Ongoing Strategies to Improve Antimicrobial Utilization in Hospitals across the Middle East and North Africa (MENA): Findings and Implications
Abstract
1. Introduction
2. Results
2.1. Current Antimicrobial Utilisation Patterns across the MENA Region
2.2. Current Length of Antibiotic Prescribing Postoperatively to Prevent SSIs
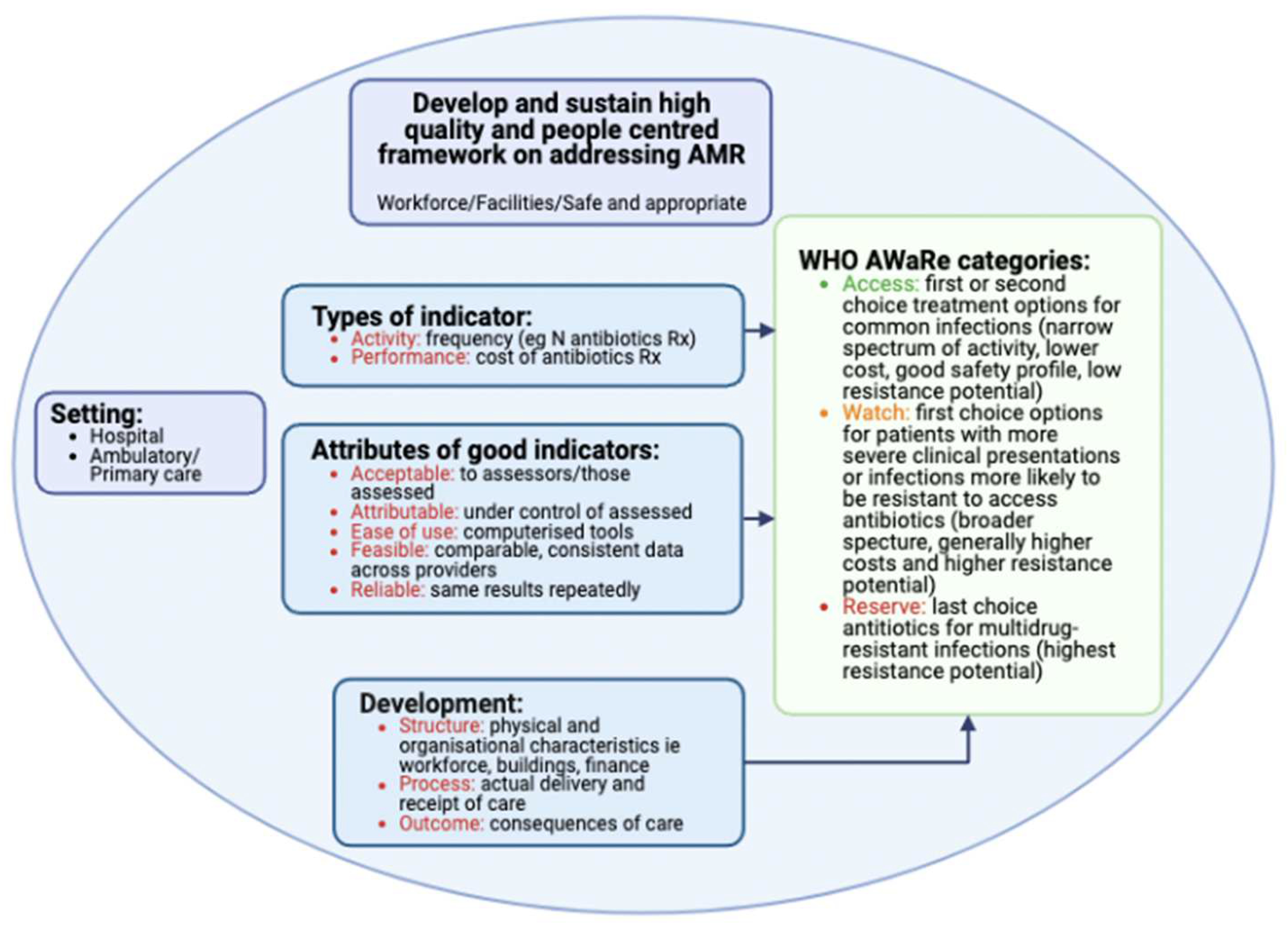
2.3. Prescribing and Quality Indicators
2.4. Antimicrobial Stewardship Programmes
2.5. Knowledge, Attitude, and Perceptions of Key Stakeholders towards Antibiotics and ASPs and Suggested Activities to Improve Future Antibiotic Prescribing in Hospitals
3. Discussion
4. Materials and Methods
4.1. Current Antimicrobial Utilisation Patterns among Hospitals across the MENA Region
4.2. Antibiotic Prophylaxis to Prevent Surgical Site Infections
4.3. Prescribing and Quality Indicators
4.4. Antimicrobial Stewardship Programs and Subsequent
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar]
- Hofer, U. The cost of antimicrobial resistance. Nat. Rev. Microbiol. 2019, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A. Antimicrobial Resistance: The Next Probable Pandemic. JNMA J. Nepal. Med. Assoc. 2022, 60, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug. Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- WHO Implementation Handbook for National Action Plans on Antimicrobial Resistance: Guidance for the Human Health Sector. 2022. Available online: https://www.who.int/publications/i/item/9789240041981 (accessed on 3 April 2023).
- OECD Health Policy Studies. Stemming the Superbug Tide. 2018. Available online: https://www.oecd-ilibrary.org/sites/9789264307599-en/index.html?itemId=/content/publication/9789264307599-en&mimeType=text/html (accessed on 2 April 2023).
- World Bank Group. Pulling Together to Beat Superbugs Knowledge and Implementation Gaps in Addressing Antimicrobial Resistance. 2019. Available online: https://openknowledge.worldbank.org/bitstream/handle/10986/32552/Pulling-Together-to-Beat-Superbugs-Knowledge-and-Implementation-Gaps-in-Addressing-Antimicrobial-Resistance.pdf?sequence=1&isAllowed=y (accessed on 3 April 2023).
- Willemsen, A.; Reid, S.; Assefa, Y. A review of national action plans on antimicrobial resistance: Strengths and weaknesses. Antimicrob. Resist. Infect. Control 2022, 11, 90. [Google Scholar] [CrossRef]
- Torumkuney, D.; Behbehani, N.; van Hasselt, J.; Hamouda, M.; Keles, N. Country data on AMR in Kuwait in the context of community-acquired respiratory tract infections: Links between antibiotic susceptibility, local and international antibiotic prescribing guidelines, access to medicine and clinical outcome. J. Antimicrob. Chemother. 2022, 77 (Suppl. 1), i77–i83. [Google Scholar] [CrossRef]
- Torumkuney, D.; Dolgum, S.; van Hasselt, J.; Abdullah, W.; Keles, N. Country data on AMR in Saudi Arabia in the context of community-acquired respiratory tract infections: Links between antibiotic susceptibility, local and international antibiotic prescribing guidelines, access to medicine and clinical outcome. J. Antimicrob. Chemother. 2022, 77 (Suppl. 1), i70–i76. [Google Scholar] [CrossRef]
- Iwu, C.D.; Patrick, S.M. An insight into the implementation of the global action plan on antimicrobial resistance in the WHO African region: A roadmap for action. Int. J. Antimicrob. Agents 2021, 58, 106411. [Google Scholar] [CrossRef]
- Ministry of Health and Prevention United Arab Emirates. National Strategy and Action Plan for Combatting Antimicrobial Resistance (NAP-AMR) United Arab Emirates: 2019–2023. 2019. Available online: https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/nap-library/uae_nap-amr-english.pdf?sfvrsn=83bb9e84_1&download=true (accessed on 3 April 2023).
- Charani, E.; Mendelson, M.; Pallett, S.J.C.; Ahmad, R.; Mpundu, M.; Mbamalu, O.; Bonaconsa, C.; Nampoothiri, V.; Singh, S.; Peiffer-Smadja, N.; et al. An analysis of existing national action plans for antimicrobial resistance—Gaps and opportunities in strategies ptimizing antibiotic use in human populations. Lancet Glob. Health 2023, 11, e466–e474. [Google Scholar] [CrossRef]
- World Bank. Middle East and North Africa. 2023. Available online: https://www.worldbank.org/en/region/mena (accessed on 3 April 2023).
- Sadeghi, H.; Khoei, S.G.; Bakht, M.; Rostamani, M.; Rahimi, S.; Ghaemi, M.; Mirzaei, B. A retrospective cross-sectional survey on nosocomial bacterial infections and their antimicrobial susceptibility patterns in hospitalized patients in northwest of Iran. BMC Res. Notes 2021, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Ennab, R.; Al-Momani, W.; Al-Titi, R.; Elayan, A. Antibiotic Profile of Pathogenic Bacteria Isolated from Postsurgical Site Infections in Public Hospitals in Northern Jordan. Infect. Drug. Resist. 2022, 15, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Truppa, C.; Abo-Shehada, M.N. Antimicrobial resistance among GLASS pathogens in conflict and non-conflict affected settings in the Middle East: A systematic review. BMC Infect. Dis. 2020, 20, 936. [Google Scholar] [CrossRef]
- Al-Orphaly, M.; Hadi, H.A.; Eltayeb, F.K.; Al-Hail, H.; Samuel, B.G.; Sultan, A.A.; Skariah, S. Epidemiology of Multidrug-Resistant Pseudomonas aeruginosa in the Middle East and North Africa Region. Msphere 2021, 6, e00202-21. [Google Scholar] [CrossRef] [PubMed]
- Alhomoud, F.; Aljamea, Z.; Almahasnah, R.; Alkhalifah, K.; Basalelah, L.; Alhomoud, F.K. Self-medication and self-prescription with antibiotics in the Middle East-do they really happen? A systematic review of the prevalence, possible reasons, and outcomes. Int. J. Infect. Dis. 2017, 57, 3–12. [Google Scholar] [CrossRef]
- Nimer, N.A. Nosocomial Infection and Antibiotic-Resistant Threat in the Middle East. Infect. Drug. Resist. 2022, 15, 631–639. [Google Scholar] [CrossRef]
- Moghnieh, R.; Araj, G.F.; Awad, L.; Daoud, Z.; Mokhbat, J.E.; Jisr, T.; Abdallah, D.; Azar, N.; Irani-Hakimeh, N.; Balkis, M.M.; et al. A compilation of antimicrobial susceptibility data from a network of 13 Lebanese hospitals reflecting the national situation during 2015–2016. Antimicrob. Resist. Infect. Control 2019, 8, 41. [Google Scholar] [CrossRef]
- Devi, S. AMR in the Middle East: A perfect storm. Lancet 2019, 394, 1311–1312. [Google Scholar] [CrossRef]
- Khalifeh, M.M.; Moore, N.D.; Salameh, P.R. Self-medication misuse in the Middle East: A systematic literature review. Pharmacol. Res. Perspect. 2017, 5, e00323. [Google Scholar] [CrossRef]
- Bert, F.; Previti, C.; Calabrese, F.; Scaioli, G.; Siliquini, R. Antibiotics Self Medication among Children: A Systematic Review. Antibiotics 2022, 11, 1583. [Google Scholar] [CrossRef]
- Sami, R.; Salehi, K.; Sadegh, R.; Solgi, H.; Atashi, V. Barriers to rational antibiotic prescription in Iran: A descriptive qualitative study. Antimicrob. Resist. Infect. Control 2022, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, A.; Hasan, A.J.; Baker, K.I.; Seaton, R.A.; Ramzi, Z.S.; Sneddon, J.; Godman, B. A multicentre point prevalence survey of hospital antibiotic prescribing and quality indices in the Kurdistan regional government of Northern Iraq: The need for urgent action. Expert Rev. Anti-Infect. Ther. 2021, 19, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Yaacoub, S.G.; Lahoud, N.A.; Francis, N.J.; Rahme, D.W.; Murr, T.H.; Maison, P.F.; Saleh, N.G. Antibiotic Prescribing Rate in Lebanese Community Pharmacies: A Nationwide Patient-Simulated Study of Acute Bacterial Rhinosinusitis. J. Epidemiol. Glob. Health 2019, 9, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Al-Halawa, D.A.; Seir, R.A.; Qasrawi, R. Antibiotic Resistance Knowledge, Attitudes, and Practices among Pharmacists: A Cross-Sectional Study in West Bank, Palestine. J. Environ. Public Health 2023, 2023, 2294048. [Google Scholar] [CrossRef]
- Rabayah, R.; Alsayed, R.B.; Abu Taha, A.; Salameh, H.; Amer, R.; Sabateen, A.; Aiesh, B.M.; Zyoud, S.H. Microbial spectrum and drug resistance profile in solid malignancies in a large tertiary hospital from Palestine. BMC Infect. Dis. 2022, 22, 385. [Google Scholar] [CrossRef]
- Handal, R.; Qunibi, L.; Sahouri, I.; Juhari, M.; Dawodi, R.; Marzouqa, H.; Hindiyeh, M. Characterization of Carbapenem-Resistant Acinetobacter baumannii Strains Isolated from Hospitalized Patients in Palestine. Int. J. Microbiol. 2017, 2017, 8012104. [Google Scholar] [CrossRef]
- Alshammari, M.K.; Alotaibi, M.A.; AlOtaibi, A.S.; Alosaime, H.T.; Aljuaid, M.A.; Alshehri, B.M.; AlOtaibi, Y.B.; Alasmari, A.A.; Alasmari, G.A.; Mohammed, M.H.; et al. Prevalence and Etiology of Community- and Hospital-Acquired Pneumonia in Saudi Arabia and Their Antimicrobial Susceptibility Patterns: A Systematic Review. Medicina 2023, 59, 760. [Google Scholar] [CrossRef]
- Karimi, G.; Kabir, K.; Farrokhi, B.; Abbaszadeh, E.; Esmaeili, E.D.; Khodamoradi, F.; Sarbazi, E.; Azizi, H. Prescribing pattern of antibiotics by family physicians in primary health care. J. Pharm. Policy Pract. 2023, 16, 11. [Google Scholar] [CrossRef]
- Alkhaldi, S.M.; Yaseen, N.A.; Bataineh, E.A.; Al-Rawashdeh, B.; Albadaineh, M.A.; Mubarak, S.M.; Jaras, R.E.; Taha, H.A. Patterns of antibiotic prescribing and appropriateness for respiratory tract infections in a teaching hospital in Jordan. Int. J. Clin. Pract. 2021, 75, e14113. [Google Scholar] [CrossRef]
- Mahmood, R.K.; Gillani, S.W.; Saeed, M.W.; Hafeez, M.U.; Gulam, S.M. Systematic Review: Study of the Prescribing Pattern of Antibiotics in Outpatients and Emergency Departments in the Gulf Region. Front. Pharmacol. 2020, 11, 585051. [Google Scholar] [CrossRef]
- Lakkis, N.A.; Alameddine, R.; Issa, H.G.; Mahmassani, D.; Osman, M.H. Prescribing antibiotics in adults with respiratory tract infections in Lebanon. Int. J. Clin. Pract. 2021, 75, e14514. [Google Scholar] [CrossRef] [PubMed]
- Al-Yamani, A.; Khamis, F.; Al-Zakwani, I.; Al-Noomani, H.; Al-Noomani, J.; Al-Abri, S. Patterns of Antimicrobial Prescribing in a Tertiary Care Hospital in Oman. Oman Med. J. 2016, 31, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Albuhairi, S.; Farhan, M.A.; Alanazi, S.; Althaqib, A.; Albeladi, K.; Alarfaj, S.; Alhezemy, R.; Ali, M.G.; Faraz, A.; Alsudais, M.; et al. Antibiotic Prescribing Patterns for Hospitalized children with Community-Acquired Pneumonia in a Secondary Care Center. J. Infect. Public Health 2021, 14, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, F.; Godman, B.; Yarimanesh, P.; Kebriaeezadeh, A. Prescribing patterns of physicians working in both the direct and indirect treatment sectors in Iran; findings and implications. J. Pharm. Health Serv. Res. 2019, 10, 407–413. [Google Scholar] [CrossRef]
- Saleem, Z.; Hassali, M.A.; Godman, B.; Versporten, A.; Hashmi, F.K.; Saeed, H.; Saleem, F.; Salman, M.; Rehman, I.U.; Khan, T.M. Point prevalence surveys of antimicrobial use: A systematic review and the implications. Expert Rev. Anti-Infect. Ther. 2020, 18, 897–910. [Google Scholar] [CrossRef]
- Al-Yaqoubi, W.S.; Al-Maqbali, N.S. Patterns of Prescribing Co-Amoxiclav to Children in Ibri Polyclinic, Oman. Sultan Qaboos Univ. Med. J. 2021, 21, e72–e76. [Google Scholar] [CrossRef]
- Alanazi, M.Q.; AlQahtani, H.; Almangour, T.A.; Aleanizy, F.S.; Alqahtani, F.Y. Evaluation of the Clinical Outcome and Cost Analysis of Antibiotics in the Treatment of Acute Respiratory Tract Infections in the Emergency Department in Saudi Arabia. Antibiotics 2022, 11, 1478. [Google Scholar] [CrossRef]
- Alneyadi, A.M.; Alketbi, L. Antibiotic prescription rates for upper respiratory tract infections at the Alain ambulatory healthcare centers, United Arab Emirates (2014–2016). Res. Sq. 2022, preprint. [Google Scholar] [CrossRef]
- Pauwels, I.; Versporten, A.; Drapier, N.; Vlieghe, E.; Goossens, H.; Koraqi, A.; Hoxha, I.; Tafaj, S.; Cornistein, W.; Quiros, R.; et al. Hospital antibiotic prescribing patterns in adult patients according to the WHO Access, Watch and Reserve classification (AWaRe): Results from a worldwide point prevalence survey in 69 countries. J. Antimicrob. Chemother. 2021, 76, 1614–1624. [Google Scholar] [CrossRef]
- Godman, B.; Haque, M.; McKimm, J.; Abu Bakar, M.; Sneddon, J.; Wale, J.; Campbell, S.; Martin, A.P.; Hoxha, I.; Abilova, V.; et al. Ongoing strategies to improve the management of upper respiratory tract infections and reduce inappropriate antibiotic use particularly among lower and middle-income countries: Findings and implications for the future. Curr. Med. Res. Opin. 2020, 36, 301–327. [Google Scholar] [CrossRef]
- Sharland, M.; Pulcini, C.; Harbarth, S.; Zeng, M.; Gandra, S.; Mathur, S.; Magrini, N. Classifying antibiotics in the WHO Essential Medicines List for optimal use—Be AWaRe. Lancet Infect. Dis. 2018, 18, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Sharland, M.; Gandra, S.; Huttner, B.; Moja, L.; Pulcini, C.; Zeng, M.; Mendelson, M.; Cappello, B.; Cooke, G.; Magrini, N.; et al. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use—The new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect. Dis. 2019, 19, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- WHO. The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book. 2022. Available online: https://www.who.int/publications/i/item/9789240062382 (accessed on 3 April 2023).
- Sharland, M.; Zanichelli, V.; Ombajo, L.A.; Bazira, J.; Cappello, B.; Chitatanga, R.; Chuki, P.; Gandra, S.; Getahun, H.; Harbarth, S.; et al. The WHO essential medicines list AWaRe book: From a list to a quality improvement system. Clin. Microbiol. Infect. 2022, 28, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- Hsia, Y.; Lee, B.R.; Versporten, A.; Yang, Y.; Bielicki, J.; Jackson, C.; Newland, J.; Goossens, H.; Magrini, N.; Sharland, M.; et al. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): An analysis of paediatric survey data from 56 countries. Lancet Glob. Health 2019, 7, e861–e871. [Google Scholar] [CrossRef] [PubMed]
- Zanichelli, V.; Sharland, M.; Cappello, B.; Moja, L.; Getahun, H.; Pessoa-Silva, C.; Sati, H.; van Weezenbeek, C.; Balkhy, H.; Simão, M.; et al. The WHO AWaRe (Access, Watch, Reserve) antibiotic book and prevention of antimicrobial resistance. Bull. World Health Organ. 2023, 101, 290–296. [Google Scholar] [CrossRef]
- Alothman, A.; Al Thaqafi, A.; Al Ansary, A.; Zikri, A.; Fayed, A.; Khamis, F.; Al Salman, J.; Al Dabal, L.; Khalife, N.; AlMusawi, T.; et al. Prevalence of infections and antimicrobial use in the acute-care hospital setting in the Middle East: Results from the first point-prevalence survey in the region. Int. J. Infect. Dis. 2020, 101, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Nassr, O.A.; Abd Alridha, A.M.; Naser, R.A.; Abbas, R.S. Antibiotic prescribing in the acute care in Iraq. Int. J. Pharmacol. Pharm. Sci. 2018, 12, 485–489. [Google Scholar]
- Haseeb, A.; Faidah, H.S.; Algethamy, M.; Alghamdi, S.; Alhazmi, G.A.; Alshomrani, A.O.; Alqethami, B.R.; Alotibi, H.S.; Almutiri, M.Z.; Almuqati, K.S.; et al. Antimicrobial Usage and Resistance in Makkah Region Hospitals: A Regional Point Prevalence Survey of Public Hospitals. Int. J. Environ. Res. Public Health 2021, 19, 254. [Google Scholar] [CrossRef]
- Rizk, N.A.; Moghnieh, R.; Haddad, N.; Rebeiz, M.-C.; Zeenny, R.M.; Hindy, J.-R.; Orlando, G.; Kanj, S.S. Challenges to Antimicrobial Stewardship in the Countries of the Arab League: Concerns of Worsening Resistance during the COVID-19 Pandemic and Proposed Solutions. Antibiotics 2021, 10, 1320. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Alshaikh, F.S.; Godman, B.; Sindi, O.N.; Seaton, R.A.; Kurdi, A. Prevalence of bacterial coinfection and patterns of antibiotics prescribing in patients with COVID-19: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0272375. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, K.; Shafiq, S.; Raees, I.; Mustafa, Z.U.; Salman, M.; Khan, A.H.; Meyer, J.C.; Godman, B. Co-Infections, Secondary Infections, and Antimicrobial Use in Patients Hospitalized with COVID-19 during the First Five Waves of the Pandemic in Pakistan; Findings and Implications. Antibiotics 2022, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Ameen, L.; Assaggaf, H.; Alsafi, R.; Minshawi, F.; Alghamdi, S.; Alharbi, A.; Qashqari, F.; Makhdoom, H.; Refaat, B.; Alsaif, B.; et al. Analysis of the Clinical Characteristics of COVID-19 Patient Severity Amongst Saudi Hospital Admission in 2020. J. Umm Al-Qura Univ. Med. Sci. 2022, 8, 18–23. [Google Scholar] [CrossRef]
- Alsaeed, O.M.; Bukhari, A.A.; Alshehri, A.A.; Alsumairi, F.A.; Alnami, A.M.; Elsheikh, H.A. The Use of Antibiotics for the Prevention of Surgical Site Infections in Two Government Hospitals in Taif, Saudi Arabia: A Retrospective Study. Cureus 2022, 14, e26731. [Google Scholar] [CrossRef] [PubMed]
- Alkaaki, A.; Al-Radi, O.O.; Khoja, A.; Alnawawi, A.; Alnawawi, A.; Maghrabi, A.; Altaf, A.; Aljiffry, M. Surgical site infection following abdominal surgery: A prospective cohort study. Can. J. Surg. 2019, 62, 111–117. [Google Scholar] [CrossRef]
- Alnajjar, M.S.; Alashker, D.A. Surgical site infections following caesarean sections at Emirati teaching hospital: Incidence and implicated factors. Sci. Rep. 2020, 10, 18702. [Google Scholar] [CrossRef]
- Saleem, Z.; Godman, B.; Cook, A.; Khan, M.A.; Campbell, S.M.; Seaton, R.A.; Siachalinga, L.; Haseeb, A.; Amir, A.; Kurdi, A.; et al. Ongoing Efforts to Improve Antimicrobial Utilization in Hospitals among African Countries and Implications for the Future. Antibiotics 2022, 11, 1824. [Google Scholar] [CrossRef]
- Jalil, M.H.A.; Hammour, K.A.; Alsous, M.; Hadadden, R.; Awad, W.; Bakri, F.; Fram, K. Noncompliance with surgical antimicrobial prophylaxis guidelines: A Jordanian experience in cesarean deliveries. Am. J. Infect. Control 2018, 46, 14–19. [Google Scholar] [CrossRef]
- Mwita, J.C.; Ogunleye, O.O.; Olalekan, A.; Kalungia, A.C.; Kurdi, A.; Saleem, Z.; Sneddon, J.; Godman, B. Key Issues Surrounding Appropriate Antibiotic Use for Prevention of Surgical Site Infections in Low- and Middle-Income Countries: A Narrative Review and the Implications. Int. J. Gen. Med. 2021, 14, 515–530. [Google Scholar] [CrossRef]
- Field, A.M.; Seabury, R.W.; Kufel, W.D.; Darko, W.; Miller, C.D.; Mastro, K.A.; Steele, J.M. Single-Dose Antibiotic Prophylaxis with Ertapenem Increases Compliance with Recommendations for Surgical Antibiotic Prophylaxis in Elective Colorectal Surgery: A Retrospective, Single-Center Analysis. Surg. Infect. 2023, 24, 177–182. [Google Scholar] [CrossRef]
- Alshehhi, H.S.; Ali, A.A.; Jawhar, D.S.; Aly, E.M.; Swamy, S.; Fattah, M.A.; Drweesh, K.A.; Alsaadi, A. Assessment of implementation of antibiotic stewardship program in surgical prophylaxis at a secondary care hospital in Ras Al Khaimah, United Arab Emirates. Sci. Rep. 2021, 11, 1042. [Google Scholar] [CrossRef] [PubMed]
- Vippadapu, P.; Gillani, S.W.; Thomas, D.; Ahmed, F.; Gulam, S.M.; Mahmood, R.K.; Menon, V.; Abdi, S.; Rathore, H.A. Choice of Antimicrobials in Surgical Prophylaxis—Overuse and Surgical Site Infection Outcomes from a Tertiary-Level Care Hospital. Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, D.; Varghese, D.; Stephens, J.; Ansari, W.; Martin, S.; Charbonneau, C. Value of hospital antimicrobial stewardship programs [ASPs]: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 35. [Google Scholar] [CrossRef]
- Shallal, A.; Lahoud, C.; Merhej, D.; Youssef, S.; Verkler, J.; Kaljee, L.; Prentiss, T.; Joshi, S.; Zervos, M.; Matar, M. The Impact of a Post-Prescription Review and Feedback Antimicrobial Stewardship Program in Lebanon. Antibiotics 2022, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Sarang, B.; Tiwary, A.; Gadgil, A.; Roy, N. Implementing antimicrobial stewardship to reduce surgical site infections: Experience and challenges from two tertiary-care hospitals in Mumbai, India. J. Glob. Antimicrob. Resist. 2020, 20, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Siachalinga, L.; Mufwambi, W.; Lee, L.-H. Impact of antimicrobial stewardship interventions to improve antibiotic prescribing for hospital inpatients in Africa: A systematic review and meta-analysis. J. Hosp. Infect. 2022, 129, 124–143. [Google Scholar] [CrossRef]
- Cox, J.A.; Vlieghe, E.; Mendelson, M.; Wertheim, H.; Ndegwa, L.; Villegas, M.V.; Gould, I.; Hara, G.L. Antibiotic stewardship in low- and middle-income countries: The same but different? Clin. Microbiol. Infect. 2017, 23, 812–818. [Google Scholar] [CrossRef]
- Kalungia, A.C.; Mwambula, H.; Munkombwe, D.; Marshall, S.; Schellack, N.; May, C.; Jones, A.S.C.; Godman, B. Antimicrobial stewardship knowledge and perception among physicians and pharmacists at leading tertiary teaching hospitals in Zambia: Implications for future policy and practice. J. Chemother. 2019, 31, 378–387. [Google Scholar] [CrossRef]
- Kamere, N.; Garwe, S.T.; Akinwotu, O.O.; Tuck, C.; Krockow, E.M.; Yadav, S.; Olawale, A.G.; Diyaolu, A.H.; Munkombwe, D.; Muringu, E.; et al. Scoping Review of National Antimicrobial Stewardship Activities in Eight African Countries and Adaptable Recommendations. Antibiotics 2022, 11, 1149. [Google Scholar] [CrossRef]
- Niaz, Q.; Godman, B.; Campbell, S.; Kibuule, D. Compliance to prescribing guidelines among public health care facilities in Namibia; findings and implications. Pharm. Weekbl. 2020, 42, 1227–1236. [Google Scholar] [CrossRef]
- Afriyie, D.K.; Sefah, I.A.; Sneddon, J.; Malcolm, W.; McKinney, R.; Cooper, L.; Kurdi, A.; Godman, B.; Seaton, R.A. Antimicrobial point prevalence surveys in two Ghanaian hospitals: Opportunities for antimicrobial stewardship. JAC-Antimicrob. Resist. 2020, 2, dlaa001. [Google Scholar] [CrossRef] [PubMed]
- Olaoye, O.; Tuck, C.; Khor, W.P.; McMenamin, R.; Hudson, L.; Northall, M.; Panford-Quainoo, E.; Asima, D.M.; Ashiru-Oredope, D. Improving Access to Antimicrobial Prescribing Guidelines in 4 African Countries: Development and Pilot Implementation of an App and Cross-Sectional Assessment of Attitudes and Behaviour Survey of Healthcare Workers and Patients. Antibiotics 2020, 9, 555. [Google Scholar] [CrossRef] [PubMed]
- Gebretekle, G.B.; Mariam, D.H.; Taye, W.A.; Fentie, A.M.; Degu, W.A.; Alemayehu, T.; Beyene, T.; Libman, M.; Fenta, T.G.; Yansouni, C.P.; et al. Half of Prescribed Antibiotics Are Not Needed: A Pharmacist-Led Antimicrobial Stewardship Intervention and Clinical Outcomes in a Referral Hospital in Ethiopia. Front. Public Health 2020, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Ababneh, M.A.; Nasser, S.A.; Rababa’h, A.M. A systematic review of Antimicrobial Stewardship Program implementation in Middle Eastern countries. Int. J. Infect. Dis. 2021, 105, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Al Salman, J.; Al Dabal, L.; Bassetti, M.; Alfouzan, W.A.; Al Maslamani, M.; Alraddadi, B.; Elhoufi, A.; Khamis, F.; Mokkadas, E.; Romany, I.; et al. Promoting cross-regional collaboration in antimicrobial stewardship: Findings of an infectious diseases working group survey in Arab countries of the Middle East. J. Infect. Public Health 2021, 14, 978–984. [Google Scholar] [CrossRef]
- Sadeq, A.A.; Shamseddine, J.M.; Babiker, Z.O.E.; Nsutebu, E.F.; Moukarzel, M.B.; Conway, B.R.; Hasan, S.S.; Conlon-Bingham, G.M.; Aldeyab, M.A. Impact of Multidisciplinary Team Escalating Approach on Antibiotic Stewardship in the United Arab Emirates. Antibiotics 2021, 10, 1289. [Google Scholar] [CrossRef]
- Basu, S.; Copana, R.M.; Morales, R.J.B.; Anugulruengkitt, S.; Puthanakit, T.M.; Maramba-Lazarte, C.; Williams, P.; Musembi, J.; Boga, M.; Issack, M.F.; et al. Keeping It Real: Antibiotic Use Problems and Stewardship Solutions in Low- and Middle-income Countries. Pediatr. Infect. Dis. J. 2022, 41, S18–S25. [Google Scholar] [CrossRef]
- Le Maréchal, M.; Tebano, G.; Monnier, A.A.; Adriaenssens, N.; Gyssens, I.C.; Huttner, B.; Milanič, R.; Schouten, J.; Stanić Benić, M.; Versporten, A.; et al. Quality indicators assessing antibiotic use in the outpatient setting: A systematic review followed by an international multidisciplinary consensus procedure. J. Antimicrob. Chemother. 2018, 73 (Suppl. 6), vi40–vi49. [Google Scholar] [CrossRef]
- Almeleebia, T.M.; Alhifany, A.A.; Almutairi, F.; Alshibani, M.; Alhossan, A.M. Regulating antimicrobial sales in Saudi Arabia: Achievements and challenges. Int. J. Clin. Pract. 2021, 75, e13833. [Google Scholar] [CrossRef]
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.-F.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H.; Koraqi, A.; et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health 2018, 6, e619–e629. [Google Scholar] [CrossRef]
- van den Bosch, C.M.; Hulscher, M.E.; Natsch, S.; Wille, J.; Prins, J.M.; Geerlings, S.E. Applicability of generic quality indicators for appropriate antibiotic use in daily hospital practice: A cross-sectional point-prevalence multicenter study. Clin. Microbiol. Infect. 2016, 22, 888.e1–888.e9. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, C.M.; Geerlings, S.E.; Natsch, S.; Prins, J.M.; Hulscher, M.E. Quality indicators to measure appropriate antibiotic use in hospitalized adults. Clin. Infect. Dis. 2015, 60, 281–291. [Google Scholar] [CrossRef]
- Kallen, M.C.; Prins, J.M. A Systematic Review of Quality Indicators for Appropriate Antibiotic Use in Hospitalized Adult Patients. Infect. Dis. Rep. 2017, 9, 13–17. [Google Scholar] [CrossRef]
- Campbell, S.M.; Braspenning, J.; Hutchinson, A.; Marshall, M. Research methods used in developing and applying quality indicators in primary care. Qual. Saf. Health Care 2002, 11, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.M.; Kontopantelis, E.; Hannon, K.; Burke, M.; Barber, A.; E Lester, H. Framework and indicator testing protocol for developing and piloting quality indicators for the UK quality and outcomes framework. BMC Fam. Pract. 2011, 12, 85. [Google Scholar] [CrossRef]
- Campbell, S.M.; Godman, B.; Diogene, E.; Fürst, J.; Gustafsson, L.L.; MacBride-Stewart, S.; Malmström, R.E.; Pedersen, H.; Selke, G.; Vlahović-Palčevski, V.; et al. Quality indicators as a tool in improving the introduction of new medicines. Basic Clin. Pharmacol. Toxicol. 2015, 116, 146–157. [Google Scholar] [CrossRef]
- WHO. Global Action Plan on Antimicrobial Resistance. 2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1 (accessed on 3 April 2023).
- Almangour, T.A.; Alenazi, B.; Ghonem, L.; Alhifany, A.A.; Aldakheel, B.A.; Alruwaili, A. Inhaled colistin for the treatment of nosocomial pneumonia due to multidrug-resistant Gram-negative bacteria: A real-life experience in tertiary care hospitals in Saudi Arabia. Saudi Pharm. J. 2020, 28, 1009–1013. [Google Scholar] [CrossRef]
- Almangour, T.A.; Fletcher, V.; Alessa, M.; Alhifany, A.; Tabb, D. Multiple weekly dalbavancin dosing for the treatment of native vertebral osteomyelitis caused by methicillin-resistant staphylococcus aureus: A case report. Am. J. Case Rep. 2017, 18, 1315–1319. [Google Scholar] [CrossRef]
- Hadi, M.A.; Karami, N.A.; Al-Muwalid, A.S.; Al-Otabi, A.; Al-Subahi, E.; Bamomen, A.; Mohamed, M.M.; Elrggal, M.E. Community pharmacists’ knowledge, attitude, and practices towards dispensing antibiotics without prescription (DAwP): A cross-sectional survey in Makkah Province, Saudi Arabia. Int. J. Infect. Dis. 2016, 47, 95–100. [Google Scholar] [CrossRef]
- Klein, E.Y.; Milkowska-Shibata, M.; Tseng, K.K.; Sharland, M.; Gandra, S.; Pulcini, C.; Laxminarayan, R. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–2015: An analysis of pharmaceutical sales data. Lancet Infect. Dis. 2021, 21, 107–115. [Google Scholar] [CrossRef]
- Sulis, G.; Sayood, S.; Katukoori, S.; Bollam, N.; George, I.; Yaeger, L.H.; Chavez, M.A.; Tetteh, E.; Yarrabelli, S.; Pulcini, C.; et al. Exposure to World Health Organization’s AWaRe antibiotics and isolation of multidrug resistant bacteria: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Zimlichman, E.; Henderson, D.; Tamir, O.; Franz, C.; Song, P.; Yamin, C.K.; Keohane, C.; Denham, C.R.; Bates, D.W. Health care-associated infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Intern. Med. 2013, 173, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Karaali, C.; Emiroglu, M.; Atalay, S.; Sert, I.; Dursun, A.; Kose, S.; Akbulut, G.; Aydın, C. A new antibiotic stewardship program approach is effective on inappropriate surgical prophylaxis and discharge prescription. J. Infect. Dev. Ctries. 2019, 13, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.; Alotaibi, F.; Ahmed, H.; Alharbi, F.; Bukhari, O.; Youssef, A.-R. Effect of medical education on the knowledge, attitude and compliance regarding infection control measures among dental students in Makkah. J. Umm Al-Qura Univ. Med. Sci. 2021, 7, 14–17. [Google Scholar] [CrossRef]
- Ababneh, M.A.; Issa, N.; Alkhatatbeh, M. Evaluation of core elements of antimicrobial stewardship programs in Jordanian hospitals. Jordan J. Pharm. Sci. 2017, 10, 127–134. [Google Scholar] [CrossRef]
- Sallam, M.; Al-Sanafi, M.; Sallam, M. A Global Map of COVID-19 Vaccine Acceptance Rates per Country: An Updated Concise Narrative Review. J. Multidiscip. Health 2022, 15, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Sallam, M. COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines 2021, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Faezi, N.A.; Gholizadeh, P.; Sanogo, M.; Oumarou, A.; Mohamed, M.N.; Cissoko, Y.; Sow, M.S.; Keita, B.S.; Baye, Y.A.M.; Pagliano, P.; et al. Peoples’ attitude toward COVID-19 vaccine, acceptance, and social trust among African and Middle East countries. Health Promot. Perspect. 2021, 11, 171–178. [Google Scholar] [CrossRef] [PubMed]
- El-Elimat, T.; AbuAlSamen, M.M.; Almomani, B.A.; Al-Sawalha, N.A.; Alali, F.Q. Acceptance and attitudes toward COVID-19 vaccines: A cross-sectional study from Jordan. PLoS ONE 2021, 16, e0250555. [Google Scholar] [CrossRef]
- Desbois, A.P.; Garza, M.; Eltholth, M.; Hegazy, Y.M.; Mateus, A.; Adams, A.; Little, D.C.; Høg, E.; Mohan, C.V.; Ali, S.E.; et al. Systems-thinking approach to identify and assess feasibility of potential interventions to reduce antibiotic use in tilapia farming in Egypt. Aquaculture 2021, 540, 736735. [Google Scholar] [CrossRef]
- Samy, A.A.; Mansour, A.S.; Khalaf, D.D.; Khairy, E.A. Development of multidrug-resistant Escherichia coli in some Egyptian veterinary farms. Vet. World 2022, 15, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Kaviani Rad, A.; Balasundram, S.K.; Azizi, S.; Afsharyzad, Y.; Zarei, M.; Etesami, H.; Shamshiri, R.R. An Overview of Antibiotic Resistance and Abiotic Stresses Affecting Antimicrobial Resistance in Agricultural Soils. Int. J. Environ. Res. Public Health 2022, 19, 4666. [Google Scholar] [CrossRef] [PubMed]
- El Baaboua, A.; El Maadoudi, M.; Bouyahya, A.; Kounnoun, A.; Bougtaib, H.; Belmehdi, O.; Senhaji, N.S.; Abrini, J. Prevalence and antimicrobial profiling of Campylobacter spp. isolated from meats, animal, and human feces in Northern of Morocco. Int. J. Food Microbiol. 2021, 349, 109202. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, K.; Hanna, P.A.; Salameh, P.; Raad, E.B. Antibiotic consumption in non-teaching Lebanese hospitals: A cross-sectional study. J. Infect. Public Health 2016, 9, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Ashour, R.H.; Abdelkader, E.A.; Hamdy, O.; Elmetwally, M.; Laimon, W.; Abd-Elaziz, M.A. The Pattern of Antimicrobial Prescription at a Tertiary Health Center in Egypt: A Point Survey and Implications. Infect. Drug. Resist. 2022, 15, 6365–6378. [Google Scholar] [CrossRef] [PubMed]
- Fahimzad, A.; Eydian, Z.; Karimi, A.; Shiva, F.; Sayyahfar, S.; Kahbazi, M.; Rahbarimanesh, A.; Sedighi, I.; Arjmand, R.; Soleimani, G.; et al. Surveillance of antibiotic consumption point prevalence survey 2014: Antimicrobial prescribing in pediatrics wards of 16 Iranian hospitals. Arch. Iran. Med. 2016, 19, 204–209. [Google Scholar]
- Soltani, J.; Pouladfar, G.; Versporten, A.; Sharland, M.; Goossen, H.; Jafarpour, Z.; Soleimani, N. Point Prevalence Survey of Antimicrobial Prescription and Infection in Pediatric and Neonatal wards of Two Iranian Teaching Hospitals. Erciyes Med. J. 2019, 41, 25–32. [Google Scholar] [CrossRef]
- Razine, R.; Azzouzi, A.; Barkat, A.; Khoudri, I.; Hassouni, F.; Chefchaouni, A.C.; Abouqal, R. Prevalence of hospital-acquired infections in the university medical center of Rabat, Morocco. Int. Arch. Med. 2012, 5, 26. [Google Scholar] [CrossRef]
- Chiguer, M.; Alami, Z.; Lamti, S.; Abda, N. Prevalence and risk factors of healthcare-associated infections in a Moroccan teaching hospital. Can. J. Infect. Control 2018, 33. [Google Scholar]
- Alagha, H.Z.; Al Telbani, M.J. Investigating antibiotic use in Gaza Strip hospitals: A retrospective cross-sectional analysis. J. Infect. Dev. Ctries. 2022, 16, 1739–1747. [Google Scholar] [CrossRef]
- Ayed, H.B.; Yaich, S.; Trigui, M.; Jemaa, M.B.; Hmida, M.B.; Karray, R.; Kassis, M.; Mejdoub, Y.; Feki, H.; Jedidi, J.; et al. Prevalence and risk factors of health care–associated infections in a limited resources country: A cross-sectional study. Am. J. Infect. Control 2019, 47, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Maamri, H.; Ben Ayed, H.; Ketata, N.; Yaich, S.; Baklouti, M.; Karray, R.; Feki, H.; Damak, J. Prevalence survey on antimicrobial use & multidrug resistance in tertiary level university hospital. Eur. J. Public Health 2021, 31 (Suppl. 3), ckab165-490. [Google Scholar] [CrossRef]
- Kurmanji, J.; Hassali, A.; Younus, M.; Versporten, A.; Pauwels, I.; Goossens, H.; Riadh, Z. A point prevalence survey in Baghdad teaching hospital for antimicrobial prescribing pattern. Int. J. Infect. Dis. 2020, 101, 105. [Google Scholar] [CrossRef]
- Kurmanji, J.M.; Hassali, A.; Versporten, A.; Younus, M.; Pauwels, I.; Goossens, H.; Alnedawi, Z. Global Point Prevalence Survey in Five Teaching Hospitals in Baghdad, Iraq. Mediterr. J. Infect. Microbes Antimicrob./Infect. Dis. Clin. Microbiol. Spec. Soc. Turkey 2021, 10, 17. [Google Scholar] [CrossRef]
- Elhajji, F.D.; Al-Taani, G.M.; Anani, L.; Al-Masri, S.; Abdalaziz, H.; Qabba’h, S.H.; Al Bawab, A.Q.; Scott, M.; Farren, D.; Gilmore, F.; et al. Comparative point prevalence survey of antimicrobial consumption between a hospital in Northern Ireland and a hospital in Jordan. BMC Health Serv. Res. 2018, 18, 849. [Google Scholar] [CrossRef]
- Abu Hammour, K.; Al-Heyari, E.; Allan, A.; Versporten, A.; Goossens, H.; Abu Hammour, G.; Manaseer, Q. Antimicrobial consumption and resistance in a tertiary care hospital in Jordan: Results of an internet-based global point prevalence survey. Antibiotics 2020, 9, 598. [Google Scholar] [CrossRef]
- Ababneh, M.A.; Jaber, M.; Rababa’h, A.; Alabweny, E. Prevalence of antimicrobial use in a tertiary academic hospital: A venue for antimicrobial stewardship programs. Expert Rev. Anti-Infect. Ther. 2021, 19, 1047–1051. [Google Scholar] [CrossRef]
- Salman, J.; Alagha, R.; Ebrahim, Z.; Majed, M.; Taitoon, S.; Tajer, Z.; Omran, M.; Al Nashaba, F.; Al Arrayedh, A.; Radhi, A.; et al. Antibiotics Point Prevalence. Bahrain Med. Bull. 2017, 39, 220–224. [Google Scholar] [CrossRef]
- Al Matar, M.; Enani, M.; Binsaleh, G.; Roushdy, H.; Alokaili, D.; Al Bannai, A.; Khidir, Y.; Al-Abdely, H. Point prevalence survey of antibiotic use in 26 Saudi hospitals in 2016. J. Infect. Public Health 2019, 12, 77–82. [Google Scholar] [CrossRef]
- Yaser, M.; Aljabri, A.K.; Alsaadi, F.N.; Rizk, L.M.; Alahmadi, R.Y.; Aljuhani, S.R.; Aljohani, S.H.; Al Thagfan, S.S.; Alamuddin, W.A.; Alonazie, W.S.; et al. A prospective antibiotic point prevalence survey in two primary referral hospitals during and after pilgrims stay in Madinah, Saudi Arabia. Trop. J. Pharm. Res. 2020, 19, 391–399. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A.; Al-Homoud, A.H. Pattern of systemic antibiotic use among hospitalized patients in a general hospital in Saudi Arabia. Travel. Med. Infect. Dis. 2020, 36, 101605. [Google Scholar] [CrossRef] [PubMed]
- Alsaedi, A.A.; El-Saed, A.; Althaqafi, A.; Bhutta, M.J.; Abukhzam, B.; Alshamrani, M. Antimicrobial therapy, resistance, and appropriateness in healthcare-associated and community-associated infections; a point prevalence survey. J. Infect. Chemother. 2022, 28, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Alnajjar, M.S.; Jawhar, D.S.; Aburuz, S.; Saeed, D.A.; Ibrahim, A.H. Point prevalence survey of antibiotic utilization in secondary care hospital in the United Arab Emirates. Pharm. Pract. 2022, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Adekoya, I.; Maraj, D.; Steiner, L.; Yaphe, H.; Moja, L.; Magrini, N.; Cooke, G.; Loeb, M.; Persaud, N. Comparison of antibiotics included in national essential medicines lists of 138 countries using the WHO Access, Watch, Reserve (AWaRe) classification: A cross-sectional study. Lancet Infect. Dis. 2021, 21, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Alsahli, A.; Alqarzaie, A.; Alasmari, A.; AlOtaibi, M.; Aljuraisi, A.; Khojah, A.; Alzahrani, N.M.; Alaqeel, F. Awareness and Knowledge of Postoperative Surgical Site Infections in Patients from Saudi Arabia: A Multi-Regional Cross-Sectional Study. Saudi J. Med. Med. Sci. 2022, 10, 243–252. [Google Scholar] [CrossRef]
- Momattin, H.; Al-Ali, A.Y.; Mohammed, K.; Al-Tawfiq, J.A. Benchmarking of antibiotic usage: An adjustment to reflect antibiotic stewardship program outcome in a hospital in Saudi Arabia. J. Infect. Public Health 2018, 11, 310–313. [Google Scholar] [CrossRef]
- Garcell, H.G.; Arias, A.V.; Sandoval, C.P.; Gamboa, M.E.V.; Sado, A.B.; Serrano, R.N.A. Impact of a focused antimicrobial stewardship program in adherence to antibiotic prophylaxis and antimicrobial consumption in appendectomies. J. Infect. Public Health 2017, 10, 415–420. [Google Scholar] [CrossRef]
- Chamieh, A.; Nawfal, T.D.; Ballouz, T.; Afif, C.; Juvelekian, G.; Hlais, S.; Rolain, J.M.; Azar, E. Control and Elimination of Extensively Drug-Resistant Acinetobacter baumanii in an Intensive Care Unit. Emerg Infect. Dis. 2019, 25, 1928–1931. [Google Scholar] [CrossRef]
- Moghnieh, R.; Awad, L.; Abdallah, D.; Jadayel, M.; Sinno, L.; Tamim, H.; Jisr, T.; El-Hassan, S.; Lakkis, R.; Dabbagh, R.; et al. Effect of a “handshake” stewardship program versus a formulary restriction policy on High-End antibiotic use, expenditure, antibiotic resistance, and patient outcome. J. Chemother. 2020, 32, 368–384. [Google Scholar] [CrossRef]
- Mahmoudi, L.; Sepasian, A.; Firouzabadi, D.; Akbari, A. The Impact of an Antibiotic Stewardship Program on the Consumption of Specific Antimicrobials and Their Cost Burden: A Hospital-wide Intervention. Risk Manag. Health Policy 2020, 13, 1701–1709. [Google Scholar] [CrossRef]
- Al-Omari, A.; Al Mutair, A.; Alhumaid, S.; Salih, S.; Alanazi, A.; Albarsan, H.; AbouRayan, M.; Al Subaie, M. The impact of antimicrobial stewardship program implementation at four tertiary private hospitals: Results of a five-years pre-post analysis. Antimicrob. Resist. Infect. Control 2020, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.A.-W.W.; Nawash, E.; Sonallah, H. Antimicrobial Stewardship: A Shared Responsibility among Primary Prescribers, Pharmacists, Infectious Disease Physicians and Microbiologists. J. Infect. Dis. Ther. 2020, 8, 420. [Google Scholar]
- Darwish, R.M.; Matar, S.G.; Abu Snaineh, A.A.; Alsharif, M.R.; Yahia, A.B.; Mustafa, H.N.; Hasabo, E.A. Impact of antimicrobial stewardship on antibiogram, consumption and incidence of multi drug resistance. BMC Infect. Dis. 2022, 22, 916. [Google Scholar] [CrossRef] [PubMed]
- Rahbarimanesh, A.; Mojtahedi, S.Y.; Sadeghi, P.; Ghodsi, M.; Kianfar, S.; Khedmat, L.; Siyahkali, S.J.M.; Yazdi, M.K.; Izadi, A. Antimicrobial stewardship program (ASP): An effective implementing technique for the therapy efficiency of meropenem and vancomycin antibiotics in Iranian pediatric patients. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 6. [Google Scholar] [CrossRef]
- GolAli, E.; Sistanizad, M.; Salamzadeh, J.; Haghighi, M.; Solooki, M. Antibiotic Prescribing Trends Before and After Implementation of an Audit and Feedback Program in Internal Ward of a Tertiary Hospital in Tehran. Iran. J. Pharm. Res. IJPR 2019, 18, 2136–2143. [Google Scholar] [CrossRef]
- Bhalla, N.; Hussein, N.; Atari, M.; Fakhri, R.; Lepora, C.; Walsh, N.; Cosgrove, S.E.; Murphy, R.A. Introducing an antibiotic stewardship program in a humanitarian surgical hospital. Am. J. Infect. Control 2016, 44, 1381–1384. [Google Scholar] [CrossRef] [PubMed]
- Alawi, M.M.; Darwesh, B.M. A stepwise introduction of a successful antimicrobial stewardship program. Saudi Med. J. 2016, 37, 1350–1358. [Google Scholar] [CrossRef]
- Yusef, D.; Hayajneh, W.A.; Issa, A.B.; Haddad, R.; Al-Azzam, S.; Lattyak, E.A.; Lattyak, W.J.; Gould, I.; Conway, B.R.; Bond, S.; et al. Impact of an antimicrobial stewardship programme on reducing broad-spectrum antibiotic use and its effect on carbapenem-resistant Acinetobacter baumannii (CRAb) in hospitals in Jordan. J. Antimicrob. Chemother. 2021, 76, 516–523. [Google Scholar] [CrossRef]
- El-Lababidi, R.M.; Mooty, M.; Bonilla, M.F.; Nusair, A.; Alatoom, A.; Mohamed, S. Implementation and outcomes of an advanced antimicrobial stewardship program at a quaternary care hospital in the United Arab Emirates. Int. Int. J. Avian Wildl. Biol. 2019, 2, 515–523. [Google Scholar] [CrossRef]
- Abdelrahman, D.H.; AbuSara, A.K.; Khabour, D.S. The Impact of Pharmacist-Led Antimicrobial Stewardship Review of Cultures in the Ambulatory Setting at a Comprehensive Cancer Center. Hosp. Pharm. 2023. [Google Scholar] [CrossRef]
- Salman, B.; Al-Hashar, A.; Al-Khirbash, A.; Al-Zakwani, I. Clinical and Cost Implications of Clinical Pharmacist Interventions on Antimicrobial Use at Sultan Qaboos University Hospital in Oman. Int. J. Infect. Dis. 2021, 109, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Kwon, K.T. Core Elements for Successful Implementation of Antimicrobial Stewardship Programs. Infect. Chemother. 2021, 53, 421. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antimicrobial Stewardship Programs in Health-Care Facilities in Low- and Middle-Income Countries: A WHO Practical Toolkit. 2019. Available online: https://www.who.int/publications/i/item/9789241515481 (accessed on 3 April 2023).
- Hajiabdolbaghi, M.; Makarem, J.; Salehi, M.; Manshadi, S.A.D.; Mohammadnejad, E.; Mazaherpoor, H.; Seifi, A. Does an antimicrobial stewardship program for Carbapenem use reduce Costs? An observation in Tehran, Iran. Casp. J. Intern. Med. 2020, 11, 329–332. [Google Scholar] [CrossRef]
- Bahrampour Juybari, K.; Vosooghi, V.; Zahmatkesh, M.; Mirmohammadkhani, M.; Paknazar, F. Compliance of imipenem and meropenem administration with the national antimicrobial stewardship program in a referral teaching hospital in Iran. Hosp. Pract. 2022, 50, 49–54. [Google Scholar] [CrossRef]
- Khasawneh, R.A.; Ababneh, M.A.; Al-Azzam, S.I. Antimicrobial stewardship programs: Perceptions and practices among Jordanian healthcare practitioners. J. Pharm. Health Serv. Res. 2021, 12, 235–241. [Google Scholar] [CrossRef]
- Sayegh, N.; Hallit, S.; Hallit, R.; Saleh, N.; Zeidan, R.K. Physicians’ attitudes on the implementation of an antimicrobial stewardship program in Lebanese hospitals. Pharm. Pract. 2021, 19, 2192. [Google Scholar] [CrossRef]
- Nasr, Z.; Babiker, A.; Elbasheer, M.; Osman, A.; Elazzazy, S.; Wilby, K.J. Practice implications of an antimicrobial stewardship intervention in a tertiary care teaching hospital, Qatar. East. Mediterr. Health J. 2019, 25, 172–180. [Google Scholar] [CrossRef]
- Sid Ahmed, M.A.; Abdel Hadi, H.; Abu Jarir, S.; Al Khal, A.L.; Al-Maslamani, M.A.; Jass, J.; Ibrahim, E.B.; Ziglam, H. Impact of an antimicrobial stewardship programme on antimicrobial utilization and the prevalence of MDR Pseudomonas aeruginosa in an acute care hospital in Qatar. JAC Antimicrob. Resist. 2020, 2, dlaa050. [Google Scholar] [CrossRef]
- Amer, M.R.; Akhras, N.S.; Mahmood, W.A.; Al-Jazairi, A.S. Antimicrobial stewardship program implementation in a medical intensive care unit at a tertiary care hospital in Saudi Arabia. Ann. Saudi Med. 2013, 33, 547–554. [Google Scholar] [CrossRef]
- Momattin, H.; Zogheib, M.; Homoud, A.; Al-Tawfiq, J.A. Safety and Outcome of Pharmacy-Led Vancomycin Dosing and Monitoring. Chemotherapy 2016, 61, 3–7. [Google Scholar] [CrossRef]
- Baraka, M.A.; Alsultan, H.; Alsalman, T.; Alaithan, H.; Islam, A.; Alasseri, A.A. Health care providers’ perceptions regarding antimicrobial stewardship programs (AMS) implementation—Facilitators and challenges: A cross-sectional study in the Eastern province of Saudi Arabia. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Faidah, H.S.; Al-Gethamy, M.; Iqbal, M.S.; Alhifany, A.A.; Ali, M.; Abuhussain, S.S.A.; Elrggal, M.E.; Almalki, W.H.; Alghamdi, S.; et al. Evaluation of Antimicrobial Stewardship Programs (ASPs) and their perceived level of success at Makkah region hospitals, Kingdom of Saudi Arabia. Saudi Pharm. J. 2020, 28, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Faidah, H.S.; Al-Gethamy, M.; Iqbal, M.S.; Barnawi, A.M.; Elahe, S.S.; Bukhari, D.N.; Al-Sulaimani, T.M.N.; Fadaaq, M.; Alghamdi, S.; et al. Evaluation of a Multidisciplinary Antimicrobial Stewardship Program in a Saudi Critical Care Unit: A Quasi-Experimental Study. Front. Pharmacol. 2021, 11, 2222. [Google Scholar] [CrossRef]
- El-Lababidi, R.; Mooty, M.; Nusair, A.; Bonilla, M.-F. Implementation and Outcomes of an Advanced Antimicrobial Stewardship Program at a Quaternary Care Hospital in the United Arab Emirates. Open Forum Infect. Dis. 2017, 4, S265. [Google Scholar] [CrossRef]
- Abu Taha, A.; Abu-Zaydeh, A.H.; Ardah, R.A.; Al-Jabi, S.W.; Sweileh, W.M.; Awang, R.; Zyoud, S.H. Public Knowledge and Attitudes Regarding the Use of Antibiotics and Resistance: Findings from a Cross-Sectional Study among Palestinian Adults. Zoonoses Public Health 2016, 63, 449–457. [Google Scholar] [CrossRef]
- Zyoud, S.H.; Abu Taha, A.; Araj, K.F.; Abahri, I.A.; Sawalha, A.F.; Sweileh, W.M.; Awang, R.; Al-Jabi, S.W. Parental knowledge, attitudes and practices regarding antibiotic use for acute upper respiratory tract infections in children: A cross-sectional study in Palestine. BMC Pediatr. 2015, 15, 176. [Google Scholar] [CrossRef]
- Alrafiaah, A.S.; Alqarny, M.H.; Alkubedan, H.Y.; AlQueflie, S.; Omair, A. Are the Saudi parents aware of antibiotic role in upper respiratory tract infections in children? J. Infect. Public Health 2017, 10, 579–585. [Google Scholar] [CrossRef]
- Saleh Faidah, H.; Haseeb, A.; Yousuf Lamfon, M.; Mohammad Almatrafi, M.; Abdullah Almasoudi, I.; Cheema, E.; Hassan Almalki, W.; EElrggal, M.; MAMohamed, M.; Saleem, F.; et al. Parents’ self-directed practices towards the use of antibiotics for upper respiratory tract infections in Makkah, Saudi Arabia. BMC Pediatr. 2019, 19, 46. [Google Scholar] [CrossRef]
- Al-Qerem, W.; Hammad, A.; Jarab, A.; Saleh, M.M.; Amawi, H.A.; Ling, J.; Alasmari, F. Knowledge, attitudes, and practice with respect to antibiotic use among pharmacy students: A cross-sectional study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3408–3418. [Google Scholar]
- Al-Taani, G.M.; Al-Azzam, S.; Karasneh, R.A.; Sadeq, A.S.; Al Mazrouei, N.; Bond, S.E.; Conway, B.R.; Aldeyab, M.A. Pharmacists’ Knowledge, Attitudes, Behaviors and Information Sources on Antibiotic Use and Resistance in Jordan. Antibiotics 2022, 11, 175. [Google Scholar] [CrossRef]
- Al-Taani, G.M.; Karasneh, R.A.; Al-Azzam, S.; Bin Shaman, M.; Jirjees, F.; Al-Obaidi, H.; Conway, B.R.; Aldeyab, M.A. Knowledge, Attitude, and Behavior about Antimicrobial Use and Resistance among Medical, Nursing and Pharmacy Students in Jordan: A Cross Sectional Study. Antibiotics 2022, 11, 1559. [Google Scholar] [CrossRef] [PubMed]
- Karasneh, R.A.; Al-Azzam, S.I.; Ababneh, M.; Al-Azzeh, O.; Al-Batayneh, O.B.; Muflih, S.M.; Khasawneh, M.; Khassawneh, A.R.M.; Khader, Y.S.; Conway, B.R.; et al. Prescribers’ Knowledge, Attitudes and Behaviors on Antibiotics, Antibiotic Use and Antibiotic Resistance in Jordan. Antibiotics. 2021, 10, 858. [Google Scholar] [CrossRef] [PubMed]
- Hashemzaei, M.; Afshari, M.; Koohkan, Z.; Bazi, A.; Rezaee, R.; Tabrizian, K. Knowledge, attitude, and practice of pharmacy and medical students regarding self-medication, a study in Zabol University of Medical Sciences; Sistan and Baluchestan province in south-east of Iran. BMC Med. Educ. 2021, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Sami, R.; Sadegh, R.; Fani, F.; Atashi, V.; Solgi, H. Assessing the knowledge, attitudes and practices of physicians on antibiotic use and antimicrobial resistance in Iran: A cross-sectional survey. J. Pharm. Policy Pract. 2022, 15, 82. [Google Scholar] [CrossRef]
- Zahreddine, L.; Hallit, S.; Shakaroun, S.; Al-Hajje, A.; Awada, S.; Lahoud, N. Knowledge of pharmacists and parents towards antibiotic use in pediatrics: A cross-sectional study in Lebanon. Pharm. Pract. 2018, 16, 1194. [Google Scholar] [CrossRef] [PubMed]
- Jabbarin, H.; Nawajah, I.; Hejaz, H.A. Knowledge, Attitude, Awareness, and Perceptions among Physicians toward Antibiotic Resistance in Hospitals in South Palestine. Avicenna J. Med. 2023, 13, 049–055. [Google Scholar] [CrossRef] [PubMed]
- Akbar, Z.; Alquwez, N.; Alsolais, A.; Thazha, S.K.; Ahmad, M.D.; Cruz, J.P. Knowledge about antibiotics and antibiotic resistance among health-related students in a Saudi University. J. Infect. Dev. Ctries. 2021, 15, 925–933. [Google Scholar] [CrossRef]
- WHO. WHO Methodology for Point Prevalence Survey on Antibiotic Use in Hospitals, Version 1.1. 2018. Available online: https://www.who.int/publications/i/item/WHO-EMP-IAU-2018.01 (accessed on 5 April 2023).
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Mustafa, Z.U.; Saleem, M.S.; Ikram, M.N.; Salman, M.; Butt, S.A.; Khan, S.; Godman, B.; Seaton, R.A. Co-infections and antimicrobial use among hospitalized COVID-19 patients in Punjab, Pakistan: Findings from a multicenter, point prevalence survey. Pathog. Glob. Health 2022, 116, 421–427. [Google Scholar] [CrossRef]
- Jampani, M.; Chandy, S.J. Increased antimicrobial use during COVID-19: The risk of advancing the threat of antimicrobial resistance. Health Sci. Rep. 2021, 4, e459. [Google Scholar] [CrossRef]
- Hsu, J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ 2020, 369, m1983. [Google Scholar] [CrossRef] [PubMed]
- El-Hassan, O.; Sharif, A.; Al Redha, M.; Blair, I. Tracking the Implementation of Electronic Medical Records in Dubai, United Arab Emirates, Using an Adoption Benchmarking Tool. Stud. Health Technol. Inform. 2017, 245, 64–68. [Google Scholar] [PubMed]
- Neamah, A.; Ghani, M.; Ahmad, A.; Alomari, E.; Nuiaa, R.R. E-Health State in Middle East Countries: An Overview. Turk. Online J. Des. Art Commun. 2018, 1, 2974–2990. [Google Scholar]
- Akpan, M.R.; Isemin, N.U.; Udoh, A.E.; Ashiru-Oredope, D. Implementation of antimicrobial stewardship programmes in African countries: A systematic literature review. J. Glob. Antimicrob. Resist. 2020, 22, 317–324. [Google Scholar] [CrossRef]
- Ogunleye, O.O.; Basu, D.; Mueller, D.; Sneddon, J.; Seaton, R.A.; Yinka-Ogunleye, A.F.; Wamboga, J.; Miljković, N.; Mwita, J.C.; Rwegerera, G.M.; et al. Response to the Novel Corona Virus (COVID-19) Pandemic Across Africa: Successes, Challenges, and Implications for the Future. Front. Pharmacol. 2020, 11, 1205. [Google Scholar] [CrossRef]
- Godman, B.; Grobler, C.; Van-De-Lisle, M.; Wale, J.; Barbosa, W.B.; Massele, A.; Opondo, P.; Petrova, G.; Tachkov, K.; Sefah, I.; et al. Pharmacotherapeutic interventions for bipolar disorder type II: Addressing multiple symptoms and approaches with a particular emphasis on strategies in lower and middle-income countries. Expert Opin. Pharmacother. 2019, 20, 2237–2255. [Google Scholar] [CrossRef]
- Godman, B.; Egwuenu, A.; Haque, M.; Malande, O.O.; Schellack, N.; Kumar, S.; Saleem, Z.; Sneddon, J.; Hoxha, I.; Islam, S.; et al. Strategies to Improve Antimicrobial Utilization with a Special Focus on Developing Countries. Life 2021, 11, 528. [Google Scholar] [CrossRef]
- Godman, B.; Egwuenu, A.; Wesangula, E.; Schellack, N.; Kalungia, A.C.; Tiroyakgosi, C.; Kgatlwane, J.; Mwita, J.C.; Patrick, O.; Niba, L.L.; et al. Tackling antimicrobial resistance across sub-Saharan Africa: Current challenges and implications for the future. Expert Opin. Drug. Saf. 2022, 21, 1089–1111. [Google Scholar] [CrossRef]
- WHO. Anatomical Therapeutic Chemical (ATC) Classification. 2021. Available online: https://www.who.int/tools/atc-ddd-toolkit/atc-classification (accessed on 2 February 2023).
- Cooper, L.; Sneddon, J.; Afriyie, D.K.; Sefah, I.A.; Kurdi, A.; Godman, B.; Seaton, R.A. Supporting global antimicrobial stewardship: Antibiotic prophylaxis for the prevention of surgical site infection in low- and middle-income countries (LMICs): A scoping review and meta-analysis. JAC-Antimicrob. Resist. 2020, 2, dlaa070. [Google Scholar] [CrossRef]
- Abdel Jalil, M.H.; Abu Hammour, K.; Alsous, M.; Awad, W.; Hadadden, R.; Bakri, F.; Fram, K. Surgical site infections following caesarean operations at a Jordanian teaching hospital: Frequency and implicated factors. Sci. Rep. 2017, 7, 12210. [Google Scholar] [CrossRef]
- Hamza, W.S.; Salama, M.F.; Morsi, S.S.; Abdo, N.M.; Al-Fadhli, M.A. Benchmarking for surgical site infections among gastrointestinal surgeries and related risk factors: Multicenter study in Kuwait. Infect. Drug. Resist. 2018, 11, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Godman, B.; Basu, D.; Pillay, Y.; Mwita, J.C.; Rwegerera, G.M.; Paramadhas, B.D.A.; Tiroyakgosi, C.; Okwen, P.M.; Niba, L.L.; Nonvignon, J.; et al. Review of Ongoing Activities and Challenges to Improve the Care of Patients with Type 2 Diabetes Across Africa and the Implications for the Future. Front. Pharmacol. 2020, 11, 108. [Google Scholar] [CrossRef] [PubMed]
| Country | Author and Year | No. of Hospitals | PPS Timing (Period) | PPS Protocol | Study Duration (Period) | AM Use Rate n (%) | 1st AM ATC Code (%) and AWaRe Classification | 2nd AM ATC Code (%) and AWaRe Classification | 3rd AM ATC Code (%) and AWaRe Classification | Used for Prophylaxis(%) | Used for Treatment (%) | Total Antimicrobials Delivered (Per/Patient) for Their Infections |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Middle-Income Countries * | ||||||||||||
| Egypt | Ashour et al., 2022 [112] | 1 | Daily two weeks | ECDC | During July 2019 | 300 (79.15%) | Aminopenicillins+ Beta-lactamase inhibitors (A) J01CR (43.3%) | 3rd Generation cephalosporins (W) J01DD (29.0%) | Fluroquinolones (W) J01MA (8.7%) | 62.3 | 37.7 | 1.9 |
| Egypt/Various | Alothman et al., 2020 [52] | 11 | One day | ECDC | 3 April 2018 | 1586 (84.9%) | Cephalosporins (W) J01D (32.4%) | Carbapenems (W) J01DH (18.8%) | Glycopeptides (W) J01XA (16.9%) | - | 28.3 | - |
| Iran | Fahimzad et al., 2016 [113] | 16 | Daily over one week | Standard | January 2014 and February 2014 | 571 (66.66%) | Ceftriaxone (W) J01DD04 (36.6%) | Metronidazole (A) J01XD01 (23.8%) | Vancomycin (W) J01XA01 (18.8%) | 26.77 | 73.1 | - |
| Iran | Soltani et al., 2018 [114] | 2 | Two weeks | ARPEC-web PPS | October 2011 and 2012 | 252 (64%) | Ceftriaxone (W) J01DD04 (19.9%) | Ampicillin (A) J01CA01 (14.3%) | Vancomycin (W) J01XA01 (13.3%) | 7.5 | 88 | - |
| Morocco | Razine et al., 2012 [115] | 1 | - | - | January 2010 | 392 (32.8%) | Amoxicillin Clavulanic acid (A) J01CR02 (32%) | 3rd Generation Cephalosporins (W) J01DD (13%) | Gentamycin (A) J01GB03 (10%) | - | - | - |
| Morocco | Chiguer et al., 2018 [116] | 1 | - | ECDC | 5 June to 19 July 2017 | - | 3rd generation cephalosporins (W) J01DD (38.2%) | - | - | - | - | - |
| Palestine | Alagha et al., 2022 [117] | 3 | - | WHO | July 2019 to January 2020 | 1400 (68.2%) | Ceftriaxone (W) J01DD04 (47.5%) | Cefazolin (A) J01DB04 (10.6%) | Ciprofloxacin (W) J01MA02 (W) (8.6%) | - | - | 1.26 |
| Tunisia | Ayed et al., 2019 [118] | 2 | One Week | Self | 3–10 July 2017 | 371 (49.2%) | - | - | - | 20 | 80 | - |
| Tunisia | Maamri et al., 2021 [119] | 2 | - | - | 2019 | 410 (39.2%) | Penicillin (A) J01C (26.7%) | Fluroquinolones (W) J01MA (20%) | 3rd generation cephalosporins (W) J01DD (16.5%) | 24.9 | - | - |
| Upper Middle-Income Countries * | ||||||||||||
| Iraq | Nassr et al., 2018 [53] | 4 | Period | Descriptive statical analysis | October 2017 and April 2018 | 177 (88.5%) | Parenteral 3rd generation cephalosporins (W) J01DD (54.3%) | Nitroimidazole P01AB (29.4%) | Quinolones (W) J01M (7.5%) | - | - | 1.65 |
| Iraq | Kurdi et al., 2020 [27] | 3 | Period | Global PPS | Sep-Dec 2019 | 192 (93.7%) | Cephalosporins 3rd generation J01DD (52.6%) | Imidazole derivatives J01XD (16.9%) | Beta-lactam Penicillin J01C (10.5%) | 17.3 | 82.7 | 1.38 |
| Iraq | Kurmanji et al., 2021 [120] | 1 | Period | Global PPS | January–April 2019 | 402 (66.2%) | Ceftriaxone (W) J01DD04 (70%) | - | - | - | - | - |
| Iraq | Kurmanji et al, 2021 [121] | 5 | Period | Global PPS | January 2019 to April 2019 | 808 (66.7%) | Ceftriaxone (W) J01DD04 (31.5%) | Metronidazole (A) J01XD01 (20.9) | Meropenem (W) J01DH02 (10.1) | 51.1 | 42.4 | - |
| Jordan | Elhajji et al., 2018 [122] | 1 | One day | Global PPS | 2015 | 85(78.2%) | 3rd generation Cephalosporins (W) J01DD (26.2%) | Fluroquinolones (W) J01MA (18.5%) | Carbapenems (R) J01DH (15.4%) | 40 | 60 | 1.42 |
| Jordan | Abu Hannour et al., 2020 [123] | 1 | Period | Global PPS | June–July 2018 | 488 (98.1%) | Cephalosporins J01D (50.6%) | Carbapenems (R) J01DH (39.6%) | Imidazole derivatives (A) J01XD (22.4%) | - | - | 1.57 |
| Jordan | Ababneh et al., 2021 [124] | 1 | One day | Standard | 13 August 2018 | 144 (21.1%) | - | - | - | - | - | - |
| High-Income Countries * | ||||||||||||
| Bahrain | Al Salman et al., 2017 [125] | 1 | - | Global PPS | 1 Feburary 2015 to 30 April 2015 | 263 (70.7%) | Beta-lactam Other than penicillin (W) J01D (42.5%) | Penicillin (A) J01C (15.3%) | - | - | - | - |
| Qatar | Saleem et al., 2020 [40] | 1 | Repeated | ECDC | April–May 2012 | 25 (43.0%) | Penicillin plus beta-lactamase inhibitors (W) J01CR (39.4%) | Carbapenems (RW) J01DH (15.2%) | Fluroquinolones (W) J01 MA (9.1%) | 6.1 | 93.9 | 1.32 |
| Saudi Arabia | Al Matar et al., 2018 [126] | 26 | One Day | Global PPS | May 2016 | 2182(46.9%) | 3rd generation cephalosporins J01DD (17.2%) | Penicillin J01C (9.4%) | Penicillin and enzyme inhibitor J01CR (8.8%) | 34.6 | 61.4 | 1.4 |
| Saudi Arabia | Alahmadi et al., 2020 [127] | 2 | Two-week period | ECDC | September 2016 and November 2016 | 332(49.18%) | 3rd generation cephalosporins (W) J01DD (16.5%) | 2nd generation cephalosporins (W) J01DC (8.6%) | Fluroquinolones (W) J01MA (8.6%) | - | - | 1.5 |
| Saudi Arabia | Al-Tawfiq et al. [128] | 1 | Period | - | January 2017 to January 2019 | 200 (40%) | Meropenem (W) J01DH02 (18%) | Cefazolin (A) J01DB04 (10%) | Ceftriaxone (W) J01DD04 (8%) | 8.5 | 89.5 | 1.3 |
| Saudi Arabia | Haseeb et al., 2021 [54] | 6 | Period | Global PPS | January 2019 to July 2019 | 447 (61.9%) | Ceftriaxone (W) J01DD04 (15%) | Piperacillin (W) J01CA12 (10.9%) | Metronidazole (A) J01XD01 (7.1%) | 7.9 | 92.1 | 1.7 |
| Saudi Arabia | Alsaedi et al., 2022 [129] | 6 | One day | Standard | 2017 | 240(14.4%) | Carbapenems (R) J01DH (19.6%) | Cephalosporins J01D (14.8%) | Vancomycin (W) J01XA01 (13.2%) | - | - | - |
| United Arab Emirates | Alnajjar et al., 2022 [130] | 1 | One day | ESAC | 26 January 2020 | 41(32.8%) | Combinations of penicillin (W) J01CR50 (31.5%) | Amoxicillin-clavulanic acid (A) J01CR02 (22.2%) | Piperacillin-tazobactam (W) J01CR05 (9.3%) | 29.6 | 70.4 | 1.3 |
| Country | Author and Year | Findings including Number of Patients Where Documented |
|---|---|---|
| Low Middle-Income Countries * | ||
| Egypt | Ashour et al., 2022 [112] |
|
| Tunisia | Ayed et al., 2019 [118] | Out of 371 admitted patients, 73 (20%) were administered, with a duration > 1 day. |
| Upper Middle-income Countries * | ||
| Iraq | Nassr et al., 2018 [53] | All patients (77) undergoing SAP were prescribed antibiotics >1 day. |
| Iraq | Kurmanji et al., 2021 [120] | 89% of patients received SAP for more >1 day for the different surgical categories, including obstetric and gynecological indications. |
| Iraq | Kurnanji et al., 2021 [121] | 65.5% of surgical and medical prophylactic antibiotics were used for >1 day, especially ceftriaxone, across five teaching hospitals. |
| Jordan | Abu Hammour et al., 2020 [123] |
|
| High-Income Countries * | ||
| Saudi Arabia | Al Matar et al., 2019 [126] |
|
| Saudi Arabia | Al-Tawfiq et al., 2020 [128] | Duration of antibiotic administration for SAP was typically >1 day. |
| Saudi Arabia | Haseeb et al., 2021 [54] | 20.9% of patients undergoing SAP received a single dose of antibiotics, 35.2% for one day and 43.9% for >1 day. |
| United Arab Emirates | Alnajjar et al., 2020 [130] | 10/16 patients (62.5%) received antibiotics for more than one day to prevent SSIs. |
| United Arab Emirates | Alshehhi et al. 2021 [67] |
|
| United Arab Emirates | Vippadapu et al., 2022 [68] | Almost all patients undergoing SAP to prevent SSIs were prescribed discharge antimicrobials (99%) |
| Indicator | Reference |
|---|---|
| Activity (Process/Performance) Indicators | |
| Defined daily doses (DDDs) and DDDs/100 or 1000 bed-days/patient days | [124,133,134,135,136,137,138,139,140] |
| % of inpatients prescribed antibiotics/antimicrobials | [27,40,86,112,114,125,126] |
| Days of therapy per 1000 study patient days | [70] |
| Average number of antibiotics prescribed per in-patient | [53] |
| % of patients’ notes where the indication for administering antibiotics is documented/stop or review dates recorded | [27,53,86] |
| % of patients prescribed antibiotics postoperatively for SAP for longer than 24 h after surgery/duration of SAP postoperatively | [67,123,126,134] |
| % Empiric prescribing vs. targeted antibiotic prescribing (following culture and sensitivity testing results) | [27,86,113,114,141] |
| % of “Access” versus “Watch” or “Reserve” antibiotics or % reduction in targeted antibiotics | [44,82,137,142,143] |
| % decreased prescribing of restricted antibiotics | [144,145,146] |
| % compliance with current guidelines | [54,86,126,134,139,142] |
| % de-escalation, including IV to oral switches and their timing | [82,133,139,142,147] |
| % reduction in length of stay | [67,141] |
| % reduction in expenditure of (targeted) antimicrobials | [137,138,143,146,148] |
| Outcome indicators | |
| % of patients postoperatively getting SSIs | [60,61,62] |
| % reduction in resistance rates to targeted pathogens | [135,140,145,146] |
| % reduction in re-admission rates | [82] |
| % decrease in healthcare-associated infections | [138,139,146] |
| % reduction in mortality rates | [82,136,141,142,144] |
| Country | Author, and Year | Intervention and Aim | Impact of the Intervention |
|---|---|---|---|
| Lower Middle-Income Countries * | |||
| Iran | GolAli et al., 2017 [142] |
|
|
| Iran | Rahbarimanesh et al., 2019 [141] |
|
|
| Iran | Mahmoudi et al., 2020 [137] |
|
|
| Iran | Hajiabdolbaghi et al., 2020 [151] |
|
|
| Iran | Bahrampour Juybari et al., 2022 [152] |
|
|
| Jordan/Various including Yemen | Bhalla et al., 2016 [143] |
|
|
| Jordan | Yusef et al., 2021 [145] |
|
|
| Jordan | Khasawneh et. al., 2021 [153] |
|
|
| Jordan | Darwish et al., 2022 [140] |
|
|
| Upper Middle-Income Countries * | |||
| Lebanon | Chamieh et al., 2019 [135] |
|
|
| Lebanon | Moghnieh et al., 2020 [136] | Implementation of an ASP based on the “handshake” strategy for 2 years in this hospital. |
|
| Lebanon | Sayegh et al., 2021 [154] |
|
|
| Lebanon | Shallal et al., 2022 [70] |
|
|
| High Income Countries * | |||
| Qatar | Garcell et al., 2017 [134] |
|
|
| Qatar | Nasr et al., 2019 [155] |
|
|
| Qatar | Shaukat et al., 2020 [139] |
|
|
| Qatar | Sid Ahmed et al., 2020 [156] |
|
|
| Saudi Arabia | Amer et al., 2013 [157] |
|
|
| Saudi Arabia | Alawi et al., 2016 [144] |
|
|
| Saudi Arabia | Momattin et al., 2018 [133] |
|
|
| Saudi Arabia | Baraka et al., 2019 [159] |
|
|
| Saudi Arabia | Haseeb et al., 2020 [160] |
|
|
| Saudi Arabia | Haseeb et al., 2021 [161] |
|
|
| Saudi Arabia | Al-Omari et al., 2020 [138] |
|
|
| United Arab Emirates | El-Lababidi et al., 2017 [162] |
|
|
| United Arab Emirates | El-Lababidi et al., 2019 [146] |
|
|
| Alshehhi et al., 2021 [67] |
|
| |
| Sadeq et al., 2021 [82] |
|
| |
| Country | Author, Year | Study Design | Population | Objectives | Knowledge | Attitude/Perception | Practice | Inference |
|---|---|---|---|---|---|---|---|---|
| Jordan | Al-Qerem et al., 2022 [167] | Quantitative | Pharmacy students | AMR is a major health threat and efforts should be intensified to reduce its burden. Healthcare providers, especially pharmacists, can be actively involved in the reduction of AMR. | Appreciable number of students have knowledge of antibiotics (>60%). | Positive attitude | Overall good knowledge. However, concerns with practice in some areas, including the use of antibiotics and their disposal. | Universities should ensure that pharmacy students acquire adequate education about antibiotics, including their use. |
| Jordan | Al-Tani et al., 2022 [168] | Quantitative | Pharmacists | To assess the knowledge, opportunity, motivation, and behavior of pharmacists and their information. | Respondents score highly on effective use of antibiotics and side effects (87%). One-third reported no knowledge of any initiatives on antibiotics or AMR. | Positive attitude | Pharmacists indicated an interest in receiving more information on AMR and medical conditions where antibiotics are appropriate. | Require more knowledge of antibiotics, their appropriate use, and AMR. |
| Jordan | Al-Tani et al., 2022 [169] | Quantitative | Medicine, nursing, and Pharmacy students | Survey students’ knowledge, attitude, and practice regarding antimicrobial use and AMR. | Knowledge of more than three-quarters of respondents was good regarding antibiotics and side effects. | Positive attitude | Low awareness of the national action plan on AMR. | Require more information regarding antibiotic use and AMR. |
| Jordan | Karasneh et al., 2021 [170] | Quantitative | Physicians and dentists | Ascertain prescribers’ knowledge, attitudes, and behaviors about antibiotic use and AMR. | Prescribers had good knowledge of antibiotics, indications, and side effects (>90%). Lower knowledge of AMR (62.2%). | Positive attitude | Strategies not implemented effectively. | Educate practitioners and update them about local and global antibiotic use status and AMR. |
| Iran | Hashemzaei et al., 2021 [171] | Quantitative | Pharmacy and medical students | To investigate the knowledge, attitude, and practice of pharmacy and medical students toward self-medication. | There was no difference in the level of knowledge, mostly associated with years of study. Pharmacy students had better knowledge. | Pharmacy students had more negative attitudes than medical students. | Concerns with following national protocols. | The high prevalence of self-medication and the overuse of antibiotics can pose a significant risk of AMR. |
| Iran | Sami et al., 2022 [172] | Quantitative | Physicians | To assess KAP in Physicians. | 97.2% were aware of the AMR problem in Iran. | 95.6% agreed that prescribing of antimicrobials was not appropriate in Iran. | 65.9% said before prescribing, they used local/ international guidelines <50% were in contact with a microbiology laboratory to guide prescribing. | Physicians’ level of knowledge about AMR and antimicrobial stewardship is poor. Consequently, a need to increase training on AMR and ASPs. |
| Lebanon | Zahreddine et al., 2018 [173] | Quantitative | Pharmacists and parents | To assess knowledge of both parents of children and pharmacist on antibiotics and AMR. | One-third of pharmacists did not know which factors were associated with AMR. Parents with university education had better knowledge of antibiotics. | Positive attitude | Misuse of antibiotics mostly involves parents, physicians, and pharmacists and not adhering to guidelines. | Implement educational campaigns in order to increase awareness of antibiotics, their misuse, and their implications on AMR. |
| Palestine | Al-Halawa et al., 2023 [29] | Quantitative | Community pharmacists | Evaluate the knowledge, attitude, and practices of community pharmacists to AMR. | Appreciable number (92.1%) said that inappropriate use increases resistance, and 86.2% disagreed that patients should stop antibiotics when symptoms improve. | Generally positive, with 75.6% agreeing that AMR is a serious public health issue. | Need to design programs to further improve the education of pharmacists and decrease dispensing of antibiotics without a prescription. | Re-design education and training, and strengthen legislation to reduce dispensing of antibiotics without a prescription. |
| Palestine | Jabbarin et al. [174] | Quantitative | Physicians | Evaluate the knowledge, attitude, awareness, and perceptions of AMR among physicians; and the correlation between their knowledge of AMR and experience. | Variable knowledge and perceptions of AMR. Senior specialists/consultants more knowledgeable about AMR. | Generally positive attitude to AMR, with 69.3% perceiving AMR as a very important problem worldwide and 54.7% a very important problem in the country. | A need to increase education on AMR among physicians with an emphasis on junior physicians Instigate activities to raise physicians’ awareness regarding AMR and its consequences on public health. | Implement educational programs among both practicing physicians and students to enhance knowledge of AMR and its public health importance. |
| Saudi Arabia | Akbar et al., 2021 [175] | Quantitative | Clinical laboratory science, nurses, and pharmacy students | Evaluate the knowledge of future HCWs in Saudi Arabia on antibiotics, antibiotic use, and antibiotic resistance. | Students have above-average knowledge of antibiotics and AMR However, misconceptions about antibiotics/ their use. | Happy to change. | Need improvements, especially in antibiotic use. | Curricular contents must be reviewed and enhanced to suit the specific learning needs of students. |
| Short to Medium Term (1–5 Years) |
|---|
|
| Long Term (5–10 Years) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haseeb, A.; Saleem, Z.; Maqadmi, A.F.; Allehyani, R.A.; Mahrous, A.J.; Elrggal, M.E.; Kamran, S.H.; AlGethamy, M.; Naji, A.S.; AlQarni, A.; et al. Ongoing Strategies to Improve Antimicrobial Utilization in Hospitals across the Middle East and North Africa (MENA): Findings and Implications. Antibiotics 2023, 12, 827. https://doi.org/10.3390/antibiotics12050827
Haseeb A, Saleem Z, Maqadmi AF, Allehyani RA, Mahrous AJ, Elrggal ME, Kamran SH, AlGethamy M, Naji AS, AlQarni A, et al. Ongoing Strategies to Improve Antimicrobial Utilization in Hospitals across the Middle East and North Africa (MENA): Findings and Implications. Antibiotics. 2023; 12(5):827. https://doi.org/10.3390/antibiotics12050827
Chicago/Turabian StyleHaseeb, Abdul, Zikria Saleem, Aseel Fayk Maqadmi, Roaa Abdulrahman Allehyani, Ahmad J. Mahrous, Mahmoud E. Elrggal, Sairah Hafeez Kamran, Manal AlGethamy, Asem Saleh Naji, Abdullmoin AlQarni, and et al. 2023. "Ongoing Strategies to Improve Antimicrobial Utilization in Hospitals across the Middle East and North Africa (MENA): Findings and Implications" Antibiotics 12, no. 5: 827. https://doi.org/10.3390/antibiotics12050827
APA StyleHaseeb, A., Saleem, Z., Maqadmi, A. F., Allehyani, R. A., Mahrous, A. J., Elrggal, M. E., Kamran, S. H., AlGethamy, M., Naji, A. S., AlQarni, A., Alhariqi, K. W., Khan, M. A., Ibrahim, K., Raees, F., Azmat, A., Cook, A., Campbell, S. M., Lorenzetti, G., Meyer, J. C., ... Moore, C. E. (2023). Ongoing Strategies to Improve Antimicrobial Utilization in Hospitals across the Middle East and North Africa (MENA): Findings and Implications. Antibiotics, 12(5), 827. https://doi.org/10.3390/antibiotics12050827










