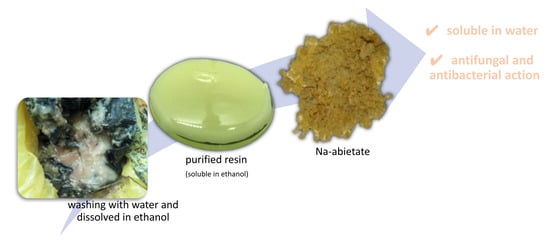
Green Synthesis of Na abietate Obtained from the Salification of Pinus elliottii Resin with Promising Antimicrobial Action
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition (CHN, ED, S and MS)
2.2. Vibrational Spectroscopy (FTIR)
2.3. Ionic Properties and Zeta Potential (ζ)
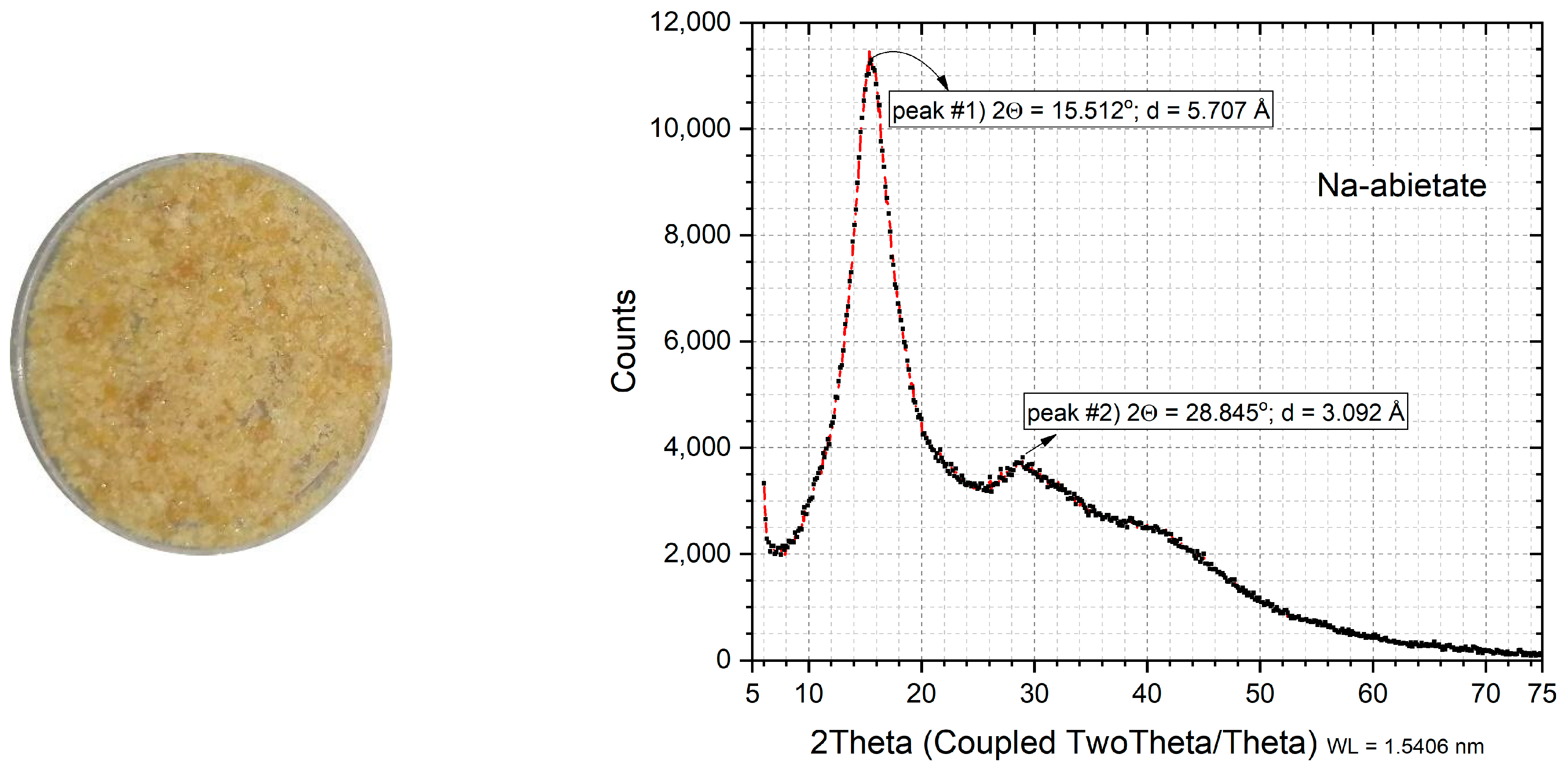
2.4. X-ray Diffractometry (XRD)
2.5. Antimicrobial Tests
2.5.1. MIC/MBC/MFC Methods
2.5.2. Disk Diffusion Method
3. Material and Methods
3.1. Reagents
3.2. Na abietate Synthesis
3.3. Characterization
3.3.1. Composition by CHN, MS, and EDS
3.3.2. Vibrational Spectroscopy (FTIR)
3.3.3. Zeta Potential (ζ)
3.3.4. X-ray Diffractometry (XRD)
3.4. Antimicrobiological Tests
3.4.1. Minimum Inhibitory Concentration (MIC)
3.4.2. Minimum Bactericidal Concentration (MBC) and Minimum Fungicidal Concentration (MFC)
3.4.3. Disk Diffusion Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 17 December 2022).
- OMS: Relatório Alerta Para Aumento de Resistência a Antibióticos Em Infecções Bacterianas. ONU News, 9 December 2022. Available online: https://news.un.org/pt/story/2022/12/1806582 (accessed on 17 December 2022).
- Menezes, M. Detecção de Bactérias Resistentes a Antibióticos Triplicou Na Pandemia; Fiocruz: Rio de Janeiro, Brazil, 2021. [Google Scholar]
- VALÉCIO, M. OMS Alerta: Em 2050 Superbactérias Devem Matar Uma Pessoa a Cada Três Segundos. Available online: https://ictq.com.br/farmacia-clinica/3166-oms-alerta-em-2050-superbacterias-matarao-uma-pessoa-a-cada-tres-segundos (accessed on 12 December 2022).
- Li, X.Q.; Chen, Y.; Dai, G.C.; Zhou, B.B.; Yan, X.N.; Tan, R.X. Abietic Acid Ameliorates Psoriasis-like Inflammation and Modulates Gut Microbiota in Mice. J. Ethnopharmacol. 2021, 272, 113934. [Google Scholar] [CrossRef] [PubMed]
- Helfenstein, A.; Vahermo, M.; Nawrot, D.A.; Demirci, F.; İşcan, G.; Krogerus, S.; Yli-Kauhaluoma, J.; Moreira, V.M.; Tammela, P. Antibacterial Profiling of Abietane-Type Diterpenoids. Bioorg. Med. Chem. 2017, 25, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Kurnaz, L.B.; Luo, Y.; Yang, X.; Alabresm, A.; Leighton, R.; Kumar, R.; Hwang, J.; Decho, A.W.; Nagarkatti, P.; Nagarkatti, M.; et al. Facial Amphiphilicity Index Correlating Chemical Structures with Antimicrobial Efficacy. Bioact. Mater. 2023, 20, 519–527. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, M.S. Abietic Acid and (+)-Sclareolide as Raw Material for the Preparation of the Key Intermediate and Synthesis of Labdanic Diterpenes Biologic Active; Universidade Estadual de Campinas: Campinas, Brazil, 2007. [Google Scholar]
- Correa, J.S.; Primo, J.O.; Bittencourt, C.; Horsth, D.F.L.; Radovanovic, E.; Silveira, A.T., Jr.; Toma, H.E.; Zanette, C.M.; Anaissi, F.J. Ecofriendly Synthesis of Zn-Abietate Complex Derived from Pinus Elliottii Resin and Its Application as an Antibacterial Pigment against S. Aureus and E. Coli. Dye. Pigment. 2022, 197, 109946. [Google Scholar] [CrossRef]
- Schons, A.B.; Correa, J.S.; Appelt, P.; Meneguzzi, D.; Cunha, M.A.A.; Bittencourt, C.; Toma, H.E.; Anaissi, F.J. Eco-Friendly Synthesis of an Oxovanadium(IV)-Bis(Abietate) Complex with Antimicrobial Action. Molecules 2022, 27, 6679. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Jin, W.T.; Weng, W.Z.; Zhou, Z.H. Interactions of Vanadium with Amino Acids-Monodentate Coordination of Vanadyl Proline, Lysine and Histidine and Catalytic Degradations of Methyl Orange. Polyhedron 2019, 159, 375–381. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal–Carboxylate Interactions in Metal–Alginate Complexes Studied with FTIR Spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. Software Update: The ORCA Program System, Version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Haroon, M.; Akhtar, T.; Khalid, M.; Zahra, S.S.; Haq, I.; Assiri, M.A.; Imran, M.; Braga, A.A.C. Synthesized Thiazole-Based Hydrazides and Their Spectral Characterization along with Biological Studies: Promising Quantum Chemical Insights. J. Mol. Struct. 2022, 1270, 133923. [Google Scholar] [CrossRef]
- Silva, M.G.L.; Silva, L.Y.S.; Freitas, T.S.; Rocha, J.E.; Pereira, R.L.S.; Tintino, S.R.; Oliveira, M.R.C.; Martins, A.O.B.P.B.; Lima, M.C.P.; Hora, G.C.A.; et al. Antibacterial effect and evaluation of the inhibitory effect against efflux pump in Staphylococcus aureus by abietic acid: In vitro and in silico assays. Process Biochem. 2022, 122, 363–372. [Google Scholar] [CrossRef]
- Adam, M.S.S.; El-Hady, O.M.; Makhlouf, M.M.; Bayazeed, A.; El-Metwaly, N.M.; Mohamad, A.D.M. Effect of Oxy-Vanadium (IV) and Oxy-Zirconium (IV) Ions in O,N-Bidentate Arylhydrazone Complexes on Their Catalytic and Biological Potentials That Supported via Computerized Usages. J. Taiwan Inst. Chem. Eng. 2022, 132, 104168. [Google Scholar] [CrossRef]
- Appelt, P.; Fagundes, F.D.; Facchin, G.; Gabriela Kramer, M.; Back, D.F.; Cunha, M.A.A.; Sandrino, B.; Wohnrath, K.; De Araujo, M.P. Ruthenium (II) Complexes Containing 2-Mercaptothiazolinates as Ligands and Evaluation of Their Antimicrobial Activity. Inorganica Chim. Acta 2015, 436, 152–158. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Daud, F.; Pande, G.; Joshi, M.; Pathak, R.; Wankhede, S.S. A Study of Antibacterial Effect of Some Selected Essential Oils and Medicinal Herbs Against Acne Causing Bacteria. Int. J. Pharm. Sci. Invent. 2013, 2, 27–34. [Google Scholar]
- Helal, A.-M.M.; Ali, S.; Aly, S.M.; Al-Debasi, Y. Antibacterial activity of sider (ziziphus spina- christi), leaves extract against selected pathogenic bacteria. Eur. J. Pharm. Med. Res. 2016, 3, 138–144. [Google Scholar]
- Abdel-Rahman, L.H.; Adam, M.S.S.; Al-Zaqri, N.; Shehata, M.R.; El-Sayed Ahmed, H.; Mohamed, S.K. Synthesis, Characterization, Biological and Docking Studies of ZrO(II), VO(II) and Zn(II) Complexes of a Halogenated Tetra-Dentate Schiff Base. Arab. J. Chem. 2022, 15, 103737. [Google Scholar] [CrossRef]
- Cabezas-Pizarro, J.; Redondo-Solano, M.; Umaña-Gamboa, C.; Arias-Echandi, M.L. Antimicrobial Activity of Different Sodium and Potassium Salts of Carboxylic Acid against Some Common Foodborne Pathogens and Spoilage-Associated Bacteria. Rev. Argent. Microbiol. 2018, 50, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2008; ISBN 610.688.0700. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 6th ed.; NCCLS document M7-A6, Suite 1400; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2003; ISBN 610.688.0700. [Google Scholar]
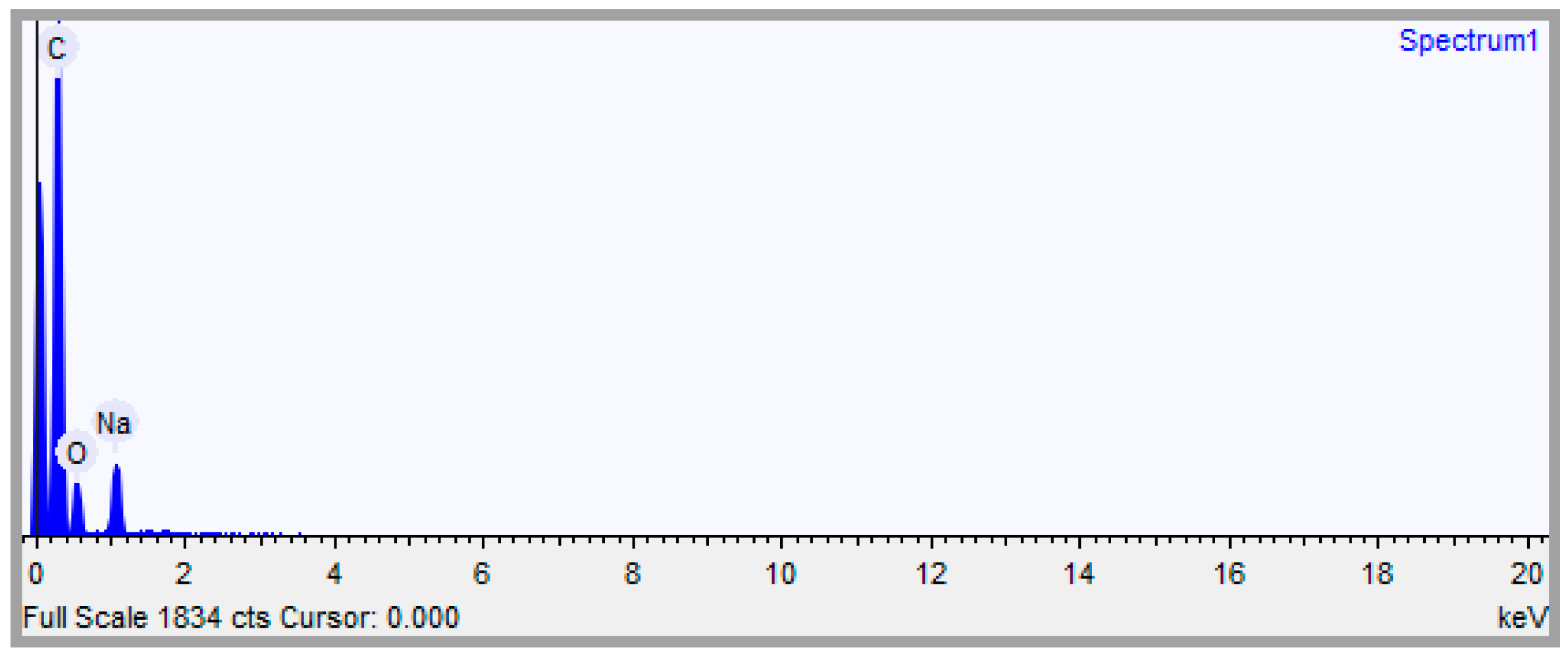





| Sodium (Na) | Carbon (C) | Hydrogen (H) | Oxygen (O) | C/H Ratio | |
|---|---|---|---|---|---|
| Mass (%) theoretical | 7.0861 | 74.0412 | 9.0096 | 9.8630 | 8.218 |
| Mass (%) found | --- | 64.33 | 9.13 | --- | 7.04 |
| Composition by EDS | 4.806 | 73.833 | --- | 21.361 | --- |
| Theoretical (cm−1) | Experimental (cm−1) | ΔTh-Ex (cm−1) | Assignments |
|---|---|---|---|
| 3087 | 2926 | 161 | ν O-H/νC-H (sp3) [11] * |
| 3055 | 2865 | 190 | ν O-H/ν C-H |
| 1547 | 1544 | 3 | νass COO− [10,16] |
| 1394 | 1397 | 3 | νsim COO− [10,16] |
| Mass (g) | Concentration (g mol−1) | pH | Conductivity (mS/cm) |
|---|---|---|---|
| 0.5 | 1.54 × 10−3 | 9.76 | 132.7 |
| 1.0 | 3.08 × 10−3 | 9.87 | 140.6 |
| 2.0 | 6.16 × 10−3 | 9.93 | 150.6 |
| Sample | Size (d. nm) | ζ Potential (mV) | Mobility (μm cm/Vs) | Conductivity (mS/cm) |
|---|---|---|---|---|
| 1 | 266.50 | −43.5 | −3.410 | 0.183 |
| 2 | 257.70 | −45.9 | −3.602 | 0.184 |
| 3 | 206.08 | −44.0 | −3.451 | 0.187 |
| Average | 243.42 | −44.5 | −3.487 | 0.1846 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schons, A.B.; Appelt, P.; Correa, J.S.; Cunha, M.A.A.; Rodrigues, M.G.; Anaissi, F.J. Green Synthesis of Na abietate Obtained from the Salification of Pinus elliottii Resin with Promising Antimicrobial Action. Antibiotics 2023, 12, 514. https://doi.org/10.3390/antibiotics12030514
Schons AB, Appelt P, Correa JS, Cunha MAA, Rodrigues MG, Anaissi FJ. Green Synthesis of Na abietate Obtained from the Salification of Pinus elliottii Resin with Promising Antimicrobial Action. Antibiotics. 2023; 12(3):514. https://doi.org/10.3390/antibiotics12030514
Chicago/Turabian StyleSchons, Aline B., Patrícia Appelt, Jamille S. Correa, Mário A. A. Cunha, Mauricio G. Rodrigues, and Fauze J. Anaissi. 2023. "Green Synthesis of Na abietate Obtained from the Salification of Pinus elliottii Resin with Promising Antimicrobial Action" Antibiotics 12, no. 3: 514. https://doi.org/10.3390/antibiotics12030514
APA StyleSchons, A. B., Appelt, P., Correa, J. S., Cunha, M. A. A., Rodrigues, M. G., & Anaissi, F. J. (2023). Green Synthesis of Na abietate Obtained from the Salification of Pinus elliottii Resin with Promising Antimicrobial Action. Antibiotics, 12(3), 514. https://doi.org/10.3390/antibiotics12030514