11-Year Trend in Antibiotic Consumption in a South-Eastern European Country; the Situation in Albania and the Implications for the Future
Abstract
1. Introduction
2. Results
2.1. Utilization Patterns
2.2. Potential Influencing Factors
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Bank Group. Pulling Together to Beat Superbugs Knowledge and Implementation Gaps in Addressing Antimicrobial Resistance. 2019. Available online: https://openknowledge.worldbank.org/bitstream/handle/10986/32552/Pulling-Together-to-Beat-Superbugs-Knowledge-and-Implementation-Gaps-in-Addressing-Antimicrobial-Resistance.pdf?sequence=1&isAllowed=y (accessed on 28 April 2023).
- OECD Health Policy Studies. Stemming the Superbug Tide. 2018. Available online: https://www.oecd-ilibrary.org/sites/9789264307599-en/index.html?itemId=/content/publication/9789264307599-en&mimeType=text/html (accessed on 26 April 2023).
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations—The Review on Antimicrobial Resistance. 2016. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 26 April 2023).
- Hofer, U. The cost of antimicrobial resistance. Nat. Rev. Microbiol. 2019, 17, 3. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Michele Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Gautam, A. Antimicrobial Resistance: The Next Probable Pandemic. JNMA J. Nepal Med. Assoc. 2022, 60, 225–228. [Google Scholar] [CrossRef]
- Charani, E.; Mendelson, M.; Pallett, S.J.C.; Ahmad, R.; Mpundu, M.; Mbamalu, O.; Bonaconsa, C.; Nampoothiri, V.; Singh, S.; Peiffer-Smadja, N.; et al. An analysis of existing national action plans for antimicrobial resistance-gaps and opportunities in strategies optimising antibiotic use in human populations. Lancet Glob. Health 2023, 11, e466–e474. [Google Scholar] [CrossRef]
- Godman, B.; Egwuenu, A.; Wesangula, E.; Schellack, N.; Kalungia, A.C.; Tiroyakgosi, C.; Kgatlwane, J.; Mwita, J.C.; Patrick, O.; Niba, L.L.; et al. Tackling antimicrobial resistance across sub-Saharan Africa: Current challenges and implications for the future. Expert Opin. Drug Saf. 2022, 21, 1089–1111. [Google Scholar] [CrossRef]
- Nabadda, S.; Kakooza, F.; Kiggundu, R.; Walwema, R.; Bazira, J.; Mayito, J.; Mugerwa, I.; Sekamatte, M.; Kambugu, A.; Lamorde, M.; et al. Implementation of the World Health Organization Global Antimicrobial Resistance Surveillance System in Uganda, 2015–2020: Mixed-Methods Study Using National Surveillance Data. JMIR Public Health Surveill. 2021, 7, e29954. [Google Scholar] [CrossRef]
- WHO Implementation Handbook for National Action Plans on Antimicrobial Resistance: Guidance for the Human Health Sector. 2022. Available online: https://www.who.int/publications/i/item/9789240041981 (accessed on 5 April 2023).
- WHO. Global Action Plan on Antimicrobial Resistance—Report by the Secretariat. 2016. Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA69/A69_24-en.pdf (accessed on 5 April 2023).
- Sharland, M.; Pulcini, C.; Harbarth, S.; Zeng, M.; Gandra, S.; Mathur, S.; Magrini, N. Classifying antibiotics in the WHO Essential Medicines List for optimal use-be AWaRe. Lancet Infect. Dis. 2018, 18, 18–20. [Google Scholar] [CrossRef]
- Sharland, M.; Gandra, S.; Huttner, B.; Moja, L.; Pulcini, C.; Zeng, M.; Mendelson, M.; Cappello, B.; Cooke, G.; Magrini, N.; et al. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use-the new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect. Dis. 2019, 19, 1278–1280. [Google Scholar] [CrossRef]
- WHO. The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book. 2022. Available online: https://www.who.int/publications/i/item/9789240062382 (accessed on 5 April 2023).
- Sharland, M.; Zanichelli, V.; Ombajo, L.A.; Bazira, J.; Cappello, B.; Chitatanga, R.; Chuki, P.; Gandra, S.; Getahun, H.; Harbarth, S.; et al. The WHO essential medicines list AWaRe book: From a list to a quality improvement system. Clin. Microbiol. Infect. 2022, 28, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- Sulis, G.; Sayood, S.; Katukoori, S.; Bollam, N.; George, I.; Yaeger, L.H.; Chavez, M.A.; Tetteh, E.; Yarrabelli, S.; Pulcini, C.; et al. Exposure to World Health Organization’s AWaRe antibiotics and isolation of multidrug resistant bacteria: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 1193–1202. [Google Scholar] [CrossRef]
- Klein, E.Y.; Milkowska-Shibata, M.; Tseng, K.K.; Sharland, M.; Gandra, S.; Pulcini, C.; Laxminarayan, R. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–2015: An analysis of pharmaceutical sales data. Lancet Infect. Dis. 2021, 21, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Fürst, J.; Čižman, M.; Mrak, J.; Kos, D.; Campbell, S.; Coenen, S.; Gustafsson, L.L.; Fürst, L.; Godman, B. The influence of a sustained multifaceted approach to improve antibiotic prescribing in Slovenia during the past decade: Findings and implications. Expert Rev. Anti Infect. Ther. 2015, 13, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Bojanić, L.; Marković-Peković, V.; Škrbić, R.; Stojaković, N.; Ðermanović, M.; Bojanić, J.; Fürst, J.; Kurdi, A.; Godman, B. Recent Initiatives in the Republic of Srpska to Enhance Appropriate Use of Antibiotics in Ambulatory Care; Their Influence and Implications. Front. Pharmacol. 2018, 9, 442. [Google Scholar] [CrossRef]
- Abilova, V.; Kurdi, A.; Godman, B. Ongoing initiatives in Azerbaijan to improve the use of antibiotics; findings and implications. Expert Rev. Anti Infect. Ther. 2018, 16, 77–84. [Google Scholar] [CrossRef]
- Robertson, J.; Iwamoto, K.; Hoxha, I.; Ghazaryan, L.; Abilova, V.; Cvijanovic, A.; Pyshnik, H.; Darakhvelidze, M.; Makalkina, L.; Jakupi, A.; et al. Antimicrobial Medicines Consumption in Eastern Europeand Central Asia—An Updated Cross-National Study and Assessment of Quantitative Metrics for Policy Action. Front. Pharmacol. 2018, 9, 1156. [Google Scholar] [CrossRef]
- EVIPNet Europe. Tackling Antimicrobial Resistance in Primary Health Care through Promoting the Appropriate Use of Antibiotics in Estonia. 2022. Available online: https://apps.who.int/iris/bitstream/handle/10665/351528/WHO-EURO-2022-4779-44542-63078-eng.pdf (accessed on 8 April 2023).
- Ministry of Health Republic of Latvia. Latvia’s Experience on AMR Policy Development. Meeting of the subgroup of the AMR One Health Network on AMR NAPs 31 May 2022–01 June 2022. Available online: https://health.ec.europa.eu/system/files/2022-06/amr_20220531_co10_en.pdf (accessed on 15 April 2023).
- Tesar, T.; Masarykova, L.; Lehocka, L.; Porubcova, S.; Cicova, M.; Wawruch, M. Consumption of Antibacterials for Systemic Use in Slovakia: A National Study and the Quality Indicators for Outpatient Antibiotic Use. Antibiotics 2021, 10, 1180. [Google Scholar] [CrossRef]
- Wojkowska-Mach, J.; Godman, B.; Glassman, A.; Kurdi, A.; Pilc, A.; Rozanska, A.; Skoczyński, S.; Wałaszek, M.; Bochenek, T. Antibiotic consumption and antimicrobial resistance in Poland; findings and implications. Antimicrob. Resist. Infect. Control 2018, 7, 136. [Google Scholar] [CrossRef]
- Rolfe, R., Jr.; Kwobah, C.; Muro, F.; Ruwanpathirana, A.; Lyamuya, F.; Bodinayake, C.; Nagahawatte, A.; Piyasiri, B.; Sheng, T.; Bollinger, J.; et al. Barriers to implementing antimicrobial stewardship programs in three low- and middle-income country tertiary care settings: Findings from a multi-site qualitative study. Antimicrob. Resist. Infect. Control 2021, 10, 60. [Google Scholar] [CrossRef]
- Bosley, H.; Henshall, C.; Appleton, J.V.; Jackson, D. A systematic review to explore influences on parental attitudes towards antibiotic prescribing in children. J. Clin. Nurs. 2018, 27, 892–905. [Google Scholar] [CrossRef]
- Kenyon, C.; Manoharan-Basil, S.S. Cultural Drivers of Antibiotic Consumption in High-Income Countries: A Global Ecological Analysis. Microb. Drug Resist. 2020, 26, 1063–1070. [Google Scholar] [CrossRef]
- Borg, M.A.; Camilleri, L. Broad-spectrum antibiotic use in Europe: More evidence of cultural influences on prescribing behaviour. J. Antimicrob. Chemother. 2019, 74, 3379–3383. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Puglisi, F.; Agodi, A. Socio-economic, governance and health indicators shaping antimicrobial resistance: An ecological analysis of 30 european countries. Glob. Health 2023, 19, 12. [Google Scholar] [CrossRef]
- Mueller, T.; Östergren, P.O. The correlation between regulatory conditions and antibiotic consumption within the WHO European Region. Health Policy 2016, 120, 882–889. [Google Scholar] [CrossRef]
- Collignon, P.; Athukorala, P.C.; Senanayake, S.; Khan, F. Antimicrobial resistance: The major contribution of poor governance and corruption to this growing problem. PLoS ONE 2015, 10, e0116746. [Google Scholar] [CrossRef]
- Rönnerstrand, B.; Lapuente, V. Corruption and use of antibiotics in regions of Europe. Health Policy 2017, 121, 250–256. [Google Scholar] [CrossRef]
- INSTAT. Annual Average of Total Population by Age_Group, Gender, Type and Year—Albania. 2023. Available online: http://databaza.instat.gov.al/pxweb/en/DST/START__DE/NewPOP_0001/table/tableViewLayout1/ (accessed on 4 April 2023).
- Transparency International. Corruption Perceptions Index. Albania. 2022. Available online: https://www.transparency.org/en/cpi/2022/index/alb (accessed on 4 April 2023).
- Hoxha, I.; Malaj, A.; Kraja, B.; Bino, S.; Oluka, M.; Marković-Peković, V.; Godman, B. Are pharmacists’ good knowledge and awareness on antibiotics taken for granted? The situation in Albania and future implications across countries. J. Glob. Antimicrob. Resist. 2018, 13, 240–245. [Google Scholar] [CrossRef]
- Kleva, S.; Elona, K.; Edit, X.; Anis, T.; Neada, H.; Suida, K. Approach to the Current Rational Use of Antibiotics among the Albanian Dentist Community. J. Pharm. Bioallied Sci. 2022, 14, 106–113. [Google Scholar] [CrossRef]
- WHO. Joint External Evaluation of IHR Core Capacities of the Republic of Albania—Mission Report: September 2016. Available online: https://www.who.int/countries/alb/ (accessed on 4 April 2023).
- Hoxha, I.; Malaj, A.; Tako, R.; Malaj, L. Survey on how antibiotics are dispensed in community pharmacies in Albania. Int. J. Pharm. Pharm. Sci. 2015, 7, 449–450. [Google Scholar]
- Kaae, S.; Malaj, A.; Hoxha, I. Antibiotic knowledge, attitudes and behaviours of Albanian health care professionals and patients—A qualitative interview study. J. Pharm. Policy Pract. 2017, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Marković-Peković, V.; Grubiša, N.; Burger, J.; Bojanić, L.; Godman, B. Initiatives to Reduce Nonprescription Sales and Dispensing of Antibiotics: Findings and Implications. J. Res. Pharm. Pract. 2017, 6, 120–125. [Google Scholar] [PubMed]
- Nika, D.; Zarb, P. Antimicrobial consumption in Albanian reference teaching hospital (2012–2015). J. Infect. Dev. Ctries. 2017, 11, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Vlahović-Palčevski, V.; Iwamoto, K.; Högberg, L.D.; Godman, B.; Monnet, D.L.; Garner, S.; Weist, K.; ESAC-Net Study Group; WHO Europe AMC Network Study Group. Variations in the Consumption of Antimicrobial Medicines in the European Region, 2014–2018: Findings and Implications from ESAC-Net and WHO Europe. Front. Pharmacol. 2021, 12, 639207. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, I.; Malaj, A.; Malaj, L. Antibiotic use in Albania between 2011 and 2012. J. Infect. Dev. Ctries. 2015, 9, 94–98. [Google Scholar] [CrossRef] [PubMed]
- WHO. Albania: National Action Plan against Antimicrobial Resistance 2017. 25 November 2022. Available online: https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/nap-library/albania-amr-nap-2017.pdf?sfvrsn=8ba07901_3&download=true (accessed on 6 April 2023).
- Bednarčuk, N.; Golić Jelić, A.; Stoisavljević Šatara, S.; Stojaković, N.; Marković Peković, V.; Stojiljković, M.P.; Popović, N.; Škrbić, R. Antibiotic Utilization during COVID-19: Are We Over-Prescribing? Antibiotics 2023, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hasan, S.S.; Bond, S.E.; Conway, B.R.; Aldeyab, M.A. Antimicrobial consumption in patients with COVID-19: A systematic review and meta-analysis. Expert Rev. Anti Infect. Ther. 2022, 20, 749–772. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, F.S.; Godman, B.; Sindi, O.N.; Seaton, R.A.; Kurdi, A. Prevalence of bacterial coinfection and patterns of antibiotics prescribing in patients with COVID-19: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0272375. [Google Scholar] [CrossRef]
- Nandi, A.; Pecetta, S.; Bloom, D.E. Global antibiotic use during the COVID-19 pandemic: Analysis of pharmaceutical sales data from 71 countries, 2020–2022. eClinicalMedicine 2023, 57, 101848. [Google Scholar] [CrossRef]
- Sulis, G.; Batomen, B.; Kotwani, A.; Pai, M.; Gandra, S. Sales of antibiotics and hydroxychloroquine in India during the COVID-19 epidemic: An interrupted time series analysis. PLoS Med 2021, 18, e1003682. [Google Scholar] [CrossRef]
- World Bank. GDP per Capita (US$). 2021. Available online: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD (accessed on 7 April 2023).
- Zanichelli, V.; Sharland, M.; Cappello, B.; Moja, L.; Getahun, H.; Pessoa-Silva, C.; Sati, H.; van Weezenbeek, C.; Balkhy, H.; Simão, M.; et al. The WHO AWaRe (Access, Watch, Reserve) antibiotic book and prevention of antimicrobial resistance. Bull. World Health Organ. 2023, 101, 290–296. [Google Scholar] [CrossRef]
- Godman, B.; Haque, M.; McKimm, J.; Abu Bakar, M.; Sneddon, J.; Wale, J.; Campbell, S.; Martin, A.P.; Hoxha, I.; Abilova, V.; et al. Ongoing strategies to improve the management of upper respiratory tract infections and reduce inappropriate antibiotic use particularly among lower and middle-income countries: Findings and implications for the future. Curr. Med. Res. Opin. 2020, 36, 301–327. [Google Scholar] [CrossRef]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planet Health 2018, 2, e398–e405. [Google Scholar] [CrossRef]
- Mwita, J.C.; Ogunleye, O.O.; Olalekan, A.; Kalungia, A.C.; Kurdi, A.; Saleem, Z.; Sneddon, J.; Godman, B. Key Issues Surrounding Appropriate Antibiotic Use for Prevention of Surgical Site Infections in Low- and Middle-Income Countries: A Narrative Review and the Implications. Int. J. Gen. Med. 2021, 14, 515–530. [Google Scholar] [CrossRef]
- Saleem, Z.; Ahsan, U.; Haseeb, A.; Altaf, U.; Batool, N.; Rani, H.; Jaffer, J.; Shahid, F.; Hussain, M.; Amir, A.; et al. Antibiotic Utilization Patterns for Different Wound Types among Surgical Patients: Findings and Implications. Antibiotics 2023, 12, 678. [Google Scholar] [CrossRef]
- Cox, J.A.; Vlieghe, E.; Mendelson, M.; Wertheim, H.; Ndegwa, L.; Villegas, M.V.; Gould, I.; Hara, G.L. Antibiotic stewardship in low- and middle-income countries: The same but different? Clin. Microbiol. Infect. 2017, 23, 812–818. [Google Scholar] [CrossRef]
- GolAli, E.; Sistanizad, M.; Salamzadeh, J.; Haghighi, M.; Solooki, M. Antibiotic Prescribing Trends Before and After Implementation of an Audit and Feedback Program in Internal Ward of a Tertiary Hospital in Tehran. Iran J. Pharm. Res. 2019, 18, 2136–2143. [Google Scholar]
- Dellsperger, S.; Kramer, S.; Stoller, M.; Burger, A.; Geissbühler, E.; Amsler, I.; Hirsig, A.; Weyer, L.; Hebeisen, U.; Aebi, P.; et al. Early Switch from Intravenous to Oral Antibiotics in Skin and Soft Tissue Infections: An Algorithm-Based Prospective Multicenter Pilot Trial. Open Forum Infect. Dis. 2022, 9, ofac197. [Google Scholar] [CrossRef]
- Mustafa, Z.U.; Khan, A.H.; Harun, S.N.; Salman, M.; Godman, B. Antibiotic Overprescribing among Neonates and Children Hospitalized with COVID-19 in Pakistan and the Implications. Antibiotics 2023, 12, 646. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Mukokinya, M.M.A.; Opanga, S.; Oluka, M.; Godman, B. Dispensing of Antimicrobials in Kenya: A Cross-sectional Pilot Study and Its Implications. J. Res. Pharm. Pract. 2018, 7, 77–82. [Google Scholar] [PubMed]
- Opanga, S.R.N.; Wamaitha, A.; Abebrese Sefah, I.; Godman, B.B. Availability of Medicines in Community Pharmacy to Manage Patients with COVID-19 in Kenya; Pilot Study and Implications. Sch. Acad. J. Pharm. 2021, 3, 36–42. [Google Scholar] [CrossRef]
- Kibuule, D.N.L.; Sefah, I.A.; Kurdi, A.; Phuong, T.N.T.; Kwon, H.-Y.; Godman, B. Activities in Namibia to limit the prevalence and mortality from COVID-19 including community pharmacy activities and the implications. Sch. Acad. J. Pharm. 2021, 5, 82–92. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe Antimicrobial Medicines Consumption (AMC) Network. AMC Data 2019. Copenhagen: WHO Regional Office for Europe. 2022. Available online: https://apps.who.int/iris/bitstream/handle/10665/363394/9789289058278-eng.pdf?sequence=1&isAllowed=y (accessed on 7 April 2023).
- Versporten, A.; Bolokhovets, G.; Ghazaryan, L.; Abilova, V.; Pyshnik, G.; Spasojevic, T.; Korinteli, I.; Raka, L.; Kambaralieva, B.; Cizmovic, L.; et al. Antibiotic use in eastern Europe: A cross-national database study in coordination with the WHO Regional Office for Europe. Lancet Infect. Dis. 2014, 14, 381–387. [Google Scholar] [CrossRef]
- WHO. Anatomical Therapeutic Chemical (ATC) Classification. 2021. Available online: https://www.who.int/tools/atc-ddd-toolkit/atc-classification (accessed on 25 March 2023).
- Tang, Y.; Liu, C.; Zhang, Z.; Zhang, X. Effects of prescription restrictive interventions on antibiotic procurement in primary care settings: A controlled interrupted time series study in China. Cost Eff. Resour. Alloc. 2018, 16, 1. [Google Scholar] [CrossRef]
- Hider-Mlynarz, K.; Cavalié, P.; Maison, P. Trends in analgesic consumption in France over the last 10 years and comparison of patterns across Europe. Br. J. Clin. Pharmacol. 2018, 84, 1324–1334. [Google Scholar] [CrossRef]
- Godman, B.; Egwuenu, A.; Haque, M.; Malande, O.O.; Schellack, N.; Kumar, S.; Saleem, Z.; Sneddon, J.; Hoxha, I.; Islam, S.; et al. Strategies to Improve Antimicrobial Utilization with a Special Focus on Developing Countries. Life 2021, 11, 528. [Google Scholar] [CrossRef]
- Oppong, R.; Smith, R.D.; Little, P.; Verheij, T.; Butler, C.C.; Goossens, H.; Coenen, S.; Jowett, S.; Roberts, T.E.; Achana, F.; et al. Cost-effectiveness of internet-based training for primary care clinicians on antibiotic prescribing for acute respiratory tract infections in Europe. J. Antimicrob. Chemother. 2018, 73, 3189–3198. [Google Scholar] [CrossRef]
- Oppong, R.; Smith, R.D.; Little, P.; Verheij, T.; Butler, C.C.; Goossens, H.; Coenen, S.; Moore, M.; Coast, J. Cost effectiveness of amoxicillin for lower respiratory tract infections in primary care: An economic evaluation accounting for the cost of antimicrobial resistance. Br. J. Gen. Pract. 2016, 66, e633–e639. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Smit, C.C.H.; De Vos, S.; Benko, R.; Llor, C.; Paget, W.J.; Briant, K.; Pont, L.; Van Dijk, L.; Taxis, K.; et al. A systematic literature review and meta-analysis of community pharmacist-led interventions to optimise the use of antibiotics. Br. J. Clin. Pharmacol. 2022, 88, 2617–2641. [Google Scholar] [CrossRef]
- Parveen, S.; Garzon-Orjuela, N.; Amin, D.; McHugh, P.; Vellinga, A. Public Health Interventions to Improve Antimicrobial Resistance Awareness and Behavioural Change Associated with Antimicrobial Use: A Systematic Review Exploring the Use of Social Media. Antibiotics 2022, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- Allegranzi, B.; Aiken, A.M.; Zeynep Kubilay, N.; Nthumba, P.; Barasa, J.; Okumu, G.; Mugarura, R.; Elobu, A.; Jombwe, J.; Maimbo, M.; et al. A multimodal infection control and patient safety intervention to reduce surgical site infections in Africa: A multicentre, before-after, cohort study. Lancet Infect. Dis. 2018, 18, 507–515. [Google Scholar] [CrossRef]
- Saied, T.; Hafez, S.F.; Kandeel, A.; El-kholy, A.; Ismail, G.; Aboushady, M.; Attia, E.; Hassaan, A.; Abdel-Atty, O.; Elfekky, E.; et al. Antimicrobial stewardship to optimize the use of antimicrobials for surgical prophylaxis in Egypt: A multicenter pilot intervention study. Am. J. Infect. Contro. 2015, 43, e67–e71. [Google Scholar] [CrossRef]
- Shankar, R. Implementation of the WHO Surgical Safety Checklist at a teaching hospital in India and evaluation of the effects on perioperative complications. Int. J. Health Plan. Manag. 2018, 33, 836–846. [Google Scholar] [CrossRef]
- Mahmoudi, L.; Ghouchani, M.; Mahi-Birjand, M.; Bananzadeh, A.; Akbari, A. Optimizing compliance with surgical antimicrobial prophylaxis guidelines in patients undergoing gastrointestinal surgery at a referral teaching hospital in southern Iran: Clinical and economic impact. Infect. Drug Resist. 2019, 12, 2437–2444. [Google Scholar] [CrossRef]
- Aiken, A.M.; Wanyoro, A.K.; Mwangi, J.; Juma, F.; Mugoya, I.K.; Scott, J.A. Changing use of surgical antibiotic prophylaxis in Thika Hospital, Kenya: A quality improvement intervention with an interrupted time series design. PLoS ONE 2013, 8, e78942. [Google Scholar] [CrossRef]
- Ntumba, P.; Mwangi, C.; Barasa, J.; Aiken, A.; Kubilay, Z.; Allegranzi, B. Multimodal approach for surgical site infection prevention—Results from a pilot site in Kenya. Antimicrob. Resist. Infect. Control. 2015, 4, P87. [Google Scholar] [CrossRef]
- Kim, R.Y.; Kwakye, G.; Kwok, A.C.; Baltaga, R.; Ciobanu, G.; Merry, A.F.; Funk, L.M.; Lipsitz, S.R.; Gawande, A.A.; Berry, W.R.; et al. Sustainability and long-term effectiveness of the WHO surgical safety checklist combined with pulse oximetry in a resource-limited setting: Two-year update from Moldova. JAMA Surg. 2015, 150, 473–479. [Google Scholar] [CrossRef]
- Abubakar, U.; Syed Sulaiman, S.A.; Adesiyun, A.G. Impact of pharmacist-led antibiotic stewardship interventions on compliance with surgical antibiotic prophylaxis in obstetric and gynecologic surgeries in Nigeria. PLoS ONE 2019, 14, e0213395. [Google Scholar] [CrossRef] [PubMed]
- Butt, S.Z.; Ahmad, M.; Saeed, H.; Saleem, Z.; Javaid, Z. Post-surgical antibiotic prophylaxis: Impact of pharmacist’s educational intervention on appropriate use of antibiotics. J. Infect. Public Health 2019, 12, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Brink, A.J.; Messina, A.P.; Feldman, C.; Richards, G.A.; van den Bergh, D. From guidelines to practice: A pharmacist-driven prospective audit and feedback improvement model for peri-operative antibiotic prophylaxis in 34 South African hospitals. J. Antimicrob. Chemother. 2017, 72, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, F.; Kaya, S.; Tekin, R.; Gulsun, S.; Deveci, O.; Dayan, S.; Hoşoglu, S. Analysis of antimicrobial consumption and cost in a teaching hospital. J. Infect. Public Health 2014, 7, 161–169. [Google Scholar] [CrossRef]
- Karaali, C.; Emiroglu, M.; Atalay, S.; Sert, I.; Dursun, A.; Kose, S.; Akbulut, G.; Aydın, C.A. A new antibiotic stewardship program approach is effective on inappropriate surgical prophylaxis and discharge prescription. J. Infect. Dev. Ctries. 2019, 13, 961–967. [Google Scholar] [CrossRef]
- Teng, C.L.; Achike, F.I.; Phua, K.L.; Nurjahan, M.I.; Mastura, I.; Asiah, H.N.; Mariam, A.M.; Narayanan, S.; Norsiah, A.; Sabariah, I.; et al. Modifying antibiotic prescribing: The effectiveness of academic detailing plus information leaflet in a Malaysian primary care setting. Med. J. Malays. 2006, 61, 323–331. [Google Scholar]
- Awad, A.I.; Eltayeb, I.B.; Baraka, O.Z. Changing antibiotics prescribing practices in health centers of Khartoum State, Sudan. Eur. J. Clin. Pharmacol. 2006, 62, 135–142. [Google Scholar] [CrossRef]
- Chowdhury, A.K.; Khan, O.F.; Matin, M.A.; Begum, K.; Galib, M.A. Effect of standard treatment guidelines with or without prescription audit on prescribing for acute respiratory tract infection (ARI) and diarrhoea in some thana health complexes (THCs) of Bangladesh. Bangladesh Med. Res. Counc. Bull. 2007, 33, 21–30. [Google Scholar]
- Kafle, K.K.; Bhuju, G.B.; Karkee, S.B.; Prasad, R.R.; Shrestha, N.; Shrestha, A.D.; Das, P.L.; Chataut, B.D.; Daud, M. An intervention improving prescribing practices and monitoring drugs availability in a district. Nepal Med. Coll. J. 2009, 11, 217–221. [Google Scholar]
- Yip, W.; Powell-Jackson, T.; Chen, W.; Hu, M.; Fe, E.; Hu, M.; Hsiao, W.C. Capitation combined with pay-for-performance improves antibiotic prescribing practices in rural China. Health Aff. 2014, 33, 502–510. [Google Scholar] [CrossRef]
- Boonyasiri, A.; Thamlikitkul, V. Effectiveness of multifaceted interventions on rational use of antibiotics for patients with upper respiratory tract infections and acute diarrhea. J. Med. Assoc. Thail. 2014, 97 (Suppl. 3), S13–S19. [Google Scholar]
- Egger, J.R.; Stankevitz, K.; Korom, R.; Angwenyi, P.; Sullivan, B.; Wang, J.; Hatfield, S.; Smith, E.; Popli, K.; Gross, J. Evaluating the effects of organizational and educational interventions on adherence to clinical practice guidelines in a low-resource primary-care setting in Kenya. Health Policy Plan. 2017, 32, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Korom, R.R.; Onguka, S.; Halestrap, P.; McAlhaney, M.; Adam, M. Brief educational interventions to improve performance on novel quality metrics in ambulatory settings in Kenya: A multi-site pre-post effectiveness trial. PLoS ONE 2017, 12, e0174566. [Google Scholar] [CrossRef] [PubMed]
- Dehn Lunn, A. Reducing inappropriate antibiotic prescribing in upper respiratory tract infection in a primary care setting in Kolkata, India. BMJ Open Qual. 2018, 7, e000217. [Google Scholar] [CrossRef]
- Tay, K.H.; Ariffin, F.; Sim, B.L.; Chin, S.Y.; Sobry, A.C. Multi-Faceted Intervention to Improve the Antibiotic Prescriptions among Doctors for Acute URI and Acute Diarrhoea Cases: The Green Zone Antibiotic Project. Malays. J. Med. Sci. MJMS 2019, 26, 101–109. [Google Scholar] [CrossRef]
- Brinkmann, I.; Kibuule, D. Effectiveness of antibiotic stewardship programmes in primary health care settings in developing countries. Res. Soc. Adm. Pharm. 2020, 16, 1309–1313. [Google Scholar] [CrossRef]
- Chalker, J.; Ratanawijitrasin, S.; Chuc, N.T.; Petzold, M.; Tomson, G. Effectiveness of a multi-component intervention on dispensing practices at private pharmacies in Vietnam and Thailand--a randomized controlled trial. Soc. Sci. Med. 2005, 60, 131–141. [Google Scholar] [CrossRef]
- Nguyen, T.T.P.; Do, T.X.; Nguyen, H.A.; Nguyen, C.T.T.; Meyer, J.C.; Godman, B.; Skosana, P.; Nguyen, B.T. A National Survey of Dispensing Practice and Customer Knowledge on Antibiotic Use in Vietnam and the Implications. Antibiotics 2022, 11, 1091. [Google Scholar] [CrossRef]
- Santa-Ana-Tellez, Y.; Mantel-Teeuwisse, A.K.; Dreser, A.; Leufkens, H.G.; Wirtz, V.J. Impact of over-the-counter restrictions on antibiotic consumption in Brazil and Mexico. PLoS ONE 2013, 8, e75550. [Google Scholar] [CrossRef]
- Santa-Ana-Tellez, Y.; Mantel-Teeuwisse, A.K.; Leufkens, H.G.M.; Wirtz, V.J. Seasonal Variation in Penicillin Use in Mexico and Brazil: Analysis of the Impact of Over-the-Counter Restrictions. Antimicrob. Agents Chemother. 2015, 59, 105–110. [Google Scholar] [CrossRef]
- Arparsrithongsagul, S.; Kulsomboon, V.; Zuckerman, I.H. Multidisciplinary perspective intervention with community involvement to decrease antibiotic sales in village groceries in Thailand. Asia Pac. J. Public Health 2015, 27, Np2480-8. [Google Scholar] [CrossRef] [PubMed]
- Kitutu, F.E.; Kalyango, J.N.; Mayora, C.; Selling, K.E.; Peterson, S.; Wamani, H. Integrated community case management by drug sellers influences appropriate treatment of paediatric febrile illness in South Western Uganda: A quasi-experimental study. Malar. J. 2017, 16, 425. [Google Scholar] [CrossRef] [PubMed]
- Kimathi, G.; Kiarie, J.; Njarambah, L.; Onditi, J.; Ojakaa, D. A cross-sectional study of antimicrobial use among self-medicating COVID-19 cases in Nyeri County, Kenya. Antimicrob. Resist. Infect. Control 2022, 11, 111. [Google Scholar] [CrossRef]
- Muloi, D.; Fèvre, E.M.; Bettridge, J.; Rono, R.; Ong’are, D.; Hassell, J.M.; Karani, M.K.; Muinde, P.; van Bunnik, B.; Street, A.; et al. A cross-sectional survey of practices and knowledge among antibiotic retailers in Nairobi, Kenya. J. Glob. Health 2019, 9, 010412. [Google Scholar] [CrossRef] [PubMed]
- WHO. Kenya National Action Plan on Antimicrobial Resistance: Review of Progress in the Human Health Sector. 2022. Available online: https://www.who.int/publications/i/item/9789240062689 (accessed on 5 April 2023).
- Chang, J.; Xu, S.; Zhu, S.; Li, Z.; Yu, J.; Zhang, Y.; Zu, J.; Fang, Y.; Ross-Degnan, D. Assessment of non-prescription antibiotic dispensing at community pharmacies in China with simulated clients: A mixed cross-sectional and longitudinal study. Lancet Infect. Dis. 2019, 19, 1345–1354. [Google Scholar] [CrossRef]
- Sefah, I.A.; Ogunleye, O.O.; Essah, D.O.; Opanga, S.A.; Butt, N.; Wamaitha, A.; Guantai, A.N.; Chikowe, I.; Khuluza, F.; Kibuule, D.; et al. Rapid Assessment of the Potential Paucity and Price Increases for Suggested Medicines and Protection Equipment for COVID-19 Across Developing Countries with a Particular Focus on Africa and the Implications. Front. Pharmacol. 2020, 11, 588106. [Google Scholar] [CrossRef]
- Kamati, M.; Godman, B.; Kibuule, D. Prevalence of self-medication for acute respiratory infections in young children in Namibia: Findings and implications. J. Res. Pharm. Pract. 2019, 8, 220. [Google Scholar]
- Onwunduba, A.; Ekwunife, O.; Onyilogwu, E. Impact of point-of-care C-reactive protein testing intervention on non-prescription dispensing of antibiotics for respiratory tract infections in private community pharmacies in Nigeria: A cluster randomized controlled trial. Int. J. Infect. Dis. 2023, 127, 137–143. [Google Scholar] [CrossRef]
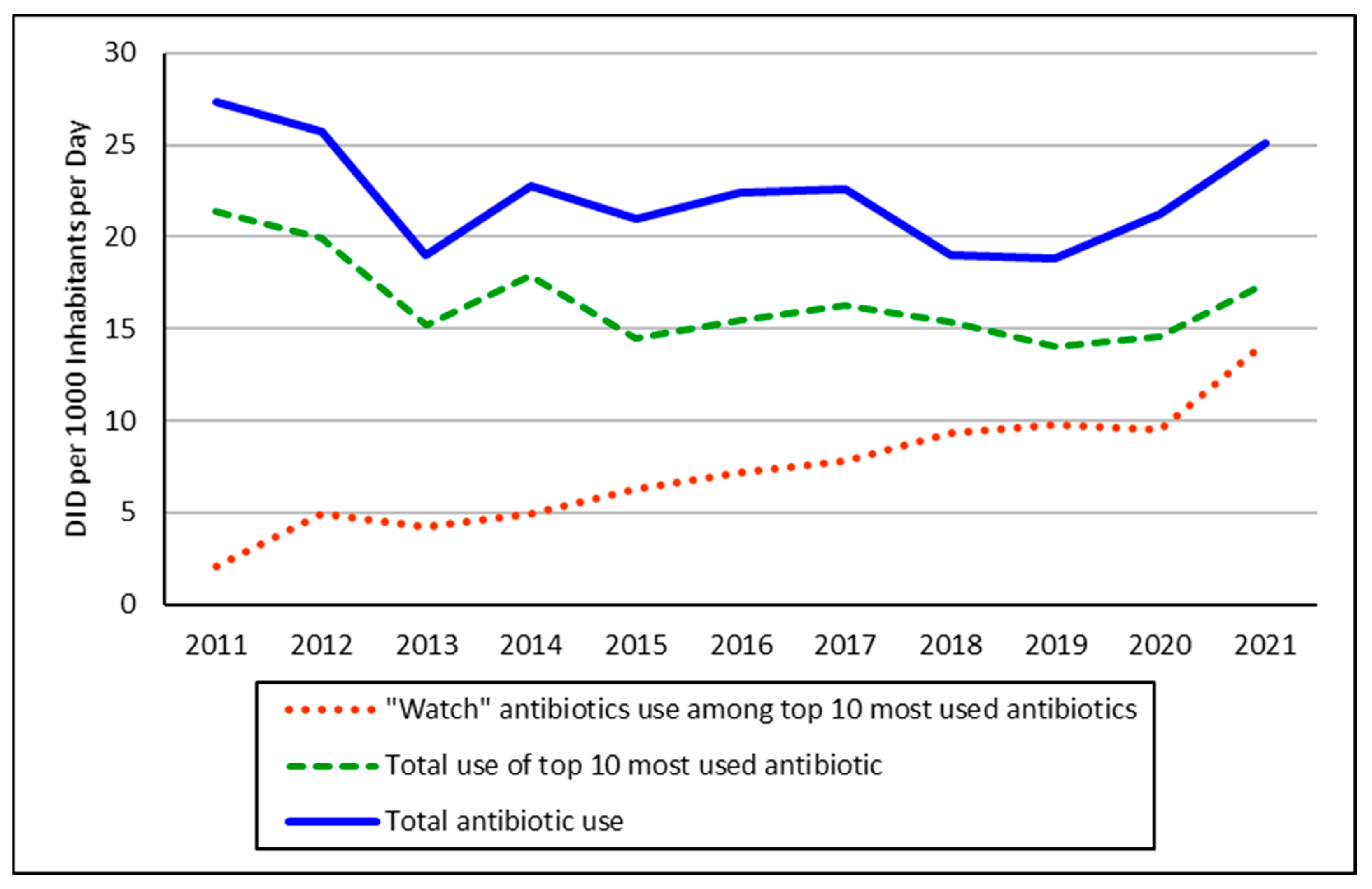
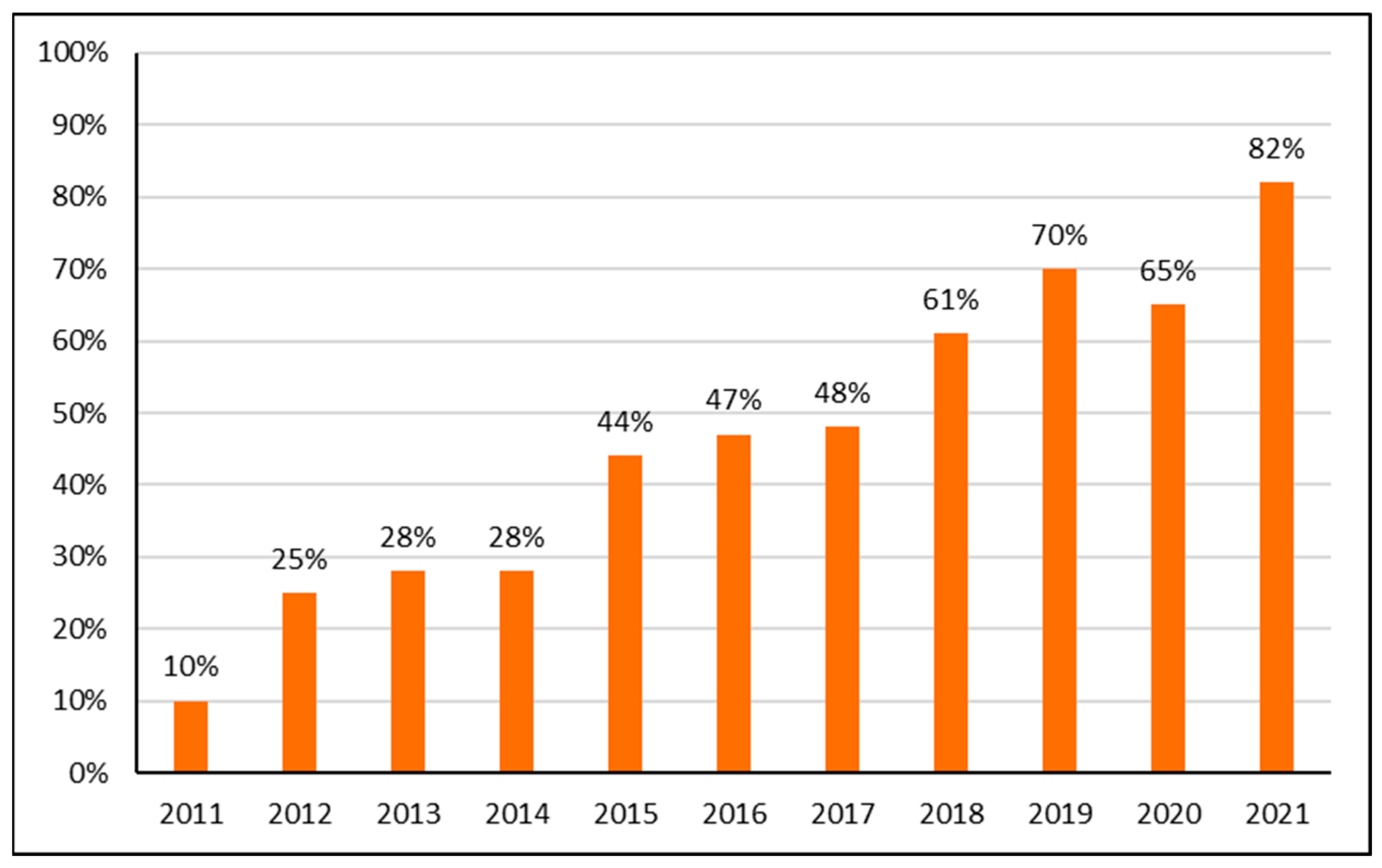

| 2011 | DID | 2012 | DID | 2013 | DID | 2014 | DID | 2015 | DID | 2016 | DID | 2018 | DID | 2018 | DID | 2019 | DID | 2020 | DID | 2021 | DID |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tetracycline | 6.5 | Amoxicillin | 6.0 | Amoxicillin | 3.9 | Amoxicillin | 5.8 | Amoxicillin | 3.1 | Co-amoxiclav | 3.9 | Co-amoxiclav | 3.4 | Co-amoxiclav | 2.6 | Co-amoxiclav | 2.3 | Azithromycin | 3.0 | Azithromycin | 4.3 |
| Amoxicillin | 5.5 | Co-amoxiclav | 3.4 | Co-amoxiclav | 3.4 | Co-amoxiclav | 3.4 | Ciprofloxacin | 2.4 | Cefuroxime | 1.9 | Amoxicillin | 2.8 | Cefuroxime | 1.9 | Cefuroxime | 2.0 | Co-amoxiclav | 2.7 | Levofloxacin | 2.7 |
| Co-amoxiclav | 2.4 | Metronidazole | 3.2 | Ciprofloxacin | 1.7 | Tetracycline | 2.1 | Co-amoxiclav | 2.0 | Amoxicillin | 1.7 | Cefuroxime | 2.2 | Amoxicillin | 1.8 | Azithromycin | 1.9 | Levofloxacin | 1.4 | Co-amoxiclav | 2.1 |
| Ciprofloxacin | 1.3 | Ciprofloxacin | 1.9 | Tetracycline | 1.2 | Ciprofloxacin | 1.7 | Tetracycline | 1.6 | Tetracycline | 1.6 | Ciprofloxacin | 2.0 | Ciprofloxacin | 1.8 | Cefaclor | 1.5 | Cefuroxime | 1.4 | Ciprofloxacin | 1.5 |
| Doxycycline | 1.3 | Moxifloxacin | 1.2 | Doxycycline | 1.2 | Doxycycline | 1.1 | Cefuroxime | 1.3 | Ciprofloxacin | 1.4 | Doxycycline | 1.5 | Tetracycline | 1.6 | Ciprofloxacin | 1.2 | Ceftriaxone | 1.4 | Cefixime | 1.5 |
| Cefazolin | 1.1 | Azithromycin | 0.9 | Azithromycin | 1.0 | Cefuroxime | 1.0 | Azithromycin | 1.3 | Azithromycin | 1.4 | Azithromycin | 1.2 | Cefaclor | 1.5 | Amoxicillin | 1.1 | Amoxicillin | 1.2 | Ceftriaxone | 1.4 |
| Cotrimoxazole | 0.9 | Cefaclor | 0.9 | Cefuroxime | 0.8 | Azithromycin | 1.0 | Cefixime | 0.8 | Clarithromycin | 1.1 | Ceftriaxone | 0.9 | Azithromycin | 1.3 | Levofloxacin | 1.1 | Tetracycline | 1.2 | Cefuroxime | 1.2 |
| Moxifloxacin | 0.9 | Cefalexin | 0.8 | Clarithromycin | 0.7 | Clarithromycin | 0.8 | Doxycycline | 0.8 | Doxycycline | 0.9 | Clarithromycin | 0.8 | Cefixime | 1.0 | Ceftriaxone | 1.1 | Ciprofloxacin | 0.9 | Tetracycline | 1.0 |
| Cefaclor | 0.8 | Cotrimoxazole | 0.8 | Ampicillin | 0.7 | Ampicillin | 0.6 | Clarithromycin | 0.6 | Ceftriaxone | 0.8 | Cefixime | 0.8 | Levofloxacin | 1.0 | Cefixime | 1.0 | Cefixime | 0.8 | Clarithromycin | 0.9 |
| Ampicillin | 0.7 | Doxycycline | 0.7 | Cotrimoxazole | 0.5 | Ceftriaxone | 0.5 | Nitrofurantoin | 0.6 | Levofloxacin | 0.7 | Nitrofurantoin | 0.7 | Ceftriaxone | 0.8 | Doxycycline | 0.8 | Cefaclor | 0.7 | Cefaclor | 0.9 |
| Total | 21.4 | 19.9 | 15.2 | 17.9 | 14.5 | 15.4 | 16.3 | 15.3 | 14.0 | 14.6 | 17.5 | ||||||||||
| Grand Total | 27.4 | 25.7 | 19.0 | 22.7 | 21.0 | 22.4 | 22.6 | 19.0 | 18.8 | 21.2 | 25.1 |
| Year | Antibiotic Utilization (DIDs) * | Median Age (Years) | GDP per Capita (ALL) * | Albania Corruption Index Score * |
|---|---|---|---|---|
| 2011 | 27.40 | 32.60 | 447,689 | 33 |
| 2012 | 25.70 | 33.20 | 459,526 | 31 |
| 2013 | 19.00 | 33.70 | 466,325 | 33 |
| 2014 | 22.70 | 34.20 | 482,954 | 31 |
| 2015 | 21.00 | 34.70 | 497,902 | 33 |
| 2016 | 22.40 | 35.20 | 511,971 | 36 |
| 2017 | 22.60 | 35.60 | 539,645 | 39 |
| 2018 | 19.00 | 36.10 | 571,011 | 38 |
| 2019 | 18.80 | 36.70 | 592,779 | 36 |
| 2020 | 21.20 | 37.20 | 580,521 | 35 |
| 2021 | 25.10 | 37.60 | 660,168 | 36 |
| Years | Antibiotic Utilization (DIDs) VS: | Kendall’s Tau-b Correlation Coefficient | p-Value (2-Tailed) | 95% Confidence Intervals (2-Tailed) a | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| 2011–2020 | Median age (years) | −0.494 * | 0.048 | −0.756 | −0.098 |
| GDP per capita (ALL) | −0.539 * | 0.031 | −0.781 | −0.158 | |
| Albania corruption index score | −0.262 | 0.312 | −0.663 | 0.255 | |
| 2011–2021 | Median age (years) | −0.294 | 0.212 | −0.660 | 0.185 |
| GDP per capita (ALL) | −0.330 | 0.160 | −0.682 | 0.146 | |
| Albania corruption index score | −0.216 | 0.378 | −0.610 | 0.264 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoxha, I.; Godman, B.; Malaj, A.; Meyer, J.C. 11-Year Trend in Antibiotic Consumption in a South-Eastern European Country; the Situation in Albania and the Implications for the Future. Antibiotics 2023, 12, 882. https://doi.org/10.3390/antibiotics12050882
Hoxha I, Godman B, Malaj A, Meyer JC. 11-Year Trend in Antibiotic Consumption in a South-Eastern European Country; the Situation in Albania and the Implications for the Future. Antibiotics. 2023; 12(5):882. https://doi.org/10.3390/antibiotics12050882
Chicago/Turabian StyleHoxha, Iris, Brian Godman, Admir Malaj, and Johanna C. Meyer. 2023. "11-Year Trend in Antibiotic Consumption in a South-Eastern European Country; the Situation in Albania and the Implications for the Future" Antibiotics 12, no. 5: 882. https://doi.org/10.3390/antibiotics12050882
APA StyleHoxha, I., Godman, B., Malaj, A., & Meyer, J. C. (2023). 11-Year Trend in Antibiotic Consumption in a South-Eastern European Country; the Situation in Albania and the Implications for the Future. Antibiotics, 12(5), 882. https://doi.org/10.3390/antibiotics12050882







