Tyndallized Bacteria Preferentially Induce Human Macrophage M1 Polarization: An Effect Useful to Balance Allergic Immune Responses and to Control Infections
Abstract
1. Introduction
2. Results
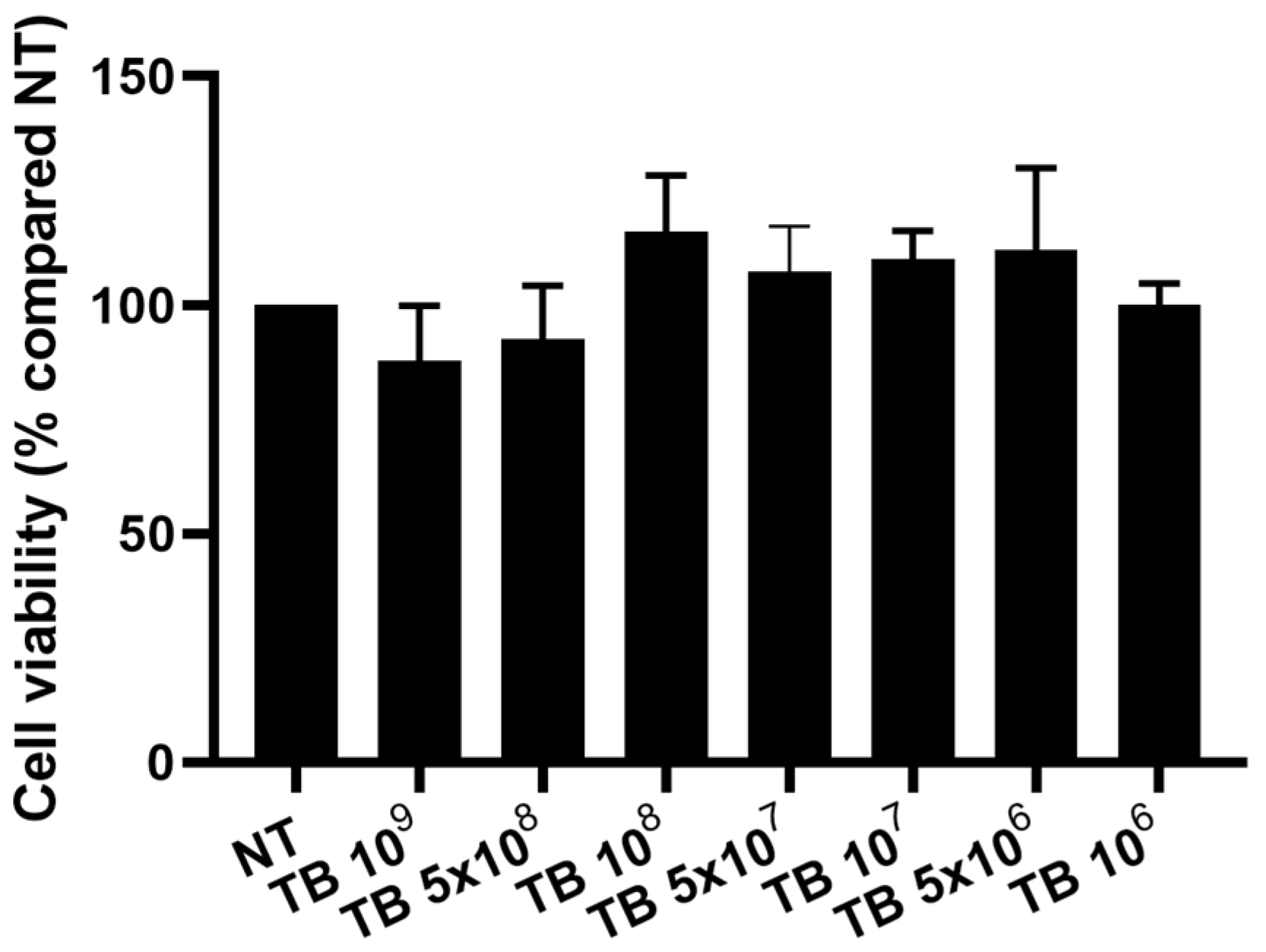
2.1. Effect of TB on the Viability of THP-1 Derived Macrophages
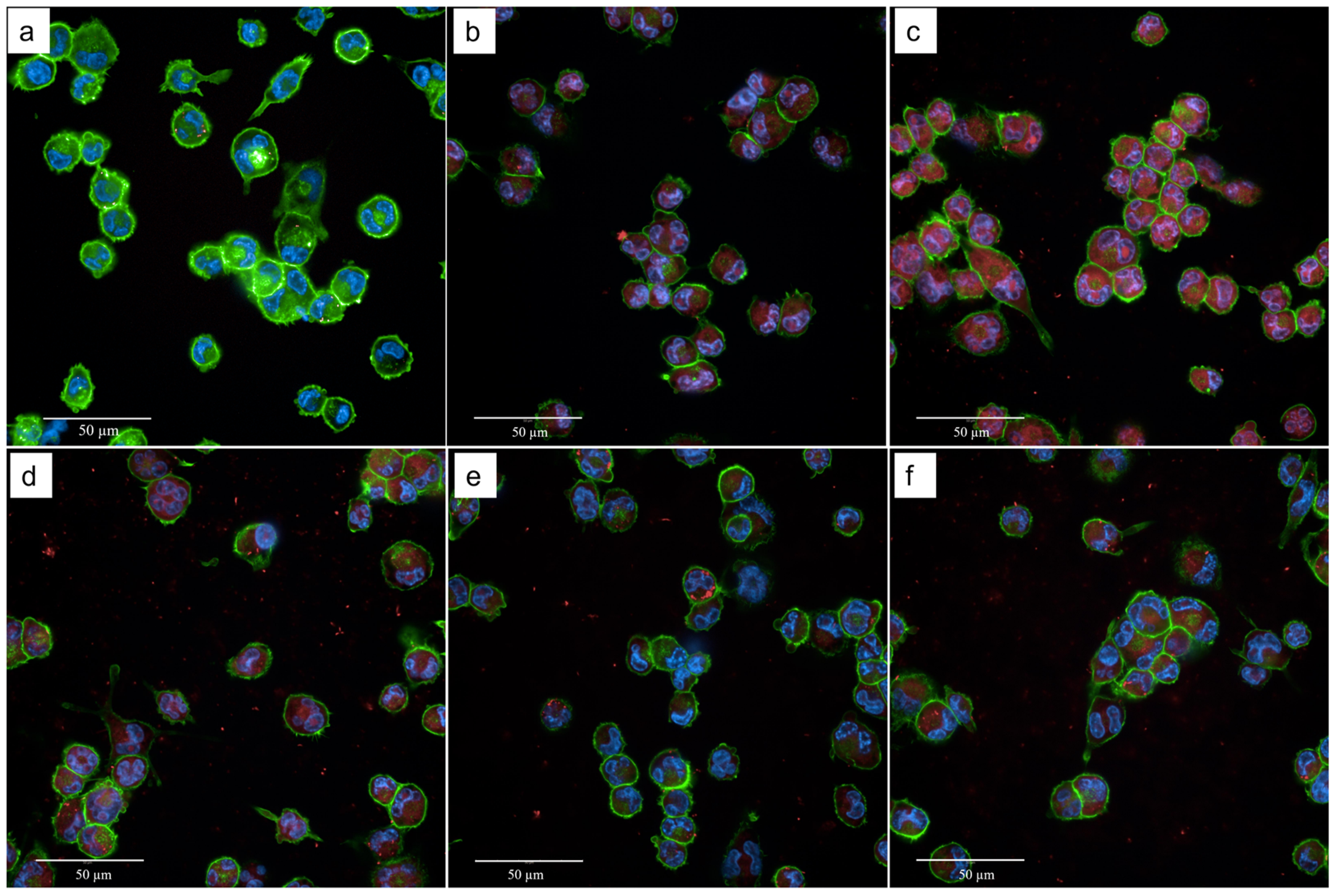
2.2. Effect of TB on the Phagocytosis of THP-1-Derived Macrophages
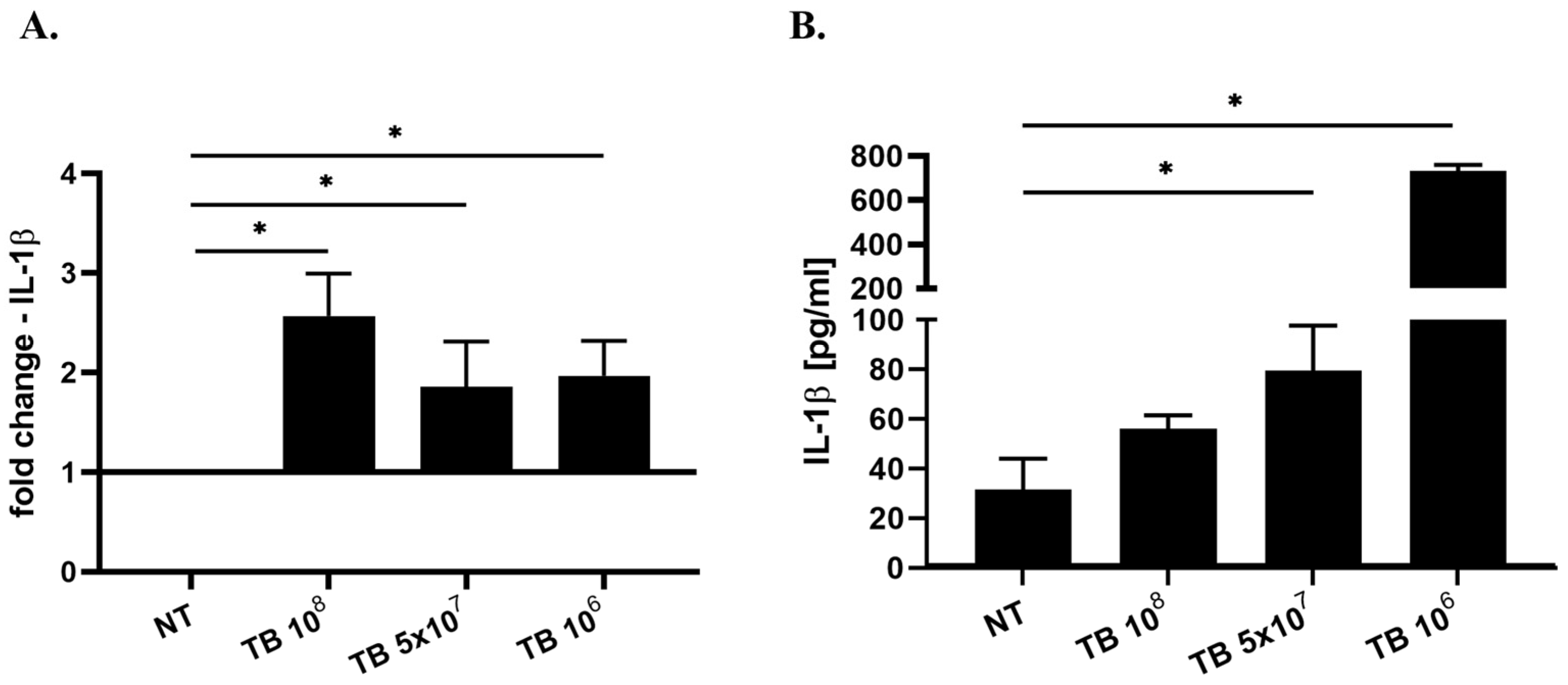
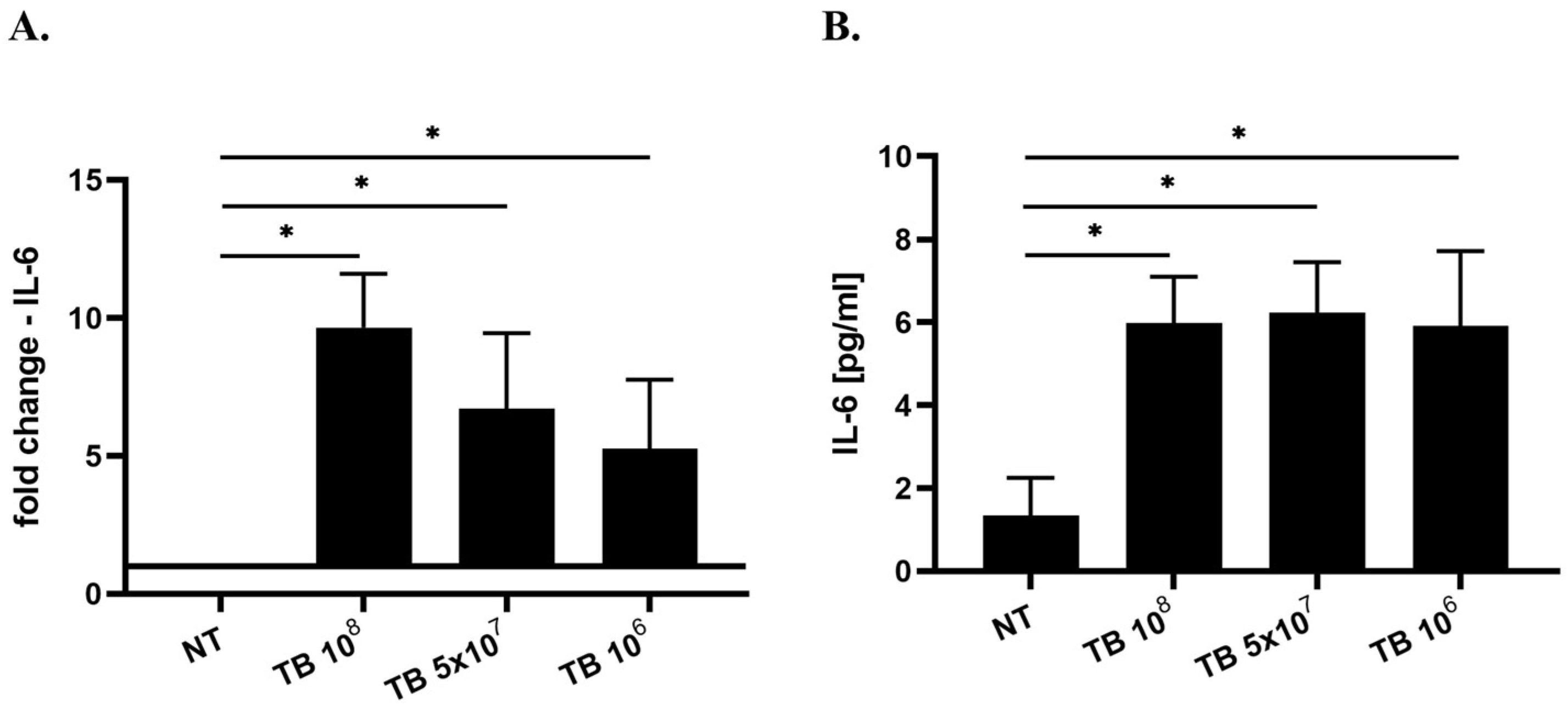
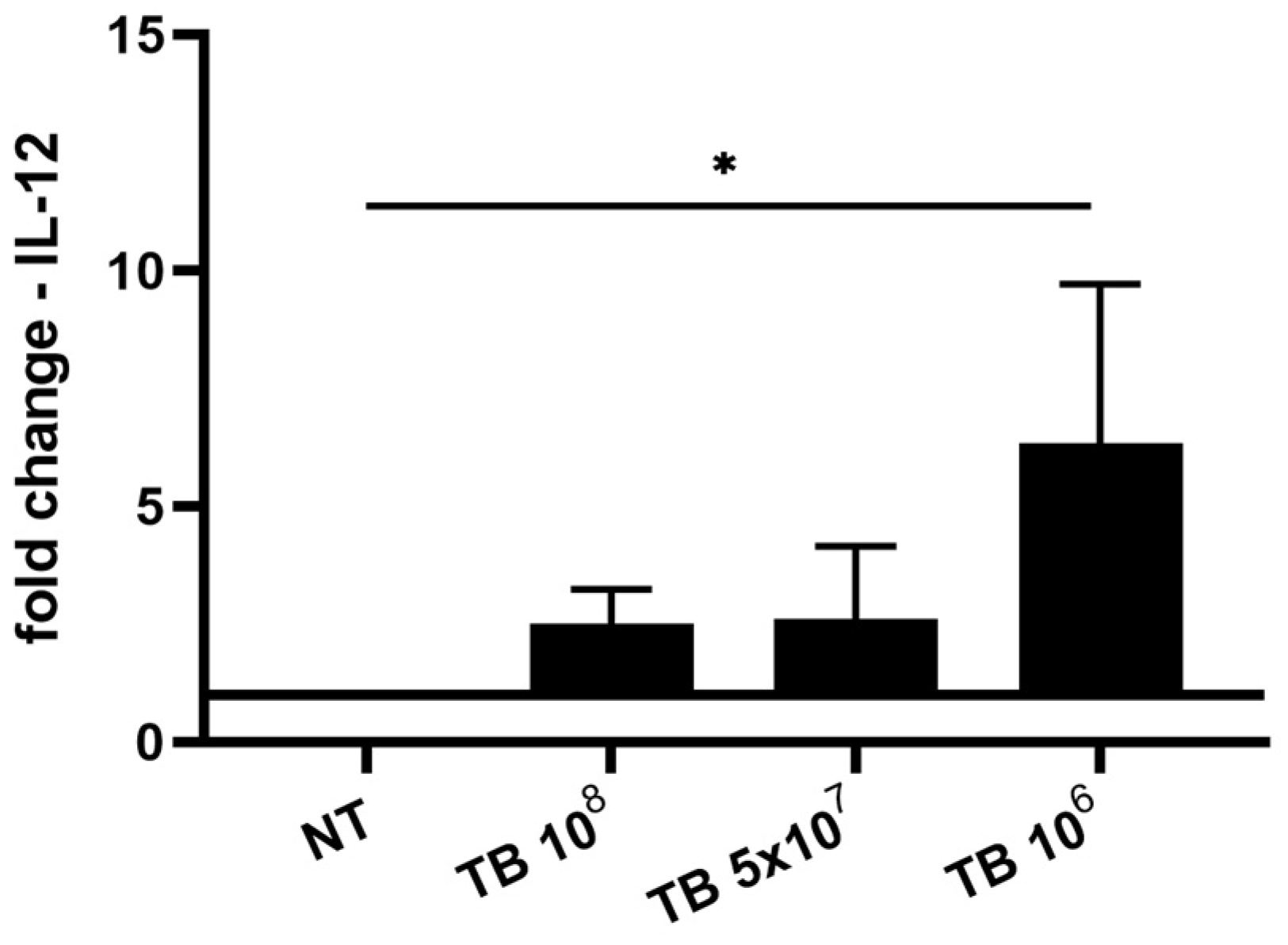
2.3. Effect of TB on the Gene Expression and Release of Cytokines Associated with M1 Polarization (IL-8, IL-1β, IL-6 and IL-12) by THP-1-Derived Macrophages
2.4. Effect of TB on the Gene Expression and Release of Cytokines Associated with M2 Macrophages (TGF-β1) by THP-1-Derived Macrophages
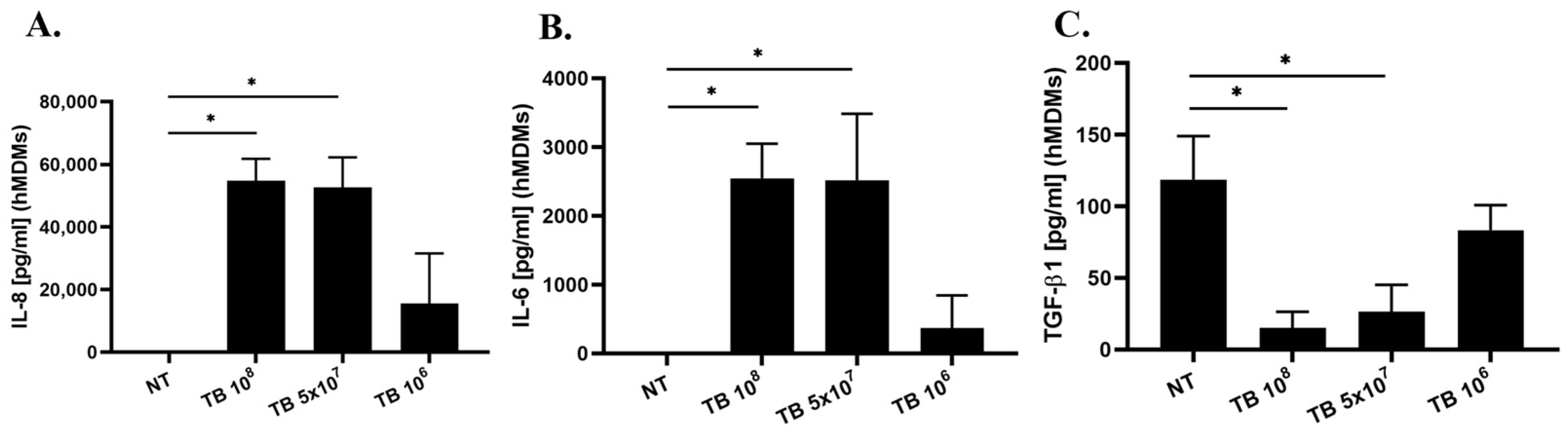
2.5. Effect of TB on the Release of IL-8, IL-6, IL-1β and TGF-β1 by hMDMs
3. Discussion
4. Materials and Methods
4.1. THP-1-Derived Macrophage Cultures
4.2. Human Monocyte-Derived Macrophages (hMDMs)
4.3. Cell Treatment
4.4. Cell Viability Assay
4.5. Staining of TB with SytoRed and Phagocytosis of TB
4.6. Real-Time PCR
4.7. ELISA
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IL | interleukin |
| TGFb | transforming growth factor |
| M1 | classically activated macrophage |
| M2 | alternatively activated macrophages |
| TB | tyndallized bacteria |
| hMDMs | human monocyte-derived macrophages |
References
- Fehervari, Z. Alveolar macrophages in asthma. Nat. Immunol. 2015, 16, 64. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Bosco, M.C. Macrophage polarization: Reaching across the aisle? J. Allergy Clin. Immunol. 2019, 143, 1348–1350. [Google Scholar] [CrossRef]
- Gerasimova, E.V.; Popkova, T.V.; Gerasimova, D.A.; Kirichenko, T.V. Macrophage dysfunction in autoimmune rheumatic diseases and atherosclerosis. Int. J. Mol. Sci. 2022, 23, 4513. [Google Scholar] [CrossRef]
- Liu, Z.; Kuang, W.; Zhou, Q.; Zhang, Y. TGF-β1 secreted by M2 phenotype macrophages enhances the stemness and migration of glioma cells via the SMAD2/3 signalling pathway. Int. J. Mol. Med. 2018, 42, 3395–3403. [Google Scholar] [CrossRef]
- Roszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Saradna, A.; Do, D.C.; Kumar, S.; Fu, Q.-L.; Gao, P. Macrophage polarization and allergic asthma. Transl. Res. 2018, 191, 1–14. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, A.R.; Seo, H.S.; Baik, J.E.; Ahn, K.B.; Yun, C.-H.; Han, S.H. Lipoproteins in Streptococcus gordonii are critical in the infection and inflammatory responses. Mol. Immunol. 2018, 101, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Paveljšek, D.; Ivičak-Kocjan, K.; Treven, P.; Benčina, M.; Jerala, R.; Rogelj, I. Distinctive probiotic features share common TLR2-dependent signalling in intestinal epithelial cells. Cell. Microbiol. 2021, 23, e13264. [Google Scholar] [CrossRef]
- Maeß, M.B.; Wittig, B.; Cignarella, A.; Lorkowski, S. Reduced PMA enhances the responsiveness of transfected THP-1 macrophages to polarizing stimuli. J. Immunol. Methods 2014, 402, 76–81. [Google Scholar] [CrossRef]
- Galdeano, C.M.; Cazorla, S.I.; Dumit, J.M.L.; Vélez, E.; Perdigón, G. Beneficial effects of probiotic consumption on the immune system. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar]
- Kothari, D.; Patel, S.; Kim, S.K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharm. 2019, 111, 537–547. [Google Scholar] [CrossRef]
- Pique, N.; Berlanga, M.; Minana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Yang, T.-Y.; Hsu, C.-C.; Wei, Y.-H.; Wu, C.-C.; Tsai, Y.-C. Lactobacillus paragasseri BBM171 Ameliorates Allergic Airway Inflammation Induced by Ovalbumin in Mice via Modulating the Th1/Th2 Balance. Microorganisms 2022, 10, 2041. [Google Scholar] [CrossRef]
- Velez, E.M.; Galdeano, C.M.; Carmuega, E.; Weill, R.; Bonet, M.E.B.; Perdigón, G. Probiotic fermented milk consumption modulates the allergic process induced by ovoalbumin in mice. Br. J. Nutr. 2015, 114, 566–576. [Google Scholar] [CrossRef]
- Schnyder, J.; Baggiolini, M. Role of phagocytosis in the activation of macrophages. J. Exp. Med. 1978, 148, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, D.; Iida, T.; Nakase, H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef]
- Tarique, A.A.; Logan, J.; Thomas, E.; Holt, P.G.; Sly, P.D.; Fantino, E. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am. J. Respir. Cell Mol. Biol. 2015, 53, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Moretti, J.; Blander, J.M. Insights into phagocytosis-coupled activation of pattern recognition receptors and inflammasomes. Curr. Opin. Immunol. 2014, 26, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Fernando, M.R.; Reyes, J.L.; Iannuzzi, J.; Leung, G.; McKay, D.M. The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PLoS ONE 2014, 9, e94188. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yan, W.; Zheng, H.; Du, Q.; Zhang, L.; Ban, Y.; Li, N.; Wei, F. Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000Research 2015, 4, 1465. [Google Scholar] [CrossRef] [PubMed]
- Haspeslagh, E.; Heyndrickx, I.; Hammad, H.; Lambrecht, B.N. The hygiene hypothesis: Immunological mechanisms of airway tolerance. Curr. Opin. Immunol. 2018, 54, 102–108. [Google Scholar] [CrossRef]
- Yang, A.; Liao, Y.; Zhu, J.; Zhang, J.; Wu, Z.; Li, X.; Tong, P.; Chen, H.; Wang, S.; Liu, Z. Screening of anti-allergy Lactobacillus and its effect on allergic reactions in BALB/c mice sensitized by soybean protein. J. Funct. Foods 2021, 87, 104858. [Google Scholar] [CrossRef]
- Zyrek, A.A.; Cichon, C.; Helms, S.; Enders, C.; Sonnenborn, U.; Schmidt, M.A. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCζ redistribution resulting in tight junction and epithelial barrier repair. Cell. Microbiol. 2007, 9, 804–816. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Won, T.H.; Li, T.T.; Yano, H.; Digumarthi, S.; Heras, A.F.; Zhang, W.; Parkhurst, C.N.; Kashyap, S.; Jin, W.B.; et al. Inulin fibre promotes microbiota-derived bile acids and type 2 inflammation. Nature 2022, 611, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, Y.; Chen, L.; Xu, Q.; Wang, Y.; Xie, M.; Liu, X.; Zhao, J.; Wang, C.-Y. Regulatory T cells, a viable target against airway allergic inflammatory responses in asthma. Front. Immunol. 2022, 13, 902318. [Google Scholar] [CrossRef]
- Thomas, C.A.; Li, Y.; Kodama, T.; Suzuki, H.; Silverstein, S.C.; El Khoury, J. Protection from lethal Gram-positive infection by macrophage scavenger receptor–dependent phagocytosis. J. Exp. Med. 2000, 191, 147–156. [Google Scholar] [CrossRef]
- Pace, E.; Gjomarkaj, M.; Melis, M.; Profita, M.; Spatafora, M.; Vignola, A.M.; Bonsignore, G.; Mody, C.H. Interleukin-8 induces lymphocyte chemotaxis into the pleural space: Role of pleural macrophages. Am. J. Respir. Crit. Care Med. 1999, 159, 1592–1599. [Google Scholar] [CrossRef]
- Meniailo, M.E.; Malashchenko, V.V.; Shmarov, V.A.; Gazatova, N.D.; Melashchenko, O.B.; Goncharov, A.G.; Seledtsova, G.V.; Seledtsov, V.I. Interleukin-8 favors pro-inflammatory activity of human monocytes/macrophages. Int. Immunopharmacol. 2018, 56, 217–221. [Google Scholar] [CrossRef]
- Franchi, L.; Munoz-Planillo, R.; Nunez, G. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 2012, 13, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Van Den Eeckhout, B.; Tavernier, J.; Gerlo, S. Interleukin-1 as innate mediator of T cell immunity. Front. Immunol. 2021, 11, 621931. [Google Scholar] [CrossRef] [PubMed]
- Gaidt, M.M.; Ebert, T.S.; Chauhan, D.; Schmidt, T.; Schmid-Burgk, J.L.; Rapino, F.; Robertson, A.A.; Cooper, M.A.; Graf, T.; Hornung, V. Human monocytes engage an alternative inflammasome pathway. Immunity 2016, 44, 833–846. [Google Scholar] [CrossRef]
- Rincon, M.; Irvin, C.G. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int. J. Biol. Sci. 2012, 8, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Kohno, N.; Fujino, S.; Hamada, H.; Inoue, Y.; Fujioka, S.; Ishida, S.; Hiwada, K. Circulating interleukin-6 levels in patients with bronchial asthma. Am. J. Respir. Crit. Care Med. 1995, 151, 1354–1358. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Spatafora, M.; Chiappara, G.; D’Amico, D.; Volpes, D.; Melis, M.; Pace, E.; Merendino, A. Effect of indomethacin on the kinetics of tumour necrosis factor alpha release and tumour necrosis factor alpha gene expression by human blood monocytes. Pharmacol. Res. 1991, 23, 247–257. [Google Scholar] [CrossRef]
- Spatafora, M.; Chiappara, G.; D’Amico, D.; Volpes, D.; Melis, M.; Pace, E.; Merendino, A.M. Prostaglandin E2 down-regulates the expression of tumor necrosis alpha gene by human blood monocytes. Adv. Prostaglandin Thromboxane Leukot Res. 1991, 21B, 521–524. [Google Scholar]
- Zhang, S.; Wang, Q. Factors determining the formation and release of bioactive IL-12: Regulatory mechanisms for IL-12p70 synthesis and inhibition. Biochem. Biophys. Res. Commun. 2008, 372, 509–512. [Google Scholar] [CrossRef]
- Bottalico, L.A.; Wager, R.; Agellon, L.; Assoian, R.; Tabas, I. Transforming growth factor-beta 1 inhibits scavenger receptor activity in THP-1 human macrophages. J. Biol. Chem. 1991, 266, 22866–22871. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Kohro, T.; Tanaka, T.; Murakami, T.; Wada, Y.; Aburatani, H.; Hamakubo, T.; Kodama, T. A comparison of differences in the gene expression profiles of phorbol 12-myristate 13-acetate differentiated THP-1 cells and human monocyte-derived macrophage. J. Atheroscler. Thromb. 2004, 11, 88–97. [Google Scholar] [CrossRef]
- Schildberger, A.; Rossmanith, E.; Eichhorn, T.; Strassl, K.; Weber, V. Monocytes, peripheral blood mononuclear cells, and THP-1 cells exhibit different cytokine expression patterns following stimulation with lipopolysaccharide. Mediat. Inflamm. 2013, 2013, 697972. [Google Scholar] [CrossRef]
- Hijiya, N.; Miyake, K.; Akashi, S.; Matsuura, K.; Higuchi, Y.; Yamamoto, S. Possible involvement of toll-like receptor 4 in endothelial cell activation of larger vessels in response to lipopolysaccharide. Pathobiology 2002, 70, 18–25. [Google Scholar] [CrossRef]
- Buscetta, M.; Di Vincenzo, S.; Miele, M.; Badami, E.; Pace, E.; Cipollina, C. Cigarette smoke inhibits the NLRP3 inflammasome and leads to caspase-1 activation via the TLR4-TRIF-caspase-8 axis in human macrophages. FASEB J. 2020, 34, 1819–1832. [Google Scholar] [CrossRef]
- Zhang, Y.; Olson, R.M.; Brown, C.R. Macrophage LTB(4) drives efficient phagocytosis of Borrelia burgdorferi via BLT1 or BLT2. J. Lipid Res. 2017, 58, 494–503. [Google Scholar] [CrossRef]
- Sousa-Vasconcelos Pda, S.; Seguins Wda, S.; Luz Ede, S.; Pinho, R.T. Pattern of cytokine and chemokine production by THP-1 derived macrophages in response to live or heat-killed Mycobacterium bovis bacillus Calmette-Guerin Moreau strain. Memórias Do Inst. Oswaldo Cruz 2015, 110, 809–813. [Google Scholar] [CrossRef]
- Harrison, L.M.; van den Hoogen, C.; van Haaften, W.C.; Tesh, V.L. Chemokine expression in the monocytic cell line THP-1 in response to purified shiga toxin 1 and/or lipopolysaccharides. Infect. Immun. 2005, 73, 403–412. [Google Scholar] [CrossRef]
- Taverna, S.; Fontana, S.; Monteleone, F.; Pucci, M.; Saieva, L.; De Caro, V.; Cardinale, V.G.; Giallombardo, M.; Vicario, E.; Rolfo, C. Curcumin modulates chronic myelogenous leukemia exosomes composition and affects angiogenic phenotype via exosomal miR-21. Oncotarget 2016, 7, 30420. [Google Scholar] [CrossRef]
- Ferraro, M.; Gjomarkaj, M.; Siena, L.; Di Vincenzo, S.; Pace, E. Formoterol and fluticasone propionate combination improves histone deacetylation and anti-inflammatory activities in bronchial epithelial cells exposed to cigarette smoke. Biochim. Et Biophys. Acta. Mol. Basis Dis. 2017, 1863, 1718–1727. [Google Scholar] [CrossRef]
- Di Vincenzo, S.; Heijink, I.H.; Noordhoek, J.A.; Cipollina, C.; Siena, L.; Bruno, A.; Ferraro, M.; Postma, D.S.; Gjomarkaj, M.; Pace, E. SIRT1/FoxO3 axis alteration leads to aberrant immune responses in bronchial epithelial cells. J. Cell. Mol. Med. 2018, 22, 2272–2282. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Vincenzo, S.; Ferraro, M.; Taverna, S.; Malizia, V.; Buscetta, M.; Cipollina, C.; Lazzara, V.; Pinto, P.; Bassano, M.; La Grutta, S.; et al. Tyndallized Bacteria Preferentially Induce Human Macrophage M1 Polarization: An Effect Useful to Balance Allergic Immune Responses and to Control Infections. Antibiotics 2023, 12, 571. https://doi.org/10.3390/antibiotics12030571
Di Vincenzo S, Ferraro M, Taverna S, Malizia V, Buscetta M, Cipollina C, Lazzara V, Pinto P, Bassano M, La Grutta S, et al. Tyndallized Bacteria Preferentially Induce Human Macrophage M1 Polarization: An Effect Useful to Balance Allergic Immune Responses and to Control Infections. Antibiotics. 2023; 12(3):571. https://doi.org/10.3390/antibiotics12030571
Chicago/Turabian StyleDi Vincenzo, Serena, Maria Ferraro, Simona Taverna, Velia Malizia, Marco Buscetta, Chiara Cipollina, Valentina Lazzara, Paola Pinto, Marco Bassano, Stefania La Grutta, and et al. 2023. "Tyndallized Bacteria Preferentially Induce Human Macrophage M1 Polarization: An Effect Useful to Balance Allergic Immune Responses and to Control Infections" Antibiotics 12, no. 3: 571. https://doi.org/10.3390/antibiotics12030571
APA StyleDi Vincenzo, S., Ferraro, M., Taverna, S., Malizia, V., Buscetta, M., Cipollina, C., Lazzara, V., Pinto, P., Bassano, M., La Grutta, S., & Pace, E. (2023). Tyndallized Bacteria Preferentially Induce Human Macrophage M1 Polarization: An Effect Useful to Balance Allergic Immune Responses and to Control Infections. Antibiotics, 12(3), 571. https://doi.org/10.3390/antibiotics12030571






