Inhibition of Listeria monocytogenes Cocktail Culture Biofilms on Crab and Shrimp Coupons and the Expression of Biofilm-Related Genes
Abstract
1. Introduction
2. Results
2.1. MIC Determination
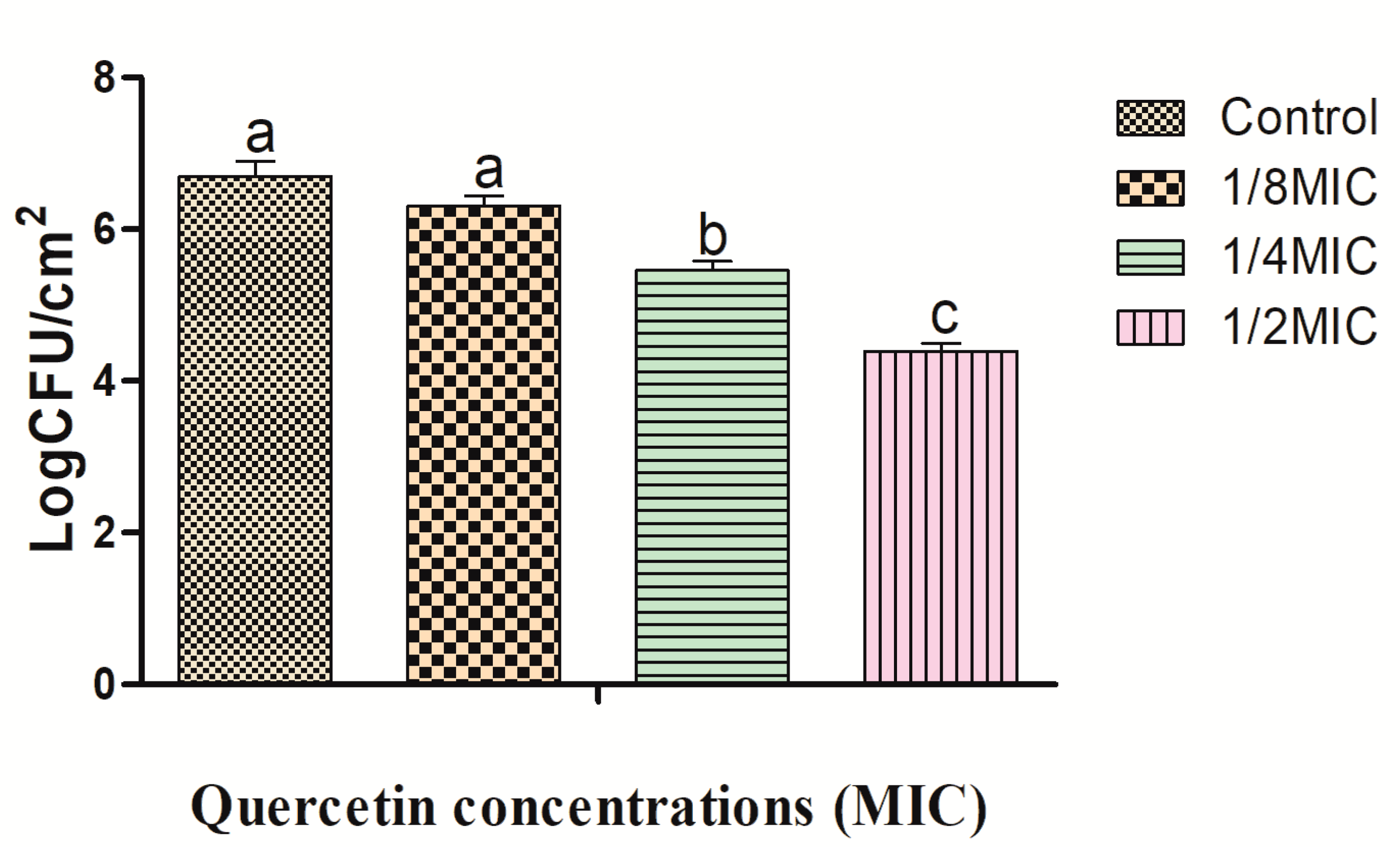
2.2. Effects of Quercetin against L. monocytogenes Cocktail Culture Biofilm on Foods
2.3. Visual Analysis of Biofilm
2.4. Relative Expression of Gene
3. Materials and Methods
3.1. Bacterial Strains and Culture
3.2. Quercetin Preparation
3.3. MIC Determination
3.4. Formation and Detachment of Biofilm on Foods
3.5. Field Emission Electron Microscope (FE-SEM) for Visual Analysis of Biofilms
3.6. Expression of Genes Analysis by Real-Time PCR (RT-PCR)
3.7. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hossain, M.I.; Mizan, M.F.R.; Roy, P.K.; Nahar, S.; Toushik, S.H.; Ashrafudoulla, M.; Jahid, I.K.; Lee, J.; Ha, S.D. Listeria monocytogenes biofilm inhibition on food contact surfaces by application of postbiotics from Lactobacillus curvatus B.67 and Lactobacillus plantarum M.2. Food Res. Int. 2021, 148, 110595. [Google Scholar] [CrossRef]
- Hossain, M.I.; Kim, K.; Mizan, M.F.R.; Toushik, S.H.; Ashrafudoulla, M.; Roy, P.K.; Nahar, S.; Jahid, I.K.; Choi, C.; Park, S.H.; et al. Comprehensive molecular, probiotic, and quorum-sensing characterization of anti-listerial lactic acid bacteria, and application as bioprotective in a food (milk) model. J. Dairy Sci. 2021, 104, 6516–6534. [Google Scholar] [CrossRef]
- Hossain, M.I.; Mizan, M.F.R.; Toushik, S.H.; Roy, P.K.; Jahid, I.K.; Park, S.H.; Ha, S.D. Antibiofilm effect of nisin alone and combined with food-grade oil components (thymol and eugenol) against Listeria monocytogenes cocktail culture on food and food-contact surfaces. Food Control 2022, 135, 108796. [Google Scholar] [CrossRef]
- Lemon, K.P.; Freitag, N.E.; Kolter, R. The virulence regulator PrfA promotes biofilm formation by Listeria monocytogenes. J. Bacteriol. 2010, 192, 3969–3976. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Armenta, F.J.; Hernandez-Onate, M.A.; Martinez-Tellez, M.A.; Lopez-Zavala, A.A.; Gonzalez-Aguilar, G.A.; Gutierrez-Pacheco, M.M.; Ayala-Zavala, J.F. Quercetin repressed the stress response factor (sigB) and virulence genes (prfA, actA, inlA, and inlC), lower the adhesion, and biofilm development of L. monocytogenes. Food Microbiol. 2020, 87, 103377. [Google Scholar] [CrossRef] [PubMed]
- Toushik, S.H.; Roy, A.; Alam, M.; Rahman, U.H.; Nath, N.K.; Nahar, S.; Matubber, B.; Uddin, M.J.; Roy, P.K. Pernicious attitude of microbial biofilms in agri-farm industries: Acquisitions and challenges of existing antibiofilm approaches. Microorganisms 2022, 10, 2348. [Google Scholar] [CrossRef] [PubMed]
- Nahar, S.; Jeong, H.L.; Kim, Y.; Ha, A.J.W.; Roy, P.K.; Park, S.H.; Ashrafudoulla, M.; Mizan, M.F.R.; Ha, S.D. Inhibitory effects of Flavourzyme on biofilm formation, quorum sensing, and virulence genes of foodborne pathogens Salmonella Typhimurium and Escherichia coli. Food Res. Int. 2021, 147, 110461. [Google Scholar] [CrossRef]
- Li, Y.; Dong, R.Y.; Ma, L.; Qian, Y.L.; Liu, Z.Y. Combined anti-biofilm enzymes strengthen the eradicate effect of Vibrio parahaemolyticus biofilm: Mechanism on cpsa-j expression and application on different carriers. Foods 2022, 11, 1305. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Ulrich, M.S.I.; Toushik, S.H.; Nahar, S.; Roy, P.K.; Mizan, F.R.; Park, S.H.; Ha, S.D. Challenges and opportunities of non-conventional technologies concerning food safety. World Poult. Sci. J. 2023, 79, 3–26. [Google Scholar] [CrossRef]
- Meireles, A.; Borges, A.; Giaouris, E.; Simoes, M. The current knowledge on the application of anti-biofilm enzymes in the food industry. Food Res. Int. 2016, 86, 140–146. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, J.Y.; Roy, P.K.; Mizan, M.F.R.; Hossain, M.I.; Park, S.H.; Ha, S.D. Viability of Salmonella Typhimurium biofilms on major food-contact surfaces and eggshell treated during 35 days with and without water storage at room temperature. Poult. Sci. 2020, 99, 4558–4565. [Google Scholar] [CrossRef] [PubMed]
- Toushik, S.H.; Park, J.H.; Kim, K.; Ashrafudoulla, M.; Ulrich, M.S.I.; Mizan, M.F.R.; Roy, P.K.; Shim, W.B.; Kim, Y.M.; Park, S.H.; et al. Antibiofilm efficacy of Leuconostoc mesenteroides J.27-derived postbiotic and food-grade essential oils against Vibrio parahaemolyticus, Pseudomonas aeruginosa, and Escherichia coli alone and in combination, and their application as a green preservative in the seafood industry. Food Res. Int. 2022, 156, 111163. [Google Scholar] [CrossRef] [PubMed]
- Toushik, S.H.; Kim, K.; Ashrafudoulla, M.; Mizan, M.F.R.; Roy, P.K.; Nahar, S.; Kim, Y.; Ha, S.D. Korean kimchi-derived lactic acid bacteria inhibit foodborne pathogenic biofilm growth on seafood and food processing surface materials. Food Control 2021, 129, 108276. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Zhu, W.X.; Cao, Y.; Gao, J.Z.; Jin, T.; Qin, N.B.; Xia, X.D. Punicalagin inhibits biofilm formation and virulence gene expression of Vibrio parahaemolyticus. Food Control 2022, 139, 109045. [Google Scholar] [CrossRef]
- Song, M.G.; Roy, P.K.; Jeon, E.B.; Kim, S.H.; Heu, M.S.; Lee, J.S.; Choi, J.S.; Kim, J.S.; Park, S.Y. Effect of Dielectric Barrier discharge plasma against Listeria monocytogenes mixed-culture biofilms on food-contact surfaces. Antibiotics 2023, 12, 609. [Google Scholar] [CrossRef]
- Roy, P.K.; Park, S.H.; Song, M.G.; Park, S.Y. Antimicrobial efficacy of quercetin against Vibrio parahaemolyticus biofilm on food surfaces and downregulation of virulence genes. Polymers 2022, 14, 3847. [Google Scholar] [CrossRef]
- Roy, P.K.; Song, M.G.; Jeon, E.B.; Kim, S.H.; Park, S.Y. antibiofilm efficacy of quercetin against Vibrio parahaemolyticus biofilm on food-contact surfaces in the food industry. Microorganisms 2022, 10, 1902. [Google Scholar] [CrossRef]
- Galie, S.; Garcia-Gutierrez, C.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Baptista, R.C.; Horita, C.N.; Sant’Ana, A.S. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: A review. Food Res. Int. 2020, 127, 108762. [Google Scholar] [CrossRef]
- Upadhyay, A.; Upadhyaya, I.; Kollanoor-Johny, A.; Venkitanarayanan, K. Combating pathogenic microorganisms using plant-derived antimicrobials: A minireview of the mechanistic basis. Biomed. Res. Int. 2014, 2014, 761741. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Hossain, M.I.; Roy, P.K. Advances in plant-based green synthesis of nanoparticles. J. Multidiscip. Sci. 2022, 4, 17–27. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Song, M.G.; Park, S.Y. The inhibitory effect of quercetin on biofilm formation of Listeria monocytogenes mixed culture and repression of virulence. Antioxidants 2022, 11, 1733. [Google Scholar] [CrossRef]
- Upadhyay, A.; Johny, A.K.; Amalaradjou, M.A.R.; Baskaran, S.A.; Kim, K.S.; Venkitanarayanan, K. Plant-derived antimicrobials reduce Listeria monocytogenes virulence factors in vitro, and down-regulate expression of virulence genes. Int. J. Food Microbiol. 2012, 157, 88–94. [Google Scholar] [CrossRef]
- Borges, A.; Saavedra, M.J.; Simoes, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef]
- Quecan, B.X.V.; Santos, J.T.C.; Rivera, M.L.C.; Hassimotto, N.M.A.; Almeida, F.A.; Pinto, U.M. Effect of quercetin rich onion extracts on bacterial quorum sensing. Front. Microbiol. 2019, 10, 867. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Domingues, F. The antimicrobial action of resveratrol against Listeria monocytogenes in food-based models and its antibiofilm properties. J. Sci. Food Agric. 2016, 96, 4531–4535. [Google Scholar] [CrossRef]
- Hegazy, W.A.H.; Salem, I.M.; Alotaibi, H.F.; Khafagy, E.; Ibrahim, D. Terazosin interferes with quorum sensing and type three secretion system and diminishes the bacterial espionage to mitigate the Salmonella Typhimurium pathogenesis. Antibiotics 2022, 11, 465. [Google Scholar] [CrossRef]
- Khayyat, A.N.; Hegazy, W.A.H.; Shaldam, M.A.; Mosbah, R.; Almalki, A.J.; Ibrahim, T.S.; Khayat, M.T.; Khafagy, E.S.; Soliman, W.E.; Abbas, H.A. Xylitol inhibits growth and blocks virulence in serratia marcescens. Microorganisms 2021, 9, 1083. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial activity of quercetin: An approach to its mechanistic principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- Kim, Y.K.; Roy, P.K.; Ashrafudoulla, M.; Nahar, S.; Toushik, S.H.; Hossain, M.I.; Mizan, M.F.R.; Park, S.H.; Ha, S.D. Antibiofilm effects of quercetin against Salmonella enterica biofilm formation and virulence, stress response, and quorum-sensing gene expression. Food Control 2022, 137, 108964. [Google Scholar] [CrossRef]
- He, Z.Y.; Zhang, X.; Song, Z.C.; Li, L.; Chang, H.S.; Li, S.L.; Zhou, W. Quercetin inhibits virulence properties of Porphyromas gingivalis in periodontal disease. Sci. Rep. 2020, 10, 18313. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.Q.; Zeng, H.; Chen, W. Quercetin inhibits biofilm formation by decreasing the production of EPS and altering the composition of eps in Staphylococcus epidermidis. Front. Microbiol. 2021, 12, 631058. [Google Scholar] [CrossRef]
- Park, Y.; Lee, J. Thermal properties of poly(lactic acid) film containing antibacterial quercetin. Polym. Korea 2022, 46, 223–228. [Google Scholar] [CrossRef]
- Diao, Y.J.; Yu, X.Q.; Zhang, C.H.; Jing, Y.J. Quercetin-grafted chitosan prepared by free radical grafting: Characterization and evaluation of antioxidant and antibacterial properties. J. Food Sci. Technol. 2020, 57, 2259–2268. [Google Scholar] [CrossRef]
- Ong, K.S.; Mawang, C.I.; Daniel-Jambun, D.; Lim, Y.Y.; Lee, S.M. Current anti-biofilm strategies and potential of antioxidants in biofilm control. Expert Rev. Anti-Infect. 2018, 16, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.U.; Khurram, M.; Khattak, B.; Khan, J. Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus. BMC Complement. Altern. Med. 2015, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.N.; Yao, J.Y.; Zhou, B.; Yang, J.X.; Chaudry, M.T.; Wang, M.; Xiao, F.L.; Li, Y.; Yin, W.Z. Bacteriostatic effect of quercetin as an antibiotic alternative In Vivo and its antibacterial mechanism In Vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Sun, F.; Feng, W.; Sun, Y.; Qiu, X.; Xiong, L.; Liu, Y.; Chen, Y. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J. Appl. Microbiol. 2016, 120, 966–974. [Google Scholar] [CrossRef]
- Vazquez-Armenta, F.J.; Bernal-Mercado, A.T.; Tapia-Rodriguez, M.R.; Gonzalez-Aguilar, G.A.; Lopez-Zavala, A.A.; Martinez-Tellez, M.A.; Hernandez-Onate, M.A.; Ayala-Zavala, J.F. Quercetin reduces adhesion and inhibits biofilm development by Listeria monocytogenes by reducing the amount of extracellular proteins. Food Control 2018, 90, 266–273. [Google Scholar] [CrossRef]
- Roy, P.K.; Song, M.G.; Park, S.Y. Impact of quercetin against Salmonella Typhimurium biofilm formation on food-contact surfaces and molecular mechanism pattern. Foods 2022, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Ha, A.J.W.; Mizan, M.F.R.; Hossain, M.I.; Ashrafudoulla, M.; Toushik, S.H.; Nahar, S.; Kim, Y.K.; Ha, S.D. Effects of environmental conditions (temperature, pH, and glucose) on biofilm formation of Salmonella enterica serotype Kentucky and virulence gene expression. Poult. Sci. 2021, 100, 101209. [Google Scholar] [CrossRef]
- Roy, P.K.; Mizan, M.F.R.; Hossain, M.I.; Han, N.; Nahar, S.; Ashrafudoulla, M.; Toushik, S.H.; Shim, W.B.; Kim, Y.M.; Ha, S.D. Elimination of Vibrio parahaemolyticus biofilms on crab and shrimp surfaces using ultraviolet C irradiation coupled with sodium hypochlorite and slightly acidic electrolyzed water. Food Control 2021, 128, 108179. [Google Scholar] [CrossRef]
- Cho, J.; Kim, G.; Qamar, A.Y.; Fang, X.; Roy, P.K.; Tanga, B.M.; Bang, S.; Kim, J.K.; Galli, C.; Perota, A.; et al. Improved efficiencies in the generation of multigene-modified pigs by recloning and using sows as the recipient. Zygote 2022, 30, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Qamar, A.Y.; Fang, X.; Hassan, B.M.S.; Cho, J. Effects of cobalamin on meiotic resumption and developmental competence of growing porcine oocytes. Theriogenology 2020, 154, 24–30. [Google Scholar] [CrossRef]
- Gopu, V.; Meena, C.K.; Shetty, P.H. Quercetin Influences Quorum Sensing in Food Borne Bacteria: In-Vitro and In-Silico Evidence. PLoS ONE 2015, 10, e0134684. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Na, K.W.; Hossain, M.I.; Mizan, M.F.R.; Nahar, S.; Toushik, S.H.; Roy, P.K.; Park, S.H.; Ha, S.D. Molecular and pathogenic characterization of Vibrio parahaemolyticus isolated from seafood. Mar. Pollut. Bull. 2021, 172, 112927. [Google Scholar] [CrossRef]
- Gambino, M.; Cappitelli, F. Mini-review: Biofilm responses to oxidative stress. Biofouling 2016, 32, 167–178. [Google Scholar] [CrossRef]
- Sreelatha, S.; Jayachitra, A. Targeting biofilm inhibition using Quercetin—Interaction with bacterial cell membrane and ROS mediated biofilm control. Funct. Foods Health Dis. 2018, 8, 292–306. [Google Scholar]



| Primer Name | Primer Sequences (5′-3′) | Amplicon Size (bp) | Accession Number |
|---|---|---|---|
| 16S rRNA | F: GGAGCATGTGGTTTAATTCG R: CCAACTAAATGCTGGCAACT | 199 | CP016470.1 |
| agrA | F: ATGAAGCAAGCGGAAGAAC R: ACGACCTGTGACAACGATAAA | 239 | CP076669.1 |
| prfA | F: CAATGGGATCCACAAGAATA R: AGCCTGCTCGCTAATGACTT | 186 | CP093220.1 |
| fbp | F: GCCTGGTCTAAACTGGATTT R: CGCCATAAAGAGCGATACTT | 189 | CP090057.1 |
| flaA | F: TGGTTCTACAGTTGCTGGTT R: TTTAGTTGCGATGGATTGGT | 184 | CP087264.1 |
| hlyA | F: GCAATTTCGAGCCTAACCTA R: ACTGCGTTGTTAACGTTTGA | 188 | CP093220.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, P.K.; Kim, S.H.; Jeon, E.B.; Park, E.H.; Park, S.Y. Inhibition of Listeria monocytogenes Cocktail Culture Biofilms on Crab and Shrimp Coupons and the Expression of Biofilm-Related Genes. Antibiotics 2023, 12, 1008. https://doi.org/10.3390/antibiotics12061008
Roy PK, Kim SH, Jeon EB, Park EH, Park SY. Inhibition of Listeria monocytogenes Cocktail Culture Biofilms on Crab and Shrimp Coupons and the Expression of Biofilm-Related Genes. Antibiotics. 2023; 12(6):1008. https://doi.org/10.3390/antibiotics12061008
Chicago/Turabian StyleRoy, Pantu Kumar, So Hee Kim, Eun Bi Jeon, Eun Hee Park, and Shin Young Park. 2023. "Inhibition of Listeria monocytogenes Cocktail Culture Biofilms on Crab and Shrimp Coupons and the Expression of Biofilm-Related Genes" Antibiotics 12, no. 6: 1008. https://doi.org/10.3390/antibiotics12061008
APA StyleRoy, P. K., Kim, S. H., Jeon, E. B., Park, E. H., & Park, S. Y. (2023). Inhibition of Listeria monocytogenes Cocktail Culture Biofilms on Crab and Shrimp Coupons and the Expression of Biofilm-Related Genes. Antibiotics, 12(6), 1008. https://doi.org/10.3390/antibiotics12061008








