Hypoalbuminemia and Pharmacokinetics: When the Misunderstanding of a Fundamental Concept Leads to Repeated Errors over Decades
Abstract
Take-home message:
- The misinterpretation of the influence of hypoalbuminemia on drug pharmacokinetics and clinical effects is still a problem in 2022.
- Two major levers can be used to reduce this endemic error: (i) improve the way in which the protein-binding concept is taught to medical/pharmacy students; and (ii) systematically quantify the unbound/free form for drugs highly bound to plasma proteins for clinical trials as well as for therapeutic drug monitoring.
1. Introduction
2. Results
- -
- The correct explanations:
- -
- The ambiguous explanations reported in five different cases:
| Cases | Ambiguous Sentences | Explanations |
|---|---|---|
| A | “Calculation of the unbound concentrations, assuming 95% protein binding, may therefore result in considerable overdosing, in particular in critically ill patients with hypoalbuminaemia and renal impairment. In the present study, the inter-individual unbound plasma fraction of flucloxacillin varied widely from 1.1% to 64.7%, showing a substantially higher median value (11%) than reported for healthy individuals (5%).” [32] | Combining the impact of hypoalbuminemia and a change in kidney/liver and drug-drug interactions in the same sentence makes it difficult to identify clinical repercussions. Indeed, hypoalbuminemia does not alter unbound/free concentrations and cannot have any clinical impact. On the contrary, a defective excretory system reduces unbound/free/intrinsic clearance, leading to greater unbound/free exposure and, therefore, impacts the clinical effect of the drug. Moreover, when two drugs (A and B) are administered concomitantly, drug A can modify the protein-binding of drug B without altering the latter’s unbound/free concentration. However, it cannot be ruled out that drug A will also decrease the clearance of drug B, thereby potentially increasing the unbound/free exposure (i.e., concentrations) of drug B. Two mechanisms are, therefore, involved in the drug A–drug B interaction. |
| B | “The binding of beta-lactams to albumin and plasma proteins determines the free fraction, which is the biologically active fraction that diffuses across biological membranes to tissues. The free fraction is also the fraction that is eliminated by renal and liver clearance. When plasma protein amount decreases, the capacity of beta-lactams to bind to protein decreases and beta-lactam-free fraction increases. Previous studies have shown that the binding of beta-lactams to plasma proteins in ICU patients is highly variable and is more altered for antibiotics highly bound to plasma proteins in conditions of homeostasis (e.g., ceftriaxone, cefazolin, or ertapenem). As a result, plasma concentration of beta-lactam antibiotics may be lowered and more unpredictable in patients with severe hypoalbuminemia.” [38] | There is confusion of the terms, “unbound/free form” and “unbound/free fraction” as in the sentence “the unbound/free fraction is the pharmacologically active fraction”. A number (i.e., unbound/free fraction) cannot be pharmacologically active, unlike the drug form. Indeed, the unbound/free form of the drug is the pharmacologically active form regardless of the albuminemia and unbound/free fraction. Moreover, the value of the unbound/free fraction is misleading because it increases with the hypoalbuminemia and thus incorrectly suggests that the unbound/free concentration also rises, thereby suggesting an enhanced clinical effect with the drug in question. |
| C | “SAFE Study Investigator reported that approximately 40% of critically ill patients presented with hypoalbuminemia, because cefoperazone, meropenem, and imipenem are highly bound to albumin, which could increase the unbound fraction significantly. Therefore, various pathological characteristics in critically ill patients may induce a wide discrepancy in the unbound fraction concentrations.” [34] | Use of the term, “unbound/free fraction” instead of “unbound/free concentration” is confusing because the unbound/free fraction changes with hypoalbuminemia, while the unbound/free concentration is unchanged. Consequently, the variation in terms of the unbound/free fraction sheds no light on the clinical effect of the drug. |
| D | “Probably due to this dramatic increase in the ƒu, our patients exhibit a higher ceftriaxone CL than healthy volunteers or critically ill patients with sepsis, septic shock, and different degrees of renal function, a CL that is dependent on albumin concentration and weight based on the results of the population PK model. Surprisingly, in spite of the augmented CL, dosing simulations show that, for an MIC ≤ 2 mg/L (the clinical breakpoint for susceptibility to ceftriaxone), a dose of 1000 mg q24 h maintains unbound ceftriaxone concentrations for a 100% of the dosing interval above the MIC regardless of albumin concentration and body weight.” [31] | Using expressions such as “mostly, usually, often, almost, potentially, may, in several cases, surprisingly…” suggests that the fact is only observed in specific cases, as indicated in the sentence, “Serum albumin concentrations may not affect unbound/free concentrations”, whereas this is always the case. |
| E | “In addition, low serum albumin concentration is frequently observed in ICU patients, leading to an increase in the free fraction of the beta-lactams highly bound to plasma proteins, such as cefazoline, ceftriaxone, or ertapenem. Thus, hypoalbuminemia may lead to increased Vd and tissue penetration, and also increased elimination, of beta-lactam antibiotics by glomerular filtration and/or metabolic clearance. This has been particularly observed for ceftriaxone or ertapenem.” [38] | The paper gives correct conclusions on total pharmacokinetic parameters of no clinical consequence. For example, hypoalbuminemia implies an increase in total clearance without affecting the unbound/free/intrinsic clearance that controls unbound/free exposure. This information misleadingly suggests to the reader that the unbound/free concentration is lower because of increased total clearance. In another example, hypoalbuminemia implies an increase in the total volume of distribution without affecting the unbound/free volume of distribution. This information incorrectly suggests to the reader that the kinetic profile of the unbound/free concentration is modified because of the increase in the total volume of distribution. |
- -
- The incorrect explanations:
| Incorrect Sentences | Explanations |
|---|---|
| “We recognise that our case series is limited and that the study design was retrospective and monocentric. Additionally, only total cefiderocol concentrations were measured, thus potential variability in protein binding commonly encountered in critically ill patients could impact on cefiderocol free levels.” [58] | Variation in unbound concentration occurs over a very short duration (probablyseconds or minutes). However, unbound/free concentration returns to the baseline level, while unbound/free fraction (fu) increases. This increase in fu leads to an increase in the clearance and the volume of distribution for total concentration but not for unbound/free concentration. |
- -
- None of the selected articles contained exclusively true assertions for a specific author;
- -
- Some authors wrote both correct and ambiguous sentences in different articles ([20]/[27]; [22]/[47]; [23]/[41]);
- -
- Some authors have remained consistent by repeating the same incorrect message over time, suggesting that this concept was not taught to them properly at medical school (references of two articles with wrong assertions: [64]/[74]; [66]/[67]; [72]/[73]).
- -
- Some authors state in articles published a few months apart (or even in the same article), facts that contradict each other (references of two articles with correct and incorrect assertions, respectively: [21]/[59]; [20]/[59]; [19]/[41]; [19]/[39]; [22]/[25]; [23]/[66]; [21]/[66]; [21]/[21]; [25]/[25]).
3. Discussion and Conclusions
4. Methods
- -
- False: The authors state that hypoalbuminemia leads to higher unbound/free concentrations and/or higher/lower unbound/free/intrinsic clearance and/or higher/lower unbound/free volume of distribution.
- -
- Ambiguous: The paper suggests, without stating it explicitly, that hypoalbuminemia can impact unbound/free concentration. Various examples of ambiguous sentences are provided below:
- Case A: Combining the impact of hypoalbuminemia and a change in kidney/liver and drug–drug interactions in the same sentence/section makes it difficult to identify clinical repercussions. Indeed, hypoalbuminemia does not alter unbound/free concentrations and cannot have any clinical impact. On the contrary, a defective excretory system reduces unbound/free/intrinsic clearance leading to greater unbound/free exposure and, therefore, impacts the clinical effect of the drug. Moreover, when two drugs (A and B) are administered concomitantly, drug A can modify the protein-binding of drug B without altering the latter’s unbound/free concentration. However, it cannot be ruled out that drug A will also decrease the clearance of drug B, thereby potentially increasing the unbound/free exposure (i.e., concentrations) of drug B. Two mechanisms are, therefore, involved in the drug A–drug B interaction.
- Case B: Confusion of the terms, “unbound/free form” and “unbound/free fraction” as in the sentence “the unbound/free fraction is the pharmacologically active fraction”. A number (i.e., unbound/free fraction) cannot be pharmacologically active, unlike the drug form. Indeed, the unbound/free form of the drug is the pharmacologically active form regardless of the albuminemia and unbound/free fraction. Moreover, the value of the unbound/free fraction is misleading because it increases with the hypoalbuminemia and thus incorrectly suggests that the unbound/free concentration also rises, thereby suggesting an enhanced clinical effect with the drug in question.
- Case C: Use of the term, “unbound/free fraction” instead of “unbound/free concentration” is confusing because the unbound/free fraction changes with hypoalbuminemia, whereas the unbound/free concentration is unchanged. Consequently, the variation in terms of the unbound/free fraction sheds no light on the clinical effect of the drug.
- Case D: Using expressions such as “mostly, usually, often, almost, potentially, may, in several cases, surprisingly…” suggests that the fact is only observed in specific cases as indicated in the sentence, “Serum albumin concentrations may not affect unbound/free concentrations”, whereas this is always the case.
- Case E: Giving correct conclusions on total pharmacokinetic parameters is of no clinical consequence. For example, hypoalbuminemia implies an increase in total clearance without affecting the unbound/free/intrinsic clearance that controls unbound/free exposure. This information misleadingly suggests to the reader that the unbound/free concentration is lower because of increased total clearance. In another example, hypoalbuminemia implies an increase in the total volume of distribution without affecting the unbound/free volume of distribution. This information incorrectly suggests to the reader that the kinetic profile of the unbound/free concentration is modified because of the increase in the total volume of distribution.
- -
- True: The sentence states explicitly that hypoalbuminemia has no impact on the unbound/free concentration.
- -
- Category 1: an article is retained when it contains only correct assertions; an article is deemed unacceptable when it contains ambiguous and/or false assertions.
- -
- Category 2: an article is retained when it contains correct or ambiguous assertions; an article is deemed unacceptable when it contains only incorrect assertions.
- Period A: From drug administration to steady state
- -
- The black squares represent albumin molecules (1 black square = 1 albumin molecule). These squares remained fixed to make this video watchable. The red dots appearing at the top of the figure represent the drug molecules (i.e., 1 red dot = 1 drug molecule) injected into the blood (central compartment) at a constant perfusion rate (i.e., X molecules per hour);
- -
- When the drug (red dots) binds to albumin (black squares), the black squares turn green to illustrate the albumin-binding of the drug. In an attempt to simplify the presentation, we illustrated the drug bound to albumin as a static relationship throughout all of the simulation periods. There is actually a continuous “bond and detachment” process that would have been difficult to represent;
- -
- All red dots travel from the top of the figure to the bottom at the same pace. This means that the drug molecules pass through the excretory organ at the same speed, thereby mimicking intrinsic clearance. This speed does not change over time (i.e., intrinsic clearance is constant and independent of time and dose/concentration);
- -
- At the beginning of the drug perfusion stage, a few red dots reach the bottom of the figure. This shows that a few drug molecules are eliminated at the start of drug perfusion. Indeed, when the first drug molecules reach the blood, they immediately bind to albumin as the drug has a high affinity for albumin. More and more black squares gradually turn green as increasing numbers of drug molecules bind to albumin;
- -
- Due to continuous perfusion, drug accumulation in the body simultaneously leads to an increase in the number of red dots in the figure (i.e., an increase in drug concentration) until a perfusion and a steady elimination rate is reached;
- -
- Once the steady state has been reached, (i) the ratio of red dots (i.e., unbound drug molecules) to green squares (i.e., bound drug molecules) is constant, (ii) the number of red dots expelled at the bottom of the figure per unit of time is constant, and (iii) the number of red dots injected into the central compartment per unit of time is equal to the number of red dots expelled at the bottom of the figure per unit of time.
- Period B: Severe hypoalbuminemia
- -
- When severe hypoalbuminemia occurs (i.e., a marked decrease in albumin molecules, despite the fact that severe hypoalbuminemia takes many days to appear), a large number of green squares disappear and an equal number of red dots appear;
- -
- This increase in red dots indicates an increase in unbound/free drug molecules as well as an increase in unbound/free drug concentrations;
- -
- The excess red dots are channeled downwards at the same speed for all the dots. This means that the surplus unbound/free drug molecules are gradually eliminated by the body, with unbound/free drug concentrations being restored to the original steady state driven by both the perfusion rate and intrinsic clearance;
- -
- Peak concentrations are transient and of short duration, as highlighted by the concentration-versus-time curve of the unbound/free drug.
- Period C: New steady state with hypoalbuminemic status
- -
- When the peak has been reached and the number of albumin molecules has decreased, the number of bound drug molecules falls along with the overall number (bound + unbound) of drug molecules. Consequently, bound and total concentrations are lower when comparing hypoalbuminemia to the values reported at steady state when the patient was not hypoalbuminemic;
- -
- The new steady state shows the same unbound/free drug concentration with lower bound and total drug concentrations.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haller, C. Hypoalbuminemia in Renal Failure: Pathogenesis and Therapeutic Considerations. Kidney Blood Press. Res. 2005, 28, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Gatta, A.; Verardo, A.; Bolognesi, M. Hypoalbuminemia. Intern. Emerg. Med. 2012, 7, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. J. Parenter. Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Toutain, P.L.; Bousquet-Melou, A. Free drug fraction vs free drug concentration: A matter of frequent confusion. J. Vet. Pharmacol. Ther. 2002, 25, 460–463. [Google Scholar] [CrossRef]
- Derendorf, H.; Schmidt, S. Rowland and Tozer’s Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications, 5th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2020. [Google Scholar]
- McElnay, J.C.; D’Arcy, P.F. Protein binding displacement interactions and their clinical importance. Drugs 1983, 25, 495–513. [Google Scholar] [CrossRef]
- Rolan, P.E. Plasma protein binding displacement interactions-why are they still regarded as clinically important? Br. J. Clin. Pharmacol. 1994, 37, 125–128. [Google Scholar] [CrossRef]
- Sansom, L.N.; Evans, A.M. What is the True Clinical Significance of Plasma Protein Binding Displacement Interactions? Drug Saf. 1995, 12, 227–233. [Google Scholar] [CrossRef]
- Benet, L.Z.; Hoener, B.A. Changes in plasma protein binding have little clinical relevance. Clin. Pharmacol. Ther. 2002, 71, 115–121. [Google Scholar] [CrossRef]
- Bjornsson, T.D.; Meffin, P.J.; Swezey, S.; Blaschke, T.F. Clofibrate displaces warfarin from plasma proteins in man: An example of a pure displacement interaction. J. Pharmacol. Exp. Ther. 1979, 210, 316–321. [Google Scholar]
- Heuberger, J.; Schmidt, S.; Derendorf, H. When is Protein Binding Important? J. Pharm. Sci. 2013, 102, 3458–3467. [Google Scholar] [CrossRef]
- T’jollyn, H.; Vermeulen, A.; Van Bocxlaer, J.; Colin, P. A Physiologically Based Pharmacokinetic Perspective on the Clinical Utility of Albumin-Based Dose Adjustments in Critically Ill Patients. Clin. Pharmacokinet. 2018, 57, 59–69. [Google Scholar] [CrossRef]
- Hiraoka, H.; Yamamoto, K.; Okano, N.; Morita, T.; Goto, F.; Horiuchi, R. Changes in Drug Plasma Concentrations of an Extensively Bound and Highly Extracted Drug, Propofol, in Response to Altered Plasma Binding. Clin. Pharmacol. Ther. 2004, 75, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, E.; Hiraoka, H.; Takizawa, D.; Goto, F. Changes in the effect of propofol in response to altered plasma protein binding during normothermic cardiopulmonary bypass. Br. J. Anaesth. 2006, 96, 179–185. [Google Scholar] [CrossRef]
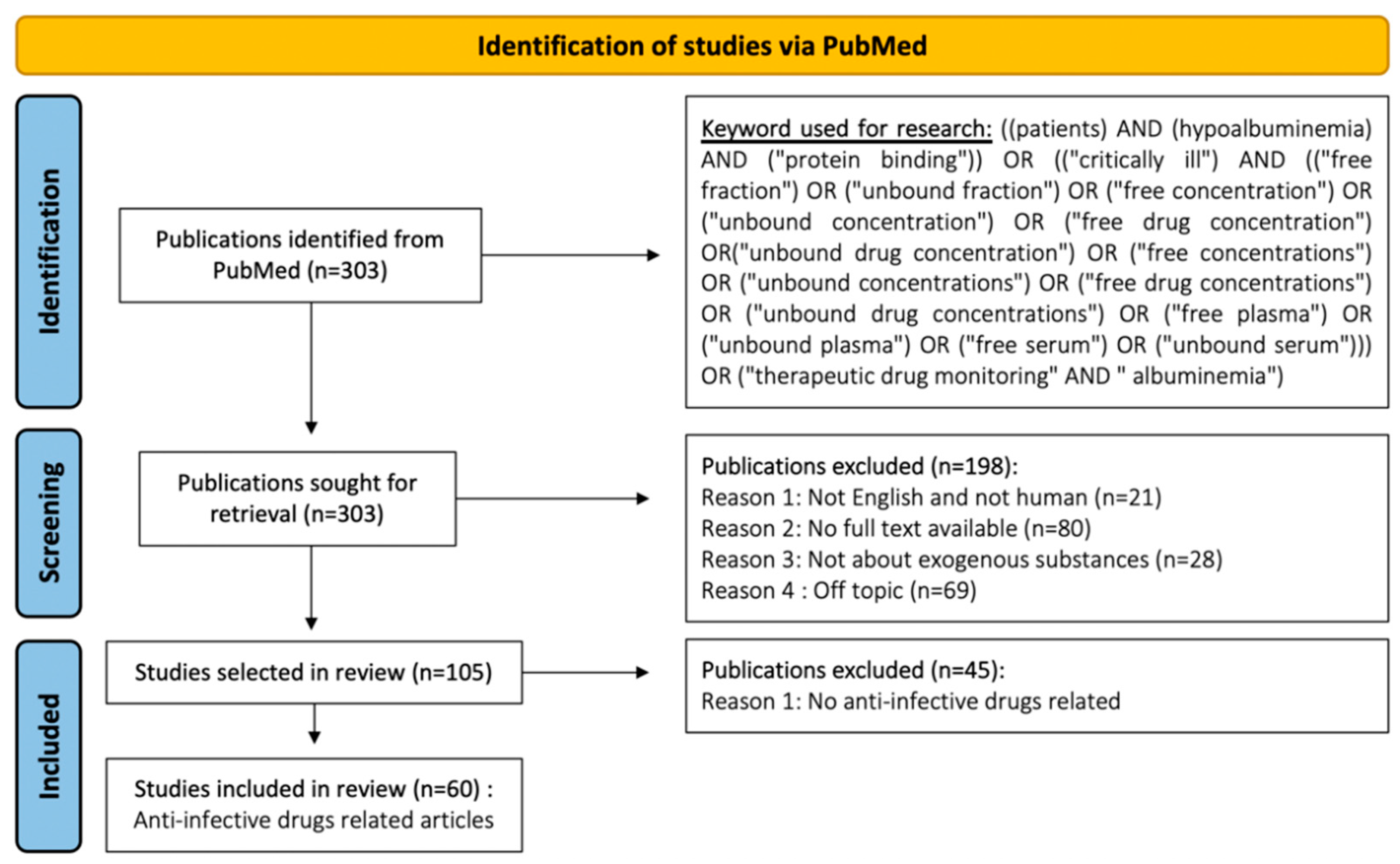
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Van Wart, S.; Sund, Z.M.; Bressler, A.M.; Khan, A.; Makley, A.T.; Hamad, Y.; Salata, R.A.; Silveira, F.P.; Sims, M.D.; et al. Pharmacokinetic and Pharmacodynamic Profiling of Minocycline for Injection following a Single Infusion in Critically Ill Adults in a Phase IV Open-Label Multicenter Study (ACUMIN). Antimicrob. Agents Chemother. 2021, 65, e01809-20. [Google Scholar] [CrossRef]
- Samura, M.; Takada, K.; Yamamoto, R.; Ito, H.; Nagumo, F.; Uchida, M.; Kurata, T.; Koshioka, S.; Enoki, Y.; Taguchi, K.; et al. Population Pharmacokinetic Analysis and Dosing Optimization Based on Unbound Daptomycin Concentration and Cystatin C in Nonobese Elderly Patients with Hypoalbuminemia and Chronic Kidney Disease. Pharm. Res. 2021, 38, 1041–1055. [Google Scholar] [CrossRef]
- Leegwater, E.; Kraaijenbrink, B.V.C.; Moes, D.J.A.R.; Purmer, I.M.; Wilms, E.B. Population pharmacokinetics of ceftriaxone administered as continuous or intermittent infusion in critically ill patients. J. Antimicrob. Chemother. 2020, 75, 1554–1558. [Google Scholar] [CrossRef]
- Sime, F.B.; Byrne, C.J.; Parker, S.; Stuart, J.; Butler, J.; Starr, T.; Pandey, S.; Wallis, S.C.; Lipman, J.; Roberts, J.A. Population pharmacokinetics of total and unbound concentrations of intravenous posaconazole in adult critically ill patients. Crit. Care 2019, 23, 205. [Google Scholar] [CrossRef]
- Wasmann, R.E.; Muilwijk, E.W.; Burger, D.M.; Verweij, P.E.; Knibbe, C.A.; Brüggemann, R.J. Clinical Pharmacokinetics and Pharmacodynamics of Micafungin. Clin. Pharmacokinet. 2018, 57, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Briscoe, S.; McWhinney, B.; Ally, M.; Ungerer, J.; Lipman, J.; Roberts, J.A. Therapeutic drug monitoring of β-lactam antibiotics in the critically ill: Direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J. Antimicrob. Chemother. 2018, 73, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
- Schleibinger, M.; Steinbach, C.L.; Töpper, C.; Kratzer, A.; Liebchen, U.; Kees, F.; Salzberger, B.; Kees, M.G. Protein binding characteristics and pharmacokinetics of ceftriaxone in intensive care unit patients: Unbound ceftriaxone in ICU patients. Br. J. Clin. Pharmacol. 2015, 80, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Carlier, M.; Stove, V.; Wallis, S.C.; De Waele, J.J.; Verstraete, A.G.; Lipman, J.; Roberts, J.A. Assays for therapeutic drug monitoring of β-lactam antibiotics: A structured review. Int. J. Antimicrob. Agents 2015, 46, 367–375. [Google Scholar] [CrossRef]
- Enokiya, T.; Muraki, Y.; Iwamoto, T.; Okuda, M. Changes in the pharmacokinetics of teicoplanin in patients with hyperglycaemic hypoalbuminaemia: Impact of albumin glycosylation on the binding of teicoplanin to albumin. Int. J. Antimicrob. Agents 2015, 46, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Kees, M.G.; Wicha, S.G.; Seefeld, A.; Kees, F.; Kloft, C. Unbound fraction of vancomycin in intensive care unit patients. J. Clin. Pharmacol. 2014, 54, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A. Usefulness of monitoring free (unbound) concentrations of therapeutic drugs in patient management. Clin. Chim. Acta 2007, 377, 1–13. [Google Scholar] [CrossRef]
- Wallenburg, E.; Ter Heine, R.; De Lange, D.W.; Van Leeuwen, H.; Schouten, J.A.; Ten Oever, J.; Kolwijck, E.; Burger, D.M.; Pickkers, P.; Gieling, E.M.; et al. High unbound flucloxacillin fraction in critically ill patients. J. Antimicrob. Chemother. 2021, 76, 3220–3228. [Google Scholar] [CrossRef]
- Aulin, L.B.S.; De Paepe, P.; Dhont, E.; De Jaeger, A.; Vande Walle, J.; Vandenberghe, W.; McWhinney, B.C.; Ungerer, J.P.J.; Van Hasselt, J.G.C.; De Cock, P.A.J.G. Population Pharmacokinetics of Unbound and Total Teicoplanin in Critically Ill Pediatric Patients. Clin. Pharmacokinet. 2021, 60, 353–363. [Google Scholar] [CrossRef]
- Gijsen, M.; Dreesen, E.; Van Daele, R.; Annaert, P.; Debaveye, Y.; Wauters, J.; Spriet, I. Pharmacokinetic/Pharmacodynamic Target Attainment Based on Measured versus Predicted Unbound Ceftriaxone Concentrations in Critically Ill Patients with Pneumonia: An Observational Cohort Study. Antibiotics 2021, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Asada, M.; Nagata, M.; Mizuno, T.; Uchida, T.; Takahashi, H.; Makita, K.; Arai, H.; Kijima, S.; Echizen, H.; Yasuhara, M. Population pharmacokinetics of cefazolin before, during and after cardiopulmonary bypass in adult patients undergoing cardiac surgery. Eur. J. Clin. Pharmacol. 2021, 77, 735–745. [Google Scholar] [CrossRef]
- Ulldemolins, M.; Bastida, C.; Llauradó-Serra, M.; Csajka, C.; Rodríguez, A.; Badia, J.R.; Martín-Loeches, I.; Soy, D. Once-daily 1 g ceftriaxone optimizes exposure in patients with septic shock and hypoalbuminemia receiving continuous veno-venous hemodiafiltration. Eur. J. Clin. Pharmacol. 2021, 77, 1169–1180. [Google Scholar] [CrossRef]
- Moser, S.; Rehm, S.; Guertler, N.; Hinic, V.; Dräger, S.; Bassetti, S.; Rentsch, K.M.; Sendi, P.; Osthoff, M. Probability of pharmacological target attainment with flucloxacillin in Staphylococcus aureus bloodstream infection: A prospective cohort study of unbound plasma and individual MICs. J. Antimicrob. Chemother. 2021, 76, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Tang Girdwood, S.C.; Tang, P.H.; Murphy, M.E.; Chamberlain, A.R.; Benken, L.A.; Jones, R.L.; Stoneman, E.M.; Kaplan, J.M.; Vinks, A.A. Demonstrating Feasibility of an Opportunistic Sampling Approach for Pharmacokinetic Studies of β-Lactam Antibiotics in Critically Ill Children. J. Clin. Pharmacol. 2021, 61, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Dang, Z.L.; Li, B.; Zhu, L.; Qin, H.Y.; Wu, X.A.; Wei, Y.H. Determination of Total and Unbound Meropenem, Imipenem/Cilastatin, and Cefoperazone/Sulbactam in Human Plasma: Application for Therapeutic Drug Monitoring in Critically Ill Patients. Ther. Drug Monit. 2020, 42, 578–587. [Google Scholar] [CrossRef]
- Wong, G.; Taccone, F.; Villois, P.; Scheetz, M.H.; Rhodes, N.J.; Briscoe, S.; McWhinney, B.; Nunez-Nunez, M.; Ungerer, J.; Lipman, J.; et al. β-Lactam pharmacodynamics in Gram-negative bloodstream infections in the critically ill. J. Antimicrob. Chemother. 2020, 75, 429–433. [Google Scholar] [CrossRef]
- Abdulla, A.; Dijkstra, A.; Hunfeld, N.G.M.; Endeman, H.; Bahmany, S.; Ewoldt, T.M.J.; Muller, A.E.; Van Gelder, T.; Gommers, D.; Koch, B.C.P. Failure of target attainment of beta-lactam antibiotics in critically ill patients and associated risk factors: A two-center prospective study (EXPAT). Crit. Care Lond. Engl. 2020, 24, 558. [Google Scholar] [CrossRef]
- Grégoire, N.; Marchand, S.; Ferrandière, M.; Lasocki, S.; Seguin, P.; Vourc’h, M.; Barbaz, M.; Gaillard, T.; Launey, Y.; Asehnoune, K.; et al. Population pharmacokinetics of daptomycin in critically ill patients with various degrees of renal impairment. J. Antimicrob. Chemother. 2019, 74, 117–125. [Google Scholar] [CrossRef]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics and the French Society of Anaesthesia and Intensive Care Medicine. Crit. Care 2019, 23, 104. [Google Scholar] [CrossRef] [PubMed]
- Sime, F.B.; Lassig-Smith, M.; Starr, T.; Stuart, J.; Pandey, S.; Parker, S.L.; Wallis, S.C.; Lipman, J.; Jason, A.; Roberts, J.A. Population Pharmacokinetics of Unbound Ceftolozane and Tazobactam in Critically Ill Patients without Renal Dysfunction. Antimicrob. Agents Chemother. 2019, 63, e01265-19. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.E.; Smith, C.L.; Scharmen, A.; Kidd, J.M.; Cooper, C.; Kuti, J.; Mitra, S.; Nicolau, D.P.; Havlichek, D.H. Pharmacokinetic and Pharmacodynamic Analysis of Ceftazidime/Avibactam in Critically Ill Patients. Surg. Infect. 2019, 20, 55–61. [Google Scholar] [CrossRef]
- Dhaese, S.A.M.; Thooft, A.D.J.; Farkas, A.; Lipman, J.; Verstraete, A.G.; Stove, V.; Roberts, J.A.; De Waele, J.J. Early target attainment of continuous infusion piperacillin/tazobactam and meropenem in critically ill patients: A prospective observational study. J. Crit. Care 2019, 52, 75–79. [Google Scholar] [CrossRef]
- Alexandre, K.; Fantin, B. Pharmacokinetics and Pharmacodynamics of Temocillin. Clin. Pharmacokinet. 2018, 57, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Hu, X.; Zhao, L.; Hu, M.; Gao, F. Clinical and pharmacological hallmarks of rifapentine’s use in diabetes patients with active and latent tuberculosis: Do we know enough? Drug Des. Devel. Ther. 2017, 11, 2957–2968. [Google Scholar] [CrossRef]
- De Cock, P.A.; Desmet, S.; De Jaeger, A.; Biarent, D.; Dhont, E.; Herck, I.; Vens, D.; Colman, S.; Stove, V.; Commeyne, S.; et al. Impact of vancomycin protein binding on target attainment in critically ill children: Back to the drawing board? J. Antimicrob. Chemother. 2017, 72, 801–804. [Google Scholar] [CrossRef]
- Tsai, D.; Stewart, P.; Goud, R.; Gourley, S.; Hewagama, S.; Krishnaswamy, S.; Wallis, S.C.; Lipman, J.; Roberts, J.A. Total and unbound ceftriaxone pharmacokinetics in critically ill Australian Indigenous patients with severe sepsis. Int. J. Antimicrob. Agents. 2016, 48, 748–752. [Google Scholar] [CrossRef]
- Laterre, P.F.; Wittebole, X.; Van de Velde, S.; Muller, A.E.; Mouton, J.W.; Carryn, S.; Tulkens, P.M.; Dugernier, T. Temocillin (6 g daily) in critically ill patients: Continuous infusion versus three times daily administration. J. Antimicrob. Chemother. 2015, 70, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Liebchen, U.; Kratzer, A.; Wicha, S.G.; Kees, F.; Kloft, C.; Kees, M.G. Unbound fraction of ertapenem in intensive care unit patients. J. Antimicrob. Chemother. 2014, 69, 3108–3111. [Google Scholar] [CrossRef]
- Vanstraelen, K.; Wauters, J.; Vercammen, I.; De Loor, H.; Maertens, J.; Lagrou, K.; Annaert, P.; Spriet, I. Impact of hypoalbuminemia on voriconazole pharmacokinetics in critically ill adult patients. Antimicrob. Agents Chemother. 2014, 58, 6782–6789. [Google Scholar] [CrossRef]
- Wong, G.; Briscoe, S.; Adnan, S.; McWhinney, B.; Ungerer, J.; Lipman, J.; Roberts, J.A. Protein Binding of β-Lactam Antibiotics in Critically Ill Patients: Can We Successfully Predict Unbound Concentrations? Antimicrob. Agents Chemother. 2013, 57, 6165–6170. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Lipman, J.; Udy, A.A.; Ng, M.; McWhinney, B.; Ungerer, J.; Lust, K.; Roberts, J.A. β-Lactam therapeutic drug monitoring in the critically ill: Optimising drug exposure in patients with fluctuating renal function and hypoalbuminaemia. Int. J. Antimicrob. Agents 2013, 41, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Vilay, A.M.; Grio, M.; Depestel, D.D.; Sowinski, K.M.; Gao, L.; Heung, M.; Salama, N.N.; Mueller, B.A. Daptomycin pharmacokinetics in critically ill patients receiving continuous venovenous hemodialysis. Crit. Care Med. 2011, 39, 19–25. [Google Scholar] [CrossRef]
- Benko, R.; Matuz, M.; Doro, P.; Peto, Z.; Molnar, A.; Hajdu, E.; Nagy, E.; Gardi, J.; Soos, G. Pharmacokinetics and pharmacodynamics of levofloxacin in critically ill patients with ventilator-associated pneumonia. Int. J. Antimicrob. Agents 2007, 30, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, O.; Kumar, V.; Katterwe, D.; Majcher-Peszynska, J.; Drewelow, B.; Derendorf, H.; Welte, T. Ertapenem in critically ill patients with early-onset ventilator-associated pneumonia: Pharmacokinetics with special consideration of free-drug concentration. J. Antimicrob. Chemother. 2006, 59, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Mimoz, O.; Rolland, D.; Adoun, M.; Marchand, S.; Breilh, D.; Brumpt, I.; Debaene, B.; Couet, W. Steady-state trough serum and epithelial lining fluid concentrations of teicoplanin 12 mg/kg per day in patients with ventilator-associated pneumonia. Intensive Care Med. 2006, 32, 775–779. [Google Scholar] [CrossRef]
- Mimoz, O.; Soreda, S.; Padoin, C.; Tod, M.; Petitjean, O.; Benhamou, D. Ceftriaxone pharmacokinetics during iatrogenic hydroxyethyl starch-induced hypoalbuminemia: A model to explore the effects of decreased protein binding capacity on highly bound drugs. Anesthesiology 2000, 93, 735–743. [Google Scholar] [CrossRef]
- Ochs, H.R.; Greenblatt, D.J.; Woo, E. Clinical pharmacokinetics of quinidine. Clin. Pharmacokinet. 1980, 5, 150–168. [Google Scholar] [CrossRef]
- Booke, H.; Frey, O.R.; Röhr, A.C.; Chiriac, U.; Zacharowski, K.; Holubec, T.; Adam, E.H. Excessive unbound cefazolin concentrations in critically ill patients receiving veno-arterial extracorporeal membrane oxygenation (vaECMO): An observational study. Sci. Rep. 2021, 20, 16981. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Bartoletti, M.; Cojutti, P.G.; Gaibani, P.; Conti, M.; Giannella, M.; Viale, P.; Pea, F. A descriptive case series of pharmacokinetic/pharmacodynamic target attainment and microbiological outcome in critically ill patients with documented severe extensively drug-resistant Acinetobacter baumannii bloodstream infection and/or ventilator-associated pneumonia treated with cefiderocol. J. Glob. Antimicrob. Resist. 2021, 27, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Jager, N.G.L.; van Hest, R.M.; Xie, J.; Wong, G.; Ulldemolins, M.; Brüggemann, R.J.M.; Lipman, J.; Roberts, J.A. Optimization of flucloxacillin dosing regimens in critically ill patients using population pharmacokinetic modelling of total and unbound concentrations. J. Antimicrob. Chemother. 2020, 75, 2641–2649. [Google Scholar] [CrossRef] [PubMed]
- Van Raaij, J.J.; Mabelis, N.J.D.; Shudofsky, K.N.; Meenks, S.D.; Le Noble, J.L.M.L.; Janssen, P.K.C. Quantification of total and unbound cefuroxime in plasma by ultra-performance liquid chromatography tandem mass spectrometry in a cohort of critically ill patients with hypoalbuminemia and renal failure. J. Clin. Lab. Anal. 2020, 34, e23100. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, S.N.; Gopalakrishnan, M.; Heil, E.L. A Population Pharmacokinetics and Pharmacodynamic Approach To Optimize Tazobactam Activity in Critically Ill Patients. Antimicrob. Agents Chemother. 2020, 64, e02093-19. [Google Scholar] [CrossRef]
- Wilkes, S.; van Berlo, I.; Ten Oever, J.; Jansman, F.; Ter Heine, R. Population pharmacokinetic modeling of total and unbound flucloxacillin in non-critically ill patients to devise a rational continuous dosing regimen. Int. J. Antimicrob. Agents 2019, 53, 310–317. [Google Scholar] [CrossRef]
- Osthoff, M.; Siegemund, M.; Balestra, G.; Abdul-Aziz, M.H.; Roberts, J.A. Prolonged administration of β-lactam antibiotics—A comprehensive review and critical appraisal. Swiss Med. Wkly. 2016, 146, w14368. [Google Scholar] [CrossRef] [PubMed]
- Brink, A.J.; Richards, G.A.; Lautenbach, E.E.G.; Rapeport, N.; Schillack, V.; Van Niekerk, L.; Lipman, J.; Roberts, J.A. Albumin concentration significantly impacts on free teicoplanin plasma concentrations in non-critically ill patients with chronic bone sepsis. Int. J. Antimicrob. Agents 2015, 45, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Udy, A.A.; Jarrett, P.; Wallis, S.C.; Hope, W.W.; Sharma, R.; Kirkpatrick, C.M.; Kruger, P.S.; Roberts, M.S.; Lipman, J. Plasma and target-site subcutaneous tissue population pharmacokinetics and dosing simulations of cefazolin in post-trauma critically ill patients. J. Antimicrob. Chemother. 2015, 70, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Stove, V.; De Waele, J.J.; Sipinkoski, B.; McWhinney, B.; Ungerer, P.J.P.; Akova, M.; Bassetti, M.; Dimopoulos, G.; Kaukonen, K.M.; et al. DALI Study Authors. Variability in protein binding of teicoplanin and achievement of therapeutic drug monitoring targets in critically ill patients: Lessons from the DALI Study. Int. J. Antimicrob. Agents 2014, 43, 423–430. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI Study. DALI: Defining antibiotic levels in intensive care unit patients: Are current β-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Eyler, R.F.; Vilay, A.M.; Nader, A.M.; Heung, M.; Pleva, M.; Sowinski, K.M.; DePestel, D.D.; Sörgel, F.; Kinzig, M.; Mueller, B.A. Pharmacokinetics of ertapenem in critically ill patients receiving continuous venovenous hemodialysis or hemodiafiltration. Antimicrob. Agents Chemother. 2014, 58, 1320–1326. [Google Scholar] [CrossRef]
- Yagi, T.; Naito, T.; Doi, M.; Nagura, O.; Yamada, T.; Maekawa, M.; Sato, S.; Kawakami, J. Plasma exposure of free linezolid and its ratio to minimum inhibitory concentration varies in critically ill patients. Int. J. Antimicrob. Agents 2013, 42, 329–334. [Google Scholar] [CrossRef]
- Roberts, J.A.; Pea, F.; Lipman, J. The clinical relevance of plasma protein binding changes. Clin. Pharmacokinet. 2013, 52, 1–8. [Google Scholar] [CrossRef]
- Wenisch, J.M.; Meyer, B.; Fuhrmann, V.; Saria, K.; Zuba, C.; Dittrich, P.; Thalhammer, F. Multiple-dose pharmacokinetics of daptomycin during continuous venovenous haemodiafiltration. J. Antimicrob. Chemother. 2012, 67, 977–983. [Google Scholar] [CrossRef]
- Ulldemolins, M.; Roberts, J.A.; Rello, J.; Paterson, D.L.; Lipman, J. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin. Pharmacokinet. 2011, 50, 99–110. [Google Scholar] [CrossRef]
- Ulldemolins, M.; Roberts, J.A.; Wallis, S.C.; Rello, J.; Lipman, J. Flucloxacillin dosing in critically ill patients with hypoalbuminaemia: Special emphasis on unbound pharmacokinetic. J. Antimicrob. Chemother. 2010, 65, 1771–1778. [Google Scholar] [CrossRef]
- Brink, A.J.; Richards, G.A.; Schillack, V.; Kiem, S.; Schentag, J. Pharmacokinetics of once-daily dosing of ertapenem in critically ill patients with severe sepsis. Int. J. Antimicrob. Agents 2009, 33, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Pea, F.; Viale, P.; Pavan, F.; Furlanut, M. Pharmacokinetic Considerations for Antimicrobial Therapy in Patients Receiving Renal Replacement Therapy. Clin. Pharmacokinet. 2007, 46, 997–1038. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.K.; McClain, C.D.; Zurakowski, D.; Dodson, B.; McManus, M.L. Total phenytoin concentrations do not accurately predict free phenytoin concentrations in critically ill children. Pediatr. Crit. Care Med. 2006, 7, 434–439. [Google Scholar] [CrossRef]
- Stanke-Labesque, F.; Concordet, D.; Djerada, Z.; Bouchet, B.; Solas, C.; Mériglier, E.; Bonnet, F.; Mourvillier, B.; Ruiz, S.; Martin-Blondel, G.; et al. Neglecting Plasma Protein Binding in COVID-19 Patients Leads to a Wrong Interpretation of Lopinavir Overexposure. Clin. Pharmacol. Ther. 2021, 109, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
| Screening | Analysis | ||||
|---|---|---|---|---|---|
| Period | Number of Articles Using Keywords | Number of Included | Category 1 1 | Category 2 2 (True + Ambigous) | At Least One False Assertion |
| 1975–1980 | 18 | 1 | 0 | 1 (0 + 1) | 0 |
| 1981–1985 | 15 | 0 | 0 | 0 | 0 |
| 1986–1990 | 19 | 0 | 0 | 0 | 0 |
| 1991–1995 | 17 | 0 | 0 | 0 | 0 |
| 1996–2000 | 15 | 1 | 0 | 0 | 1 |
| 2001–2005 | 16 | 0 | 0 | 0 | 0 |
| 2006–2010 | 32 | 8 | 1 | 4 (1 + 3) | 4 |
| 2011–2015 | 61 | 19 | 2 | 6 (2 + 4) | 13 |
| 2016–2020 | 81 | 21 | 3 | 11 (3 + 8) | 10 |
| 2021 | 29 | 11 | 1 | 4 (1 + 3) | 7 |
| Total | 303 | 61 | 7 | 26 (7 + 19) | 35 |
| % | - | - | 11 | 43 | 57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandia, P.; Decheiver, S.; Picard, M.; Guilhaumou, R.; Baklouti, S.; Concordet, D. Hypoalbuminemia and Pharmacokinetics: When the Misunderstanding of a Fundamental Concept Leads to Repeated Errors over Decades. Antibiotics 2023, 12, 515. https://doi.org/10.3390/antibiotics12030515
Gandia P, Decheiver S, Picard M, Guilhaumou R, Baklouti S, Concordet D. Hypoalbuminemia and Pharmacokinetics: When the Misunderstanding of a Fundamental Concept Leads to Repeated Errors over Decades. Antibiotics. 2023; 12(3):515. https://doi.org/10.3390/antibiotics12030515
Chicago/Turabian StyleGandia, Peggy, Sarah Decheiver, Manon Picard, Romain Guilhaumou, Sarah Baklouti, and Didier Concordet. 2023. "Hypoalbuminemia and Pharmacokinetics: When the Misunderstanding of a Fundamental Concept Leads to Repeated Errors over Decades" Antibiotics 12, no. 3: 515. https://doi.org/10.3390/antibiotics12030515
APA StyleGandia, P., Decheiver, S., Picard, M., Guilhaumou, R., Baklouti, S., & Concordet, D. (2023). Hypoalbuminemia and Pharmacokinetics: When the Misunderstanding of a Fundamental Concept Leads to Repeated Errors over Decades. Antibiotics, 12(3), 515. https://doi.org/10.3390/antibiotics12030515







