Abstract
Tuberculosis (TB) remains a major health problem worldwide, and the emergence of multi-resistant strains to first-line drugs has become the biggest obstacle to its treatment. On the other hand, the incidence of non-tuberculous mycobacteria (NTM) in humans has increased remarkably in recent years. The search for new and better treatments against mycobacterial infections is a constant at the global level. Hence, in this study, we propose to investigate the antimycobacterial effect of the extracts and major compounds of Hedeoma drummondii against clinical isolates of Mycobacterium tuberculosis and non-tuberculous mycobacteria: M. abscessus, M. fortuitum, M. intracellulare, and M. gordonae. To determine the antimycobacterial activity, a microdilution assay was used to establish the minimum inhibitory concentration (MIC) of the different strains of Mycobacterium. The methanolic extract presented the best activity against M. tuberculosis, inhibiting ten of the twelve strains analyzed at a concentration < 2500 µg/mL; meanwhile, the hexanic extract presented the best activity against non-tuberculous mycobacteria (NTM) by inhibiting eight of the ten strains studied at ≤625 µg/mL. Moreover, there is a strong positive correlation between the antimycobacterial activity of pulegone and the hexanic extract against non-tuberculous strains, so this compound could serve as a predictability marker against these types of microorganisms.
1. Introduction
Tuberculosis (TB) is a deadly and infectious lung disease caused by Mycobacterium tuberculosis, which has been threatening humanity for years and remains a major global health problem [1]. According to a 2018 global report by the World Health Organization (WHO), it was recorded that in 2017, there were an estimated 10 million new cases of TB. This is the equivalent to 133 cases per a 100,000 population [2]. The emergence of multidrug-resistant TB is mainly due to the inappropriate use of first-line anti-TB drugs, and the increased prevalence of such strains has become a major obstacle in TB treatment and a financial burden on the health sector [3]. As a result, there is an urgent need for new, cost-effective anti-TB drugs with different mechanisms of action and less opportunity to develop resistance [4].
On the other hand, non-tuberculous mycobacteria (NTM) are present on a wide diversity of surfaces, and their incidence in humans is increasing significantly worldwide [5]. It must be mentioned that progress in treating this type of mycobacteria has been slow due to the high number of NTM species and their clinical similarities, as well as low susceptibility to available antibiotics, which hinders correct diagnoses and the subsequent treatment [6]. One of the most important challenges of choosing an efficient treatment against NTMs has been the lack of correlation between in vitro susceptibility patterns and clinical responses [7]. In most non-tuberculous species, there are no evidence-based treatment recommendations, so physicians must make case-specific decisions on a case-by-case basis. Therefore, it is urgent to find a new strategy to combat mycobacterial infections [8].
Due to the resistance present in mycobacteria, a systematic search for compounds that are capable of dealing with this group of microorganisms has been carried out for years; natural products play an important role in this search, and many of these efforts are aimed at the study of medicinal plants, since they are used empirically for the treatment of various diseases, including respiratory diseases [9]. The research and standardization of medicinal plants has contributed to the development of phytopharmaceuticals, most of which have proven to be low cost and with low toxicity [10]. In relation to the statement above, in the northeastern region of Mexico, 234 species of medicinal plants used to cure respiratory diseases have been reported, highlighting the Lamiaceae family with 12 species [11]. It is within this aromatic family that Hedeoma drummondii is found. It can be described as a hairy perennial herb with an erect, mint-like shape up to 45 cm tall and the flowers are generally light to deep purple (Figure 1). The herb is used as a pleasant tea to relieve cold and cough in the north of Mexico and USA [12,13], and its extracts have already been reported to possess antibacterial activities, especially those of low polarity [14,15]. In these types of extracts and essential oils, the high content of the monoterpene pulegone stands out, while in the polar ones, rosmarinic and caffeic acids have been reported as the main bioactive compounds [15,16,17]. Therefore, the aim of this study was to assess the antimycobacterial activity from the crude extracts and major constituents from the Mexican aromatic plant H. drummondii against twelve strains of Mycobacterium tuberculosis and ten non-tuberculous strains.

Figure 1.
Inflorescences (left) and aerial parts (right) of Hedeoma drummondii.
2. Results
2.1. Determination of MIC in M. tuberculosis
Table 1 shows the antimycobacterial effect of the extracts and major compounds (Figure 2) of H. drummondii against each of the twelve strains of M. tuberculosis in a microplate assay. Within the samples studied in the present work, the MIC of the hexanic extract against M. tuberculosis HU-LIID-D-159 was 312.5 µg/mL and that of the methanolic extract was 19.53 µg/mL for M. tuberculosis strains HU-LIID 168-99 and HU-LIID 142-99 and 39.06 µg/mL for strain HU-LIID 376-98. As for the major compounds, the MIC of pulegone stands out with an activity of 125 µg/mL in strains HU-LIID 90-99 and HU-LIID 428-98, while rosmarinic and caffeic acid presented activities of 125 µg/mL for strains HU-LIID 434-98, HU-LIID 168-99, HU-LIID 142-99, and HU-LIID 376-98.

Table 1.
Antimycobacterial activity in the extracts and major compounds of H. drummondii against M. tuberculosis. In bold, we show those extracts with activity at <500 µg/mL.
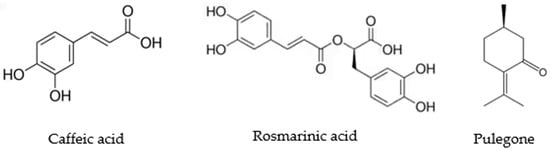
Figure 2.
Structure of the main compounds from H. drummondii analyzed against Mycobacterium strains.
2.2. Determination of MIC in NTM
Table 2 shows the antimycobacterial effect of the extracts and major compounds of H. drummondii against each of the ten NTM strains. When observing the inhibitory activity of the samples against NTMs, the hexanic extract obtained the best MIC concentrations, presenting values of 39.0625 µg/mL in M. abscessus LMMP and 78.125 µg/mL for M. intracellulare 989-3 and M. intracellulare 1105-1. On the other hand, the methanolic extract presented its best activity against M. abscessus 139-10 with an MIC of 78.125 µg/mL, followed by 312.5 µg/mL for M. abscessus LMMP, M. fortuitum 430R, M. fortuitum MLIID1, and M. gordonae A702 strains. While in the major compounds, pulegone was the one that obtained the best activity with MIC values of 15.625 µg/mL for M. abscessus 139-10 and M. abscessus LMMP as well as 31.25 µg/mL for M. gordonae A702, and in the case of rosmarinic acid, its activity was 250 µg/mL for most strains and caffeic acid stood out for its activity of 125 µg/mL against M. abscessus LMMP and M. intracellulare 142-09.

Table 2.
Antimycobacterial activity in the extracts and major compounds of H. drummondii against non-tuberculous mycobacteria. In bold, we show those extracts with activity at < 500 µg/mL.
2.3. Pearson Correlation
The Pearson’s r correlation coefficient was used to measure the association of antimycobacterial activity between the extracts of H. drummondii and their respective compounds. The results indicated that against M. tuberculosis strains, the methanolic extract presented a weak correlation (r = 0.14) with caffeic acid and a moderate correlation (r = 0.23) with rosmarinic acid, while the hexanic extract presented a negative correlation with pulegone. However, when comparing the extracts and compounds of H. drummondii against the NTM strains, it was observed that the hexanic extract presented a strong correlation (r = 0.53) with pulegone, while the methanolic extract presented negative correlations with rosmarinic acid and caffeic acid.
3. Discussion
In the present work, the antimycobacterial activity of the extracts and major compounds of H. drummondii against clinical isolates of M. tuberculosis and non-tuberculous mycobacteria was demonstrated. The use of organic solvents of different polarities is common for the extraction of bioactive compounds, since those of low polarity (hexane, chloroform, petroleum ether, and so forth) are excellent for the extraction of compounds, such as terpenes, coumarins, quinones and related molecules. Meanwhile, the polar solvents (methanol, ethanol, acetone, and so on) are used for the extraction of flavonoids, phenolic acids, and saponins, among other molecules [18]. This could explain why in our results, the methanolic (polar) extract was better against M. tuberculosis, while the hexanic (non-polar) extract presented more favorable results against non-tuberculous mycobacteria since the compounds found in them are of different chemical nature. It is worth mentioning that, similar to our results against the M. tuberculosis H37Rv strain, the nonpolar (chloroform) extracts of P. stellatum and O. integrifolia were more active than the polar (methanolic) ones [19]; however, in our research, this effect was only demonstrated against that particular strain (Table 1). Analyzing the antimicrobial effect on as many strains as possible is important to find candidate treatments that can circumvent the mechanisms of drug resistance developed by them [20].
It was observed that the two extracts studied showed an effect against ten of the twelve strains of the M. tuberculosis tested. The best effect was shown by the methanolic extract with an MIC of 19.53 µg/mL against the strains 168-99 and 142-99. The analyzed M. tuberculosis strains had resistance to rifampicin, which is a first-line drug, by presenting an MIC greater than 1 μg/mL [21].
The efficacy of the methanolic extract of H. drummondii against various strains of M. tuberculosis may be partly due to the abundance of phenolic compounds present in it, as it has previously been suggested that these compounds, such as flavones (cycloartocarpin, artocarpin, chaplashin, morusin, cudraflavone B, and cudraflavone C), play a significant role in inhibiting the production of the mycolic acid that is involved in the formation of the mycobacterial cell wall in addition to nucleic acid and protease inhibition, which affects the growth and viability of M. tuberculosis [4,22].
In this research, it was found that rosmarinic and caffeic acid showed antituberculosis activity against 9 of the 12 strains tested (Table 1). In previous research, the antimycobacterial effect of caffeic acid has already been demonstrated against a diversity of clinical isolates of M. tuberculosis, showing an MIC in the range of 128 to 1024 µg/mL [23]. On the other hand, structural derivatives of rosmarinic acid amides have been studied for the inhibition of the enzyme UDP-galactopyranose mutase (UGM), which is essential for cell wall integrity and viability; its inhibition has been presented as a cost-effective strategy for the discovery of anti-tuberculosis compounds [24]. Likewise, it has been reported that the catechol group is found in both compounds (caffeic and rosmarinic acid) and is responsible for the antimicrobial activity shown by molecules possessing this functional group [25].
As for the effect against non-tuberculous mycobacteria (NTM), it was observed that the hexanic extract was the most active, inhibiting 8 of the 10 strains studied, with the most sensitive being M. abscessus LMMP, with an MIC of 39.06 µg/mL (Table 2). The effect of essential oils obtained from a diversity of Lamiaceae against non-tuberculous mycobacterial strains has been previously demonstrated, highlighting Lavandula hybrida, which showed an effect against M. intracellulare and M. gordonae with MICs of 3200 µg/mL and 6400 µg/mL, respectively, as well as Salvia officinalis with an MIC of 6400 µg/mL for M. gordonae [26]. In the same vein, antimicrobial activity against this group of mycobacteria has already been demonstrated by organic extracts obtained with low and medium polarity solvents: the chloroplast extract of the Persea americana seed showed MICs of 12.5, 25, 50, and 100 µg/mL for M. smegmatis, M. abscessus, M. fortuitum, and M. chelonae, respectively [27], while the hexanic extract obtained from Juniperus communis showed an MIC of 64 µg/mL against M. aurum [28].
The antimicrobial activity shown by the non-polar extract of H. drummondii (hexane) against non-tuberculous strains could be related to the fact that this group of mycobacteria contains glycopeptidolipids (GPLs) that are found in the cell envelope and contribute to biofilm formation, which is related to their spread, virulence, and resistance [29,30]. These GPLs can be inhibited by compounds of lipophilic terpene nature, such as monoterpenes [31].
In relation to the statement above, pulegone, one of the major monoterpenes of H. drummondii, showed antimycobacterial activity against 8 of the 10 NTM strains tested. The most sensitive strains were M. abscessus 139-10, LMMP, and M. gordonae A702 with MICs of 15.63 µg/mL and 31.25 µg/mL, respectively (Table 2). The effect of monoterpenes against non-tuberculous strains has been reported with the effect of carvacrol against M. abscessus and M. fortuitum with an MIC of 64 µg/mL [32], and eugenol against M. kansasii, M. massilliense, M. chelonae, M. gordonae, M. smegmatis, and M. fortuitum with MICs between 3.9 and 250 µg/mL [33], in addition to α-pinene, thymol, p-cymene, limonene, myrcene, and geraniol, with MICs between 250 and 500 µg/mL for M. chelonae [34].
In our research, it was found that the hexanic extract presented a strong correlation with pulegone (r = 0.53) with NTM strains [35], which means that pulegone could possibly serve as a predictability marker. Previous investigations have been conducted in the search for chemical markers of predictability, such as the correlation of the antioxidant activity of twelve species of the Zingiberaceae family with phenolic compounds [36] and the correlation of the α-glucosidase inhibitory activity of eight species of the Lamiaceae family with phenolic acids and flavonoids [37].
However, the presence of weak correlations between the bioactivity of major compounds and pure extracts does not necessarily mean a discouraging result for the formulation of phytopharmaceuticals. In previous research, it has been mentioned that the synergy between the minority compounds present in the extracts could bring benefits by decreasing the possibility of developing resistance against a particular compound [38]. This would be useful in the polar extract of H. drummondii, where there may be other compound(s) in addition to those analyzed with effects against the different mycobacteria analyzed.
4. Materials and Methods
4.1. Extraction and Isolation
H. drummondii (Lamiaceae) was collected in Allende, Nuevo León, México. The species was identified by Dr. Marcela Gonzalez Alvarez. A dried specimen was deposited in the ethno botanical collection of the Herbarium of Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, with the voucher number: 024244.
The aerial part of the plant was dried at room temperature, and 150 g of the dried plant was extracted by static maceration with hexane first and then with methanol at room temperature. The plant–solvent ratio was 1:10, changing the solvent every 24 h 3 consecutive times, and then the extract was filtered using Whatman No. 1 filter paper. The solvent was removed from the filtered product using a rotary evaporator with reduced temperature and pressure. The concentrated organic extracts were stored at 4 °C until use.
Methanolic extract of H. drummondii was partitioned with DCM:MeOH (1:1), and the polar partition was chromatographed in Sephadex LH-20 in order to obtain five fractions. The fraction 2 was rechromatographed by HPLC semi preparative to yield a mayor phenolic acid: caffeic acid (CafAc) and rosmarinic acid (RosAc), as previously described [19]. The monoterpene (R)-(+)-Pulegone was obtained commercially from Sigma-Aldrich (No. 376388).
4.2. Mycobacterial Strains and Culture Conditions
Ten clinical isolates of NTM and twelve strains of M. tuberculosis were provided by Laboratorio Interdisciplinario y de Investigación Dermatológica (LIID) belonging to the Hospital Universitario (HU) José E. González (Monterrey, Nuevo León, México). It should be mentioned that several of the M. tuberculosis strains used are resistant to one or more first-line treatment drugs. To differentiate the strains, the following key was used: HU-LIID, which alludes to the place where they were isolated and characterized.
All strains were identified to species level by biochemical tests and PCR restriction analysis of a 441-bp sequence of the hsp65 gene. The reference isolate M. tuberculosis H37Rv ATCC 27294 was obtained from the American Type Culture Collection (ATCC). The strains were activated from frozen stocks in Lowenstein–Jensen medium by incubation at 37 °C, CO2 5% for 7 to 14 days.
4.3. Determination of the Minimum Inhibitory Concentration (MIC)
MIC of M. tuberculosis and NTM was evaluated using the 96-well plate microdilution technique according to CLSI [39]. For MIC evaluation in M. tuberculosis, solutions were prepared from the stocks of each sample with serial dilutions from 19.531 to 2500 µg/mL for the extracts and 3.906 to 500 µg/mL for the major compounds. Both cases used medium Middlebrook 7H9 supplemented with albumin–oleic acid–dextrose–catalase (OADC). While the antibiotic rifampicin was used as a control at concentrations from 0.25 to 32 µg/mL.
For each sample, 50 µL of each concentration was added horizontally in the microplate along with 50 µL of the strain to be evaluated, which had been previously adjusted to 1 on the McFarland scale, as well as a control well without treatment (M. tuberculosis strain in culture medium). This procedure was performed in duplicate and repeated for each strain in the study. The microplates were incubated at 37 °C for 5 d, and then Alamar Blue reagent was prepared in a 1:1 ratio with 10% Tween, adding 50 µL of the reagent to the wells, and the microplates were reincubated at 37 °C for 24 h [40]. MIC was defined as the lowest drug concentration that prevented a color change from blue to pink [41].
For MIC evaluation of NTM isolates, the same microdilution plate technique used for M. tuberculosis was followed; the media used are cation-adjusted Mueller–Hinton (CA-MHB) for M. abscessus and M. fortuitum and Middlebrook 7H9 medium supplemented with albumin- oleic acid- dextrose-catalase (OADC) for M. intracellulare and M. gordonae. While the control was performed with linezolid and imipenem antibiotics at serial dilutions from 0.25 to 32 µg/mL. Turbidity in the wells was measured after incubating the microplate at 37 °C for 72 h for M. abscessus and M. fortuitum strains and after 7 to 10 d for M. intracellulare and M. gordonae strains [42].
The MIC values obtained were analyzed by Pearson correlation to determine whether there was an association of antimycobacterial activity between the use of extracts and their respective compounds.
5. Conclusions
The organic extracts of H. drummondii possess antimycobacterial activities, with the hexanic one inhibiting 8 of the 10 NTM strains analyzed with an MIC between 39.0625 and 625 μg/mL, and the methanolic one inhibiting 10 of the 12 strains of M. tuberculosis with an MIC between 19.53 and 1250 μg/mL. The major compounds of H. drummondii possess antimycobacterial effects, with pulegone standing out by inhibiting 11 of the 12 M. tuberculosis strains with MICs between 125 and 500 μg/mL, as well as 8 of the 10 NTM strains tested with MICs between 15.625 and 500 μg/mL. A strong correlation was observed between the hexanic extract and the pulegone compound with its effect against non-tuberculous mycobacteria (r = 0.53), which opens the possibility of it being used as a marker of predictability for the antimycobacterial activity of the non-polar extract.
The current study indicates that crude extracts of H. drummondii can be explored for potential leads in antimycobacterial therapy. The pharmacokinetics and pharmacodynamics of these extracts need to be studied in physiologically more complex models to demonstrate their intracellular effect before they could be actually taken up further as complementary and/or alternative therapy.
Author Contributions
E.V.-V. and C.M.-T. conceived and designed the experiments; E.V.-V., C.R.-M. and C.P.-R. performed the extraction and isolation experiments and wrote the paper. C.M.-T., L.V.-C., J.O.-C. and C.P.-R. performed the antimycobacterial assays and analyzed the activity data. All the authors reviewed and commented the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work has received funds from PAICYT-UANL-288-CS-2022.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Acknowledgments
Authors thank funds from CONACYT fellowships 791651 (C.P.R.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abbas, H.S.; Baker, D.H.A. Recent Challenges in Tuberculosis Treatments: A Review. Plant Arch. 2020, 20, 3539–3547. [Google Scholar]
- Baptista, R.; Bhowmick, S.; Shen, J.; Mur, L.A.J. Molecular Docking Suggests the Targets of Anti-Mycobacterial Natural Products. Molecules 2021, 26, 475. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, M.C.; Clavera, I.; Michel de la Rosa, F.J.; Marín, B. Epidemiología de La Tuberculosis. An. Sist. Sanit. Navar. 2007, 30, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Mazlun, M.H.; Sabran, S.F.; Mohamed, M.; Abu Bakar, M.F.; Abdullah, Z. Phenolic Compounds as Promising Drug Candidates in Tuberculosis Therapy. Molecules 2019, 24, 2449. [Google Scholar] [CrossRef]
- To, K.; Cao, R.; Yegiazaryan, A.; Owens, J.; Venketaraman, V. General Overview of Nontuberculous Mycobacteria Opportunistic Pathogens: Mycobacterium avium and Mycobacterium abscessus. J. Clin. Med. 2020, 9, 2541. [Google Scholar] [CrossRef] [PubMed]
- Loddenkemper, R.; Lipman, M.; Zumla, A. Clinical Aspects of Adult Tuberculosis. Cold Spring Harb. Perspect. Med. 2016, 6, 17848. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An Official ATS/IDSA Statement: Diagnosis, Treatment, and Prevention of Nontuberculous Mycobacterial Diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef]
- Bento, C.M.; Gomes, M.S.; Silva, T. Looking beyond Typical Treatments for Atypical Mycobacteria. Antibiotics 2020, 9, 18. [Google Scholar] [CrossRef]
- Zambrano-Intriago, L.F.; Buenaño-Allauca, M.P.; Mancera-Rodríguez, N.J.; Jiménez-Romero, E. Estudio Etnobotánico de Plantas Medicinales Utilizadas Por Los Habitantes Del Área Rural de La Parroquia San Carlos, Quevedo, Ecuador. Univ. Y Salud 2015, 17, 97–111. [Google Scholar]
- Gallegos-Zurita, M. Las Plantas Medicinales: Principal Alternativa Para El Cuidado de La Salud, En La Población Rural de Babahoyo, Ecuador. An. Fac. Med. 2016, 77, 327. [Google Scholar] [CrossRef]
- Macouzet, M.V.; Estrada, E.; Jiménez, J.; José Angel, V.; Herrera, M.C. Plantas Medicinales de Miquihuana, Tamaulipas, 1st ed.; Universidad Autónoma de Nuevo León: Monterrey, Mexico, 2013; pp. 11–13. [Google Scholar]
- Vestal, P.A. The Ethnobotany of the Ramah Navaho, 4th ed.; Papers of the Peabody Museum of American Archeology and Ethnology: Cambridge, MA, USA, 1952; p. 94. [Google Scholar]
- Estrada, E.; Villarreal, J.A.; Cantú, C.; Cabral, I.; Scout, L.; Yen, C. Ethnobotany in the Cumbres de Monterrey National Park, Nuevo León, Mexico. J. Ethnobiol. Ethnomed. 2007, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Tovar, J.C. Composición Química, Actividad Antibacteriana y Tóxica de Aceites Esenciales de Seis Especies Medicinales de Lamiaceae en El Estado de Hidalgo. Licentiate Thesis, Universidad Autónoma del Estado de Hidalgo, Pachuca, Mexico, 2007. [Google Scholar]
- Viveros-Valdez, E.; Rivas-Morales, C.; Oranday-Cárdenas, A.; Verde-Star, M.J.; Carranza-Rosales, P. Antimicrobial Activity of Hedeoma drummondii against Opportunistic Pathogens. Pakistan J. Biol. Sci. 2011, 14, 305–307. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stéphane, F.F.; Jules, B.K.; Batiha, G.E.; Ali, I.; Bruno, L.N. Extraction of Bioactive Compounds from Medicinal Plants and Herbs. In Natural Medicine Plants, 1st ed.; El-Shemy, H., Ed.; IntechOpen: London, UK, 2021; Volume 1, p. 147. [Google Scholar]
- Kahaliw, W.; Aseffa, A.; Abebe, M.; Teferi, M.; Engidawork, E. Evaluation of the Antimycobacterial Activity of Crude Extracts and Solvent Fractions of Selected Ethiopian Medicinal Plants. BMC Complement. Altern. Med. 2017, 17, 143. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.; Shin, S.J. Importance of Differential Identification of Mycobacterium Tuberculosis Strains for Understanding Differences in Their Prevalence, Treatment Efficacy, and Vaccine Development. J. Microbiol. 2018, 56, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Viveros-Valdez, E.; Rivas-Morales, C.; Carranza-Rosales, P.; Mendoza, S.; Schmeda-Hirschmann, G. Free Radical Scavengers from the Mexican Herbal Tea “Poleo” (Hedeoma drummondii). Z. Für Naturforsch. C 2008, 63, 341–346. [Google Scholar] [CrossRef]
- Viveros-Valdez, E.; Rivas-Morales, C.; Oranday-Cárdenas, A.; Castro-Garza, J.; Carranza-Rosales, P. Antiproliferative Effect from the Mexican Poleo (Hedeoma drummondii). J. Med. Food 2010, 13, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Getahun, M.; Blumberg, H.M.; Ameni, G.; Beyene, D.; Kempker, R.R. Minimum Inhibitory Concentrations of Rifampin and Isoniazid among Multidrug and Isoniazid Resistant Mycobacterium tuberculosis in Ethiopia. PLoS ONE 2022, 17, e0274426. [Google Scholar] [CrossRef] [PubMed]
- Rabaan, A.A.; Alhumaid, S.; Albayat, H.; Alsaeed, M.; Alofi, F.S.; Al-Howaidi, M.H.; Turkistani, S.A.; Alhajri, S.M.; Alahmed, H.E.; Alzahrani, A.B.; et al. Promising Antimycobacterial Activities of Flavonoids against Mycobacterium sp. Drug Targets: A Comprehensive Review. Molecules 2022, 27, 5335. [Google Scholar] [CrossRef]
- Dey, D.; Ray, R.; Hazra, B. Antimicrobial Activity of Pomegranate Fruit Constituents against Drug-Resistant Mycobacterium tuberculosis and β -Lactamase Producing Klebsiella pneumoniae. Pharm. Biol. 2015, 53, 1474–1480. [Google Scholar] [CrossRef]
- Fu, J.; He, Z.; Fu, H.; Xia, Y.; N’Go, I.; Lou, H.; Wu, J.; Pan, W.; Vincent, S.P. Synthesis and Evaluation of Inhibitors of Mycobacterium tuberculosis UGM Using Bioisosteric Replacement. Bioorg. Med. Chem. 2022, 69, 116896. [Google Scholar] [CrossRef]
- Razaviamri, S.; Wang, K.; Liu, B.; Lee, B.P. Catechol-Based Antimicrobial Polymers. Molecules 2021, 26, 559. [Google Scholar] [CrossRef] [PubMed]
- Peruč, D.; Gobin, I.; Abram, M.; Broznić, D.; Svalina, T.; Štifter, S.; Staver, M.M.; Tićac, B. Antimycobacterial Potential of the Juniper Berry Essential Oil in Tap Water. Arh. Hig. Rada Toksikol. 2018, 69, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Arellanes, A.; Luna-Herrera, J.; Ruiz-Nicolás, R.; Cornejo-Garrido, J.; Tapia, A.; Yépez-Mulia, L. Antiprotozoal and Antimycobacterial Activities of Persea americana Seeds. BMC Complement. Altern. Med. 2013, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Gordien, A.Y.; Gray, A.I.; Franzblau, S.G.; Seidel, V. Antimycobacterial Terpenoids from Juniperus communis L. (Cuppressaceae). J. Ethnopharmacol. 2009, 126, 500–505. [Google Scholar] [CrossRef]
- Tran, T.; Bonham, A.J.; Chan, E.D.; Honda, J.R. A Paucity of Knowledge Regarding Nontuberculous Mycobacterial Lipids Compared to the Tubercle Bacillus. Tuberculosis 2019, 115, 96–107. [Google Scholar] [CrossRef]
- Schorey, J.S.; Sweet, L. The Mycobacterial Glycopeptidolipids: Structure, Function, and Their Role in Pathogenesis. Glycobiology 2008, 18, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Polyudova, T.V.; Eroshenko, D.V.; Pimenova, E.V. The Biofilm Formation of Nontuberculous Mycobacteria and Its Inhibition by Essential Oils. Int. J. Mycobacteriol. 2021, 10, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Marini, E.; Di Giulio, M.; Ginestra, G.; Magi, G.; Di Lodovico, S.; Marino, A.; Facinelli, B.; Cellini, L.; Nostro, A. Efficacy of Carvacrol against Resistant Rapidly Growing Mycobacteria in the Planktonic and Biofilm Growth Mode. PLoS ONE 2019, 14, e0219038. [Google Scholar] [CrossRef]
- Almeida, A.L.; Caleffi-Ferracioli, K.R.; de L Scodro, R.B.; Baldin, V.P.; Montaholi, D.C.; Spricigo, L.F.; Nakamura-Vasconcelos, S.S.; Hegeto, L.A.; Sampiron, E.G.; Costacurta, G.F.; et al. Eugenol and Derivatives Activity against Mycobacterium tuberculosis, Nontuberculous Mycobacteria and Other Bacteria. Future Microbiol. 2019, 14, 331–344. [Google Scholar] [CrossRef]
- Bueno-Sánchez, J.G.; Martínez-Morales, J.R.; Stashenko, E. Actividad Antimicobacteriana de Terpenos. Rev. La Univ. Ind. Santander. Salud 2009, 41, 231–235. [Google Scholar]
- Wang, D.; Le, X.H.; Luque, A.E. Identifying Effective Approaches for Dissemination of Clinical Evidence--Correlation Analyses on Promotional Activities and Usage of a Guideline-Driven Interactive Case Simulation Tool in a Statewide HIV-HCV-STD Clinical Education Program. Stud. Health Technol. Inform. 2015, 216, 515–519. [Google Scholar]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.-C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef]
- Skrypnik, L.; Golovin, A.; Savina, T. Effect of Salicylic Acid on Phenolic Compounds, Antioxidant and Antihyperglycemic Activity of Lamiaceae Plants Grown in a Temperate Climate. Front. Biosci. 2022, 14, 3. [Google Scholar] [CrossRef]
- Willcox, M.L.; Graz, B.; Falquet, J.; Diakite, C.; Giani, S.; Diallo, D. A “Reverse Pharmacology” Approach for Developing an Anti-Malarial Phytomedicine. Malar. J. 2011, 10, S8. [Google Scholar] [CrossRef] [PubMed]
- Clinical & Laboratory Standards Institute. Susceptibility Testing of Mycobacteria, Nocardia Spp., and Other Aerobic Actinomycetes, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; pp. 13–40. [Google Scholar]
- Martin, A.; Portaels, F.; Palomino, J.C. Colorimetric Redox-Indicator Methods for the Rapid Detection of Multidrug Resistance in Mycobacterium tuberculosis: A Systematic Review and Meta-Analysis. J. Antimicrob. Chemother. 2007, 59, 175–183. [Google Scholar] [CrossRef]
- Collins, L.; Franzblau, S.G. Microplate Alamar Blue Assay versus BACTEC 460 System for High-Throughput Screening of Compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 1997, 41, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, X.; Jin, J.; Wu, J.; Zhang, X.; Chen, J.; Zhang, W. In vitro Susceptibility of Mycobacterium abscessus and Mycobacterium fortuitum Isolates to 30 Antibiotics. Biomed. Res. Int. 2018, 2018, 4902941. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).