Virulence Characteristics and Molecular Typing of Carbapenem-Resistant ST15 Klebsiella pneumoniae Clinical Isolates, Possessing the K24 Capsular Type
Abstract
1. Introduction
2. Results
2.1. Bacterial Identification and Antimicrobial Susceptibility Testing
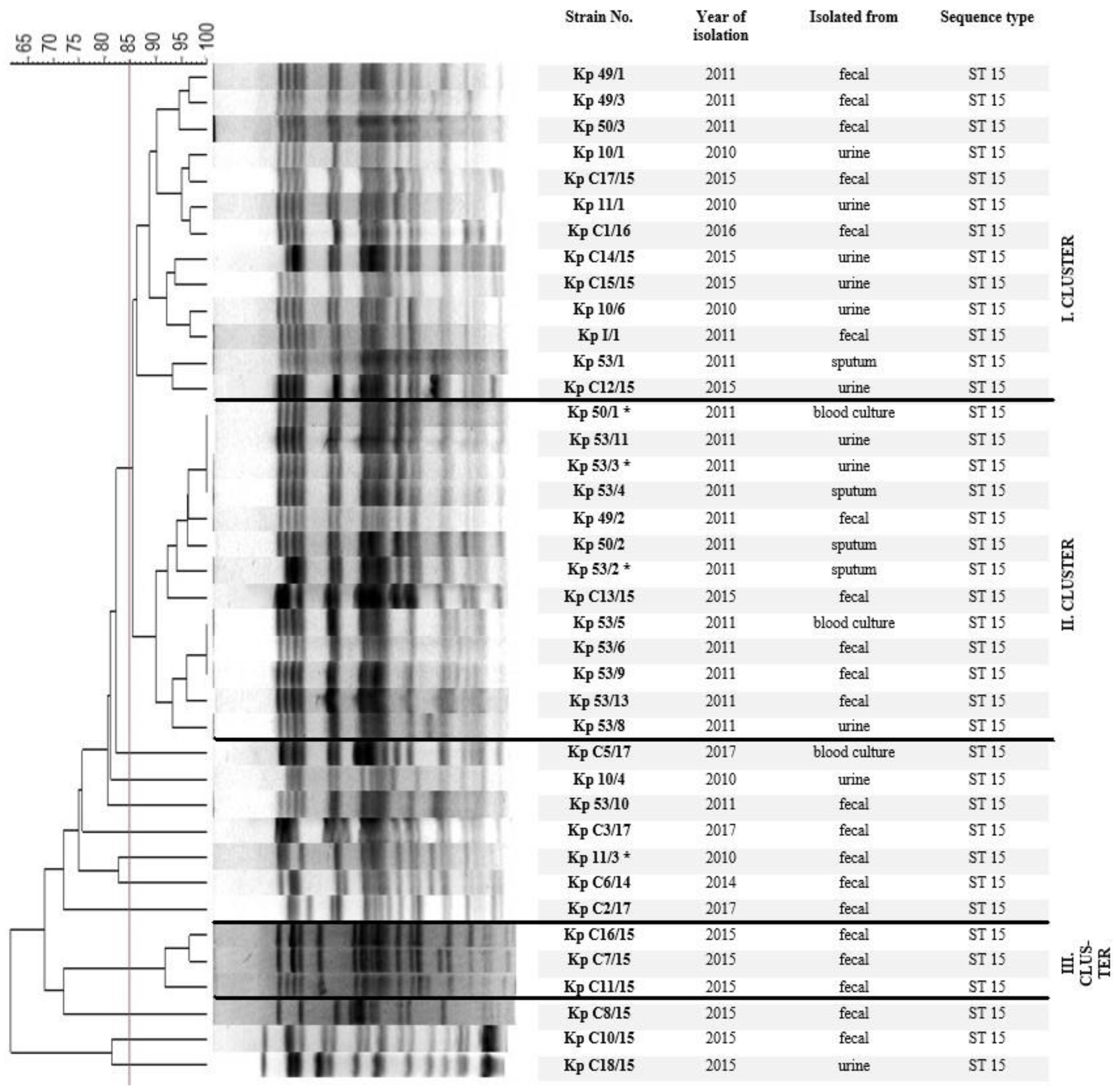
2.2. Chromosomal Macro-Restriction Fragment Polymorphism Analysis with PFGE
2.3. Presence of Virulence-Associated Genes
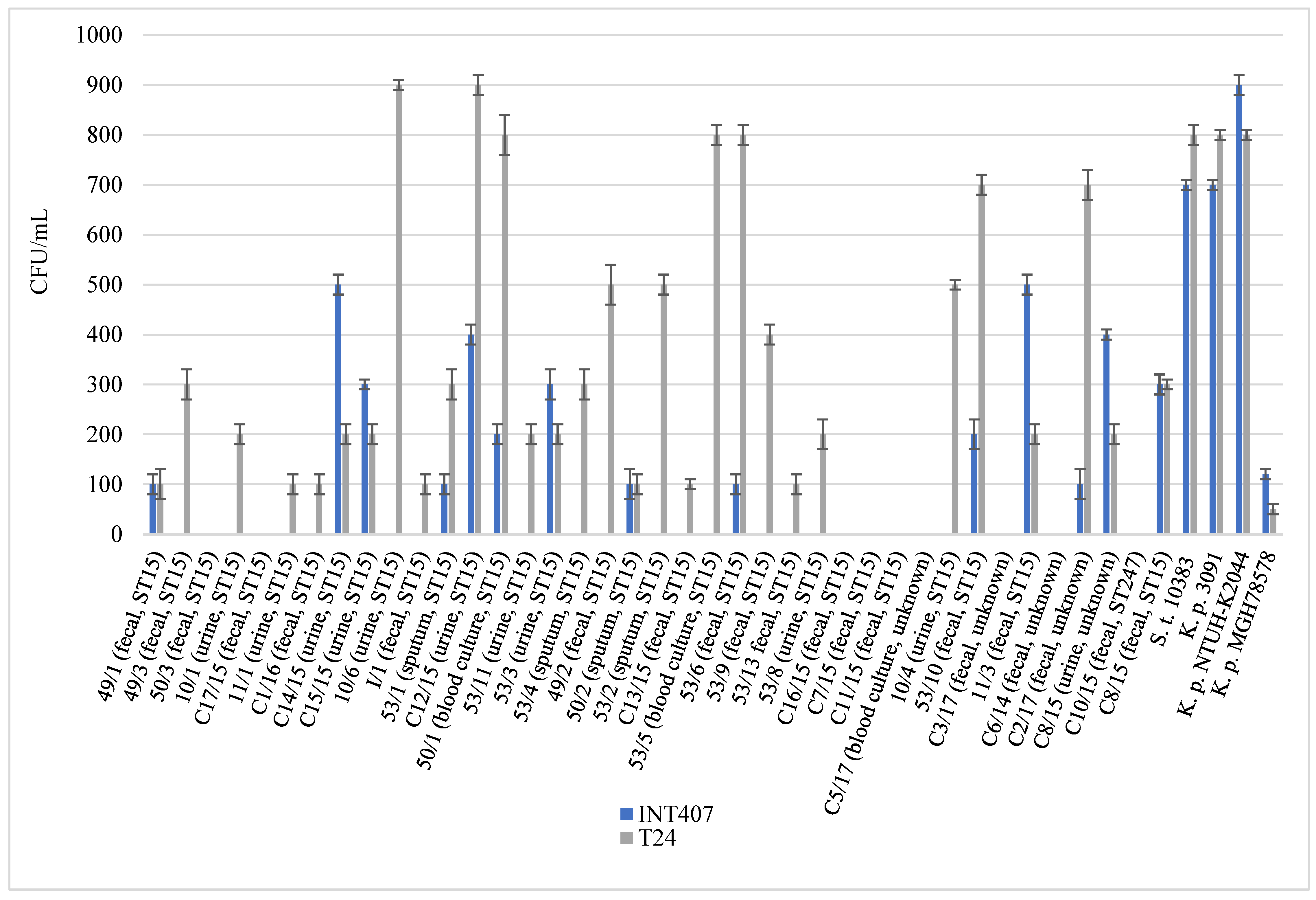
2.4. Virulence Associated Phenotypic Assays
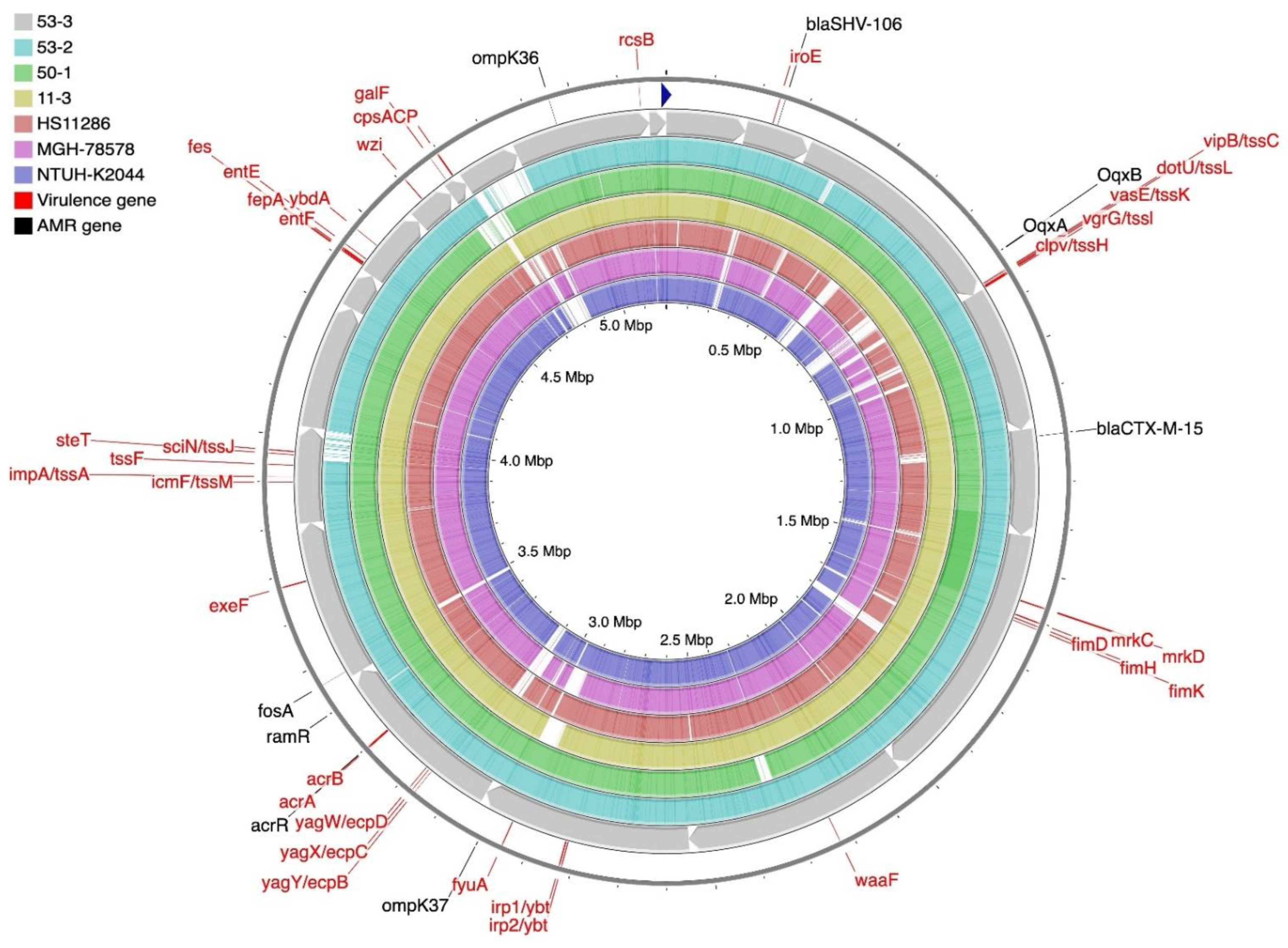
2.5. Genome Sequencing and Bioinformatic Analysis of K. pneumoniae Isolates
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates, Growth Conditions
4.2. Antimicrobial Susceptibility Testing
4.3. Chromosomal Macro-Restriction Fragment Polymorphism Analysis with Pulsed-Field Gel Electrophoresis (PFGE)
4.4. Multilocus Sequence Typing (MLST) Analysis
4.5. PCR Detection of Virulence-Associated Genes
4.6. Phenotypic Tests
4.6.1. Type 1 Fimbriae Assay (Mannose Sensitive Haemagglutination, MSHA)
4.6.2. Type 3 Fimbriae Assay (Mannose-Resistant Haemagglutination, MRHA)
4.6.3. Biofilm Assay
4.6.4. Siderophores Production Assay
4.6.5. Serum Bactericidal Assay
4.6.6. Hypermucoviscosity (HMV) Testing
4.7. Whole-Genome Sequencing
4.8. Cell Internalization Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Decré, D.; Verdet, C.; Emirian, A.; Le Gourrierec, T.; Petit, J.-C.; Offenstadt, G.; Maury, E.; Brisse, S.; Arlet, G. Emerging severe and fatal infections due to Klebsiella pneumoniae in two University Hospitals in France. J. Clin. Microbiol. 2011, 49, 3012–3014. [Google Scholar] [CrossRef]
- Schroll, C.; Barken, K.B.; Krogfelt, K.A.; Struve, C. Role of type 1 and type 3 fimbriae in Klebsiella pneumoniae biofilm formation. BMC Microbiol. 2010, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Albiger, B.; Glasner, C.; Struelens, M.J.; Grundmann, H.; Monnet, D.L. Carbapenemase-producing Enterobacteriaceae in Europe: Assessment by national experts from 38 countries. Eurosurveillance 2015, 20, 45. [Google Scholar] [CrossRef]
- Kwak, Y.G.; Choi, S.-H.; Choo, E.J.; Chung, J.-W.; Jeong, J.-Y.; Kim, N.J.; Woo, J.-H.; Ryu, J.; Kim, Y.S. Risk factors for the acquisition of carbapenem-resistant Klebsiella pneumoniae among hospitalized patients. Microb. Drug Resist. 2005, 11, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Simmonds, A.; Uhlemann, A.-C. Clinical implications of genomic adaptation and evolution of carbapenem-resistant Klebsiella pneumoniae. J. Infect. Dis. 2017, 215, S18–S27. J. Infect. Dis. 2017, 215, S18–S27. [Google Scholar] [CrossRef]
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K.; et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Struve, C.; Krogfelt, K.A. Pathogenic potential of environmental Klebsiella pneumoniae isolates. Environ. Microbiol. 2004, 6, 584–590. [Google Scholar] [CrossRef]
- Selden, R.S.; Lee, S.; Wang, W.L.L.; Bennett, J.V.; Eickhoff, T.C. Nosocomial Klebsiella infections: Intestinal colonization as a reservoir. Ann. Intern. Med. 1971, 74, 657–664. [Google Scholar] [CrossRef]
- Johnson, J.G.; Clegg, S. Role of MrkJ, a phosphodiesterase, in type 3 fimbrial expression and biofilm formation in Klebsiella pneumoniae. J. Bacteriol. 2010, 192, 3944–3950. [Google Scholar] [CrossRef]
- Potera, C. Forging a link between biofilms and disease. Science 1999, 283, 1837–1839. [Google Scholar] [CrossRef]
- Stickler, D.J.; Morris, N.S.; McLean, R.J.C.; Fuqua, C. Biofilms on indwelling urethral catheters produce quorum-sensing signal molecules in situ and in vitro. Appl. Environ. Microbiol. 1998, 64, 3486–3490. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O.; Dhir, A.; Miller, G.C.; Raad, G.C.; Musher, D.M. Vancomycin penetration into biofilm covering infected prostheses and effect on bacteria. J. Infect. Dis. 1994, 170, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, P.; Cafferini, N.; Joly, B.; Darfeuille-Michaud, A. Klebsiella pneumoniae type 3 pili facilitate adherence and biofilm formation on abiotic surfaces. Res. Microb. 2003, 154, 9–16. [Google Scholar] [CrossRef]
- Nassif, X.; Sansonetti, P.J. Correlation of the virulence of Klebsiella pneumoniae K1 and K2 with the presence of a plasmid encoding aerobactin. Infect. Immun. 1986, 54, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Wyres, K.L.; Nguyen, T.N.T.; Lam, M.M.C.; Judd, L.M.; Chau, N.; Van, V.; Dance, D.A.B.; Ip, M.; Karkey, A.; Ling, C.L.; et al. Genomic surveillance for hypervirulence and multi-drug resistance in invasive Klebsiella pneumoniae from South and Southeast Asia. Genome Med. 2020, 12, 11. [Google Scholar] [CrossRef]
- Andrade, L.N.; Novais, Â.; Stegani, L.M.M.; Ferreira, J.C.; Rodrigues, C.; Darini, A.L.C.; Peixe, L. Virulence genes, capsular and plasmid types of multidrug-resistant CTX-M (-2, -8, -15) and KPC-2-producing Klebsiella pneumoniae isolates from four major hospitals in Brazil. Diagn. Microbiol. Infect. Dis. 2018, 91, 164–168. [Google Scholar] [CrossRef]
- Ma, Y.; Bao, C.; Liu, J.; Hao, X.; Cao, J.; Ye, L. Microbiological characterization of Klebsiella pneumoniae isolates causing bloodstream infections from five tertiary hospitals in Bejing, China. J. Glob. Antimicrob. Resist. 2018, 12, 162–166. [Google Scholar] [CrossRef]
- Damjanova, I.; Tóth, Á.; Pászti, J.; Hajbel-Vékony, G.; Jakab, M.; Berta, J.; Milch, H.; Füzi, M. Expansion and countrywide dissemination of ST11, ST15 and ST147 ciprofloxacin-resistant CTX-M-15-type β-lactamase-producing Klebsiella pneumoniae epidemic clones in Hungary in 2005—The new ‘MRSAs’? J. Antimicrob. Chemother. 2008, 62, 978–985. [Google Scholar] [CrossRef]
- El Fertas-Assiani, R.; Messai, Y.; Alouache, S.; Bakour, R. Virulence profiles and antibiotic susceptibility petterns of Klebsiella pneumoniae strains isolated from different clinical specimens. Pathol. Biol. 2013, 61, 209–216. [Google Scholar] [CrossRef]
- Effah, C.Y.; Sun, T.; Liu, S.; Wu, Y. Klebsiella pneumoniae: An increasing threat to public health. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1. [Google Scholar] [CrossRef]
- Caneiras, C.; Lito, L.; Melo-Cristino, J.; Duarte, A. Community- and hospital-acquired Klebsiella pneumoniae urinary tract infections in Portugal: Virulence and antibiotic resistance. Microorganisms 2019, 7, 138. [Google Scholar] [CrossRef]
- Gupta, N.; Limbago, B.M.; Patel, J.B.; Kallen, A.J. Carbapenem-resistant Enterobacteriaceae: Epidemiology and prevention. Clin. Infect. Dis. 2011, 53, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Bush, K. Alarming β-lactamase-mediated resistance in multidrug-resistant Enterobacteriaceae. Curr. Opin. Microbiol. 2010, 13, 558–564. [Google Scholar] [CrossRef]
- Quenan, E.M.; Bush, K. Carbapenemases: The versatile beta-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef]
- Papagiannitsis, C.C.; Malli, E.; Florou, Z.; Sarrou, S.; Hrabak, J.; Mantzarlis, K.; Zakynthinos, E.; Petinaki, E. Emergence of sequence type 11 Klebsiella pneumoniae coproducing NDM-1 and VIM-1 metallo-beta-lactamases in Greek hospital. Diagn. Microbiol. Infect. Dis. 2017, 87, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, Ø.; Toleman, M.A.; Hasseltvedt, V.; Fuursted, K.; Leegaard, T.M.; Walsh, A.; Sundsfjord, A.; Giske, C.G. Molecular characterization of VIM-producing Klebsiella pneumoniae from Scandinavia reveals genetic relatedness with international clonal complexes encoding transferable multidrug resistance. Clin. Microbiol. Infect. 2011, 17, 1811–1816. [Google Scholar] [CrossRef]
- Shi, W.; Li, K.; Ji, Y.; Jiang, Q.; Wang, Y.; Shi, M.; Mi, Z. Carbapenem and cefoxitin resistance of Klebsiella pneumoniae strains associated with porin OmpK36 loss and DHA-1 β-lactamase production. Braz. J. Microbiol. 2013, 44, 435–442. [Google Scholar] [CrossRef]
- Mouloudi, E.; Protonariou, E.; Zagorianou, A.; Iosifidis, E.; Karapanagiotou, A.; Giasnetsova, T.; Tsioka, A.; Roilides, E.; Sofianou, D.; Gritsi-Gerogianni, N. Bloodstream infections caused by metallo-β-lactamase/Klebsiella pneumoniae carbapenemase-producing K. pneumoniae among intensive care unit patient in Greece: Risk factors for infection and impact of type of resistance on outcomes. Infect. Control. Hosp. Epidemiol. 2010, 31, 1250–1256. [Google Scholar] [CrossRef]
- Chong, Y.; Ito, Y.; Kamimura, T. Genetic evolution and clinical impact in extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect. Gnetics Evol. 2011, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.; Shimoda, S.; Shimono, N. Current epidemiology, genetic evolution, and clinical impact of extended-spectrum B-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect. Gnetics. Evol. 2018, 61, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-C.; Lu, M.-C.; Hsueh, P.-R. Hypervirulence and carbapenem resistance: Two distinct evolutionary directions that led high-risk K. pneumoniae clones to epidemic success. Taylor Fr. 2019, 19, 825–837. [Google Scholar] [CrossRef]
- Li, X.; Ma, W.; Quin, Q.; Liu, S.; Ye, L.; Yang, J.; Li, B. Nosocomial spread of OXA-232-producing Klebsiella pneumoniae ST15 in a teaching hospital, Shanghai, China. MBC Microbiol. 2019, 19, 235. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Sousa, C.; Lopes, J.A.; Novais, Â.; Peixea, L. A front line on Klebsiella pneumoniae capsular polysaccharide knowledge: Fourier transform infrared spectroscopy as an accurate and fast typing tool. Am. Soc. Microbiol. 2020, 5, e00386-19. [Google Scholar] [CrossRef]
- Du, Y.; Mu, S.; Liu, Y.; Yuan, Y.; Zhu, Y.; Ma, M.; Wang, Q.; Zhu, Z.; Liu, Y.; Wang, S. The genomic characterization of KPC-producing Klebsiella pneumoniae from the ICU of a Teaching Hospital in Shanghai, China. Infect. Drug Resist. 2022, 15, 69–81. [Google Scholar] [CrossRef]
- MacKenzie, F.M.; Forbes, K.J.; Dorai-John, T.; Amyes, S.G.; Gould, I.M. Emergence of a carbapenem-resistant Klebsiella pneumoniae. Lancet 1997, 350, 783. [Google Scholar] [CrossRef]
- Liu, S.W.; Chang, H.J.; Chia, J.H.; Kuo, A.J.; Wu, T.L.; Lee, M.H. Outcomes and characteristics of ertapenem-non-susceptible Klebsiella pneumoniae bacteremia at a university hospital in Northern Taiwan: A matched case-control study. J. Microbiol. Immunol. Infect. 2012, 45, 113–119. [Google Scholar] [CrossRef]
- Gasink, L.B.; Edelstein, P.H.; Lautenbach, E.; Synnestvedt, M.; Fishman, N.O. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect. Control. Hosp. Epidemiol. 2009, 30, 1180–1185. [Google Scholar] [CrossRef]
- Ben-David, D.; Kordevani, R.; Keller, N.; Tal, I.; Marzel, A.; Gal-Mor, O.; Maor, Y.; Rahav, G. Outcomes of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin. Microbiol. Infect. 2012, 18, 54–60. [Google Scholar] [CrossRef]
- Dai, G.; Xu, Y.; Kong, H.; Xie, W.; Wang, H. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection and associated clinical outcomes. Am. J. Transl. Res. 2021, 13, 7276–7281. [Google Scholar] [PubMed]
- Chen, J.; Ma, H.; Huang, X.; Cui, Y.; Peng, W.; Zhu, F.; Ma, S.; Rao, M.; Zhang, P.; Yang, H.; et al. Risk factors and mortality of carbapenem-resistant Klebsiella pneumoniae bloodstream infection in a tertiary-care hospital in China: An eight-year retrospetcive study. Antimicrob. Resist. Infect. Control. 2022, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Lou, T.; Du, X.; Zhang, P.; Shi, Q.; Han, X.; Lan, P.; Yan, R.; Hu, H.; Wang, Y.; Wu, X.; et al. Risk factors for infection and mortality caused by carbapenem-resistant Klebsiella pneumoniae: A large multicentre case–control and cohort study. J. Infect. 2022, 84, 5. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.M.; Li, L.H.; Yan, J.J.; Tsao, N.; Liao, T.L.; Tsai, H.-C.; Fung, C.-P.; Chen, H.-J.; Liu, Y.-M.; Wang, J.-T.; et al. Genome sequencing and comparative analysis of Klebsiella pneumoniae NTUH-K2044, a strain causing liver abscess and meningitis. J. Bacteriol. 2009, 191, 4492–4501. [Google Scholar] [CrossRef]
- Fang, C.T.; Chuang, Y.P.; Shun, C.T.; Chang, S.C.; Wang, J.T. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J. Exp. Med. 2004, 5, 697–705. [Google Scholar] [CrossRef]
- Tsai, Y.-K.; Fung, C.-P.; Lin, J.-C.; Chen, J.-H.; Chang, F.-Y.; Chen, T.-L.; Siu, L.K. Klebsiella pneumoniae outer membrane porins ompK35 and ompK36 play roles in both antimicrobial resistance and virulence. Antimicrob. Agents Chemothe. 2011, 55, 1485–1493. [Google Scholar] [CrossRef]
- He, F.; Fu, Y.; Chen, Q.; Ruan, Z.; Hua, X.; Zhou, H.; Yu, Y. Tigecyclne suscebility and the role of efflux pumps in tigecycline resisnatnce in KPC-producing Klebsiella pneumoniae. PLoS ONE 2015, 10, e0119064. [Google Scholar] [CrossRef]
- Schneiders, T.; Amyes, S.G.B.; Levy, S.B. Role of AcrR and RamA in fluoroquinolone rsistance in clinical Klebsiella pneumoniae isolates from Singapore. Antimicrob. Agents Chemother. 2013, 47, 9. [Google Scholar] [CrossRef]
- Rosenblum, R.; Khan, E.; Gonzalez, G.; Hasan, R.; Schneiders, A. Genetic regulation of the ramA locus and its expression in clinical isolates of Klebsiella pneumoniae. Int. J. Antimicrob. Agents 2011, 38, 39–45. [Google Scholar] [CrossRef]
- Struve, C.; Bojer, M.; Krogfelt, K.A. Identification of a conserved chromosomal region encoding Klebsiella pneumoniae Type 1 and Type 3 fimbriae and assessment of the role of fimbriae in pathogenicity. Infect. Immun. 2009, 77, 5016–5024. [Google Scholar] [CrossRef]
- Eghbalpoor, F.; Habibi, M.; Azizi, O.; Karam, M.R.A.; Bouzar, S. Antibiotic resistance, virulence and genetic diversity of Klebsiella pneumoniae in community- and hospital-acquired urinary tract infections in Iran. Acta Microbiol. Et. Immunol. Hung. 2019, 66, 3. [Google Scholar] [CrossRef]
- Maharjan, G.; Khadka, P.; Shilpakar, G.S.; Chapagain, G.; Dhungana, G.R. Catheter-associated urinary tract infection and obstinate biofilm producers. Can. J. Infect. Dis. 2018, 2018, 7624857. [Google Scholar] [CrossRef]
- Zadeh, F.M.; Zarei, H.; Jahromy, S.H. Type 1 and 3 fimbriae phenotype and genotype as suitable markers for uropathogenic bacterial pathogenesis via attachment, cell surface hydrophobicity, and biofilm formation in catheter-associated urinary tract infections (CAUTIs). Iran. J. Basic Med. Sci. 2021, 24, 1098–1106. [Google Scholar] [CrossRef]
- Khonsari, M.S.; Behzadi, P.; Foroohi, F. The prevalence of type 3 fimbriae in uropathogenic Escherichia coli isolated from clinical urine samples. Meta Gene 2021, 28, 100881. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ranjbar, R.; Behzadi, P.; Mohammadian, T. Virulence factors, antibiotic resistance patterns, and molecular types of clinical isolates of Klebsiella pneumoniae. Expert Rev. Anti-Infect. Ther. 2022, 20, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Podschun, R.; Sievers, D.; Fischer, A.; Ullmann, U. Serotypes, hemagglutinins, siderophore synthesis, and serum resistance of Klebsiella isolates causing human urinary tract infections. J. Infect. Dis. 1993, 168, 1415–1421. [Google Scholar] [CrossRef]
- Koczura, R.; Kaznowski, A. Occurrence of the Yersinia high-pathogenicity island and iron uptake systems in clinical isolates of Klebsiella pneumoniae. Microb. Pathog. 2003, 35, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Remya, P.A.; Shanthi, M.; Sekar, U. Characterisation of virulence genes associated with pathogenicity in Klebsiella pneumoniae. Indian J. Med. Microbiol. 2020, 37, 210–218. [Google Scholar] [CrossRef]
- Daoud, L.; Al-Marzooq, F.; Moubareck, C.A.; Ghazawi, A.; Collyns, T. Elucidating the effect of iron acquisition systems in Klebsiella pneumoniae on susceptibility to the novel siderophore-cephalosporin cefiderocol. PLoS ONE 2022, 17, e0277946. [Google Scholar] [CrossRef]
- Lam, M.M.C.; Wick, R.R.; Wyres, K.L.; Gorrie, C.; Judd, L.M.; Brisse, S.; Jenney, A.; Holt, K.E. Frequent emergence of pathogenic lineages of Klebsiella pneumoniae via mobilisation of yersiniabactin and colibactin. Microb. Genom. 2018, 4, 1–14. [Google Scholar] [CrossRef]
- Choby, J.E.; Howard-Anderson, J.; Weiss, D.S. Hypervirulent Klebsiella pneumoniae—Clinical and molecular perspectives. J. Intern. Med. 2020, 287, 283–300. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, T.; Chen, L.; Du, H. Virulence factors in hypervirulent Klebsiella pneumoniae. Front. Microbiol. 2021, 12, 642484. [Google Scholar] [CrossRef]
- Sahly, H.; Podschun, R.; Oelschlaeger, T.A.; Greiwe, M.; Parolis, H.; Hasty, D.; Kekow, J.; Ullmann, U.; Ofek, I.; Sela, S. Capsule impedes adhesion to and invasion of epithelial cells by Klebsiella pneumoniae. Infect. Immun. 2000, 68, 6744–6749. [Google Scholar] [CrossRef]
- Hsieh, P.-F.; Lu, Y.-R.; Lin, T.-Z.; Lai, L.-Y.; Wang, J.-T. Klebsiella pneumoniae type VI secretion system contributes to bacterial competition, cell invasion, type-1 fimbriae expression, and in vivo colonization. J. Infect. Dis. 2019, 219, 637–647. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, Y.Y.; Choi, J.Y.; Wi, Y.M.; Ko, K.S. Two distinct genotypes of KPC-2-producing Klebsiella pneumoniae isolates from South Korea. Antibiotics 2021, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.Y.; Kim, J.H.; Kim, H.; Lee, J.; Im, S.J.; Ko, K.S. Comparison of virulence between two main clones (ST11 and ST307) of Klebsiella pneumoniae isolates from South Korea. Microorganisms 2022, 10, 1827. [Google Scholar] [CrossRef] [PubMed]
- Catalán-Nájera, J.C.; Garza-Ramos, U.; Barrios-Camacho, H. Hypervirulence and hypermucoviscosity: Two different but complementary Klebsiella spp. phenotypes? Virulence 2017, 8, 1111–1123. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, X.; Chan, E.W.C.; Chen, S. The hypermucoviscosity of hypervirulent K. pneumoniae confers the ability to evade neutrophil-mediated phagocytosis. Virulence 2021, 12, 2050–2059. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.A.; Miller, V.L. The intersection of capsule gene expression, hypermucoviscosity and hypervirulence in Klebsiella pneumoniae. Curr. Opin. Microbiol. 2020, 54, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.; Chakrabortty, A.; Kapoor, A.; Warrier, A.; Nag, V.L.; Sivashanmugam, K.; Shankar, M. Unusual hypermucoviscous clinical isolate of Klebsiella pneumoniae with no known determinants of hypermucoviscosity. Microbiol. Spectr. 2022, 10, 3. [Google Scholar] [CrossRef]
- Short, F.L.; Di Sario, G.; Reichmann, N.T.; Kleanthous, C.; Parkhill, J.; Taylor, P.W. Genomic profiling reveals distinct routes to complement resistance in Klebsiella pneumoniae. Am. Soc. Microbiol. 2020, 88, e00043-20. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Machado, E.; Ramos, H.; Peixe, L.; Novais, Â. Expansion of ESBL-producing Klebsiella pneumoniae in hospitalized patients: A successful story of international clones (ST15, ST147, ST336) and epidemic plasmids (IncR, IncFIIκ). Int. J. Med. Microbiol. 2014, 304, 1100–1108. [Google Scholar] [CrossRef]
- Tang, M.; Kong, X.; Hao, J.; Liu, J. Epidemiological characteristics and formation mechanisms of multidrug-resistant hypervirulent Klebsiella pneumoniae. Front. Microbiol. 2020, 11, 581543. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Yang, X.; Chan, E.W.-C.; Zhang, R.; Chen, S. Klebsiella species: Taxonomy, hypervirulence and multidrug resistance. eBioMedicine 2022, 79, 103998. [Google Scholar] [CrossRef]
- Diep, B.A.; Carleton, H.A.; Chang, R.F.; Sensabaugh, G.F.; Perdreau-Remington, F. Roles of 34 virulence genes in the evolution of hospital- and community-associated strains of methicillin-resistant Staphylococcus aureus. J. Inf. Dis. 2006, 193, 1495–1503. [Google Scholar] [CrossRef]
- Zhan, X.-Y.; Zhu, Q.-Y. Molecular evolution of virulence genes and non-virulence genes in clinical, natural and artificial environmental Legionella pneumophila isolates. PeerJ 2017, 5, e4114. [Google Scholar] [CrossRef][Green Version]
- European Society of Clinical Microbiology and Infectious Diseases, version 12.0. In The European Committee on Antimicrobial Susceptibility Testing: Breakpoint Tables for Interpretation of MICs and Zone Diameters; EUCAST: Växjö, Sweden, 2022; Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf (accessed on 26 January 2022).
- Van der Zwaluw, K.; de Haan, A.; Pluister, G.N.; Bootsma, H.J.; de Neeling, A.J.; Schouls, L.M. The Carbapenem Inactivation Method (CIM), a simple and low-cost alternative for the Carba NP test to assess phenotypic carbapenemase activity in gram-negative rods. PLoS ONE 2015, 10, e0123690. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.B.; Vauterin, P.; Lambert-Fair, M.A.; Van Duyne, M.S.; Kubota, K.; Graves, L.; Wrigley, D. Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: Converting the national databases to the new size standard. J. Clin. Microb. 2005, 43, 3. [Google Scholar] [CrossRef]
- Tenover, F.C.; Arbeit, R.G.; Goering, R.V.; Mickelsen, P.A.; Murray, B.E.; Persing, D.H.; Swaminathan, B. Interpreting chromosomal DNA restriction patterns produced by pulse-field gel electrophoresis: Criteria for bacterial strain typing. J. Clin. Microbiol. 1995, 33, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- PubMLST. Available online: https://pubmlst.org/bigsdb?db=pubmlst_mlst_seqdef&page=schemeInfo&scheme_id=13 (accessed on 25 January 2022).
- Primer-BLAST. Available online: https://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 10 June 2021).
- Schembri, M.A.; Blom, J.; Krogfelt, K.A.; Klemm, P. Capsule and fimbria interaction in Klebsiella pneumoniae. Am. Soc. Microbiol. 2005, 73, 4626–4633. [Google Scholar] [CrossRef]
- Podschun, R.; Sahly, H. Hemagglutinins of Klebsiella pneumoniae and K. oxytoca isolated from different sources. Zenralbl. Hyg. Umweltmed. 1991, 191, 46–52. [Google Scholar]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef]
- Hantke, K. Dihydroxybenzoylserine—A siderophore for E. coli. FEMS Microbiol. Lett. 1990, 67, 5–8. [Google Scholar] [CrossRef][Green Version]
- Wiskur, B.J.; Hunt, J.J.; Callegan, M.C. Hypermucoviscosity as a virulence factor in experimental Klebsiella pneumoniae endophthalmitis. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4931–4938. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.C.; Lin, H.H.; Sabharwal, A.; Haase, E.M.; Scannapieco, F.A. MyPro: A seamless pipeline for automated prokaryotic genome assembly and annotation. J. Microbiol. Methods 2015, 113, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef]
- NCBI Nucleotide BLAST. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 25 January 2019).
- ResFinder 4.1. Available online: https://cge.cbs.dtu.dk/services/ResFinder/ (accessed on 19 November 2021).
- Pathogen Wach. Available online: https://pathogen.watch/ (accessed on 29 November 2021).
- Virulence Factor Database (VFDB). Available online: http://www.mgc.ac.cn/VFs/ (accessed on 15 November 2021).
- CGView Server Beta. Available online: http://cgview.ca/ (accessed on 13 December 2021).
- Elsinghorst, E.A.; Baron, L.S.; Kopecko, D.J. Penetration of human intestinal epihtelial cells by Salmonella: Molecular cloning and expression of Salmonella typhi invasion determinants in Escherichia coli. Proc. Natl. Acad. Sci. USA 1989, 86, 5173–5177. [Google Scholar] [CrossRef]
- Backert, S.; Hofreuter, D. Molecular methods to investigate adhesion, transmigration, invasion and intracellular survival of the foodborne pathogen Campylobacter jejuni. J. Microbiol. Methods 2013, 95, 8–23. [Google Scholar] [CrossRef]
- Oelschlaeger, T.A.; Tall, B.D. Invasion of cultured human epithelial cells by Klebsiella pneumoniae isolated from the urinary tract. Infect. Immun. 1997, 65, 2950–2958. [Google Scholar] [CrossRef]
- Abdulhasan, G.A.; Alzubaidy, S.K.; Abed, I.J. Effect of sub-inhibitory and inhibitory concentrations of some antibiotics and resemary essential oil (Rosemarinus officinalis L.) on biofilm formation of Klebsiella pneumoniae. World J. Exp. Biosci. 2016, 4, 130–135, ISSN 2313-3937. [Google Scholar]
- Chiang, T.-T.; Yang, Y.-S.; Yeh, K.-M.; Chiu, S.-K.; Wang, N.-C.; Lin, T.-Y.; Huang, L.-Y.; Chang, F.-Y.; Siu, L.K.; Lin, J.-C.; et al. Quantification and comparision of virulence and characteristics of differnt variants of carbapenemase-producing Klebsiella pneumoniae clinical isolates from Taiwan and the United States. J. Microbiol. Immunol. Infect. 2016, 49, 83–90. [Google Scholar] [CrossRef]
- Regué, M.; Hita, B.; Piqué, N.; Izquierdo, L.; Merino, S.; Fresno, S.; Benedí, V.J.; Tomás, J.M. A gene, uge, is essential for Klebsiella pneumoniae virulence. Infect. Immun. 2004, 72, 54–61. [Google Scholar] [CrossRef]
- Wilksch, J.J.; Yang, J.; Clements, A.; Gabbe, J.L.; Short, K.; Cao, H.; Cavaliere, R.; James, C.E.; Whitchurch, C.; Schembri, M.; et al. MrkH, a Novel c-di-GMP-Dependent Transcriptional Activator, Controls Klebsiella pneumoniae Biofilm Formation by Regulating Type 3 Fimbriae Expression. PLoS Pathog. 2011, 7, e1002204. [Google Scholar] [CrossRef]
| K. pneumoniae Isolates | PCR Amplification | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fimH-1 | mrkD | mrkA | mrk J | cf29a | allS | entB | iutA | traT | rmpA | uge | magA | wziK24 | |
| 53/1 | + | + | + | + | - | + | + | + | + | - | + | - | + |
| 53/2 | + | + | + | + | - | + | + | - | - | - | + | - | + |
| 53/3 | + | + | + | + | - | + | + | - | + | + | + | + | + |
| 53/4 | + | - | - | - | - | + | + | - | + | - | + | - | + |
| 53/5 | + | + | + | + | - | + | + | - | + | - | + | - | + |
| 53/6 | + | + | + | + | - | + | + | - | - | - | + | - | + |
| 53/8 | + | + | + | + | - | + | + | - | - | - | + | - | + |
| 53/9 | + | + | + | + | - | + | + | - | - | - | + | - | + |
| 53/10 | + | + | + | + | - | + | + | - | - | - | + | - | + |
| 53/11 | + | + | + | + | - | + | + | - | + | + | + | + | + |
| 53/13 | + | + | + | + | - | + | + | - | - | - | + | - | + |
| 50/1 | + | + | + | + | - | + | + | - | - | - | + | - | + |
| 50/2 | + | + | + | + | - | + | + | - | - | - | + | - | + |
| 50/3 | + | + | + | + | - | + | + | - | + | + | + | + | + |
| I/1 | + | + | + | + | - | + | + | - | - | - | + | - | + |
| 49/1 | + | + | + | + | - | + | + | - | - | - | + | - | + |
| 49/2 | + | - | - | - | - | + | - | - | + | - | + | - | + |
| 49/3 | + | + | + | + | - | + | + | - | - | - | + | - | + |
| 10/1 | + | + | + | + | - | + | + | - | - | - | + | - | + |
| 10/4 | - | - | - | - | - | + | + | - | - | - | + | - | + |
| 10/6 | + | + | + | + | - | + | + | - | - | - | + | - | + |
| 11/1 | + | + | + | + | - | + | + | - | + | + | + | + | + |
| 11/3 | + | + | + | + | - | + | + | - | - | - | + | - | + |
| C6/14 | + | + | + | + | - | + | + | - | + | - | + | - | + |
| C7/15 | + | + | + | + | + | + | + | - | + | - | + | - | + |
| C8/15 | - | + | + | + | - | + | + | + | - | - | + | - | + |
| C10/15 | + | + | + | + | - | + | + | - | - | - | + | - | + |
| C11/15 | + | + | + | + | + | + | + | - | + | - | + | - | + |
| C12/15 | + | + | + | + | - | + | + | - | + | - | + | - | + |
| C13/15 | + | + | + | + | + | + | + | - | + | - | + | - | + |
| C14/15 | + | + | + | + | - | + | + | - | - | - | + | - | + |
| C15/15 | + | + | + | + | - | + | + | - | + | - | + | - | + |
| C16/15 | + | + | + | + | + | + | + | - | + | - | + | - | + |
| C17/15 | + | + | + | + | - | + | + | - | + | - | + | - | + |
| C18/15 | + | + | + | + | - | + | + | - | - | - | + | - | + |
| C1/16 | + | + | + | + | - | + | - | - | - | - | + | - | + |
| C2/17 | + | + | + | + | - | + | + | - | + | - | + | - | + |
| C3/17 | + | + | + | + | - | + | + | - | + | - | + | - | + |
| C5/17 | + | + | + | + | - | + | + | - | - | - | + | - | + |
| NTUH-K2044 | + | + | + | + | + | + | + | + | + | + | + | + | - |
| MGH 78578 | + | + | + | + | + | + | - | - | - | - | + | - | - |
| K. pneumoniae Isolates | Type 1 Fimbriae | Type 3 Fimbriae | Biofilm Production | Siderophores Production | Serum Resistance | HMV | |
|---|---|---|---|---|---|---|---|
| Enterobactin | Aerobactin | ||||||
| 53/1 | + | + | high | + | + | + | - |
| 53/2 | + | + | high | + | - | - | - |
| 53/3 | + | + | high | + | - | + | + |
| 53/4 | + | - | poor | + | - | + | - |
| 53/5 | + | + | high | + | - | + | - |
| 53/6 | + | + | high | + | - | - | - |
| 53/8 | + | + | high | + | - | - | - |
| 53/9 | + | + | high | + | - | - | - |
| 53/10 | + | + | high | + | - | - | - |
| 53/11 | + | + | high | + | - | + | + |
| 53/13 | + | + | high | + | - | - | - |
| 50/1 | + | + | high | + | - | - | - |
| 50/2 | + | + | high | + | - | - | - |
| 50/3 | + | + | high | + | - | + | + |
| I/1 | + | + | high | + | - | - | - |
| 49/1 | + | + | high | + | - | - | - |
| 49/2 | + | - | poor | - | - | + | - |
| 49/3 | + | + | high | + | - | - | - |
| 10/1 | + | + | high | + | - | - | - |
| 10/4 | - | - | poor | + | - | - | - |
| 10/6 | + | + | high | + | - | - | - |
| 11/1 | + | + | high | + | - | + | + |
| 11/3 | + | + | high | + | - | - | - |
| C6/14 | + | + | high | + | - | + | - |
| C7/15 | + | + | high | + | - | + | - |
| C8/15 | - | + | medium | + | + | - | - |
| C10/15 | + | + | high | + | - | - | - |
| C11/15 | + | + | high | + | - | + | - |
| C12/15 | + | + | high | + | - | + | - |
| C13/15 | + | + | high | + | - | + | - |
| C14/15 | + | + | high | + | - | - | - |
| C15/15 | + | + | high | + | - | + | - |
| C16/15 | + | + | high | + | - | + | - |
| C17/15 | + | + | high | + | - | + | - |
| C18/15 | + | + | high | + | - | - | - |
| C1/16 | + | + | high | - | - | - | - |
| C2/17 | + | + | high | + | - | + | - |
| C3/17 | + | + | medium | + | - | + | - |
| C5/17 | + | + | high | + | - | - | - |
| NTUH-K2044 | + | + | high | + | + | + | + |
| MGH 78578 | + | + | high | - | - | - | - |
| K. pneumoniae Strain 11/3 (Faecal) | K. pneumoniae Strain 50/1 (Blood Culture) | K. pneumoniae Strain 53/2 (Sputum) | K. pneumoniae Strain 53/3 (Urine) | |
|---|---|---|---|---|
| Genome length (bp) | 5,517,254 | 5,379,211 | 5,269,114 | 5,201,283 |
| No. Contigs | 41 | 18 | 18 | 19 |
| Average contig length (bp) | 134,567 | 298,845 | 292,728 | 273,751 |
| Average GC content (%) | 57.3 | 57.4 | 57.4 | 57.4 |
| Genes | 5180 | 5022 | 4908 | 4844 |
| tRNA | 81 | 73 | 81 | 63 |
| CDS | 5098 | 4948 | 4826 | 4780 |
| Plasmid types | IncFIB(K), IncFII(K), ColpVC | IncFII(K) | ColpVC | IncFII(K) |
| Mutation found | acrR, ramR, ompK35, ompK36 | acrR, ramR, ompK35, ompK36 | acrR, ompK35, ompK36 | acrR, ramR, ompK35, ompK36 |
| Antimicrobial resistance genotypes | ompK35, ompK36, blaCTX-M-15, blaSHV-106, blaVIM-4, ramR, acrR | ompK35, ompK36, blaCTX-M-15, blaSHV-106, ramR, acrR | ompK35, ompK36, blaCTX-M-15, blaSHV-106, blaVIM-4, acrR | ompK35, ompK36, blaCTX-M-15, blaSHV-106, fosA, oqxA, oqxB, ramR, acrR |
| Special virulence genes | manB, manC, wbbM | mrkB, mrkH, mrkJ | – | cpsAPC, galF, irp1/ybt, irp2/ybt, wzi |
| Accession number | JAJTNS000000000 | JAJTNT000000000 | JAJTNR000000000 | JACTNU010000000 |
| Strain No. | Year of Isolation | Isolated from | ST |
|---|---|---|---|
| 10/1 | 2010 | urine | ST 15 |
| 10/4 | 2010 | urine | ST 15 |
| 10/6 | 2010 | urine | ST 15 |
| 11/1 | 2010 | urine | ST 15 |
| 11/3 | 2010 | faecal | ST 15 |
| 53/1 | 2011 | sputum | ST 15 |
| 53/2 | 2011 | sputum | ST 15 |
| 53/3 | 2011 | urine | ST 15 |
| 53/4 | 2011 | sputum | ST 15 |
| 53/5 | 2011 | blood culture | ST 15 |
| 53/6 | 2011 | faecal | ST 15 |
| 53/8 | 2011 | urine | ST 15 |
| 53/9 | 2011 | faecal | ST 15 |
| 53/10 | 2011 | faecal | ST 15 |
| 53/11 | 2011 | urine | ST 15 |
| 53/13 | 2011 | faecal | ST 15 |
| 50/1 | 2011 | blood culture | ST 15 |
| 50/2 | 2011 | sputum | ST 15 |
| 50/3 | 2011 | faecal | ST 15 |
| I/1 | 2011 | faecal | ST 15 |
| 49/1 | 2011 | faecal | ST 15 |
| 49/2 | 2011 | faecal | ST 15 |
| 49/3 | 2011 | faecal | ST 15 |
| C6/14 | 2014 | faecal | ST 15 |
| C7/15 | 2015 | faecal | ST 15 |
| C8/15 | 2015 | faecal | ST 15 |
| C10/15 | 2015 | faecal | ST 15 |
| C11/15 | 2015 | faecal | ST 15 |
| C12/15 | 2015 | urine | ST 15 |
| C13/15 | 2015 | faecal | ST 15 |
| C14/15 | 2015 | urine | ST 15 |
| C15/15 | 2015 | urine | ST 15 |
| C16/15 | 2015 | faecal | ST 15 |
| C17/15 | 2015 | faecal | ST 15 |
| C18/15 | 2015 | urine | ST 15 |
| C1/16 | 2016 | faecal | ST 15 |
| C2/17 | 2017 | faecal | ST 15 |
| C3/17 | 2017 | faecal | ST 15 |
| C5/17 | 2017 | blood culture | ST 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horváth, M.; Kovács, T.; Kun, J.; Gyenesei, A.; Damjanova, I.; Tigyi, Z.; Schneider, G. Virulence Characteristics and Molecular Typing of Carbapenem-Resistant ST15 Klebsiella pneumoniae Clinical Isolates, Possessing the K24 Capsular Type. Antibiotics 2023, 12, 479. https://doi.org/10.3390/antibiotics12030479
Horváth M, Kovács T, Kun J, Gyenesei A, Damjanova I, Tigyi Z, Schneider G. Virulence Characteristics and Molecular Typing of Carbapenem-Resistant ST15 Klebsiella pneumoniae Clinical Isolates, Possessing the K24 Capsular Type. Antibiotics. 2023; 12(3):479. https://doi.org/10.3390/antibiotics12030479
Chicago/Turabian StyleHorváth, Marianna, Tamás Kovács, József Kun, Attila Gyenesei, Ivelina Damjanova, Zoltán Tigyi, and György Schneider. 2023. "Virulence Characteristics and Molecular Typing of Carbapenem-Resistant ST15 Klebsiella pneumoniae Clinical Isolates, Possessing the K24 Capsular Type" Antibiotics 12, no. 3: 479. https://doi.org/10.3390/antibiotics12030479
APA StyleHorváth, M., Kovács, T., Kun, J., Gyenesei, A., Damjanova, I., Tigyi, Z., & Schneider, G. (2023). Virulence Characteristics and Molecular Typing of Carbapenem-Resistant ST15 Klebsiella pneumoniae Clinical Isolates, Possessing the K24 Capsular Type. Antibiotics, 12(3), 479. https://doi.org/10.3390/antibiotics12030479






