Shikonin Alleviates Gentamicin-Induced Renal Injury in Rats by Targeting Renal Endocytosis, SIRT1/Nrf2/HO-1, TLR-4/NF-κB/MAPK, and PI3K/Akt Cascades
Abstract
1. Introduction
2. Results
2.1. Shikonin Mitigates Gentamicin-Triggered Renal Dysfunction
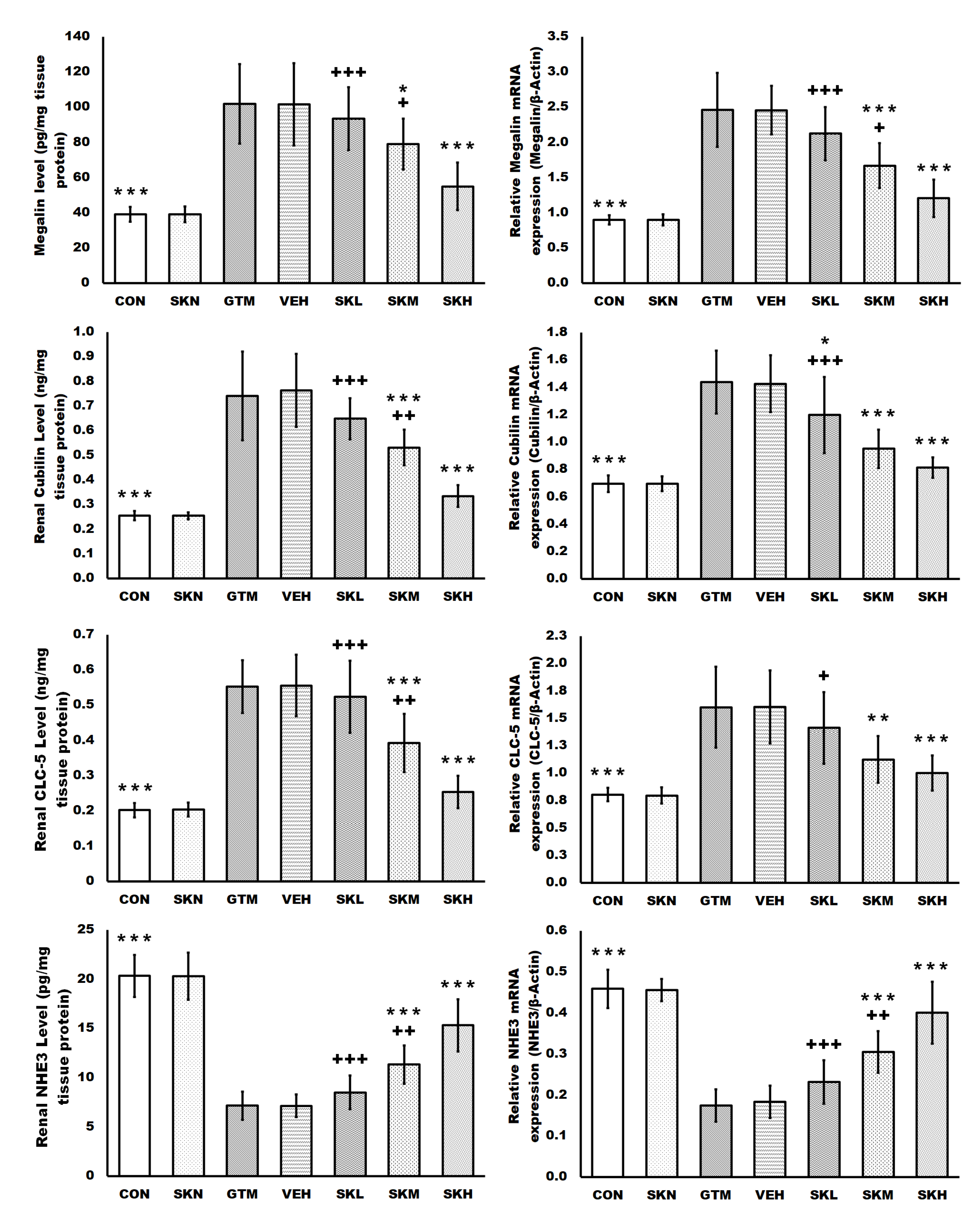
2.2. Shikonin Restored the Endocytosis Function of Proximal Tubule Epithelial Cells Stimulated by Gentamicin
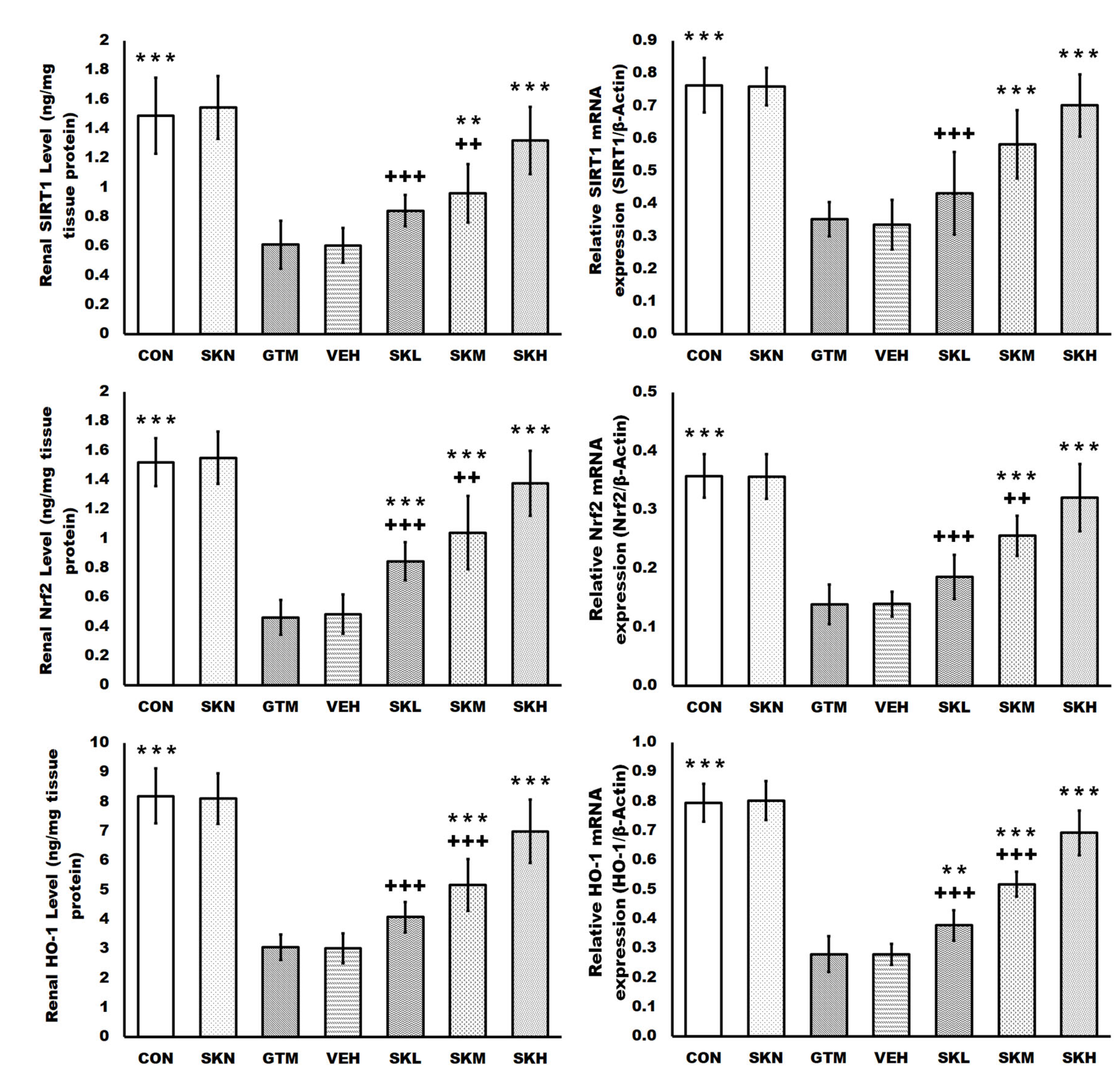
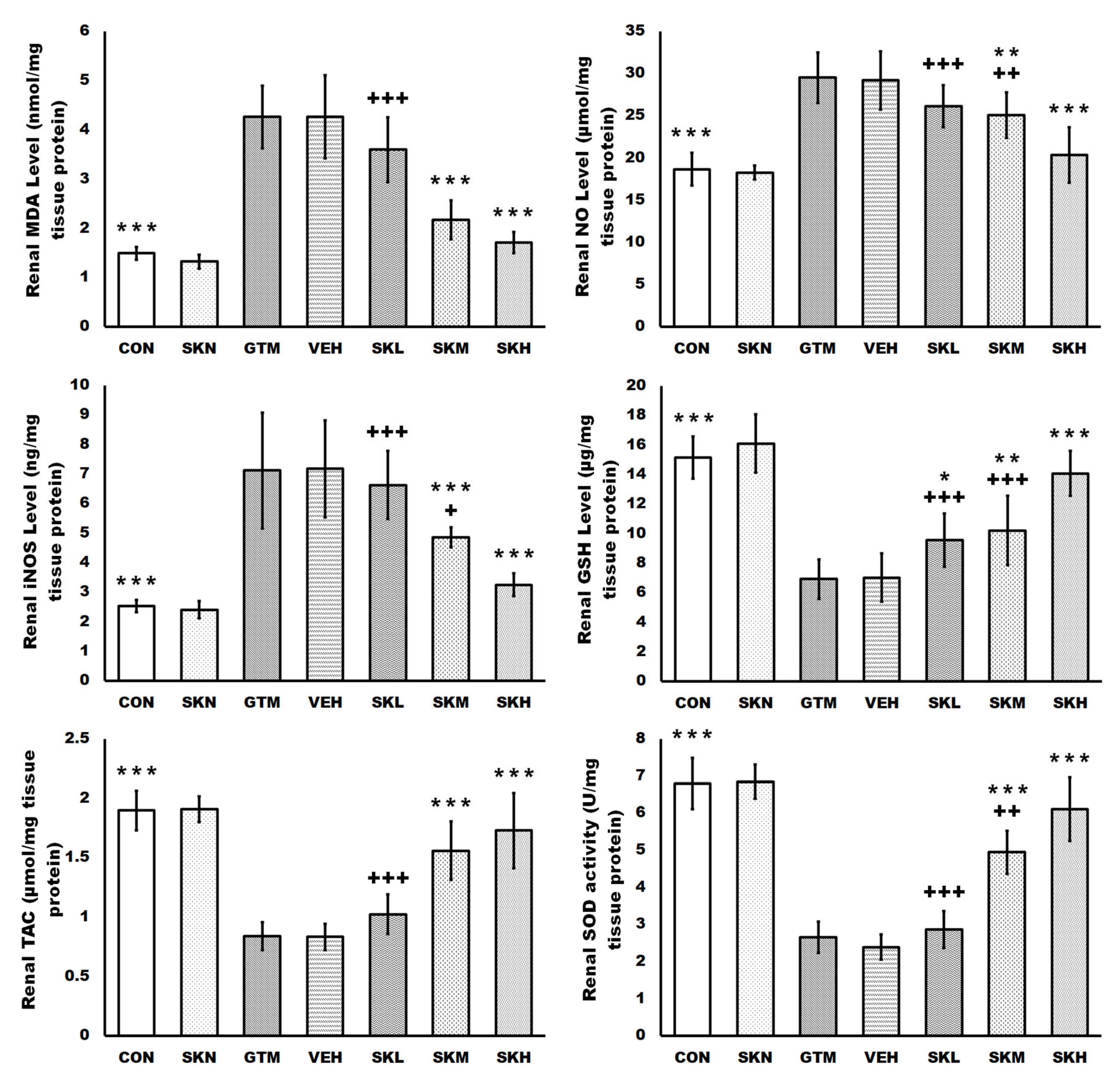
2.3. Shikonin Defended against Renal Oxidative Stress and Activated the SIRT1/Nrf2/HO-1 Cascades in Rats with Gentamicin-Induced Renal Damage
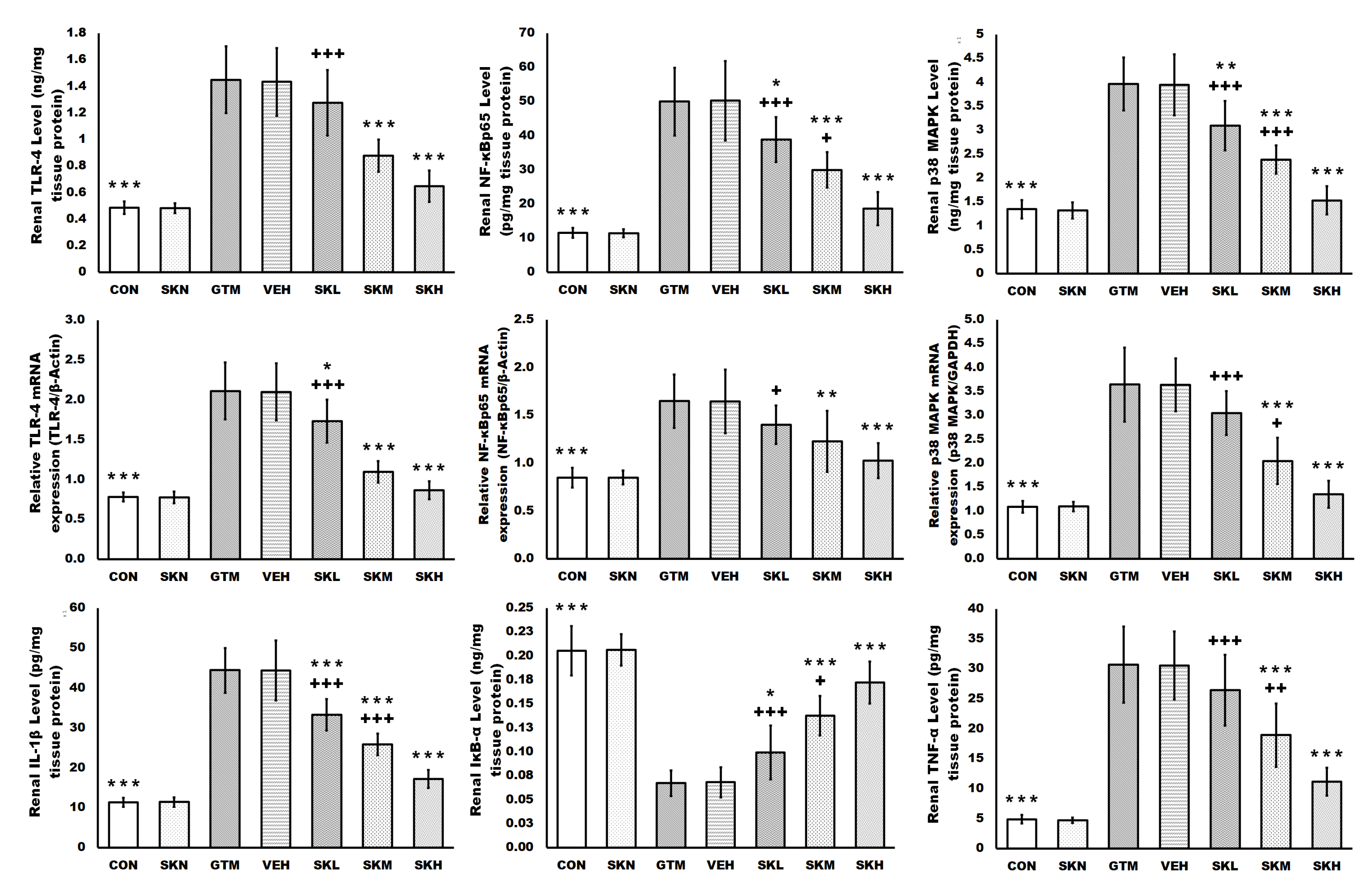
2.4. Shikonin Amended the Gentamicin-Induced Renal Inflammatory Signals
2.5. Shikonin Inhibited the Gentamicin-Induced Renal Apoptosis
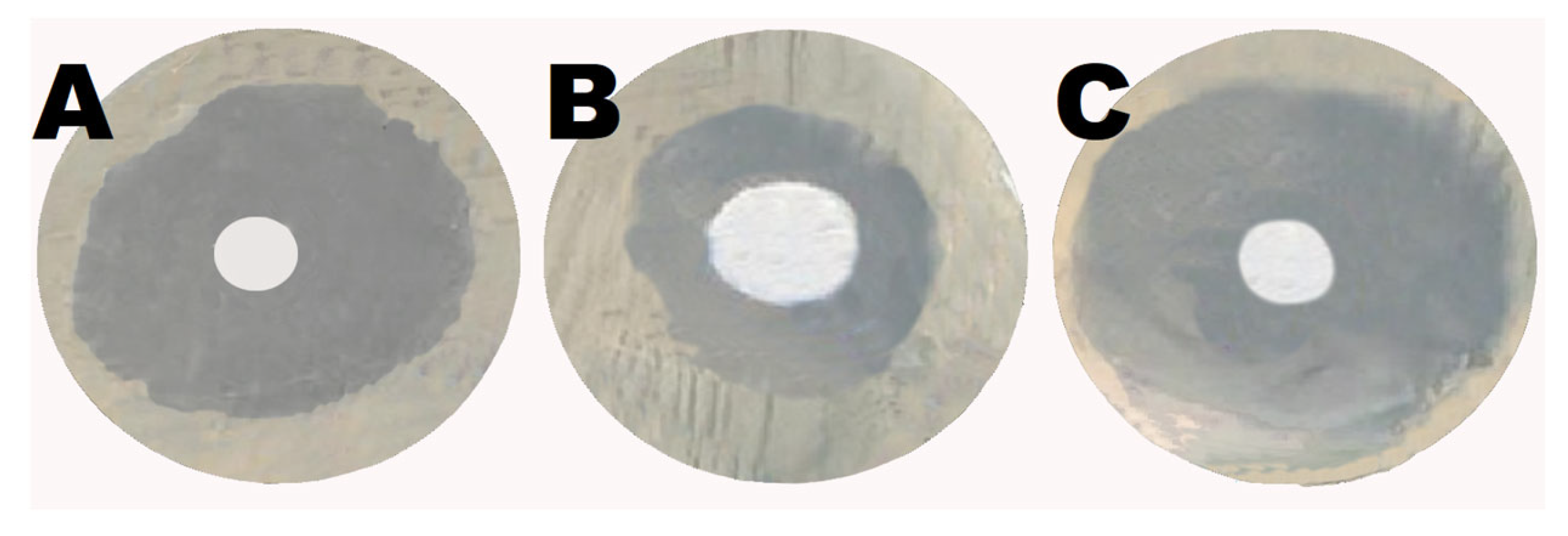
2.6. Shikonin Augmented the Antibacterial Activity of Gentamicin
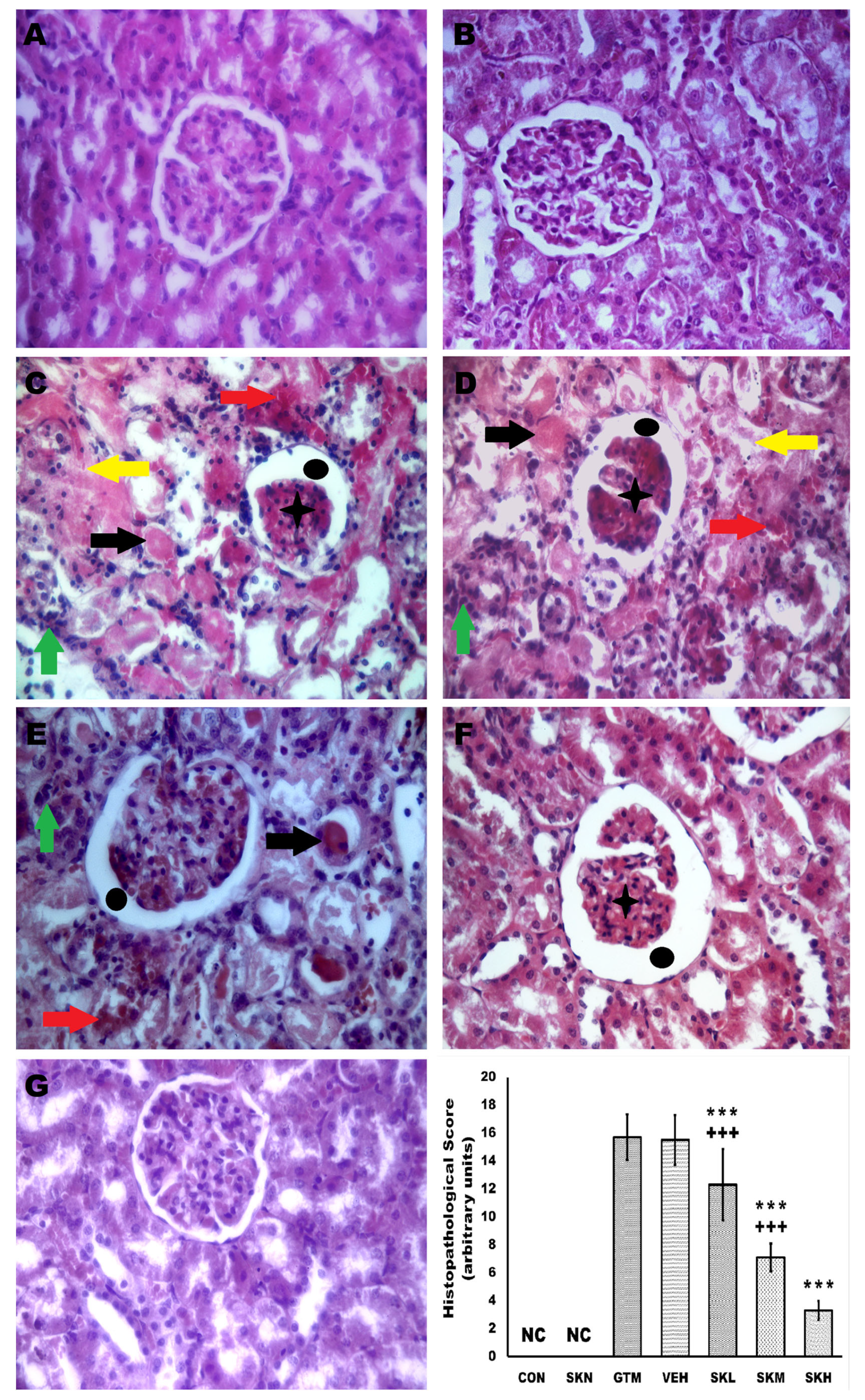
2.7. Shikonin Reversed the Renal Histopathological Alterations Induced by Gentamicin Injection
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Experimental Protocol
4.4. Evaluation of Total Protein Content
4.5. Evaluation of the Renal Functions
4.6. Evaluation of Proximal Tubule Epithelial Cells Endocytosis Function
4.7. Evaluation of SIRT1/Nrf2/HO-1 Pathway
4.8. Evaluation of Oxidative Stress Markers
4.9. Evaluation of Inflammatory Signals
4.10. Evaluation of Apoptosis Markers
4.11. Evaluation of mRNA Expressions
4.12. Evaluation of Shikonin’s Antibacterial Activity
4.13. Histopathological Evaluation
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azouz, A.A.; Hanna, D.A.; Abo-Saif, A.A.; Anwar Shehata Messiha, B. Interference with megalin expression/endocytic function by montelukast mitigates gentamicin nephrotoxicity: Downregulation of ClC-5 expression. Saudi Pharm. J. 2022, 30, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Jado, J.C.; Humanes, B.; González-Nicolás, M.Á.; Camaño, S.; Lara, J.M.; López, B.; Cercenado, E.; García-Bordas, J.; Tejedor, A.; Lázaro, A. Nephroprotective effect of cilastatin against gentamicin-induced renal injury in vitro and in vivo without altering its bactericidal efficiency. Antioxidants 2020, 9, 821. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.; Davey, P.; Nathwani, D.; Marwick, C.; Vadiveloo, T.; Sneddon, J.; Patton, A.; Bennie, M.; Fleming, S.; Donnan, P.T. Risk of AKI with gentamicin as surgical prophylaxis. J. Am. Soc. Nephrol. 2014, 25, 2625–2632. [Google Scholar] [CrossRef] [PubMed]
- Wargo, K.A.; Edwards, J.D. Aminoglycoside-induced nephrotoxicity. J. Pharm. Pract. 2014, 27, 573–577. [Google Scholar] [CrossRef]
- Randjelovic, P.; Veljkovic, S.; Stojiljkovic, N.; Sokolovic, D.; Ilic, I. Gentamicin nephrotoxicity in animals: Current knowledge and future perspectives. EXCLI J. 2017, 16, 388–399. [Google Scholar] [CrossRef]
- Trimarchi, H.; Ceol, M.; Gianesello, L.; Priante, G.; Iotti, A.; Del Prete, D. Downregulation of megalin, cubilin, ClC-5 and podocin in Fabry nephropathy: Potential implications in the decreased effectiveness of enzyme replacement therapy. J. Nephrol. 2021, 34, 1307–1314. [Google Scholar] [CrossRef]
- De, S.; Kuwahara, S.; Saito, A. The endocytic receptor megalin and its associated proteins in proximal tubule epithelial cells. Membranes 2014, 4, 333–355. [Google Scholar] [CrossRef]
- Schmitz, C.; Hilpert, J.; Jacobsen, C.; Boensch, C.; Christensen, E.I.; Luft, F.C.; Willnow, T.E. Megalin deficiency offers protection from renal aminoglycoside accumulation. J. Biol. Chem. 2002, 277, 618–622. [Google Scholar] [CrossRef]
- Karasawa, T.; Steyger, P.S. Intracellular mechanisms of aminoglycoside-induced cytotoxicity. Integr. Biol. 2011, 3, 879–886. [Google Scholar] [CrossRef]
- Abdel-Fattah, M.M.; Elgendy, A.; Mohamed, W.R. Xanthenone, ACE2 activator, counteracted gentamicin-induced nephrotoxicity in rats: Impact on oxidative stress and ACE2/Ang-(1-7) signaling. Life Sci. 2021, 275, 119387. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Abd El-Ghafar, O.A.M.; Alzoghaibi, M.A.; Hassanein, E.H.M. Agomelatine prevents gentamicin nephrotoxicity by attenuating oxidative stress and TLR-4 signaling, and upregulating PPARgamma and SIRT1. Life Sci. 2021, 278, 119600. [Google Scholar] [CrossRef]
- Sun, Q.; Gong, T.; Liu, M.; Ren, S.; Yang, H.; Zeng, S.; Zhao, H.; Chen, L.; Ming, T.; Meng, X.; et al. Shikonin, a naphthalene ingredient: Therapeutic actions, pharmacokinetics, toxicology, clinical trials and pharmaceutical researches. Phytomedicine 2022, 94, 153805. [Google Scholar] [CrossRef]
- Wu, J.; Rong, S.; Zhou, J.; Yuan, W. The role and mechanism of PKM2 in the development of LPS-induced acute kidney injury. Histol. Histopathol. 2021, 36, 845–852. [Google Scholar] [CrossRef]
- Alquraishi, M.; Chahed, S.; Alani, D.; Puckett, D.L.; Dowker, P.D.; Hubbard, K.; Zhao, Y.; Kim, J.Y.; Nodit, L.; Fatima, H. Podocyte specific deletion of PKM2 ameliorates LPS-induced podocyte injury through beta-catenin. Cell Commun. Signal. 2022, 20, 76. [Google Scholar] [CrossRef]
- Kawara, M.; Matsunaga, R.; Yamamoto, Y.; Yoneda, G.; Fujino, R.; Nishi, K.; Jono, H.; Saito, H. Nephropreventive effect of shikonin on murine LPS-induced septic acute kidney injury via Nrf2 activation with antioxidative responses. J. Clin. Exp. Nephrol. 2016, 1, 19. [Google Scholar] [CrossRef]
- Tong, Y.; Chuan, J.; Bai, L.; Shi, J.; Zhong, L.; Duan, X.; Zhu, Y. The protective effect of shikonin on renal tubular epithelial cell injury induced by high glucose. Biomed. Pharmacother. 2018, 98, 701–708. [Google Scholar] [CrossRef]
- Xie, M.; Yu, Y.; Kang, R.; Zhu, S.; Yang, L.; Zeng, L.; Sun, X.; Yang, M.; Billiar, T.R.; Wang, H.; et al. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat. Commun. 2016, 7, 13280. [Google Scholar] [CrossRef]
- Yang, X.; Xu, W.; Huang, K.; Zhang, B.; Wang, H.; Zhang, X.; Gong, L.; Luo, Y.; He, X. Precision toxicology shows that troxerutin alleviates ochratoxin A–induced renal lipotoxicity. FASEB J. 2019, 33, 2212–2227. [Google Scholar] [CrossRef]
- Nagai, J.; Takano, M. Entry of aminoglycosides into renal tubular epithelial cells via endocytosis-dependent and endocytosis-independent pathways. Biochem. Pharmacol. 2014, 90, 331–337. [Google Scholar] [CrossRef]
- Gburek, J.; Konopska, B.; Golab, K. Renal Handling of Albumin-From Early Findings to Current Concepts. Int. J. Mol. Sci. 2021, 22, 5809. [Google Scholar] [CrossRef]
- Devuyst, O.; Luciani, A. Chloride transporters and receptor-mediated endocytosis in the renal proximal tubule. J. Physiol. 2015, 593, 4151–4164. [Google Scholar] [CrossRef] [PubMed]
- Elsakka, E.G.E.; Mokhtar, M.M.; Hegazy, M.; Ismail, A.; Doghish, A.S. Megalin, a multi-ligand endocytic receptor, and its participation in renal function and diseases: A review. Life Sci. 2022, 308, 120923. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.K.; Lee, J.; Park, J.W.; Bae, E.H.; Ma, S.K.; Kim, S.H.; Kim, S.W. Decreased Expression of Na/K-ATPase, NHE3, NBC1, AQP1 and OAT in Gentamicin-induced Nephropathy. Korean J. Physiol. Pharmacol. 2008, 12, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Fuster, D.G.; Bobulescu, I.A.; Zhang, J.; Wade, J.; Moe, O.W. Characterization of the regulation of renal Na+/H+ exchanger NHE3 by insulin. Am. J. Physiol. Ren. Physiol. 2007, 292, F577–F585. [Google Scholar] [CrossRef]
- Christensen, E.I.; Nielsen, R. Role of megalin and cubilin in renal physiology and pathophysiology. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2007; Volume 158, pp. 1–22. [Google Scholar] [CrossRef]
- Udupa, V.; Prakash, V. Gentamicin induced acute renal damage and its evaluation using urinary biomarkers in rats. Toxicol. Rep. 2019, 6, 91–99. [Google Scholar] [CrossRef]
- Arab, H.H.; Ashour, A.M.; Eid, A.H.; Arafa, E.A.; Al Khabbaz, H.J.; Abd El-Aal, S.A. Targeting oxidative stress, apoptosis, and autophagy by galangin mitigates cadmium-induced renal damage: Role of SIRT1/Nrf2 and AMPK/mTOR pathways. Life Sci. 2022, 291, 120300. [Google Scholar] [CrossRef]
- Luft, F.C. Biomarkers and predicting acute kidney injury. Acta Physiol. 2021, 231, e13479. [Google Scholar] [CrossRef]
- Cardenas-Gonzalez, M.; Jacobo Estrada, T.; Rodriguez-Munoz, R.; Barrera-Chimal, J.; Bobadilla, N.A.; Barbier, O.C.; Del Razo, L.M. Sub-chronic exposure to fluoride impacts the response to a subsequent nephrotoxic treatment with gentamicin. J. Appl. Toxicol. 2016, 36, 309–319. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Mueller-Steiner, S.; Chen, L.-F.; Kwon, H.; Yi, S.; Mucke, L.; Gan, L. SIRT1 protects against microglia-dependent amyloid-β toxicity through inhibiting NF-κB signaling. J. Biol. Chem. 2005, 280, 40364–40374. [Google Scholar] [CrossRef]
- Naushad, M.; Urooj, M.; Ahmad, T.; Husain, G.M.; Kazmi, M.H.; Zakir, M. Nephroprotective effect of Apium graveolens L. against Cisplatin-induced nephrotoxicity. J. Ayurveda Integr. Med. 2021, 12, 607–615. [Google Scholar] [CrossRef]
- El-Maadawy, W.H.; Hassan, M.; Abdou, R.M.; El-Dine, R.S.; Aboushousha, T.; El-Tanbouly, N.D.; El-Sayed, A.M. 6-Paradol alleviates Diclofenac-induced acute kidney injury via autophagy enhancement-mediated by AMPK/AKT/mTOR and NLRP3 inflammasome pathways. Environ. Toxicol. Pharmacol. 2022, 91, 103817. [Google Scholar] [CrossRef]
- Botros, S.R.; Matouk, A.I.; Anter, A.; Khalifa, M.M.A.; Heeba, G.H. Protective effect of empagliflozin on gentamicin-induced acute renal injury via regulation of SIRT1/NF-kappaB signaling pathway. Environ. Toxicol. Pharmacol. 2022, 94, 103907. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Q.; Mo, W.; Yu, Q.; Xu, S.; Li, J.; Li, S.; Feng, J.; Wu, L.; Lu, X.; et al. The protective effects of shikonin on hepatic ischemia/reperfusion injury are mediated by the activation of the PI3K/Akt pathway. Sci. Rep. 2017, 7, 44785. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Wu, F.; Yuan, X.; Hu, J. Shikonin inhibits TNF-alpha-induced growth and invasion of rat aortic vascular smooth muscle cells. Can. J. Physiol. Pharmacol. 2015, 93, 615–624. [Google Scholar] [CrossRef]
- Liu, S.; Yan, W.; Hu, Y.; Wu, H. Shikonin Alleviates Endothelial Cell Injury Induced by ox-LDL via AMPK/Nrf2/HO-1 Signaling Pathway. Evid.-Based Complement. Altern. Med. 2021, 2021, 5881321. [Google Scholar] [CrossRef]
- Guo, H.; Sun, J.; Li, D.; Hu, Y.; Yu, X.; Hua, H.; Jing, X.; Chen, F.; Jia, Z.; Xu, J. Shikonin attenuates acetaminophen-induced acute liver injury via inhibition of oxidative stress and inflammation. Biomed. Pharmacother. 2019, 112, 108704. [Google Scholar] [CrossRef]
- Reisman, S.A.; Chertow, G.M.; Hebbar, S.; Vaziri, N.D.; Ward, K.W.; Meyer, C.J. Bardoxolone methyl decreases megalin and activates nrf2 in the kidney. J. Am. Soc. Nephrol. 2012, 23, 1663–1673. [Google Scholar] [CrossRef]
- Ali, F.E.M.; Sayed, A.M.; El-Bahrawy, A.H.; Omar, Z.M.M.; Hassanein, E.H.M. Targeting KEAP1/Nrf2, AKT, and PPAR-gamma signals as a potential protective mechanism of diosmin against gentamicin-induced nephrotoxicity. Life Sci. 2021, 275, 119349. [Google Scholar] [CrossRef]
- Gonzalez-Guerrero, C.; Cannata-Ortiz, P.; Guerri, C.; Egido, J.; Ortiz, A.; Ramos, A.M. TLR4-mediated inflammation is a key pathogenic event leading to kidney damage and fibrosis in cyclosporine nephrotoxicity. Arch. Toxicol. 2017, 91, 1925–1939. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Abduldaium, Y.S.; Younis, N.S. Ameliorative Effect of Linalool in Cisplatin-Induced Nephrotoxicity: The Role of HMGB1/TLR4/NF-kappaB and Nrf2/HO1 Pathways. Biomolecules 2020, 10, 1488. [Google Scholar] [CrossRef]
- Araujo, M.; Welch, W.J. Oxidative stress and nitric oxide in kidney function. Curr. Opin. Nephrol. Hypertens. 2006, 15, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, E.; Cekmen, M.; Ilbey, Y.O.; Simsek, A.; Polat, E.C.; Somay, A. Atorvastatin prevents gentamicin-induced renal damage in rats through the inhibition of p38-MAPK and NF-kB pathways. Ren. Fail. 2009, 31, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Shang, X.; Ni, Z.; Shi, G. Shikonin inhibits inflammation and chondrocyte apoptosis by regulation of the PI3K/Akt signaling pathway in a rat model of osteoarthritis. Exp. Ther. Med. 2016, 12, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; He, L.; Wang, J.; Wang, Q.; Sun, C.; Li, Y.; Jia, K.; Wang, J.; Xu, T.; Ming, R.; et al. Anti-angiogenic effect of Shikonin in rheumatoid arthritis by downregulating PI3K/AKT and MAPKs signaling pathways. J. Ethnopharmacol. 2020, 260, 113039. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Goudarzi, M.; Fatemi, I.; Basir, Z.; Malayeri, A.; Khalili, H. Chrysin attenuates sodium arsenite-induced nephrotoxicity in rats by suppressing oxidative stress and inflammation. Tissue Cell 2021, 73, 101657. [Google Scholar] [CrossRef]
- Bao, R.K.; Zheng, S.F.; Wang, X.Y. Selenium protects against cadmium-induced kidney apoptosis in chickens by activating the PI3K/AKT/Bcl-2 signaling pathway. Environ. Sci. Pollut. Res. Int. 2017, 24, 20342–20353. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, C.H.; Hsu, Y.H.; Chen, T.H.; Sue, Y.M.; Cheng, C.Y.; Chen, T.W. Leptin reduces gentamicin-induced apoptosis in rat renal tubular cells via the PI3K-Akt signaling pathway. Eur. J. Pharmacol. 2011, 658, 213–218. [Google Scholar] [CrossRef]
- Chen, C.H.; Chen, T.H.; Wu, M.Y.; Chen, J.R.; Hong, L.Y.; Zheng, C.M.; Chiu, I.J.; Lin, Y.F.; Hsu, Y.H. Peroxisome Proliferator-Activated Receptor alpha Protects Renal Tubular Cells from Gentamicin-Induced Apoptosis via Upregulating Na+/H+ Exchanger NHE1. Mol. Med. 2016, 21, 886–889. [Google Scholar] [CrossRef]
- Abdelrahman, R.S. Protective effect of apocynin against gentamicin-induced nephrotoxicity in rats. Hum. Exp. Toxicol. 2018, 37, 27–37. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques; E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]

| Parameter | CON | SKN | GTM | VEH | SKL | SKM | SKH |
|---|---|---|---|---|---|---|---|
| Serum | |||||||
| 0.60 ± 0.08 *** | 0.62 ± 0.09 | 3.50 ± 0.47 | 3.47 ± 0.64 | 2.90 ± 0.31 **+++ | 1.75 ± 0.19 ***+++ | 0.82 ± 0.06 *** |
| 19.98 ± 1.86 *** | 19.32 ± 1.95 | 59.64 ± 6.61 | 59.16 ± 10.03 | 51.97 ± 4.84 +++ | 40.17 ± 3.51 ***+++ | 30.03 ± 6.47 *** |
| 16.90 ± 1.85 *** | 16.89 ± 1.32 | 71.94 ± 9.69 | 71.94 ± 10.94 | 58.92 ± 10.93 *+++ | 40.59 ± 8.33 ***++ | 26.00 ± 5.83 *** |
| Renal tissue | |||||||
| 5.19 ± 0.50 *** | 5.13 ± 0.42 | 21.50 ± 4.93 | 21.21 ± 4.61 | 17.63 ± 3.02 +++ | 14.19 ± 2.62 ***+ | 9.405 ± 1.24 *** |
| Urine | |||||||
| 8.23 ± 0.48 *** | 8.27 ± 0.76 | 14.48 ± 3.06 | 14.38 ± 3.11 | 12.16 ± 1.22 + | 11.27 ± 1.75 ** | 9.15 ± 0.71 *** |
| 5.71 ± 0.34 *** | 5.74 ± 0.53 | 10.06 ± 2.12 | 9.98 ± 2.16 | 8.45 ± 0.85 + | 7.83 ± 1.22 ** | 6.36 ± 0.50 *** |
| 57.24 ± 5.40 *** | 57.71 ± 4.58 | 30.36 ± 3.08 | 30.50 ± 4.88 | 36.51 ± 3.75 +++ | 43.04 ± 5.62 ***++ | 52.57 ± 7.54 *** |
| 14.18 ± 1.05 *** | 14.07 ± 1.46 | 78.04 ± 14.82 | 78.80 ± 16.51 | 69.81 ± 7.43 +++ | 52.80 ± 7.79 ***+ | 39.10 ± 5.51 *** |
| 1.96 ± 0.36 *** | 1.98 ± 0.34 | 12.69 ± 2.07 | 12.57 ± 2.93 | 10.02 ± 1.09 **+++ | 8.49 ± 1.20 ***++ | 5.64 ± 1.12 *** |
| 4.29 ± 0.40 *** | 4.44 ± 0.42 | 11.41 ± 0.89 | 11.52 ± 1.08 | 9.547 ± 1.57 **+++ | 8.65 ± 0.81 ***++ | 6.86 ± 0.68 *** |
| GFR (mL/min) | 0.56 ± 0.12 *** | 0.55 ± 0.09 | 0.09 ± 0.03 | 0.09 ± 0.03 | 0.11 ± 0.01 +++ | 0.19 ± 0.03 *+++ | 0.41 ± 0.10 *** |
| KSI (%) | 0.35 ± 0.02 *** | 0.35 ± 0.03 | 0.49 ± 0.05 | 0.49 ± 0.05 | 0.47 ± 0.05 +++ | 0.44 ± 0.04 *+ | 0.38 ± 0.04 *** |
| mRNA | Forward | Reverse |
|---|---|---|
| Megalin | ACTGGGCAGCAGGAAATCTT | CGGGGCATATCCACTGAGAC |
| Cubilin | CTGTCCAAGGCCGTTACTGT | GATGAAAACGCCAACAGGGG |
| ClC-5 | CTTACGCCAATGGAGATCGTAGTGG | TCTTGGTTTGCCATCTGCGCTA |
| NHE3 | GACTGGCGTGGACTGTGTGAA | TGATACGCACATGCTTGGTGAA |
| SIRT1 | TGTTTCCTGTGGGATACCTGA | TGAAGAATGGTCTTGGGTCTT |
| Nrf2 | CACATCCAGACAGACACCAGT | CTACAAATGGGAATGTCTCTGC |
| HO-1 | CTATCGTGCTCGCATGAAC | CAGCTCCTCAAACAGCTCAA |
| TLR-4, | AGTGTATCGGTGGTCAGTGTGCT | AAACTCCAGCCACACATTCC |
| NF-κBp65 | TGGGACGACACCTCTACACA | GGAGCTCATCTCATAGTTGTCC |
| p38 MAPK | ACATCGTGTGGCAGTGAAGAAG | CTTTTGGCGTGAATGATGGA |
| p-AKT | AGGGCAGAATCATGAGCAAGT | AGGGTCTGCATFGGATGGCA |
| PI3K | AGCTGGTCTTCGTTTCCTGA | GAAACTTTTTCCCACCACGA |
| β-actin | GCAGATGTGGATCAGCAAGC | GGTGTAAAACGCAGCTCAGTAA |
| GAPDH | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaha, M.F.; Alamer, A.A.; Eisa, A.A.; Aljohani, H.M. Shikonin Alleviates Gentamicin-Induced Renal Injury in Rats by Targeting Renal Endocytosis, SIRT1/Nrf2/HO-1, TLR-4/NF-κB/MAPK, and PI3K/Akt Cascades. Antibiotics 2023, 12, 826. https://doi.org/10.3390/antibiotics12050826
Balaha MF, Alamer AA, Eisa AA, Aljohani HM. Shikonin Alleviates Gentamicin-Induced Renal Injury in Rats by Targeting Renal Endocytosis, SIRT1/Nrf2/HO-1, TLR-4/NF-κB/MAPK, and PI3K/Akt Cascades. Antibiotics. 2023; 12(5):826. https://doi.org/10.3390/antibiotics12050826
Chicago/Turabian StyleBalaha, Mohamed F., Ahmed A. Alamer, Alaa A. Eisa, and Hashim M. Aljohani. 2023. "Shikonin Alleviates Gentamicin-Induced Renal Injury in Rats by Targeting Renal Endocytosis, SIRT1/Nrf2/HO-1, TLR-4/NF-κB/MAPK, and PI3K/Akt Cascades" Antibiotics 12, no. 5: 826. https://doi.org/10.3390/antibiotics12050826
APA StyleBalaha, M. F., Alamer, A. A., Eisa, A. A., & Aljohani, H. M. (2023). Shikonin Alleviates Gentamicin-Induced Renal Injury in Rats by Targeting Renal Endocytosis, SIRT1/Nrf2/HO-1, TLR-4/NF-κB/MAPK, and PI3K/Akt Cascades. Antibiotics, 12(5), 826. https://doi.org/10.3390/antibiotics12050826







