Pilot Study on the Action of Thymus vulgaris Essential Oil in Treating the Most Common Bacterial Contaminants and Salmonella enterica subsp. enterica Serovar Derby in Poultry Litter
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of TEO
2.2. Bacteria Identification from Litter
2.3. Screening for Multidrug Resistance (MDR) Activity
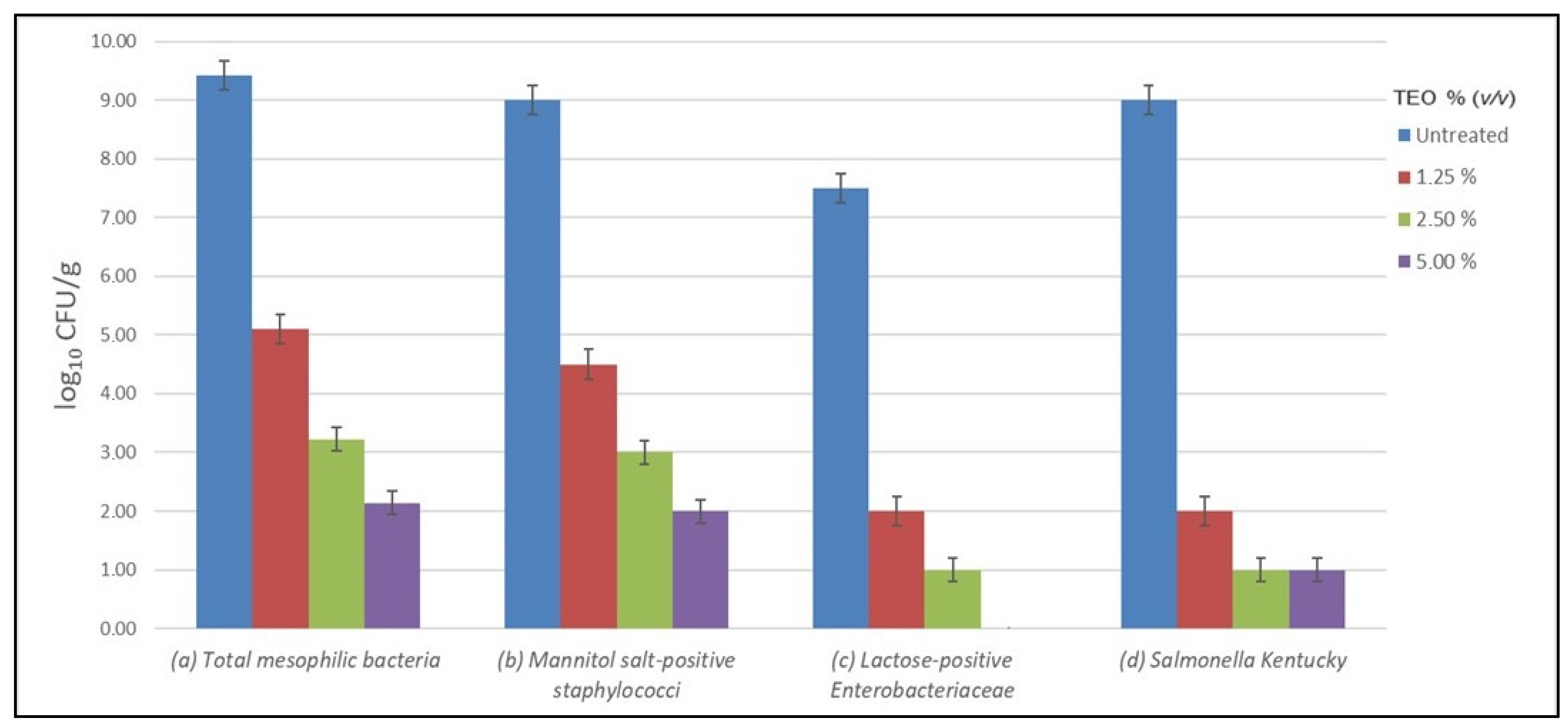
2.4. Antibacterial Activity of TEO
3. Discussion
4. Materials and Methods
4.1. Litter Bulk Sample and Bacteria Identification
4.2. Antimicrobial Susceptibility
4.3. Whole-Genome Sequencing and Strains Typing
4.4. EO: Compound Identification and Dilution Design
4.5. Experimental Design
- -
- Experimental Group A: three litter samples treated with 0.2mL TEO 5%, 2.5%, and 1.25% (v/v), respectively.
- -
- Experimental Group B: three litter samples artificially contaminated by spraying 0.2 mL of a 108 CFU/mL suspension of S. enterica subsp. enterica ser. Derby (hereafter, S. Derby) and after 12h treated with 0.2mL TEO 5%, 2.5%, and 1.25% (v/v), respectively.
- -
- Control Group: one litter sample for each group (A and B), sprayed with 0.2 mL of pure water.
4.6. Antibacterial Activity of TEO
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerber, P.F.; Gould, N.; McGahan, E. Potential Contaminants and Hazards in Alternative Chicken Bedding Materials and Proposed Guidance Levels: A Review. Poult. Sci. 2020, 99, 6664–6684. [Google Scholar] [CrossRef] [PubMed]
- Valeris-Chacin, R.; Pieters, M.; Hwang, H.; Johnson, T.J.; Singer, R.S. Association of Broiler Litter Microbiome Composition and Campylobacter Isolation. Front. Vet. Sci. 2021, 8, 654927. [Google Scholar] [CrossRef] [PubMed]
- Baykov, B. Microbial Air Pollution Caused by Intensive Broiler Chicken Breeding. FEMS Microbiol. Ecol. 1999, 29, 389–392. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, X. Microbiological Safety of Chicken Litter or Chicken Litter-Based Organic Fertilizers: A Review. Agriculture 2014, 4, 1–29. [Google Scholar] [CrossRef]
- Wales, A.; Breslin, M.; Davies, R. Assessment of Cleaning and Disinfection in Salmonella-Contaminated Poultry Layer Houses Using Qualitative and Semi-Quantitative Culture Techniques. Vet. Microbiol. 2006, 116, 283–293. [Google Scholar] [CrossRef]
- Moore, P.R.; Evenson, A.; Luckey, T.D.; McCoy, E.; Elvehjem, C.A.; Hart, E.B. Use of sulfasuxidine, streptothricin, and streptomycin in nutritional studies with the chick. J. Biol. Chem. 1946, 165, 437–441. [Google Scholar] [CrossRef]
- Starr, M.P.; Reynolds, D.M. Streptomycin Resistance of Coliform Bacteria from Turkeys Fed Streptomycin. Am. J. Public Health Nations Health 1951, 41, 1375–1380. [Google Scholar] [CrossRef]
- Ghosh, S.; LaPara, T.M. The Effects of Subtherapeutic Antibiotic Use in Farm Animals on the Proliferation and Persistence of Antibiotic Resistance among Soil Bacteria. ISME J. 2007, 1, 191–203. [Google Scholar] [CrossRef]
- Yang, Y.; Ashworth, A.J.; Willett, C.; Cook, K.; Upadhyay, A.; Owens, P.R.; Ricke, S.C.; DeBruyn, J.M.; Moore, P.A., Jr. Review of Antibiotic Resistance, Ecology, Dissemination, and Mitigation in U.S. Broiler Poultry Systems. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to Antibiotics for Maximizing Growth Performance and Feed Efficiency in Poultry: A Review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef]
- Askelson, T.E.; Flores, C.A.; Dunn-Horrocks, S.L.; Dersjant-Li, Y.; Gibbs, K.; Awati, A.; Lee, J.T.; Duong, T. Effects of Direct-Fed Microorganisms and Enzyme Blend Co-Administration on Intestinal Bacteria in Broilers Fed Diets with or without Antibiotics. Poult. Sci. 2018, 97, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Adaszyńska-Skwirzyńska, M.; Szczerbińska, D. Use of Essential Oils in Broiler Chicken Production—A Review. Ann. Anim. Sci. 2017, 17, 317–335. [Google Scholar] [CrossRef]
- Ebani, V.V.; Mancianti, F. Use of Essential Oils in Veterinary Medicine to Combat Bacterial and Fungal Infections. Vet. Sci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Naveed, R.; Hussain, I.; Mahmood, S.; Akhtar, M. Pakistan Veterinary Journal In Vitro and in Vivo Evaluation of Antimicrobial Activities of Essential Oils Extracted from Some Indigenous Spices. Pak. Vet. J. 2013, 33, 413–417. Available online: https://www.researchgate.net/publication/258952773 (accessed on 25 July 2022).
- Catella, C.; Camero, M.; Lucente, M.S.; Fracchiolla, G.; Sblano, S.; Tempesta, M.; Martella, V.; Buonavoglia, C.; Lanave, G. Virucidal and Antiviral Effects of Thymus Vulgaris Essential Oil on Feline Coronavirus. Res. Vet. Sci. 2021, 137, 44–47. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Hasan, C.M.; Dutta, D.; Nguyen, A.N.T. Revisiting Antibiotic Resistance: Mechanistic Foundations to Evolutionary Outlook. Antibiotics 2021, 11, 40. [Google Scholar] [CrossRef]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical Composition, Antimicrobial, Antioxidant and Antitumor Activity of Thymus Serpyllum L., Thymus Algeriensis Boiss. and Reut and Thymus Vulgaris L. Essential Oils. Ind. Crops. Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Miladinović, D.L.; Dimitrijević, M.V.; Mihajilov-Krstev, T.M.; Marković, M.S.; Ćirić, V.M. The Significance of Minor Components on the Antibacterial Activity of Essential Oil via Chemometrics. LWT 2021, 136, 110305. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Nehme, R.; Andrés, S.; Pereira, R.B.; ben Jemaa, M.; Bouhallab, S.; Ceciliani, F.; López, S.; Rahali, F.Z.; Ksouri, R.; Pereira, D.M.; et al. Essential Oils in Livestock: From Health to Food Quality. Antioxidants 2021, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Tertychna, O.; Svaliavchuk, L.; Mineralov, O. The Research of Litter in Poultry House and Use of Essential Oils in Broiler Production. Sci. Biol. Sci. 2018, 0, 57–61. [Google Scholar] [CrossRef]
- Nguyen, M.C.P.; Woerther, P.-L.; Bouvet, M.; Andremont, A.; Leclercq, R.; Canu, A. Escherichia Coli as Reservoir for Macrolide Resistance Genes. Emerg. Infect. Dis. 2009, 15, 1648–1650. [Google Scholar] [CrossRef]
- Hughes, D.; Andersson, D.I. Evolutionary Trajectories to Antibiotic Resistance. Annu. Rev. Microbiol. 2017, 71, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Inoue, M.; Endo, Y.; Nakajima, Y. Characteristic Expression of Three Genes, Msr (A), Mph (C) and Erm (Y), That Confer Resistance to Macrolide Antibiotics on Staphylococcus Aureus. FEMS Microbiol. Lett. 2003, 220, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Roskam, J.L.; Oude Lansink, A.G.J.M.; Saatkamp, H.W. The Relation between Technical Farm Performance and Antimicrobial Use of Broiler Farms. Poult. Sci. 2020, 99, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.P.; Park, S.-J.; Kim, E.S.; Bang, K.-M.; Kim, M.-N.; Kim, S.-H.; Lee, S.-O.; Choi, S.-H.; Jeong, J.-Y.; Woo, J.H.; et al. Prevalence of BlaZ Gene Types and the Cefazolin Inoculum Effect among Methicillin-Susceptible Staphylococcus Aureus Blood Isolates and Their Association with Multilocus Sequence Types and Clinical Outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Jena, S.; Panda, S.; Sharma, S.; Dhawan, B.; Nath, G.; Singh, N.P.; Nayak, K.C.; Singh, D.V. Antibiotic Susceptibility, Virulence Pattern, and Typing of Staphylococcus Aureus Strains Isolated From Variety of Infections in India. Front. Microbiol. 2019, 10, 2763. [Google Scholar] [CrossRef] [PubMed]
- Alekshun, M.N.; Levy, S.B. Molecular Mechanisms of Antibacterial Multidrug Resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Samia, D.; Bakir, M.; Rachid, E.; Chaffia, B.; Omar, B.; Rolain, J.-M.; Diene, S.M. Prevalence and Genotypic Characterization of Salmonella Spp. from Chicken Meats Marketed in the Province of Skikda, Algeria. J. Infect. Dev. Ctries. 2021, 15, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Aworh, M.K.; Kwaga, J.K.P.; Hendriksen, R.S.; Okolocha, E.C.; Thakur, S. Genetic Relatedness of Multidrug Resistant Escherichia Coli Isolated from Humans, Chickens and Poultry Environments. Antimicrob. Resist. Infect. Control 2021, 10, 58. [Google Scholar] [CrossRef]
- Shcherbyna, R.O.; Panasenko, O.I.; Knysh, Y.G.; Fotina, H.A.; Vashchyk, E.V.; Fotina, T.I. The Study of Antimicrobial Activity of 2-((4-R-3-(Morpholinomethylene)-4H-1,2,4-Triazole-5-Yl)Thio)Acetic Acid Salts. Zaporozhye Med. J. 2016. [Google Scholar] [CrossRef]
- Oluwatuyi, M.; Kaatz, G.; Gibbons, S. Antibacterial and Resistance Modifying Activity of. Phytochemistry 2004, 65, 3249–3254. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; di Cerbo, A.; Aloisi, P.; Manelli, M.; Pellesi, V.; Provenzano, C.; Camellini, S.; Messi, P.; Sabia, C. In Vitro Activity of Essential Oils Against Planktonic and Biofilm Cells of Extended-Spectrum β-Lactamase (ESBL)/Carbapenamase-Producing Gram-Negative Bacteria Involved in Human Nosocomial Infections. Antibiotics 2020, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Chovanová, R.; Mikulášová, M.; Vaverková, Š. Modulation of Mec A Gene Expression by Essential Oil from Salvia Sclarea and Synergism with Oxacillin in Methicillin Resistant Staphylococcus Epidermidis Carrying Different Types of Staphylococcal Chromosomal Cassette Mec. Int. J. Microbiol. 2016, 2016, 6475837. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, H.; Wang, S.; Wu, J.; Kluenter, A.-M. Potential of Essential Oils for Poultry and Pigs. Anim. Nutr. 2018, 4, 179–186. [Google Scholar] [CrossRef]
- Khan, R.U.; Naz, S.; Nikousefat, Z.; Tufarelli, V.; Laudadio, V. Thymus Vulgaris: Alternative to Antibiotics in Poultry Feed. Worlds Poult. Sci. J. 2012, 68, 401–408. [Google Scholar] [CrossRef]
- Marmion, M.; Ferone, M.T.; Whyte, P.; Scannell, A.G.M. The Changing Microbiome of Poultry Meat; from Farm to Fridge. Food Microbiol. 2021, 99, 103823. [Google Scholar] [CrossRef]
- WHO Library Cataloguing-in-Publication Data. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014.
- Markey, B.; Leonard, F.; Archambault, M.; Cullinane, A.; Maguire, D. Clinical Veterinary Microbiology; Sciences, E.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-1405158237. [Google Scholar]
- Shanmugasamy, M.; Velayutham, T.; Rajeswar, J. Inv A Gene Specific PCR for Detection of Salmonella from Broilers. Vet. World 2011, 4, 562–564. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Feldgarden, M.; Brover, V.; Haft, D.H.; Prasad, A.B.; Slotta, D.J.; Tolstoy, I.; Tyson, G.H.; Zhao, S.; Hsu, C.-H.; McDermott, P.F.; et al. Validating the AMRFinder Tool and Resistance Gene Database by Using Antimicrobial Resistance Genotype-Phenotype Correlations in a Collection of Isolates. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and Model-Centric Curation of the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef] [PubMed]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of Acquired Antimicrobial Resistance Genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In Silico Detection and Typing of Plasmids Using PlasmidFinder and Plasmid Multilocus Sequence Typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Galgano, M.; Capozza, P.; Pellegrini, F.; Cordisco, M.; Sposato, A.; Sblano, S.; Camero, M.; Lanave, G.; Fracchiolla, G.; Corrente, M.; et al. Antimicrobial Activity of Essential Oils Evaluated In Vitro against Escherichia Coli and Staphylococcus Aureus. Antibiotics 2022, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Hu, H.-W.; Chen, Q.-L.; Singh, B.K.; Yan, H.; Chen, D.; He, J.-Z. Transfer of Antibiotic Resistance from Manure-Amended Soils to Vegetable Microbiomes. Environ. Int. 2019, 130, 104912. [Google Scholar] [CrossRef]
- Camero, M.; Lanave, G.; Catella, C.; Capozza, P.; Gentile, A.; Fracchiolla, G.; Britti, D.; Martella, V.; Buonavoglia, C.; Tempesta, M. Virucidal activity of ginger essential oil against caprine alphaherpesvirus-1. Vet Microbiol 2019, 230, 150–155. [Google Scholar] [CrossRef]
- Vandendool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Koo, I.; Kim, S.; Zhang, X. Comparative analysis of mass spectral matching-based compound identification in gas chromatography-mass spectrometry. J. Chromatogr. A 2013, 1298, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectorscopy, 4th ed.; Allured Pub. Corp.: Carol Stream, IL, USA, 2007; p. viii, 804 p. [Google Scholar]
| Drugs | Bacterial Strains | ||
|---|---|---|---|
| M. lentus | E. coli | S. Derby | |
| AMP | S | S | R |
| AUG | S | S | S |
| OX | I | R | R |
| KZ | R | R | R |
| FOX | R | I | R |
| CXM | I | S | I |
| CRO | S | S | S |
| CTX | S | S | S |
| E | S | R | R |
| AK | S | S | S |
| TE | S | S | S |
| DO | S | S | S |
| CN | S | S | S |
| VA | S | R | R |
| CD | S | R | R |
| SXT | S | S | S |
| CS | R | I | R |
| IMI | S | I | R |
| Detected Bacteria | Resistance Phenotype | Detected Resistance Genes and Mutations (n) |
|---|---|---|
| M. lentus | KZ; FOX; CS | blaZ_8; mph(C)_2 |
| E. coli | OX; KZ; E; VA; CD | (Bla)AmpC1_E. coli; (Bla)Penicillin_Binding_Protein_Ecoli; baeR; (Phe)CatB4; mdtO; evgA; Ecoli_mdfA; mdtG; mdtM; emrA; emrB; Ecoli_acrA; mdtF |
| S. Derby | AMP; OX; KZ; FOX; E; VA; CD; CS; IMI | (Bla)TEM-150; aac-(6′)1; golS; mdsA; mdsB |
| Bacterial Strains | T0 |
|---|---|
| Total mesophilic count | 9.42 |
| Mannitolsalt-positive Staphylococcaceae (M. lentus) | 9.00 |
| Lactose-positive Enterobacteriaceae (E. coli) | 7.50 |
| Salmonella enterica ser. Derby (experimental infection) | 9.00 |
| T0 | TEO | 24 h | 48 h | ||
|---|---|---|---|---|---|
| log10 CFU | Reduction % | log10 CFU | Reduction % | ||
| 9.42 | 5% | 7.28 | 77.28% | 7.28 | 77.28% |
| 2.5% | 6.2 | 65.81% | 6.2 | 65.81% | |
| 1.25% | 4.32 | 45.85% | 3.89 | 41.29% | |
| TEO | Mannitol Salt-Positive Staphylococcaceae | Lactose-Positive Enterobacteriaceae | Salmonella Derby | |||
|---|---|---|---|---|---|---|
| log10 CFU/g | Reduction% | log10 CFU/g | Reduction % | log10 FCU/g | Reduction% | |
| 5% | 7.00 | 77.7 | 7.5 | 99.9 | 8.00 | 88.9 |
| 2.5% | 6.00 | 66.6 | 6.5 | 86.6 | 8.00 | 88.9 |
| 1.25% | 4.50 | 50 | 5.5 | 73.3 | 7.00 | 77.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galgano, M.; Pellegrini, F.; Fracchiolla, G.; Mrenoshki, D.; Zarea, A.A.K.; Bianco, A.; Del Sambro, L.; Capozzi, L.; Schiavone, A.; Saleh, M.S.; et al. Pilot Study on the Action of Thymus vulgaris Essential Oil in Treating the Most Common Bacterial Contaminants and Salmonella enterica subsp. enterica Serovar Derby in Poultry Litter. Antibiotics 2023, 12, 436. https://doi.org/10.3390/antibiotics12030436
Galgano M, Pellegrini F, Fracchiolla G, Mrenoshki D, Zarea AAK, Bianco A, Del Sambro L, Capozzi L, Schiavone A, Saleh MS, et al. Pilot Study on the Action of Thymus vulgaris Essential Oil in Treating the Most Common Bacterial Contaminants and Salmonella enterica subsp. enterica Serovar Derby in Poultry Litter. Antibiotics. 2023; 12(3):436. https://doi.org/10.3390/antibiotics12030436
Chicago/Turabian StyleGalgano, Michela, Francesco Pellegrini, Giuseppe Fracchiolla, Daniela Mrenoshki, Aya Attia Koraney Zarea, Angelica Bianco, Laura Del Sambro, Loredana Capozzi, Antonella Schiavone, Medhat S. Saleh, and et al. 2023. "Pilot Study on the Action of Thymus vulgaris Essential Oil in Treating the Most Common Bacterial Contaminants and Salmonella enterica subsp. enterica Serovar Derby in Poultry Litter" Antibiotics 12, no. 3: 436. https://doi.org/10.3390/antibiotics12030436
APA StyleGalgano, M., Pellegrini, F., Fracchiolla, G., Mrenoshki, D., Zarea, A. A. K., Bianco, A., Del Sambro, L., Capozzi, L., Schiavone, A., Saleh, M. S., Camero, M., Tempesta, M., Cirone, F., Buonavoglia, D., Pratelli, A., & Buonavoglia, A. (2023). Pilot Study on the Action of Thymus vulgaris Essential Oil in Treating the Most Common Bacterial Contaminants and Salmonella enterica subsp. enterica Serovar Derby in Poultry Litter. Antibiotics, 12(3), 436. https://doi.org/10.3390/antibiotics12030436









