and serological response. J. Nephrol. 2022, 35, 745–759. [Google Scholar] [CrossRef]
- Guisado-Gil, A.B.; Infante-Domínguez, C.; Peñalva, G.; Praena, J.; Roca, C.; Navarro-Amuedo, M.D.; Aguilar-Guisado, M.; Espinosa-Aguilera, N.; Poyato-Borrego, M.; Romero-Rodríguez, N.; et al. Impact of the COVID-19 Pandemic on Antimicrobial Consumption and Hospital-Acquired Can-didemia and Multidrug-Resistant Bloodstream Infections. Antibiotics 2020, 9, 816. [Google Scholar] [CrossRef]
- Pettit, N.; Nguyen, C.T.; Lew, A.; Bhagat, P.H.; Nelson, A.; Olson, G.; Ridgway, J.; Pho, M.; Pagkas-Bather, J. Reducing the use of empiric antibiotic therapy in COVID-19 on hospital admission. BMC Infect. Dis. 2021, 21, 516. [Google Scholar] [CrossRef]
- Cong, W.; Stuart, B.; AIhusein, N.; Liu, B.; Tang, Y.-S.; Wang, H.; Wang, Y.; Manchundiya, A.; Lambert, H. Antibiotic Use and Bacterial Infection in COVID-19 Patients in the Second Phase of the SARS-CoV-2 Pandemic: A Scoping Review. Antibiotics 2022, 11, 991. [Google Scholar] [CrossRef]
- Cecconi, M.; Piovani, D.; Brunetta, E.; Aghemo, A.; Greco, M.; Ciccarelli, M.; Angelini, C.; Voza, A.; Omodei, P.; Vespa, E.; et al. Early Predictors of Clinical Deterioration in a Cohort of 239 Patients Hospitalized for Covid-19 Infection in Lombardy, Italy. J. Clin. Med. 2020, 9, 1548. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group (2021). Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet (London, England) 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- Masetti, C.; Generali, E.; Colapietro, F.; Voza, A.; Cecconi, M.; Messina, A.; Omodei, P.; Angelini, C.; Ciccarelli, M.; Badalamenti, S.; et al. High mortality in COVID-19 patients with mild respiratory disease. Eur. J. Clin. Investig. 2020, 50, e13314. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.N.; Haider, N.; Stigler, F.L.; Khan, R.A.; McCoy, D.; Zumla, A.; Kock, R.A.; Uddin, J. The Global Case-Fatality Rate of COVID-19 Has Been Declining Since May 2020. Am. J. Trop. Med. Hyg. 2021, 104, 2176–2184. [Google Scholar] [CrossRef]
- De Kraker, M.; Abbas, M.; Huttner, B.; Harbarth, S. Good epidemiological practice: A narrative review of appropriate scientific methods to evaluate the impact of antimicrobial stewardship interventions. Clin. Microbiol. Infect. 2017, 23, 819–825. [Google Scholar] [CrossRef] [PubMed]
- WHO Collaborating Centre for Drug Statistics Methodology. ATC Classification Index with DDDs, 2023; WHO Collaborating Centre for Drug Statistics Methodology: Oslo, Norway, 2022. [Google Scholar]
- Benić, M.S.; Milanič, R.; A Monnier, A.; Gyssens, I.C.; Adriaenssens, N.; Versporten, A.; Zanichelli, V.; Le Maréchal, M.; Huttner, B.; Tebano, G.; et al. Metrics for quantifying antibiotic use in the hospital setting: Results from a systematic review and international multidisciplinary consensus procedure. J. Antimicrob. Chemother. 2018, 73, vi50–vi58. [Google Scholar] [CrossRef] [PubMed]
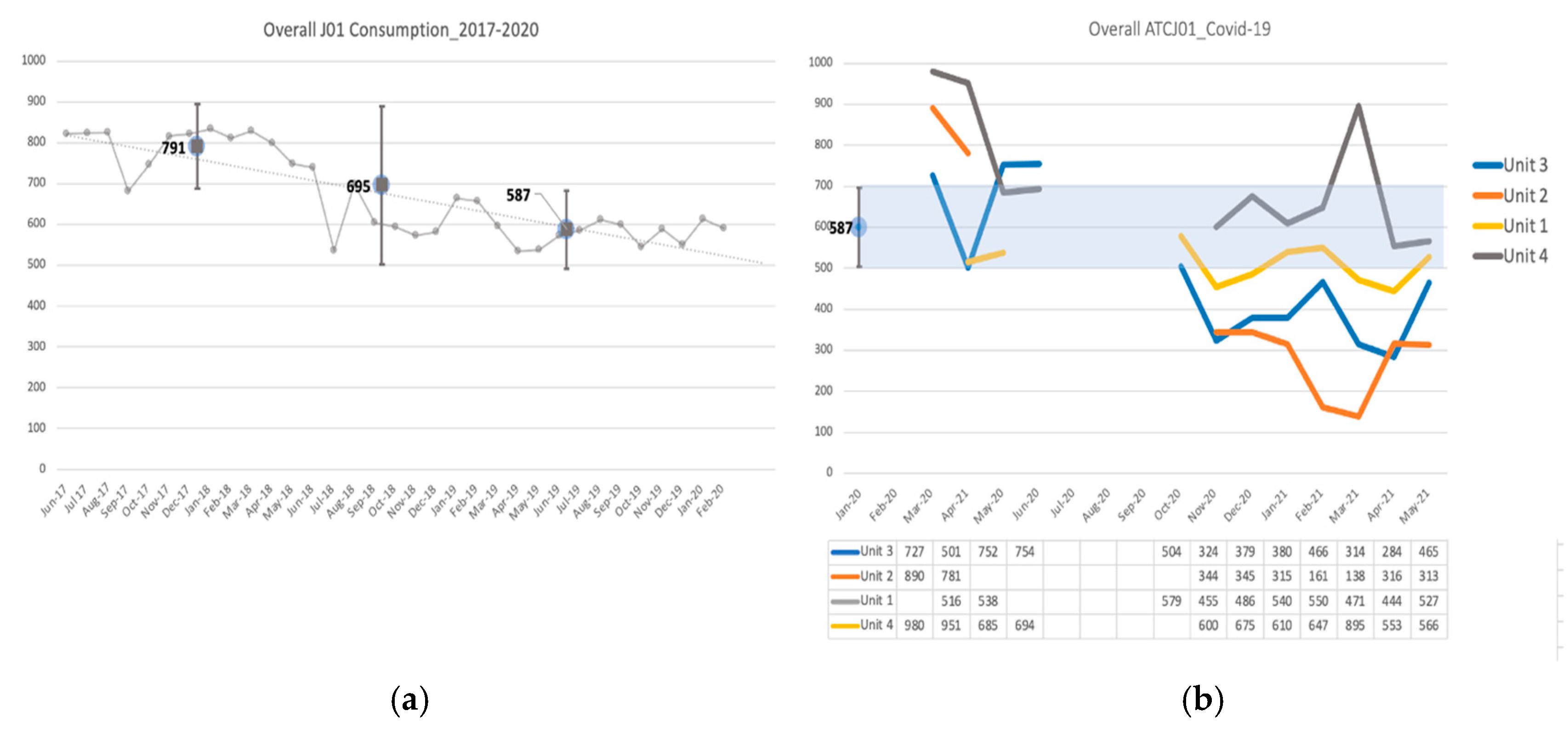
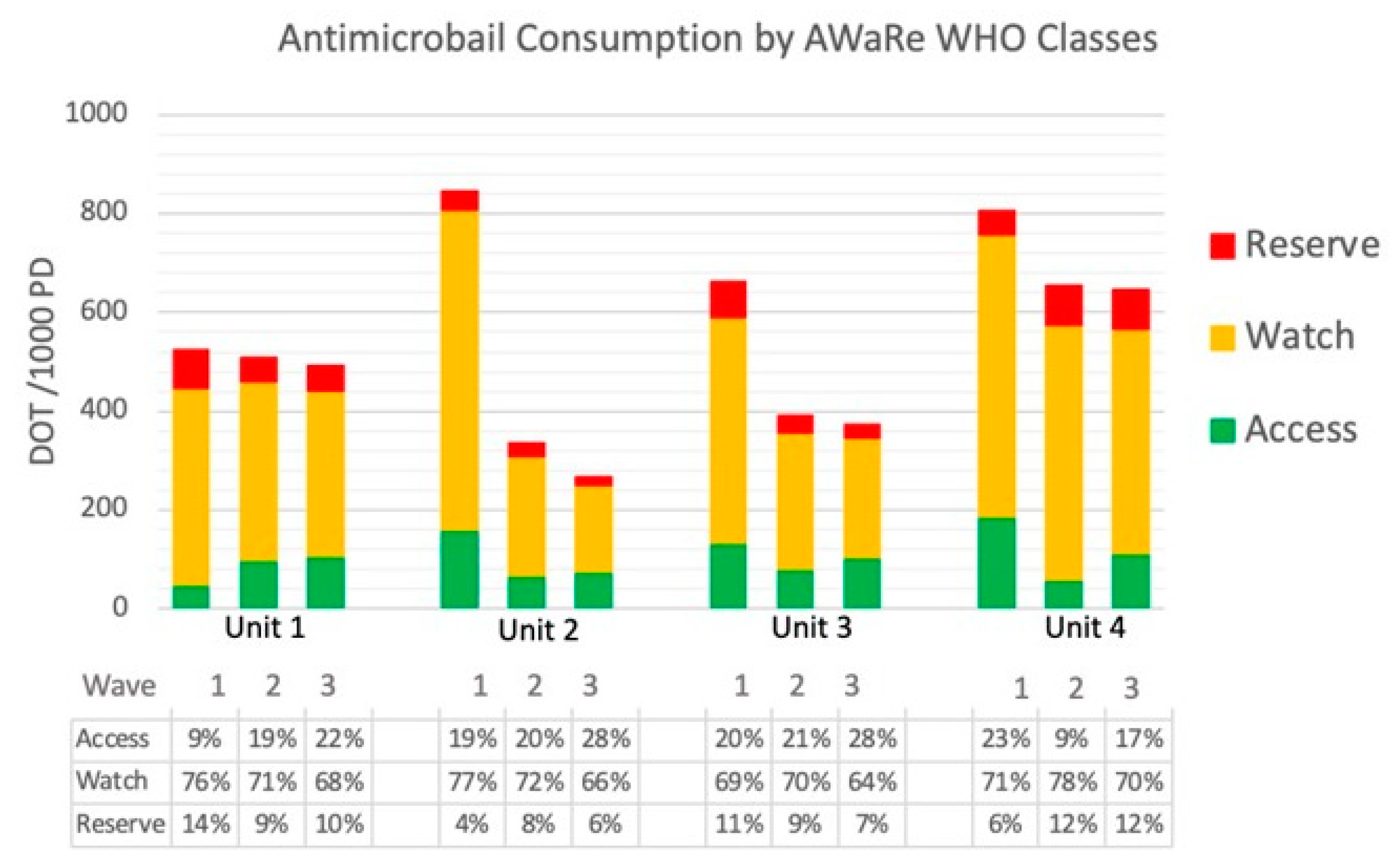
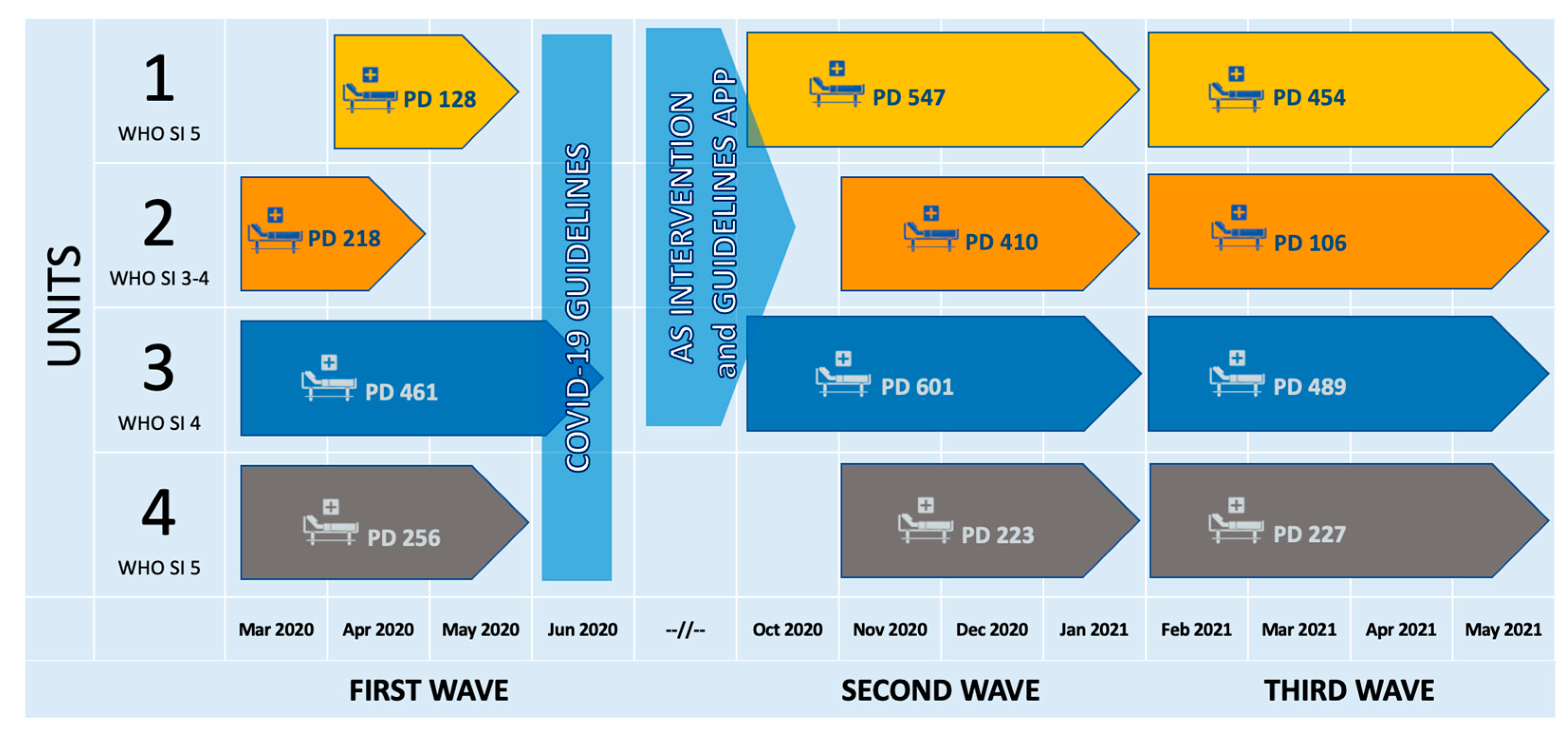
| Antimicrobial Consumption (ATC J01) DOT/1000 PD | |||||||
|---|---|---|---|---|---|---|---|
| Unit | Medical Area Desirable Consumption Estimate Mean (SD) | Wave 1 Mean (SD) | p-Value * | Wave 2 Mean (SD) | p-Value * | Wave 3 Mean (SD) | p-Value * |
| Unit 1 (WHO Scale 5) | 527 (±15.6) | 0.082 | 515 (±55.3) | 0.0168 | 498 (±49) | 0.0037 | |
| Unit 2 (WHO Scale 3–4) | 587 (±42.6) | 836 (±77.1) | <0.001 | 335 (±17.0) | <0.001 | 232 (±95.7) | <0.001 |
| Unit 3 (WHO Scale 4) | 684 (±122.3) | 0.027 | 397 (±76.1) | <0.001 | 382 (±96.9) | <0.001 | |
| Unit 4 (C) (WHO Scale 4–5) | 872 (±162.6) | <0.0001 | 628 (±40.7) | 0.1496 | 665 (±159) | 0.1236 | |
| Outcome | Unit | First Wave Mean (DS) | Second Wave Mean (DS) | Third Wave Mean (DS) | p-Value * |
|---|---|---|---|---|---|
| DOT/1000 PD | Unit 1 | 527 (±15.6) | 515 (±55.3) | 498 (±49) | <0.05 |
| Unit 2 | 836 (±77.1) | 334.7 (±17.0) | 232 (±95.7) | <0.05 | |
| Unit 3 | 684 (±122.3) | 397 (±76.1) | 382 (±96.9) | <0.05 | |
| Unit 4(C) | 872 (±162.6) | 628 (±40.7) | 665 (±159) | >0.05 | |
| DDD/1000 PD | Unit 1 | 635 (±217.1) | 575 (±94.2) | 533 (±80.9) | >0.05 |
| Unit 2 | 913 (±137.9) | 319(±34) | 219 (±96.3) | <0.05 | |
| Unit 3 | 736 (±150.4) | 408 (±67.9) | 430 (±111.7) | <0.05 | |
| Unit 4(C) | 834 (±209.8) | 576 (±42.7) | 636 (±183.0) | data | |
| LOT/1000 PD | Unit 1 | 407 (±43.8) | 444 (±49.0) | 411(±38.6) | >0.05 |
| Unit 2 | 614 (±36.1) | 294 (±15) | 201 (±74.4) | <0.05 | |
| Unit 3 | 524 (±73.5) | 327 (±55.1) | 307 (±69.7) | <0.05 | |
| Unit 4(C) | 702 (±95.6) | 532 (±50.7) | 514 (±102.8) | data | |
| ACCESS | Unit 1 | 57 (±26.2) | 101 (±23.6) | 107.5 (±8.2) | >0.05 |
| (DOT/1000 PDs) | Unit 2 | 157 (±31.8) | 67 (±16.1) | 52 (±47.9) | >0.05 |
| Unit 3 | 155 (±91.7) | 84 (±35.1) | 109 (±34.7) | >0.05 | |
| Unit 4(C) | 194 (±71.4) | 60 (±29.5) | 111 (±41.3) | >0.05 | |
| WATCH | Unit 1 | 369(±101.8) | 379 (±61.5) | 338.8 (±33.9) | >0.05 |
| (DOT/1000 PDs) | Unit 2 | 640 (±72.2) | 243 (±26.9) | 172 (67.7) | <0.05 |
| Unit 3 | 456 (±83) | 277 (±38.8) | 245 (56.5) | <0.05 | |
| Unit 4(C) | 632 (±103.3) | 513 (±116.6) | 472 (82.3) | >0.05 | |
| RESERVE | Unit 1 | 101 (±91.2) | 35 (±35.1) | 52 (±13.9) | >0.05 |
| (DOT/1000 PDs) | Unit 2 | 39 (±26.9) | 25 (±14.4) | 9 (±10.3) | >0.05 |
| Unit 3 | 72 (±9.9) | 36 (±12.3) | 29 (±22.0) | >0.05 | |
| Unit 4(C) | 46 (±22.9) | 56 (±70.4) | 81 (49.3) | >0.05 | |
| PIPERACILLIN-TAZOBACTAM | Unit 1 | 93 (±55.2) | 112 (±37.7) | 143 (±49.6) | >0.05 |
| (DOT/1000 PDs) | Unit 2 | 161 (±43.8) | 124 (±15.5) | 73.2 (±16.4) | <0.05 |
| Unit 3 | 142 (±63.6) | 114 (±23.6) | 103 (±29.9) | <0.05 | |
| Unit 4(C) | 276 (±32.8) | 225 (±34.6) | 212 (±39.0) | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sibani, M.; Canziani, L.M.; Tonolli, C.; Armellini, M.; Carrara, E.; Mazzaferri, F.; Conti, M.; SAVE Working Group; Mazzariol, A.; Micheletto, C.; et al. Antimicrobial Stewardship in COVID-19 Patients: Those Who Sow Will Reap Even through Hard Times. Antibiotics 2023, 12, 1009. https://doi.org/10.3390/antibiotics12061009
Sibani M, Canziani LM, Tonolli C, Armellini M, Carrara E, Mazzaferri F, Conti M, SAVE Working Group, Mazzariol A, Micheletto C, et al. Antimicrobial Stewardship in COVID-19 Patients: Those Who Sow Will Reap Even through Hard Times. Antibiotics. 2023; 12(6):1009. https://doi.org/10.3390/antibiotics12061009
Chicago/Turabian StyleSibani, Marcella, Lorenzo Maria Canziani, Chiara Tonolli, Maddalena Armellini, Elena Carrara, Fulvia Mazzaferri, Michela Conti, SAVE Working Group, Annarita Mazzariol, Claudio Micheletto, and et al. 2023. "Antimicrobial Stewardship in COVID-19 Patients: Those Who Sow Will Reap Even through Hard Times" Antibiotics 12, no. 6: 1009. https://doi.org/10.3390/antibiotics12061009
APA StyleSibani, M., Canziani, L. M., Tonolli, C., Armellini, M., Carrara, E., Mazzaferri, F., Conti, M., SAVE Working Group, Mazzariol, A., Micheletto, C., Dalbeni, A., Girelli, D., & Tacconelli, E. (2023). Antimicrobial Stewardship in COVID-19 Patients: Those Who Sow Will Reap Even through Hard Times. Antibiotics, 12(6), 1009. https://doi.org/10.3390/antibiotics12061009






