Disease-Specific Quality Indicators for Outpatient Antibiotic Prescribing for Respiratory Infections (ESAC Quality Indicators) Applied to Point Prevalence Audit Surveys in General Practices in 13 European Countries
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Setting
4.2. Eligibility Criteria
4.3. Data Analysis
- o
- Due to the focus on respiratory symptoms and the exclusion of those with solely ear symptoms, for ESAC Quality Indicators 3 (focusing on urinary tract infections) and 6 (focusing on otitis media) values could not be calculated.
- o
- ESAC QI for upper RTI and sinusitis were combined with the working diagnosis “cold,” as this is how it was presented on the PPAS-4 survey form (Texts S1 and S2). For the analysis of the QI, the combined diagnosis was used for both QIs [8].
- o
- Table 4 shows the antibiotic prescribing options on the PPAS survey and its allocated to ESAC Quality Indicators.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Regional Office for Europe/European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2022—2020 Data; WHO Regional Office for Europe: Copenhagen, Denmark, 2022. [Google Scholar]
- Bruyndonckx, R.; Adriaenssens, N.; Versporten, A.; Hens, N.; Monnet, D.L.; Molenberghs, G.; Goossens, H.; Weist, K.; Coenen, S.; the ESAC-Net Study Group. Consumption of antibiotics in the community, European Union/European Economic Area, 1997–2017. J. Antimicrob. Chemother. 2021, 76, ii7–ii13. [Google Scholar] [CrossRef]
- Jain, N.; Lodha, R.; Kabra, S.K. Upper respiratory tract infections. Indian J. Pediatr. 2001, 68, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Zoorob, R.; Sidani, M.A.; Fremont, R.D.; Kihlberg, C. Antibiotic use in acute upper respiratory tract infections. Am. Fam. Physician 2012, 86, 817–822. [Google Scholar] [PubMed]
- Spinks, A.; Glasziou, P.P.; Del Mar, C.B. Antibiotics for treatment of sore throat in children and adults. Cochrane Database Syst. Rev. 2021, 12, Cd000023. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, R.M. Clinical Practice. Acute Sinusitis in Adults. N. Engl. J. Med. 2016, 375, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, N.; Bartholomeeusen, S.; Ryckebosch, P.; Coenen, S. Quality of antibiotic prescription during office hours and out-of-hours in Flemish primary care, using European quality indicators. Eur. J. Gen. Pract. 2014, 20, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, N.; Coenen, S.; Tonkin-Crine, S.; Verheij, T.J.; Little, P.; Goossens, H. European Surveillance of Antimicrobial Consumption (ESAC): Disease-specific quality indicators for outpatient antibiotic prescribing. BMJ Qual. Saf. 2011, 20, 764–772. [Google Scholar] [CrossRef]
- Tyrstrup, M.; van der Velden, A.; Engstrom, S.; Goderis, G.; Molstad, S.; Verheij, T.; Coenen, S.; Adriaenssens, N. Antibiotic prescribing in relation to diagnoses and consultation rates in Belgium, the Netherlands and Sweden: Use of European quality indicators. Scand. J. Prim. Health Care 2017, 35, 10–18. [Google Scholar] [CrossRef]
- van den Broek d’Obrenan, J.; Verheij, T.J.M.; Numans, M.E.; van der Velden, A.W. Antibiotic use in Dutch primary care: Relation between diagnosis, consultation and treatment. J. Antimicrob. Chemother. 2014, 69, 1701–1707. [Google Scholar] [CrossRef]
- Glinz, D.; Leon Reyes, S.; Saccilotto, R.; Widmer, A.F.; Zeller, A.; Bucher, H.C.; Hemkens, L.G. Quality of antibiotic prescribing of Swiss primary care physicians with high prescription rates: A nationwide survey. J. Antimicrob. Chemother. 2017, 72, 3205–3212. [Google Scholar] [CrossRef]
- van der Velden, A.W.; Bax, E.A.; Bongard, E.; Munck Aabenhus, R.; Anastasaki, M.; Anthierens, S.; Balan, A.; Böhmer, F.; Bruno, P.; Chlabicz, S.; et al. Primary care for patients with respiratory tract infection before and early on in the COVID-19 pandemic: An observational study in 16 European countries. BMJ Open 2021, 11, e049257. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, A.W.; van de Pol, A.C.; Bongard, E.; Cianci, D.; Aabenhus, R.; Balan, A.; Böhmer, F.; Bralić Lang, V.; Bruno, P.; Chlabicz, S.; et al. Point-of-care testing, antibiotic prescribing, and prescribing confidence for respiratory tract infections in primary care: A prospective audit in 18 European countries. BJGP Open 2022, 6, BJGPO.2021.0212. [Google Scholar] [CrossRef] [PubMed]
- Hordijk, P.M.; Broekhuizen, B.D.; Butler, C.C.; Coenen, S.; Godycki-Cwirko, M.; Goossens, H.; Hood, K.; Smith, R.; van Vugt, S.F.; Little, P.; et al. Illness perception and related behaviour in lower respiratory tract infections—A European study. Fam. Pract. 2015, 32, 152–158. [Google Scholar] [CrossRef] [PubMed]
- van Loenen, T.; van den Berg, M.J.; Faber, M.J.; Westert, G.P. Propensity to seek healthcare in different healthcare systems: Analysis of patient data in 34 countries. BMC Health Serv. Res. 2015, 15, 465. [Google Scholar] [CrossRef] [PubMed]
- van Dulmen, S.A.; Kruse, F.M.; Burgers, J.S. Primary health care through the eyes of the general practitioner: An international study. Ned. Tijdschr. Voor Geneeskd. 2021, 165, D5419. [Google Scholar]
- Butler, C.C.; Hood, K.; Verheij, T.; Little, P.; Melbye, H.; Nuttall, J.; Kelly, M.J.; Mölstad, S.; Godycki-Cwirko, M.; Almirall, J.; et al. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: Prospective study in 13 countries. BMJ 2009, 338, b2242. [Google Scholar] [CrossRef]
- Hek, K.; Ramerman, L.; Weesie, Y.M.; Lambooij, A.C.; Lambert, M.; Heins, M.J.; Hendriksen, J.M.T.; Verheij, R.A.; Cals, J.W.L.; van Dijk, L. Antibiotic Prescribing in Dutch Daytime and Out-of-Hours General Practice during the COVID-19 Pandemic: A Retrospective Database Study. Antibiotics 2022, 11, 309. [Google Scholar] [CrossRef]
- McFarlane, A.; Sligl, W. The Value of Macrolide-Based Regimens for Community-Acquired Pneumonia. Curr. Infect. Dis. Rep. 2015, 17, 50. [Google Scholar] [CrossRef]
- Malhotra-Kumar, S.; Lammens, C.; Coenen, S.; Van Herck, K.; Goossens, H. Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide-resistant streptococci in healthy volunteers: A randomised, double-blind, placebo-controlled study. Lancet 2007, 369, 482–490. [Google Scholar] [CrossRef]
- Bruyndonckx, R.; Hens, N.; Aerts, M.; Goossens, H.; Latour, K.; Catry, B.; Coenen, S. Persistence of antimicrobial resistance in respiratory streptococci. J. Glob. Antimicrob. Resist. 2017, 8, 6–12. [Google Scholar] [CrossRef]
- O’Connor, N.; Breen, R.; Carton, M.; Mc Grath, I.; Deasy, N.; Collins, C.; Vellinga, A. Improving the quality of antibiotic prescribing through an educational intervention delivered through the out-of-hours general practice service in Ireland. Eur. J. Gen. Pract. 2020, 26, 119–125. [Google Scholar] [CrossRef]
- White, A.R.; Kaye, C.; Poupard, J.; Pypstra, R.; Woodnutt, G.; Wynne, B. Augmentin (amoxicillin/clavulanate) in the treatment of community-acquired respiratory tract infection: A review of the continuing development of an innovative antimicrobial agent. J. Antimicrob. Chemother. 2004, 53 (Suppl. S1), i3–i20. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Respiratory System Infections, Antibacterial Therapy. Available online: https://bnf.nice.org.uk/treatment-summaries/respiratory-system-infections-antibacterial-therapy/ (accessed on 10 October 2022).
- Cowling, T.; Farrah, K. Fluoroquinolones for the Treatment of Other Respiratory Tract Infections: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines. In CADTH Rapid Response Report: Summary with Critical Appraisal; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019. [Google Scholar]
- European Centre for Disease Prevention and Control. Antimicrobial Consumption in the EU/EEA (ESAC-Net)-Annual Epidemiological Report 2020; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2021.
- Little, P.; Stuart, B.; Francis, N.; Douglas, E.; Tonkin-Crine, S.; Anthierens, S.; Cals, J.W.; Melbye, H.; Santer, M.; Moore, M.; et al. Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: A multinational, cluster, randomised, factorial, controlled trial. Lancet 2013, 382, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, A.W.; Pijpers, E.J.; Kuyvenhoven, M.M.; Tonkin-Crine, S.K.; Little, P.; Verheij, T.J. Effectiveness of physician-targeted interventions to improve antibiotic use for respiratory tract infections. Br. J. Gen. Pract. 2012, 62, e801–e807. [Google Scholar] [CrossRef] [PubMed]
- Juszczyk, D.; Charlton, J.; McDermott, L.; Soames, J.; Sultana, K.; Ashworth, M.; Fox, R.; Hay, A.D.; Little, P.; Moore, M.V.; et al. Electronically delivered, multicomponent intervention to reduce unnecessary antibiotic prescribing for respiratory infections in primary care: A cluster randomised trial using electronic health records-REDUCE Trial study original protocol. BMJ Open 2016, 6, e010892. [Google Scholar] [CrossRef]
- Parveen, S.; Garzon-Orjuela, N.; Amin, D.; McHugh, P.; Vellinga, A. Public Health Interventions to Improve Antimicrobial Resistance Awareness and Behavioural Change Associated with Antimicrobial Use: A Systematic Review Exploring the Use of Social Media. Antibiotics 2022, 11, 669. [Google Scholar] [CrossRef]

| Total Consultations Recorded | Age (Years) | Comorbidity | Suspected Bacterial Aetiology * | Hospitalisation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPAS-1 | PPAS-4 | PPAS-1 | PPAS-4 | PPAS-1 | PPAS-4 | PPAS-1 | PPAS-4 | PPAS-1 | PPAS-4 | |||||||||
| N | N | Mean | SD | Mean | SD | n | % | n | % | n | % | n | % | n | % | n | % | |
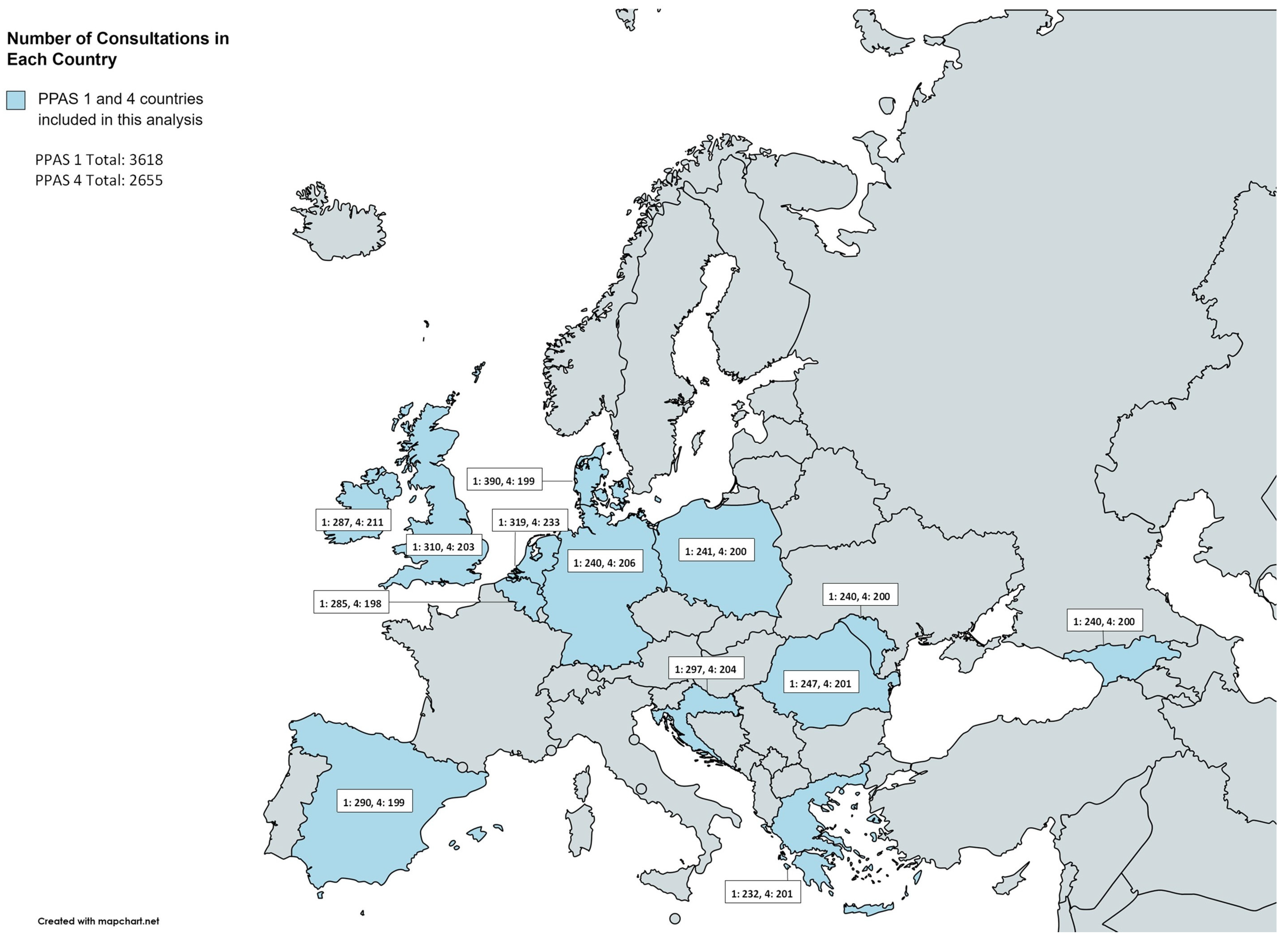
| Belgium | 285 | 198 | 37.7 | 25.0 | 28.6 | 18.3 | 78 | 27.4% | 25 | 12.6% | 46 | 16.1% | 4 | 2.0% | 5 | 1.8% | 2 | 1.0% |
| Croatia | 297 | 204 | 36.7 | 27.7 | 39.1 | 21.4 | 76 | 25.6% | 68 | 33.3% | 51 | 17.2% | 2 | 1.0% | 13 | 4.4% | 5 | 2.5% |
| Denmark | 390 | 199 | 28.8 | 23.4 | 32.5 | 27.3 | 78 | 20.0% | 75 | 37.7% | 96 | 24.6% | 34 | 17.1% | 13 | 3.3% | 12 | 6.0% |
| Georgia | 240 | 200 | 25.1 | 19.3 | 41.0 | 19.1 | 23 | 9.6% | 71 | 35.5% | 42 | 17.5% | 3 | 1.5% | 2 | 0.8% | 7 | 3.5% |
| Germany | 240 | 206 | 31.9 | 20.9 | 39.2 | 16.1 | 89 | 37.1% | 71 | 34.5% | 43 | 17.9% | 36 | 17.5% | 1 | 0.4% | 3 | 1.5% |
| Greece | 232 | 201 | 48.2 | 21.3 | 45.7 | 20.7 | 94 | 40.5% | 100 | 49.8% | 66 | 28.4% | 26 | 12.9% | 9 | 3.9% | 17 | 8.5% |
| Ireland | 287 | 211 | 25.8 | 22.9 | 25.6 | 23.6 | 133 | 46.3% | 57 | 27.0% | 98 | 34.1% | 36 | 17.1% | 10 | 3.5% | 8 | 3.8% |
| Moldova | 240 | 200 | 47.8 | 25.0 | 37.5 | 20.2 | 55 | 22.9% | 76 | 38.0% | 100 | 41.7% | 6 | 3.0% | 4 | 1.7% | 10 | 5.0% |
| Netherlands | 319 | 233 | 26.8 | 23.9 | 34.9 | 28.2 | 147 | 46.1% | 89 | 38.2% | 93 | 29.2% | 41 | 17.6% | 7 | 2.2% | 9 | 3.9% |
| Poland | 241 | 200 | 27.2 | 22.0 | 28.5 | 23.7 | 66 | 27.4% | 64 | 32.0% | 61 | 25.3% | 25 | 12.5% | 5 | 2.1% | 6 | 3.0% |
| Romania | 247 | 201 | 49.0 | 23.2 | 35.4 | 20.6 | 68 | 27.5% | 69 | 34.3% | 77 | 31.2% | 14 | 7.0% | 2 | 0.8% | 13 | 6.5% |
| Spain | 290 | 199 | 38.7 | 17.6 | 41.0 | 19.7 | 116 | 40.0% | 66 | 33.2% | 52 | 17.9% | 22 | 11.1% | 5 | 1.7% | 5 | 2.5% |
| UK | 310 | 203 | 30.9 | 23.4 | 34.2 | 26.3 | 79 | 25.5% | 63 | 31.0% | 107 | 34.5% | 80 | 39.4% | 7 | 2.3% | 2 | 1.0% |
| Total | 3618 | 2655 | 35.1 | 24.5 | 35.6 | 22.9 | 1102 | 30.5% | 894 | 33.7 | 932 | 25.8% | 329 | 12.4% | 83 | 2.3% | 99 | 3.7% |
| Rhinitis * | Sore Throat * | Cough * | Antibiotics | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPAS-1 | PPAS-4 | PPAS-1 | PPAS-4 | PPAS-1 | PPAS-4 | PPAS-1 | PPAS-4 | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Belgium | 169 | 60.1% | 136 | 69.0% | 193 | 70.7% | 113 | 57.9% | 245 | 86.6% | 142 | 72.4% | 51 | 17.9 | 5 | 2.5 |
| Croatia | 112 | 37.7% | 75 | 36.8% | 190 | 64.0% | 68 | 33.5% | 249 | 83.8% | 97 | 47.5% | 61 | 20.5 | 6 | 2.9 |
| Denmark | 211 | 58.1% | 84 | 48.3% | 171 | 51.0% | 77 | 54.2% | 323 | 84.6% | 138 | 70.4% | 95 | 24.4 | 30 | 15.1 |
| Georgia | 104 | 43.3% | 60 | 30.0% | 159 | 66.3% | 108 | 54.0% | 218 | 90.8% | 115 | 57.8% | 61 | 25.4 | 27 | 13.5 |
| Germany | 198 | 83.9% | 131 | 63.9% | 142 | 60.7% | 136 | 68.3% | 189 | 80.1% | 116 | 58.3% | 44 | 18.3 | 27 | 13.1 |
| Greece | 152 | 66.1% | 86 | 43.7% | 161 | 69.7% | 92 | 45.8% | 212 | 91.4% | 149 | 74.1% | 75 | 32.3 | 36 | 17.9 |
| Ireland | 108 | 37.9% | 93 | 47.7% | 106 | 37.9% | 89 | 45.2% | 252 | 88.1% | 154 | 74.0% | 154 | 53.7 | 63 | 29.9 |
| Moldova | 123 | 51.2% | 170 | 85.0% | 197 | 82.8% | 186 | 93.5% | 177 | 73.8% | 86 | 43.0% | 101 | 42.1 | 1 | 0.5 |
| Netherlands | 178 | 59.9% | 121 | 53.1% | 101 | 34.7% | 57 | 25.7% | 283 | 89.6% | 175 | 75.8% | 112 | 35.1 | 51 | 21.9 |
| Poland | 123 | 51.2% | 134 | 67.3% | 146 | 62.1% | 78 | 39.8% | 216 | 89.6% | 148 | 74.4% | 68 | 28.2 | 25 | 12.5 |
| Romania | 114 | 47.5% | 140 | 73.3% | 188 | 82.8% | 153 | 80.5% | 201 | 82.0% | 132 | 66.0% | 69 | 27.9 | 18 | 9 |
| Spain | 72 | 24.8% | 56 | 28.1% | 135 | 46.6% | 87 | 43.9% | 239 | 82.4% | 108 | 54.3% | 54 | 18.6 | 27 | 13.6 |
| UK | 75 | 25.2% | 59 | 30.1% | 184 | 60.5% | 78 | 41.1% | 212 | 68.8% | 166 | 82.2% | 136 | 43.9 | 98 | 48.3 |
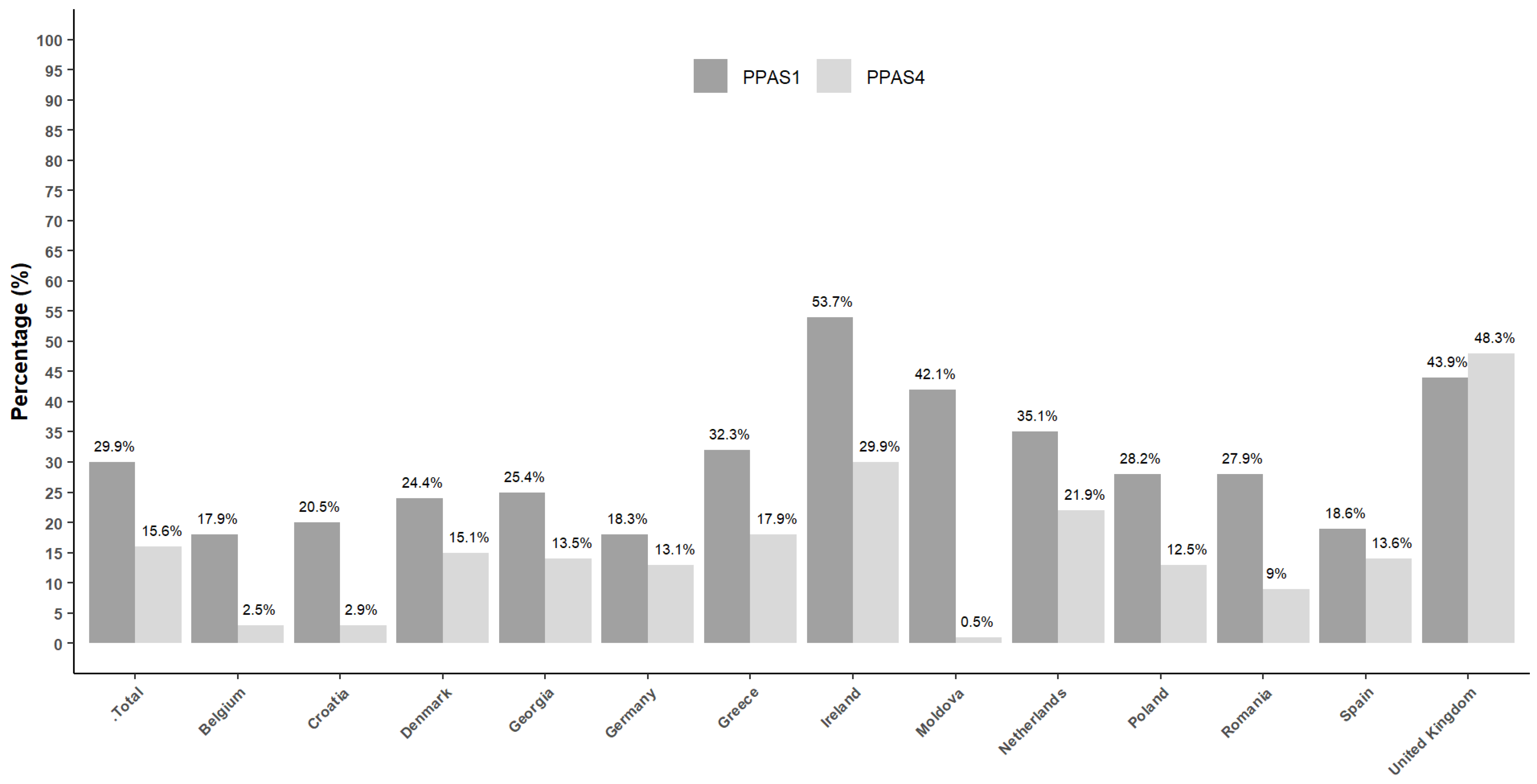
| Total | 1739 | 49.2 | 1354 | 52.0 | 2073 | 59.7 | 1322 | 52.2 | 3016 | 83.9 | 1726 | 65.5 | 1081 | 29.9 | 414 | 15.6 |
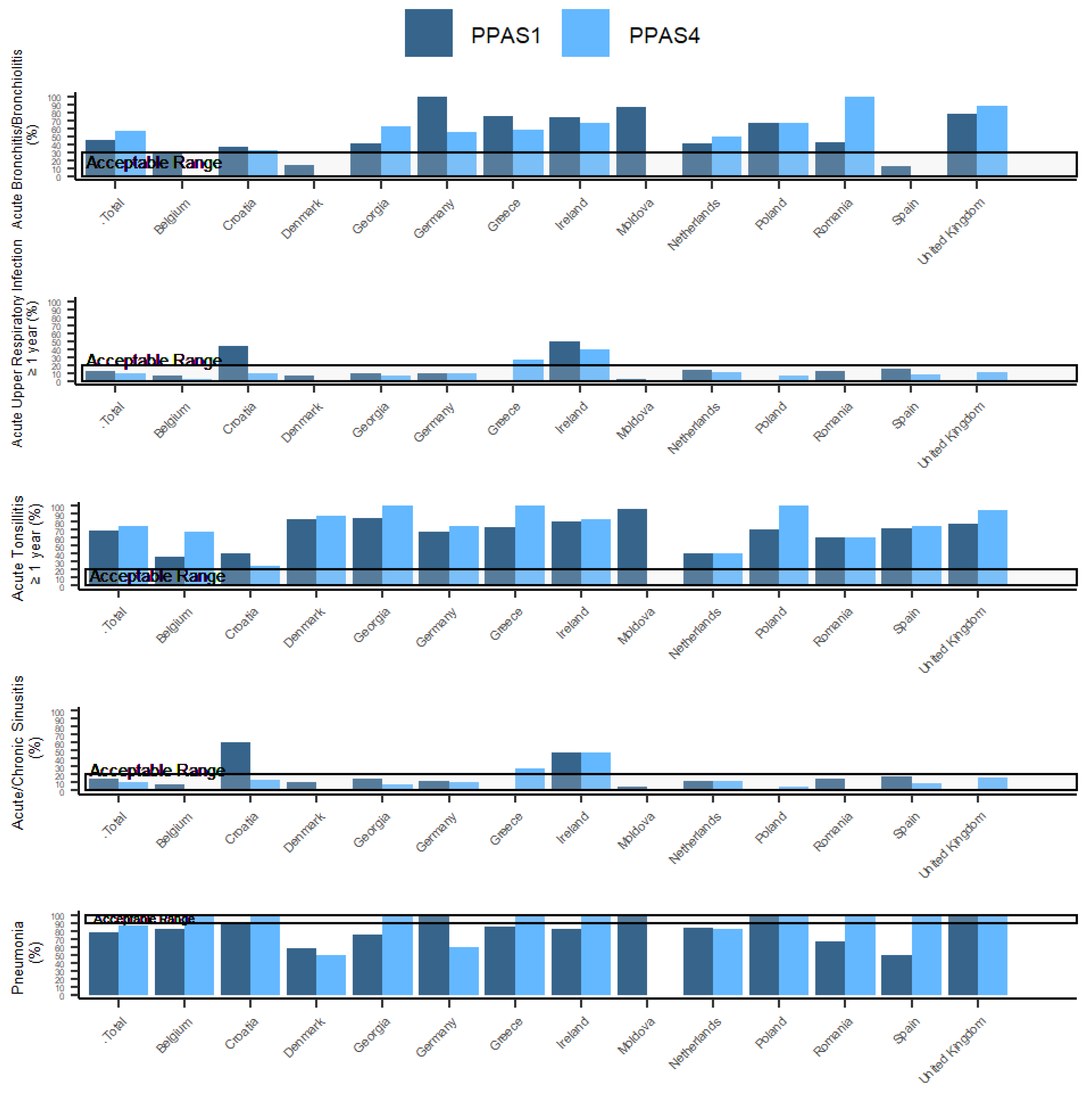
| Country | Percentage of Patients Aged 18–75 Years with Acute Bronchitis/Bronchiolitis Prescribed Antibiotics (ESAC 1A) | Percentage of Patients ≥ 1 Year with Acute Upper Respiratory Infection Prescribed Antibiotics (ESAC 2A) | Percentage of Patients ≥ 1 Year with Acute Tonsillitis Prescribed Antibiotics (ESAC 4A) | Percentage of Patients ≥ 18 Years with Acute/Chronic Sinusitis Prescribed Antibiotics (ESAC 5A) | Percentage of Patients Aged 18–65 Years with Pneumonia Prescribed Antibiotics (ESAC 7A) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acceptable Range 0–30% | Acceptable Range 0–20% | Acceptable Range 0–20% | Acceptable Range 0–20% | Acceptable Range 90–100% | ||||||||||||||||
| PPAS-1 | PPAS-4 | PPAS-1 | PPAS-4 | PPAS-1 | PPAS-4 | PPAS-1 | PPAS-4 | PPAS-1 | PPAS-4 | |||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Belgium | 9 | 31 | - | - | 5 | 7 | 1 | 2 | 16 | 36 | 2 | 67 | 4 | 8 | 1 | 2 | 9 | 82 | 1 | 100 |
| Croatia | 10 | 37 | 1 | 33 | 4 | 44 | 2 | 9 | 6 | 40 | 1 | 25 | 3 | 60 | 2 | 13 | 8 | 89 | 2 | 100 |
| Denmark | 7 | 14 | 0 | 0 | 2 | 7 | 0 | 0 | 32 | 84 | 13 | 87 | 2 | 10 | 0 | 0 | 16 | 59 | 3 | 50 |
| Georgia | 22 | 42 | 10 | 63 | 1 | 9 | 4 | 7 | 11 | 85 | 4 | 100 | 1 | 14 | 3 | 7 | 3 | 75 | 9 | 100 |
| Germany | 10 | 100 | 6 | 55 | 6 | 10 | 12 | 10 | 8 | 67 | 3 | 75 | 6 | 11 | 12 | 10 | 4 | 100 | 6 | 60 |
| Greece | 30 | 75 | 11 | 58 | - | - | 5 | 26 | 8 | 73 | 5 | 100 | - | - | 5 | 28 | 6 | 86 | 3 | 100 |
| Ireland | 26 | 74 | 4 | 67 | 24 | 49 | 22 | 40 | 21 | 81 | 10 | 83 | 19 | 48 | 14 | 47 | 9 | 82 | 3 | 100 |
| Moldova | 13 | 87 | - | - | 2 | 3 | 0 | 0 | 45 | 96 | 0 | 0 | 1 | 4 | 0 | 0 | 1 | 100 | - | - |
| Netherlands | 13 | 42 | 3 | 50 | 8 | 14 | 11 | 11 | 7 | 41 | 4 | 40 | 5 | 11 | 6 | 11 | 26 | 84 | 5 | 83 |
| Poland | 8 | 67 | 4 | 67 | 0 | 0 | 6 | 7 | 10 | 71 | 2 | 100 | 0 | 0 | 2 | 5 | 6 | 100 | 5 | 100 |
| Romania | 12 | 43 | 4 | 100 | 2 | 13 | 0 | 0 | 38 | 61 | 6 | 60 | 2 | 15 | 0 | 0 | 4 | 67 | 2 | 100 |
| Spain | 9 | 13 | 0 | 0 | 2 | 15 | 5 | 8 | 13 | 72 | 12 | 75 | 2 | 17 | 5 | 9 | 3 | 50 | 2 | 100 |
| UK | 28 | 78 | 21 | 88 | 0 | 0 | 9 | 11 | 53 | 77 | 18 | 95 | 0 | 0 | 7 | 16 | 7 | 100 | 11 | 100 |
| Total | 197 | 45 | 64 | 57 | 56 | 13 | 77 | 10 | 268 | 69 | 80 | 76 | 45 | 15 | 57 | 10 | 102 | 79 | 52 | 87 |
| ESAC Quality Indicators | Options of Antibiotic Prescribing in PPAS Survey |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vellinga, A.; Luke-Currier, A.; Garzón-Orjuela, N.; Aabenhus, R.; Anastasaki, M.; Balan, A.; Böhmer, F.; Lang, V.B.; Chlabicz, S.; Coenen, S.; et al. Disease-Specific Quality Indicators for Outpatient Antibiotic Prescribing for Respiratory Infections (ESAC Quality Indicators) Applied to Point Prevalence Audit Surveys in General Practices in 13 European Countries. Antibiotics 2023, 12, 572. https://doi.org/10.3390/antibiotics12030572
Vellinga A, Luke-Currier A, Garzón-Orjuela N, Aabenhus R, Anastasaki M, Balan A, Böhmer F, Lang VB, Chlabicz S, Coenen S, et al. Disease-Specific Quality Indicators for Outpatient Antibiotic Prescribing for Respiratory Infections (ESAC Quality Indicators) Applied to Point Prevalence Audit Surveys in General Practices in 13 European Countries. Antibiotics. 2023; 12(3):572. https://doi.org/10.3390/antibiotics12030572
Chicago/Turabian StyleVellinga, Akke, Addiena Luke-Currier, Nathaly Garzón-Orjuela, Rune Aabenhus, Marilena Anastasaki, Anca Balan, Femke Böhmer, Valerija Bralić Lang, Slawomir Chlabicz, Samuel Coenen, and et al. 2023. "Disease-Specific Quality Indicators for Outpatient Antibiotic Prescribing for Respiratory Infections (ESAC Quality Indicators) Applied to Point Prevalence Audit Surveys in General Practices in 13 European Countries" Antibiotics 12, no. 3: 572. https://doi.org/10.3390/antibiotics12030572
APA StyleVellinga, A., Luke-Currier, A., Garzón-Orjuela, N., Aabenhus, R., Anastasaki, M., Balan, A., Böhmer, F., Lang, V. B., Chlabicz, S., Coenen, S., García-Sangenís, A., Kowalczyk, A., Malania, L., Tomacinschii, A., van der Linde, S. R., Bongard, E., Butler, C. C., Goossens, H., & van der Velden, A. W. (2023). Disease-Specific Quality Indicators for Outpatient Antibiotic Prescribing for Respiratory Infections (ESAC Quality Indicators) Applied to Point Prevalence Audit Surveys in General Practices in 13 European Countries. Antibiotics, 12(3), 572. https://doi.org/10.3390/antibiotics12030572








