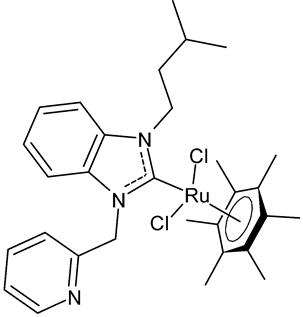
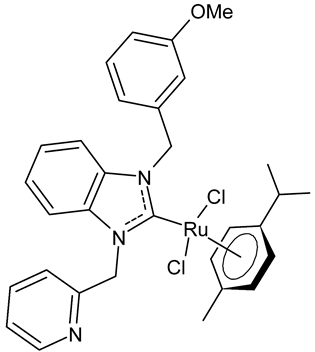
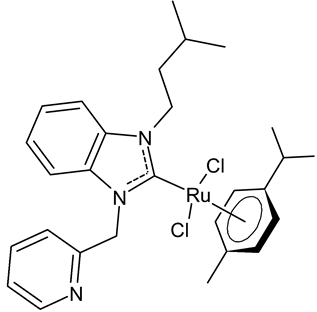
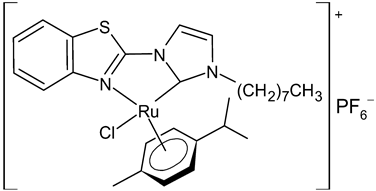
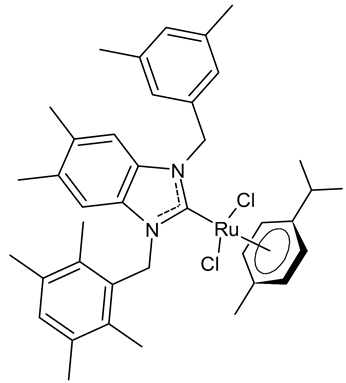
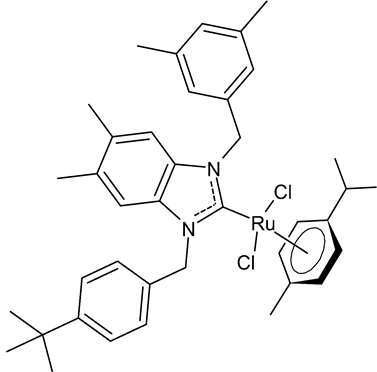
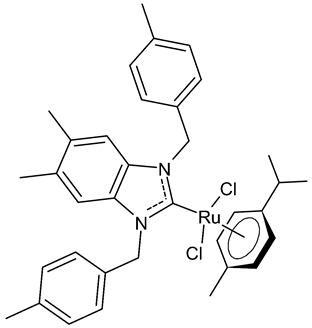
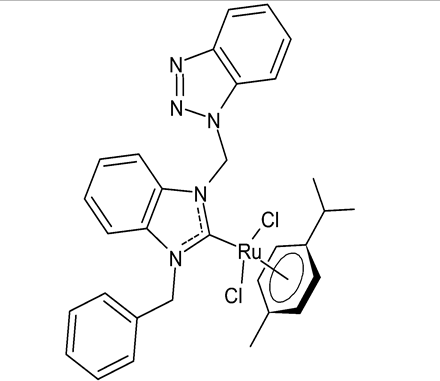
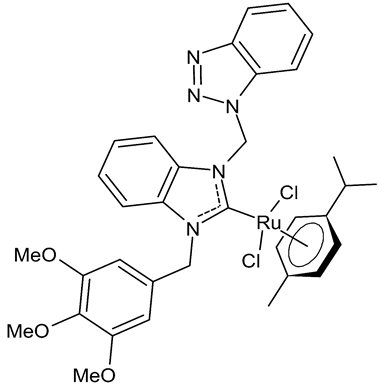
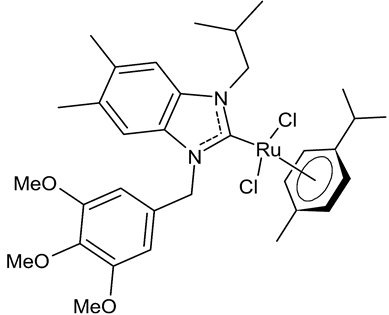
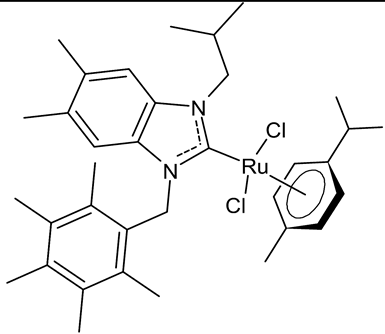
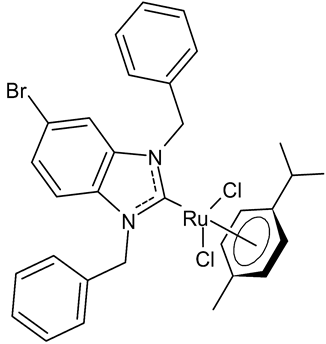
Abstract
Ruthenium N-heterocyclic carbene (NHC) complexes have unique physico-chemical properties as catalysts and a huge potential in medicinal chemistry and pharmacology, exhibiting a variety of notable biological activities. In this review, the most recent studies on ruthenium NHC complexes are summarized, focusing specifically on antimicrobial and antiproliferative activities. Ruthenium NHC complexes are generally active against Gram-positive bacteria, such as Bacillus subtilis, Staphylococcus aureus, Micrococcus luteus, Listeria monocytogenes and are seldom active against Gram-negative bacteria, including Salmonella typhimurium, Pseudomonas aeruginosa and Escherichia coli and fungal strains of Candida albicans. The antiproliferative activity was tested against cancer cell lines of human colon, breast, cervix, epidermis, liver and rat glioblastoma cell lines. Ruthenium NHC complexes generally demonstrated cytotoxicity higher than standard anticancer drugs. Further studies are needed to explore the mechanism of action of these interesting compounds.
1. Introduction
Ruthenium (II) complexes represent an important class of organometallic compounds with numerous applications in the fields of homogeneous, heterogeneous and photocatalytic catalysis [1,2], including biological stains, and in the field of therapy [3,4]. Ruthenium complexes endowed with anticancer properties [5] are attracting significant attention, due to their vast and different structural types and their ability to variously bind different ligands with the advantage of lower toxicity than platinum complexes [6,7,8]. These compounds reasonably penetrate more efficiently tumor cells and effectively bind to DNA, from which comes the suggestion of their use in different cancers [9,10], including lung [11], breast [12], ovarian [13,14,15,16] and colorectal ones [8]. Recently, nanomedicine formulations of metal complexes developed for the treatment of cancers are under study [17]. Moreover, numerous other biological activities [18], such as antioxidant, anti-inflammatory [19], and antimicrobial [3,20,21], have been described for ruthenium complexes. Recent studies are also addressed to the use of ruthenium complexes as antivirals for the treatment of COVID-19 [22]. Ruthenium complexes include those with Schiff bases [23,24,25], phosphines [26], carbazole [27], N-heterocyclic carbenes (NHCs), cycloruthenated and half-sandwiched compounds [28]. Considering the huge variety of ruthenium (II) complexes, this review will focus merely on one class of them, namely Ru-NHC complexes. Ru-NHC complexes have promising catalytic potential for a vast range of synthetic applications, including the activity in transfer hydrogenation [29,30] of ketones, metathesis reactions [31], secondary alcohol oxidation [32], N-alkylation of amines, amides, and sulfonamides [33,34] and Oppenauer-type oxidation [35,36]. The high catalytic activity of these compounds is even comparable to that of the Noels catalyst [37]. More importantly, Ru-NHC complexes exhibit notable pharmaceutical activities. The importance of these compounds is also well-established by recent computational studies [38], suggesting the structural basis of the biomolecular action of these compounds. In this review, we want to highlight the importance of the antimicrobial and antiproliferative activities of the Ru(II)-NHC complexes that may be considered significant starting points for the development of new antimicrobial and anticancer agents, providing an update of recent studies inherent this relevant topic in medicinal chemistry in the last five years.
2. N-Heterocyclic Carbenes (NHCs)
N-heterocyclic carbenes (NHCs) are a category of electron donor ligands able to form dative metal–ligand bonds, leading to a universal class of compounds in organometallic and coordination chemistry. NHCs typically mimic the chemical properties of phosphines [39]. They belong to five different families: imidazolinylidenes, imidazolylidenes, triazolinylidenes, thiazolilylidenes and pyrazolinylidenes (Figure 1) [40].
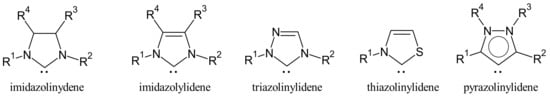
Figure 1.
Structures of NHCs.
NHCs are suitable for efficient design because they can be synthesized within a few steps, offering the possibility of the N and C functionalization for structure modification. They act as excellent σ-donors, generating a structural variety ranging from linear, square pyramidal, trigonal bipyramidal, tetrahedral and octahedral geometries of metal-NHC complexes. The essential role of NHCs is related to their ability to form complexes with metals. Metal-NHCs are widely used for organic processes, such as the formation of amide linkage, hydrogenation, isomerization, cycloisomerization, cyclopropanation, hydrosilylation, allylation and deallylation, enol-ester synthesis, heterocycle synthesis and C-C alkyne coupling [40]. Most importantly, different organometallic-NHC complexes, such as silver, gold, platinum, copper, palladium and selenium demonstrated interesting biological properties [41,42,43,44], including antimicrobial [45,46,47], anticancer [48,49,50,51,52,53,54], antiparasitic [55], hemolytic and thrombolytic activities [56,57]. Recent studies for gold and silver-NHC compounds are addressed to breast [58,59], ovarian [60] and cervical human cancer [61]. Research has been carried out in order to understand the mechanism of action of gold and silver-NHC complexes as anticancer agents, finding activity against human topoisomerases I and II [62,63], actin [64] and tubulin [65], or triggering the reactive oxygen species-dependent intrinsic apoptotic pathway [66]. Recently, gold and silver compounds with NHC have been indicated as promising compounds for the treatment of COVID-19 [67], as they demonstrated a strong inhibition of the S/ACE2 interaction and particularly of the PLpro enzymatic activity [68]. Moreover, an Ag-N-heterocyclic carbene complex bearing the hydroxyethyl ligand determined the inhibition of human carbonic anhydrase I (hCA I) and hCA II isoenzymes, α-glycosidase and AChE and BChE enzymes, thus suggesting the selection of NHC complexes for further studies in glaucoma, epilepsy and other diseases related to metabolic enzymes [69].
3. Ruthenium-NHC Complexes
Besides the importance of ruthenium-NHC complexes in organometallic catalysis [70,71] and bioinorganic chemistry, they have been described as effective anticancer and antimicrobial agents [40,43,47]. Patil et al., 2020, published the advances in the design, synthesis, characterization and biomedical applications, particularly the antimicrobial and anticancer activities, of ruthenium NHC–metal complexes and other metals (silver, gold, palladium, rhodium, iridium, and platinum), covering works published from 2015 to 2020 [39]. Several studies regard only the activity of Ru-NHC complexes as antiproliferative agents, whereas the antimicrobial activity is almost always studied along with other activities, including antiproliferative and antioxidant. The two paragraphs below summarized these studies.
3.1. Ru(II)-NHC Complexes with Antiproliferative Activity
Recent studies regarding the antiproliferative activity of Ru(II)-NHC complexes are summarized and the half-maximal (50%) inhibitory concentration (IC50) values are given, when reported in the literature.
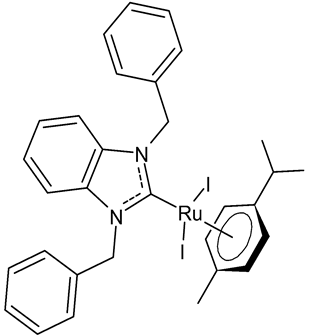
Lam et al., 2018, [72] investigated several halide-substituted benzimidazolium-derived NHC of RuII/OsII complexes, using NHCs that were symmetrically and non-symmetrically methyl- and benzyl-substituted, and reported their inhibition of the selenoenzyme thioredoxin reductase (TrxR) and antiproliferative activities. The anticancer activity was studied against human colon (HCT-116), human cervical (SiHa) and human breast cancer (NCI-H460) cells. The diiodido(1,3-dibenzylbenzimidazol-2-ylidene)(η6-p-cymene)ruthenium(II) complex 1 is a potent TrxR inhibitor and an antiproliferative agent. The authors found out that there was no clear correlation between the two activities, thus, it was suggested that TrxR inhibition was unlikely to be the main mode of action.
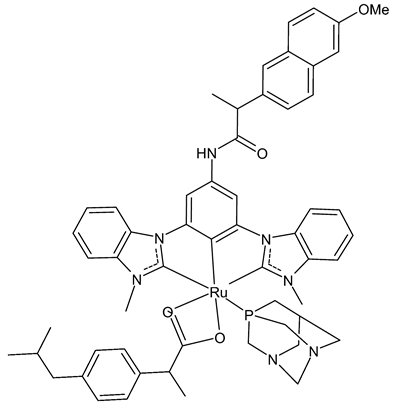
Tabrizi et al., 2019, [73] studied the in vitro antiproliferative potential and cyclooxygenase-2 (COX-2) inhibitory activity of a cyclometalated Ru(II) complex (2) containing ibuprofen (Ibu), 1,3,5-triaza-7-phosphaadamantane (PTA) and a CCC-pincer containing naproxen moiety (CCC-Nap). Antiproliferative studies were carried out on breast cancer (MCF-7 and MDA-MB-231), colon cancer (HT-29) cell lines, healthy breast cell lines (MCF-10A), and human embryonic kidney normal cells (HEK293) by means of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, after 72 h exposure. The complex 2 was quite potent, about twice as active as cisplatin against breast cancer cells, and around 14 times less active against HT-29 cell lines than cisplatin. Interestingly, the complex 2 inhibition studies against COX-2 revealed that it displayed approximately about 16- and 5-times stronger interactions than the free Ibu and CCC-Nap ligands, respectively. Moreover, it improved the production of reactive oxygen species (ROS) by 10.7-fold compared to H2O2, when used as a positive control in MCF-7 cells.
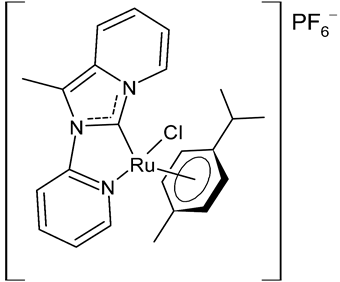
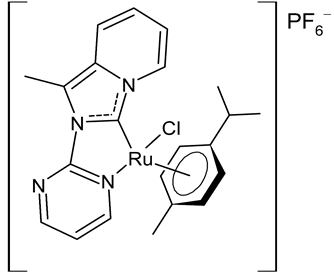
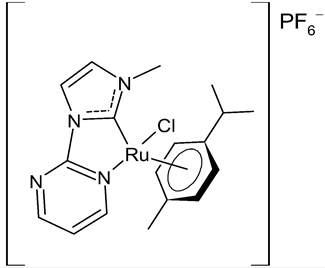
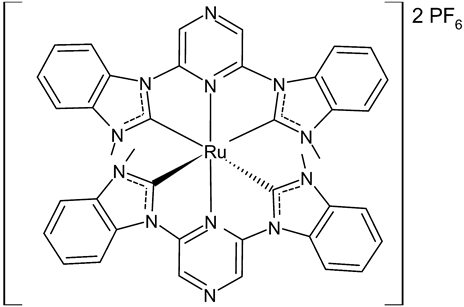
Rana et al., 2021, [74] described the synthesis and study of two pyridine and pyrimidine Ru(II)-NHC complexes, functionalized annulated NHC 3 and 4 with a half-sandwich geometry, and their in vitro cytotoxicity studies against lung (A549), colon (HCT-116) and breast (MCF-7) cancer cell lines and non-cancerous 3T3 cells (embryonic fibroblast isolated from a mouse), using cisplatin as the reference (IC50 were 64; 23.2; 14 and 64 µM, respectively) via the MTT assay. Both compounds were more active than the reference, with the exception of 3 against HCT-116 cancer cells, and, overall, compound 4 was more active than compound 3. Moreover, in silico studies, predicting the binding-sites and atomic interactions of lead molecules with B cell CLL/lymphoma (BCL-2) (PDB entry: 4lvt) and DNA dodecamer (PDB entry: 1bna), suggested that the order of reactivity of the molecules is 4 > 3. Subsequently, the same group [75] investigated a pyrimidine functionalized non-annulated half-sandwich Ru(II)-NHC complex, namely chloro(p-cymene)-1-methyl-3-pyrimidylimidazolideneruthenium(II)-hexafluorophosphate 5, derived from the non-annulated NHC precursor 1-methyl-3-pyrimidylimidazolium-hexafluorophosphate. The half-sandwich geometry of the molecule was established with single crystal X-ray diffraction. As well, this compound was more active than cisplatin against the cell lines used [74]. Particularly, 5 and 4 were 3- to 4-fold more active than 3 and cisplatin. Predicting binding affinities for 5 and 4 were −7.1 and −7.0 kcal/mol–1, respectively, for BCL-2 and −7.3 and −8.2 kcal/mol–1, respectively, for DNA. DNA cleavage activity of complex 5 confirmed the ability of ruthenium to perform a direct double-strand breaking. Moreover, molecular docking analysis suggested that complex 5 binds, with the highest binding affinity, a hydrophobic pocket in BLC-2, different from that of complexes 3 and 4. The opposite was found in the contact simulation of the complexes with a DNA strand: complexes 4 and 5 are superimposed, while complex 3 binds the other side of the DNA strand. However, the binding affinity predicted for 4 was higher than 5.
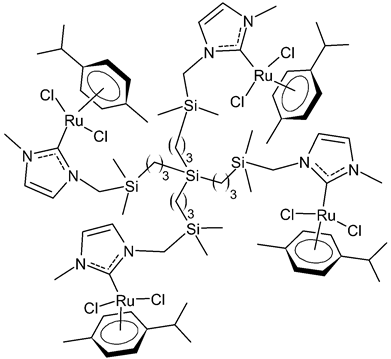
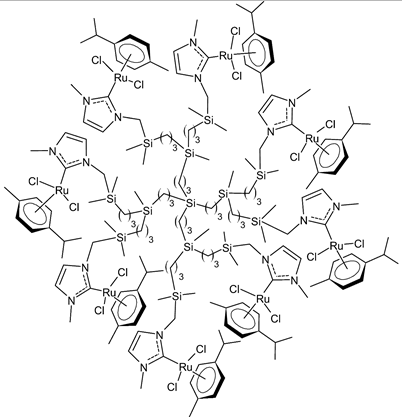
Rodriguez-Prieto et al., 2021, [76] reported the synthesis of three spherical carbosilane metallodendrimers of different generations holding Ru-NHC complexes. Compounds 6 and 7, which belong to the first- and second-generation dendrimers, respectively, are shown in Table 1, while the third-generation dendrimer is not shown. These compounds showed cytotoxic activity similar, or even better, than cisplatin against four cancer cell lines, namely advanced prostate (PC3), breast (HCC1806), cervix (HeLa), human liver (HEPG2) and the non-tumoral fibroblast (HFF-1) cell line, as determined by MTT assay. IC50 for cisplatin was referred to data of the literature: IC50 = 30.18 ± 2.58 µM (MCF-7), 11.75 ± 1.23 µM (HeLa), 17.29 ± 1.05 µM (HFF-1) [77]. The complexes have been proposed as possible candidates for cancer treatment, due to their combined double action, i.e., antitumoral and carrier for anticancer siRNA. These compounds were capable of forming dendriplexes by promoting the entrance of Mcl-1-FITC (myeloid cell leukemia-1 fluorescein labelled) small interfering RNA (siRNA) to HEPG2 cancer cells, protecting the siRNA from RNAse. Moreover, the cellular uptake of the three complexes was studied by confocal microscopy with Mcl-1-FITC siRNA. Particularly, the second-generation dendrimer 7 was more active than first-generation dendrimer 6. Compound 7 displayed promising antitumoral properties, being selective, even more than cisplatin, against cancer cell lines, with respect to the normal ones. The different activity was related to the inability of first-generation dendrimer 6 to interact with the siRNA. The compounds were internalized into the cells by endocytosis and internalization increased by generation of the dendritic system.

Table 1.
Antiproliferative activity of Ru(II)-NHC complexes.
Paşahan et al., 2022, [78] recently reported a study on Ru-NHC complexes as potential anticancer agents, examining their antiproliferative activity against rat glioblastoma (C6) and human cervix adenocarcinoma (HeLa) cell lines by ELISA assay. Four complexes, namely 8–11, were more active than cisplatin, used as the standard (IC50 = 136 ± 0.7 mM (C6) and 126 ± 0.6 mM (HeLa)).
Chen et al., 2020, [79] reported the synthesis and in vitro antiproliferative activity evaluation of a small panel of NHC-coordinated ruthenium(II) arene complexes. The compounds showed cytotoxic activities against the human ovarian A2780 cancer cells. The highest cytotoxic activities were found for 12 and 13, which were about 2-fold more potent than cisplatin. Furthermore, these compounds induced apoptosis in a caspase-dependent manner, primarily through intracellular ROS overproduction and cell cycle arrest at the G1 phase. Moreover, in a preclinical metastatic model of A2780 tumor xenograft, administration of 12 and 13 resulted in a marked inhibition of tumor progression and metastasis. A significant alleviated systemic toxicity was observed in animals for both complexes in comparison with cisplatin.
Sari et al., [80] designed four (NHC)Ru(II)(η6-p-cymene) complexes, bearing 2-morpholinoethyl and 4-vinylbenzyl substituents to the benzimidazole core and different alkyl/aryl groups to the second nitrogen atom, and studied their cytotoxic activity against MCF-7 breast cancer cells and their DNA-binding properties. The authors individuated the compound 14 as lead, showing an IC50 of 3.61 µM, and that it can bind the plasmidic DNA without exerting genotoxic effects.
A recent interesting article by Wilke et al., [81] described the study of the ruthenium complex HB324 (15), which showed promising potential as a novel anticancer agent in vitro. This complex showed good effects, even in low micromolar concentrations, especially regarding proliferation inhibition and apoptosis induction via the mitochondrial pathway on human B-cell precursor leukemia Nalm-6 cells. Moreover, of particular interest is the upregulation of the Harakiri resistance protein, which inhibits the anti-apoptotic and death repressor proteins BCL-2 and BCL-xL. Finally, compound 15 showed synergistic activity with various established anticancer drugs, including vincristine, and overcame the resistance in several cell lines, such as neuroblastoma cells.
3.2. Antimicrobial Ru(II)-NHC Complexes Possessing Other Additional Abilities (Antiproliferative, Antioxidant and Anticholinesterase)
Antimicrobic resistance is a cause of great concern worldwide and significantly affects humanity’s capacity to prevent and treat a growing number of bacterial and fungal infections [82]. In the past decade, the antimicrobial activity of ruthenium complexes has been reviewed [83] and studied as antimicrobial agents and alternatives or adjuvants to the more traditional antibiotics [84]. The recent antimicrobial studies regarding complexes of NHC-ruthenium are summarized in Table 2, and the minimal inhibitory concentrations (MICs) are reported.

Table 2.
Antimicrobial and other activities (antiproliferative, antioxidant and anticholinesterase) of Ru(II)-NHC complexes.
Roymahapatra et al., 2015, [85] described the synthesis, pro-apoptotic and antimicrobial studies of two pyrazine functionalized Ru(II)-NHC complexes of methylimidazolylidene and methylbenzimidazolylidene, namely bis-[2,6-di-(N-methylimidazol-2-ylidene)pyrazine]ruthenium(II) hexafluorophosphate (16) and bis-[2,6-di-(N-methylbenzimidazol-2-ylidene)pyrazine]ruthenium(II) hexafluorophosphate (12). Inhibition of cell proliferation was studied against human colon carcinoma cell lines (HCT15) and human epidermoid cancer cells (Hep2) by the standard MTT assay. Complex 16 was more active than complex 17 against both cancer cell lines. DNA binding and cleavage studies demonstrated that these complexes may induce cancer cell apoptosis. Moreover, complex 16 showed antimicrobial activities against Gram-positive (Staphylococcus epidermidis NCIM2493) and Gram-negative bacteria (Pseudomonas aeruginosa ATCC 27853), and antimycotic properties against Candida albicans SJ11 (unicellular fungus) by targeting their cell wall and DNA or plasmid inside the cell.
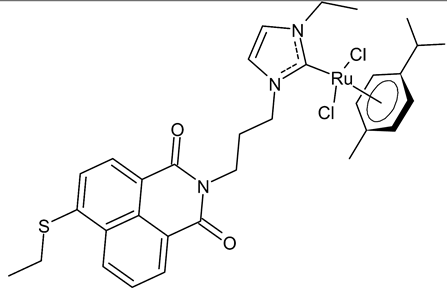
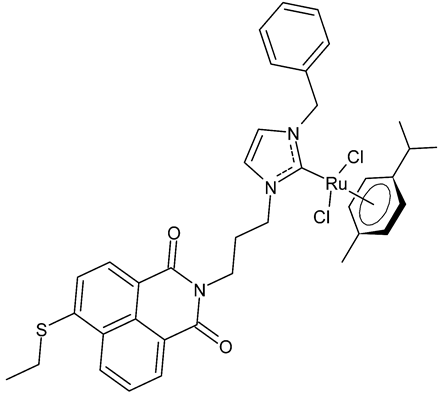
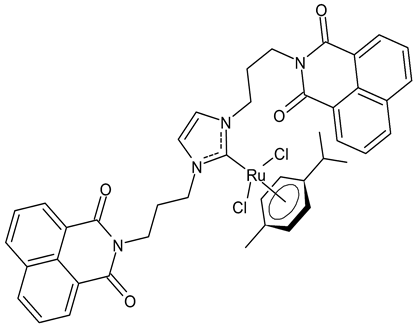
Streciwilk et al., 2018, [86] studied antimicrobial activities of three Ru(II) naphthalimide-NHC complexes against Gram-positive (Bacillus subtilis 168 DSM402; Staphylococcus aureus DSM 20231 and ATCC 43300) and Gram-negative (Escherichia coli DSM 30083, Acinetobacter baumannii DSM 30007 and P. aeruginosa DSM 50071) bacteria and the cytotoxic activity against MCF-7 human breast cancer and HT-29 colon adenocarcinoma cells. Complexes 18 and 19 showed activity only against Gram-positive bacteria, while showing no activity against Gram-negative (MIC = 256 µg/mL; 366 µM). Compound 19 also showed cytotoxicity against the two cell lines used. The results were compared to cisplatin or 5-fluorouracil (IC50 values for standards below 10 µM). DNA-binding studies were also carried out by recording the circular dichroism of B-DNA and c-Kit2 G4 in the presence of increasing amounts of 19, highlighting a strong binding of 19 to the B-DNA model and suggesting intercalation as a mode of connection. Further studies carried out by the same research group [87] with complex 19 on HCT-116 colorectal cancer cells demonstrated that it triggers apoptosis via a rapid and consistent activation of the ROS-p38 MAPK pathway. Following this, the same group [88] reported a research, similar to the previous one, regarding the antimicrobial and antiproliferative activity of a bis-naphthalimide NHC–ruthenium (II) complex (20). It showed a slight antimicrobial effect against Gram-positive bacteria, whereas it was not cytotoxic against the cell lines studied.
Boubakri et al., 2019, [89] studied a series of Ru(II)-NHC complexes against three Gram-positive bacteria (Micrococcus luteus LB14110, Listeria monocytogenes ATCC19117, S. aureus ATCC6538) and three Gram-negative bacteria (Salmonella typhimurium ATCC14028, P. aeruginosa ATCC 9189, E. coli) using the well diffusion method. The reference antibacterial drug used was ampicillin. The complex 21 and 22 were the most active against M. luteus, L. monocytogenes and S. typhimurium (ampicillin, MIC = 0.004, 0.002 and 0.625 mg/mL, respectively). The compound 22 was also active versus E. coli (for this compound only an inhibition zone value was given). Moreover, the antifungal activity of the Ru(II)–NHC complexes, with respect to C. albicans, was tested via the solid medium diffusion method. Also in this case, the compound 21 was active, showing the highest diameter of growth inhibition (34 ± 0.18 mm). Interestingly, the compound 21 also showed acetylcholinesterase inhibitory activity (AChEI), with a percentage inhibition to the order of 47.1%. Regarding the anticancer activity, the compounds 21 and 22 also showed cytotoxicity against two human breast cancer cell lines, namely MCF-7 and MDA-MB-231, as assessed by MTT assay. The compound 21 also showed antioxidant activity, as demonstrated via the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) assays, from a concentration of 1 mg/mL, showing a scavenging activity very similar to that of the two controls, i.e., gallic acid and butylated hydroxytoluene. A recent study by the same group [90] deepened the study of compound 21 and highlighted the interesting results obtained for this compound, along with its congener 23. The compound 21 showed antibacterial activity against L. monocytogenes, S. aureus and S. typhimurium, sometimes equal to, or higher than, references ampicillin (MIC = 3.9 µg/mL, 1.95 µg/mL, 3.9 µg/mL) or kanamycin (MIC = 12.5 µg/mL, 6,25 µg/mL, 12.5 µg/mL). Both the compounds 21 and 23 showed antifungal activity against C. albicans, equal to the reference fluconazole (MIC = 1.25 µg/mL). The two compounds also showed moderate cytotoxicity against MCF-7 and MDA-MB-231 cancer cell lines, as assessed by MTT assay. The compounds 21 and 23 were also studied for inhibition of acetylcholinesterase (AChE) and tyrosinase (TyrE), giving interesting results compared to galantamine (IC50 = 0.25 µg/mL against AChE) and kojic acid (IC50 = 5.05 µg/mL against TyrE). The compound 23 showed an interesting antioxidant activity, similar to that of the standard butylated hydroxytoluene (BHT: EC50 = 31.55 µg/mL, 17.41 µg/mL, 89.55 µg/m), as determined for DPPH, ABTS and β-carotene assays, respectively.
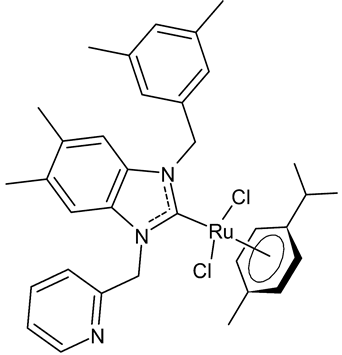
Onar et al., 2019, [91] studied four ruthenium NHC complexes for their antimicrobial, anticancer and DNA-binding activities. Antimicrobial activities of the compounds were tested against one Gram-positive (B. subtilis ATCC 21332) and one Gram-negative (E. coli ATCC 25922) bacteria and one fungal strain (C. albicans ATCC 60193). The studied compounds were slightly more active than cefotaxime, used as a reference (MIC ranging from 100 to 200 µg/mL, compared to 250 µg/mL of the standard drug). Interesting results were obtained regarding cytotoxic activity. The benzimidazole-based ruthenium complexes containing a benzyl group 24 and 25 showed cytotoxicity against human colorectal Caco-2 cancer cell lines comparable to cisplatin, whereas they were non-cytotoxic against non-cancer L-929 cell lines.
Slimani et al., 2020, [92] studied four [RuCl2(p-cymene)]2 complexes. The antibacterial activity was tested against three Gram-positive (Micrococcus luteus LB 14110, S. aureus ATCC 6538, Listeria monocytogenes ATCC 19117), and three Gram-negative (Salmonella typhimurium ATCC 14028, P. aeruginosa ATCC 49189 and E. coli) bacteria, using the well diffusion method. Ampicillin was used as a standard control drug (MIC = 0.004 mg/mL, 0.002 mg/mL and 0.625 mg/mL against M. luteus LB 14110; L. monocytogenes ATCC 19117 and S. typhimurium ATCC 14028, respectively). The compounds 26 and 27 demonstrated effective antibacterial activity against the tested bacteria; particularly, the compound 26 showed excellent activity against S. typhimurium, being more active than the reference drug. Moreover, the compound 26, from a concentration of 1 mg/mL, demonstrated a very similar scavenging activity to that of the two controls, butylated hydroxytoluene (BHT) and gallic acid (GA), as determined via DPPH assay. Finally, both 26 and 27 showed cytotoxic activity against human breast cancer cell lines MCF-7 and MDA-MB-231.
Burmeister et al., 2021, [93] studied a series of benzimidazolium cations and the corresponding organometallics of the type (p-cymene)(NHC)Ru(II)Cl2 as antibacterials against Gram-positive (B. subtilis, S. aureus DSM 20231, S. aureus ATCC 43300) and Gram-negative (E. coli, A. baumannii, P. aeruginosa) strains in a microtiter plate assay, according to CLSI guidelines, using ciprofloxacin as a reference drug. The complex (1,3-dibenzyl-5-bromo-1H-benzimidazol-2-ylidene)-dichlorido-(η6-p-cymene)ruthenium(II) 28 was the most active compound against Gram-positive bacteria, while it showed low activity against Gram-negative bacteria. This compound showed moderate inhibition of bacterial TrxR (E. coli). Thus, the inhibition of TrxR is unlikely a major mechanism of the antibacterial activity of the ruthenium NHC complexes.
4. Conclusions
NHCs have been recognized as a class of strong donating ligands, which may stabilize diverse metal complexes of catalytic importance. Among these, the NHC ruthenium complexes have been, and still are, a fruitful research, filed for various applications: catalytic, photochemical and biological. This review provides an overview of the most recent studies on ruthenium NHCs complexes with biological activities, focusing on the antimicrobial and antiproliferative properties. Most of these compounds showed interesting antimicrobial activity against Gram-positive bacteria and some are also active against Gram-negative bacteria and fungi. The antiproliferative activity was also demonstrated for several compounds belonging to this class against several types of cancer cell lines.
Author Contributions
Conceptualization, P.L., A.M. and M.S.S. writing—original draft preparation, A.C., P.L. and A.M.; writing—review and editing A.C. and P.L.; data curation, C.S. and J.C.; supervision, M.S.S. and D.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Sirignano Marco and Troiano Rubina for the useful support.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations and Cancer Cell Lines Mentioned in the Text
| ABTS | 2,2′-azinobis-3-ethylbenzothiazoline-6-sulphonic acid |
| A549 | lung cancer cells |
| BCL-2 | B cell CLL/lymphoma |
| BCL-xL | B-cell lymphoma-extra large |
| Caco-2 | colorectal cancer cells |
| C6 | rat glioblastoma cancer cells |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| HCC1806 | breast cancer cells |
| HCT15 | human colon carcinoma cell lines |
| HCT-116 | human colon cancer cells |
| HeLa | human cervix adenocarcinoma cancer cells |
| Hep2 | human epidermoid cancer cells |
| HEPG2 | human liver cancer cells |
| HEK293 | normal cells |
| HFF-1 | non-tumoral fibroblast cells |
| HT-29 | colon cancer cells |
| IC50 | half-maximal (50%) inhibitory concentration (IC50) |
| MCF-7 | breast cancer cells |
| MCF-10A | healthy breast cell line |
| MDA-MB-231 | breast cancer cells |
| MIC | minimal inhibitory concentration |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NCI-H460 | human breast cancer cells |
| PC3 | advanced prostate cancer cells |
| ROS | reactive oxygen species |
| SiHa | human cervical cancer cells |
References
- Mariconda, A.; Sirignano, M.; Costabile, C.; Longo, P. New NHC- silver and gold complexes active in A3-coupling (aldehyde-alkyne-amine) reaction. Mol. Catal. 2020, 480, 110570. [Google Scholar] [CrossRef]
- Bruno, G.; Nicolò, F.; Lo Schiavo, S.; Sinicropi, M.S.; Tresoldi, G. Synthesis and spectroscopic properties of di-2-pyridyl sulfide (dps) compounds. Crystal structure of [Ru(dps)2Cl2]. J. Chem. Soc. Dalton Trans. 1995, 1, 17–24. [Google Scholar] [CrossRef]
- Munteanu, A.-C.; Uivarosi, V. Ruthenium complexes in the fight against pathogenic microorganisms. An extensive review. Pharmaceutics 2021, 13, 874. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-N.; Zou, Y.-H.; Sun, H.-X.; Chen, Y.; Li, S.-L.; Lan, Y.-Q. Recent progress and perspectives in heterogeneous photocatalytic CO2 reduction through a solid–gas mode. Coord. Chem. Rev. 2021, 438, 213906. [Google Scholar] [CrossRef]
- Swaminathan, S.; Haribabu, J.; Balakrishnan, N.; Vasanthakumar, P.; Karvembu, R. Piano stool Ru(II)-arene complexes having three monodentate legs: A comprehensive review on their development as anticancer therapeutics over the past decade. Coord. Chem. Rev. 2022, 459, 214403. [Google Scholar] [CrossRef]
- Dragutan, I.; Dragutan, V.; Demonceau, A. Editorial of special issue ruthenium complex: The expanding chemistry of the ruthenium complexes. Molecules 2015, 20, 17244–17274. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, C.Y.; Nam, T.G. Ruthenium complexes as anticancer agents: A brief history and perspectives. Drug Des. Dev. Ther. 2020, 14, 5375–5392. [Google Scholar] [CrossRef]
- Mahmud, K.M.; Niloy, M.S.; Shakil, M.S.; Islam, M.A. Ruthenium complexes: An alternative to platinum drugs in colorectal cancer treatment. Pharmaceutics 2021, 13, 1295. [Google Scholar] [CrossRef]
- Kostova, I. Ruthenium complexes as anticancer agents. Curr. Med. Chem. 2006, 13, 1085–1107. [Google Scholar] [CrossRef]
- Pragti; Kundu, B.K.; Mukhopadhyay, S. Target based chemotherapeutic advancement of ruthenium complexes. Coord. Chem. Rev. 2021, 448, 214169. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Y.; Shi, H.; Wang, Y.; Zhang, J.; Zhang, Q. Ruthenium complexes as promising candidates against lung cancer. Molecules 2021, 26, 4389. [Google Scholar] [CrossRef]
- Popolin, C.P.; Cominetti, M.R. A review of ruthenium complexes activities on breast cancer cells. Mini Rev. Med. Chem. 2017, 17, 1435–1441. [Google Scholar] [CrossRef]
- Song, M.; Cui, M.; Liu, K. Therapeutic strategies to overcome cisplatin resistance in ovarian cancer. Eur. J. Med. Chem. 2022, 232, 114205. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, J.; Häfner, N.; Kritsch, D.; Görls, H.; Dürst, M.; Runnebaum, I.B.; Weigand, W. Highly cytotoxic osmium(II) compounds and their ruthenium(II) analogues targeting ovarian carcinoma cell lines and evading cisplatin resistance mechanisms. Int. J. Mol. Sci. 2022, 23, 4976. [Google Scholar] [CrossRef] [PubMed]
- Kljun, J.; Pavlič, R.; Hafner, E.; Lipec, T.; Moreno-Da Silva, S.; Tič, P.; Turel, I.; Büdefeld, T.; Stojan, J.; Rižner, T.L. Ruthenium complexes show potent inhibition of AKR1C1, AKR1C2, and AKR1C3 enzymes and anti-proliferative action against chemoresistant ovarian cancer cell line. Front. Pharmacol. 2022, 13, 920379. [Google Scholar] [CrossRef] [PubMed]
- Kladnik, J.; Coverdale, J.P.C.; Kljun, J.; Burmeister, H.; Lippman, P.; Ellis, F.G.; Jones, A.M.; Ott, I.; Romero-Canelón, I.; Turel, I. Organoruthenium complexes with benzo-fused pyrithiones overcome platinum resistance in ovarian cancer cells. Cancers 2021, 13, 2493. [Google Scholar] [CrossRef] [PubMed]
- Peña, Q.; Wang, A.; Zaremba, O.; Shi, Y.; Scheeren, H.W.; Metselaar, J.M.; Kiessling, F.; Pallares, R.M.; Wuttke, S.; Lammers, T. Metallodrugs in cancer nanomedicine. Chem. Soc. Rev. 2022, 51, 2544–2582. [Google Scholar] [CrossRef]
- Clarke, M.J. Ruthenium metallopharmaceuticals. Coord. Chem. Rev. 2002, 232, 69–93. [Google Scholar] [CrossRef]
- Sasahara, G.L.; Gouveia Júnior, F.S.; Rodrigues, R.O.; Zampieri, D.S.; Fonseca, S.; Gonçalves, R.C.R.; Athaydes, B.R.; Kitagawa, R.R.; Santos, F.A.; Sousa, E.H.S.; et al. Nitro-imidazole-based ruthenium complexes with antioxidant and anti-inflammatory activities. J. Inorg. Biochem. 2020, 206, 111048. [Google Scholar] [CrossRef]
- Donnici, C.L.; Araujo, M.H.; Stoianoff, M.A.R. Ruthenium complexes as antifungal agents. In Ruthenium Complexes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 293–318. [Google Scholar]
- Chen, Y.; Liu, L.; Wang, X.; Liao, Z.; Wang, R.; Xiong, Y.; Cheng, J.; Jiang, G.; Wang, J.; Liao, X. The synthesis and antibacterial activity study of ruthenium-based metallodrugs with a membrane-disruptive mechanism against Staphylococcus aureus. Dalton Trans. 2022, 51, 14980–14992. [Google Scholar] [CrossRef] [PubMed]
- Cirri, D.; Pratesi, A.; Marzo, T.; Messori, L. Metallo therapeutics for COVID-19. Exploiting metal-based compounds for the discovery of new antiviral drugs. Exp. Opin. Drug Discov. 2021, 16, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Amirthaganesan, K.; Vadivel, T.; Dhamodaran, M.; Chandraboss, V.L. In vitro antifungal studies of ruthenium (III) complex derived from chitosan Schiff bases. Mater. Today Proc. 2022, 60, 1716–1720. [Google Scholar] [CrossRef]
- Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M.S. A review on the antimicrobial activity of Schiff bases: Data collection and recent studies. Antibiotics 2022, 11, 191. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Ceramella, J.; Iacopetta, D.; Catalano, A.; Mariconda, A.; Rosano, C.; Saturnino, C.; El-Kashef, H.; Longo, P. Metal complexes with Schiff bases: Data collection and recent studies on biological activities. Int. J. Mol. Sci. 2022, 23, 14840. [Google Scholar] [CrossRef]
- Huang, M.; Li, Y.; Lan, X.-B.; Liu, J.; Zhao, C.; Liu, Y.; Ke, Z. Ruthenium(II) complexes with N-heterocyclic carbene-phosphine ligands for the N-alkylation of amines with alcohols. Org. Biomol. Chem. 2021, 19, 3451–3461. [Google Scholar] [CrossRef] [PubMed]
- Sathiya Kamatchi, T.; Mohamed Subarkhan, M.K.; Ramesh, R.; Wang, H.; Małecki, J.G. Investigation into antiproliferative activity and apoptosis mechanism of new arene Ru(II) carbazole-based hydrazone complexes. Dalton Trans. 2020, 49, 11385–11395. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, J.; Bilal, M.; Rasool, N.; Hafeez, U.; Adnan Ali Shah, S.; Imran, S.; Amiruddin Zakaria, Z. Synthesis of ruthenium complexes and their catalytic applications: A review. Arab. J. Chem. 2022, 15, 104165. [Google Scholar] [CrossRef]
- Ritleng, V.; Michon, C. Bidentate donor-functionalized N-heterocyclic carbenes: Valuable ligands for ruthenium-catalyzed transfer hydrogenation. Molecules 2022, 27, 4703. [Google Scholar] [CrossRef]
- Kaikhosravi, M.; Böth, A.D.; Sauer, M.J.; Reich, R.M.; Kühn, F.E. Synthesis and characterisation of a heterobimetallic N-heterocyclic carbene rhodium ruthenium complex as catalyst for transfer hydrogenation. J. Organomet. Chem. 2022, 979, 122498. [Google Scholar] [CrossRef]
- Ogba, O.M.; Warner, N.C.; O’Leary, D.J.; Grubbs, R.H. Recent advances in ruthenium-based olefin metathesis. Chem. Soc. Rev. 2018, 47, 4510–4544. [Google Scholar] [CrossRef]
- Malan, F.P.; Singleton, E.; van Rooyen, P.H.; Albrecht, M.; Landman, M. Synthesis, Stability, and (De)hydrogenation Catalysis by Normal and Abnormal Alkene- and Picolyl-Tethered NHC Ruthenium Complexes. Organometallics 2019, 38, 2624–2635. [Google Scholar] [CrossRef]
- Moutaoukil, Z.; Serrano-Díez, E.; Collado, I.G.; Jiménez-Tenorio, M.; Botubol-Ares, J.M. N-Alkylation of organonitrogen compounds catalyzed by methylene-linked bis-NHC half-sandwich ruthenium complexes. Org. Mol. Chem. 2022, 20, 831–839. [Google Scholar] [CrossRef] [PubMed]
- McDarmont, S.L.; Jones, M.H.; McMillen, C.D.; Smith, E.C.; Pienkos, J.A.; Joslin, E.E. Synthesis, characterization, X-ray crystallography analysis and cell viability study of (η6-p-cymene)Ru(NH2R)X2 (X = Cl, Br) derivatives. Polyhedron 2021, 200, 115130. [Google Scholar] [CrossRef]
- Böth, A.D.; Sauer, M.J.; Baratta, W.; Kühn, F.E. Abnormal NHC ruthenium catalysts: Mechanistic investigations of their preparation and steric influence on catalytic performance. Catal. Sci.Technol. 2022, 12, 5597–5603. [Google Scholar] [CrossRef]
- Pardatscher, L.; Hofmann, B.J.; Fischer, P.J.; Hölzl, S.M.; Reich, R.M.; Kühn, F.E.; Baratta, W. Highly efficient abnormal NHC ruthenium catalyst for oppenauer-type oxidation and transfer hydrogenation reactions. ACS Catal. 2019, 9, 11302–11306. [Google Scholar] [CrossRef]
- Quintin, F.; Pinaud, J.; Lamaty, F.; Bantreil, X. Mechanosynthesis of Noels-type NHC–ruthenium complexes and applications in ring-opening metathesis polymerization. Organometallics 2020, 39, 636–639. [Google Scholar] [CrossRef]
- Tolbatov, I.; Marrone, A. Reactivity of N-heterocyclic carbene half-sandwich Ru-, Os-, Rh-, and Ir-based complexes with cysteine and selenocysteine: A computational study. Inorg. Chem. 2022, 61, 746–754. [Google Scholar] [CrossRef]
- Patil, S.A.; Hoagland, A.P.; Patil, S.A.; Bugarin, A. N-heterocyclic carbene-metal complexes as bio-organometallic antimicrobial and anticancer drugs, an update (2015–2020). Future Med. Chem. 2020, 12, 2239–2275. [Google Scholar]
- Jalal, M.; Hammouti, B.; Touzani, R.; Aouniti, A.; Ozdemir, I. Metal-NHC heterocycle complexes in catalysis and biological applications: Systematic review. Mater. Today Proc. 2020, 31, S122–S129. [Google Scholar] [CrossRef]
- Roos, L.; Malan, F.P.; Landman, M. Naphthalimide-NHC complexes: Synthesis and properties in catalytic, biological and photophysical applications. Coord. Chem. Rev. 2021, 449, 214201. [Google Scholar] [CrossRef]
- Oehninger, L.; Rubbiani, R.; Ott, I. N-Heterocyclic carbene metal complexes in medicinal chemistry. Dalton Trans. 2013, 42, 3269–3284. [Google Scholar] [CrossRef] [PubMed]
- Ott, I. Metal N-heterocyclic carbene complexes in medicinal chemistry. In Advances in Inorganic Chemistry; Sadler, P.J., Van Eldik, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 75, pp. 121–148. [Google Scholar]
- Hussaini, S.Y.; Haque, R.A.; Razali, M.R. Recent progress in silver(I)-, gold(I)/(III)- and palladium(II)-N-heterocyclic carbene complexes: A review towards biological perspectives. J.Organomet. Chem. 2019, 882, 96–111. [Google Scholar] [CrossRef]
- Nomiya, K.; Morozumi, S.; Yanagawa, Y.; Hasegawa, M.; Kurose, K.; Taguchi, K.; Sakamoto, R.; Mihara, K.; Kasuga, N.C. Syntheses, structures, and antimicrobial activities of gold(I)– and copper(I)–N-heterocyclic carbene (NHC) complexes derived from basket-shaped dinuclear Ag(I)–NHC complex. Inorg. Chem. 2018, 57, 11322–11332. [Google Scholar] [CrossRef]
- Prencipe, F.; Zanfardino, A.; Di Napoli, M.; Rossi, F.; D’Errico, S.; Piccialli, G.; Mangiatordi, G.F.; Saviano, M.; Ronga, L.; Varcamonti, M.; et al. Silver (I) N-Heterocyclic carbene complexes: A winning and broad spectrum of antimicrobial properties. Int. J. Mol. Sci. 2021, 22, 2497. [Google Scholar] [CrossRef]
- Liang, J.; Sun, D.; Yang, Y.; Li, M.; Li, H.; Chen, L. Discovery of metal-based complexes as promising antimicrobial agents. Eur. J. Med. Chem. 2021, 224, 113696. [Google Scholar] [CrossRef] [PubMed]
- Karaca, Ö.; Meier-Menches, S.M.; Casini, A.; Kühn, F.E. On the binding modes of metal NHC complexes with DNA secondary structures: Implications for therapy and imaging. Chem. Commun. 2017, 53, 8249–8260. [Google Scholar] [CrossRef]
- Jakob, C.H.G.; Muñoz, A.W.; Schlagintweit, J.F.; Weiß, V.; Reich, R.M.; Sieber, S.A.; Correia, J.D.G.; Kühn, F.E. Anticancer and antibacterial properties of trinuclear Cu(I), Ag(I) and Au(I) macrocyclic NHC/urea complexes. J. Organomet. Chem. 2021, 932, 121643. [Google Scholar] [CrossRef]
- Rufino-Felipe, E.; Colorado-Peralta, R.; Reyes-Márquez, V.; Valdés, H.; Morales-Morales, D. Fluorinated-NHC transition metal complexes: Leading characters as potential anticancer metallodrugs. Anti-Cancer Agents Med. Chem. 2021, 21, 938–948. [Google Scholar] [CrossRef]
- Zou, T.; Lok, C.-N.; Wan, P.-K.; Zhang, Z.-F.; Fung, S.-K.; Che, C.-M. Anticancer metal-N-heterocyclic carbene complexes of gold, platinum and palladium. Curr. Opin. Chem. Biol. 2018, 43, 30–36. [Google Scholar] [CrossRef]
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent advances in gold–NHC complexes with biological properties. Chem. Soc. Rev. 2019, 48, 447–462. [Google Scholar] [CrossRef]
- Porchia, M.; Pellei, M.; Marinelli, M.; Tisato, F.; Del Bello, F.; Santini, C. New insights in Au-NHCs complexes as anticancer agents. Eur. J. Med. Chem. 2018, 146, 709–746. [Google Scholar] [CrossRef]
- Tialiou, A.; Chin, J.; Keppler, B.K.; Reithofer, M.R. Current developments of N-heterocyclic carbene Au(I)/Au(III) complexes toward cancer treatment. Biomedicines 2022, 10, 1417. [Google Scholar] [CrossRef]
- Touj, N.; Nasr, I.S.A.; Koko, W.S.; Khan, T.A.; Özdemir, I.; Yasar, S.; Mansour, L.; Alresheedi, F.; Hamdi, N. Anticancer, antimicrobial and antiparasitical activities of copper(I) complexes based on N-heterocyclic carbene (NHC) ligands bearing aryl substituents. J. Coord. Chem. 2020, 73, 2889–2905. [Google Scholar] [CrossRef]
- Habib, A.; Iqbal, M.A.; Bhatti, H.N.; Kamal, A.; Kamal, S. Synthesis of alkyl/aryl linked binuclear silver(I)-N-heterocyclic carbene complexes and evaluation of their antimicrobial, hemolytic and thrombolytic potential. Inorg. Chem. Commun. 2020, 111, 107670. [Google Scholar] [CrossRef]
- Kamal, A.; Iqbal, M.A.; Bhatti, H.N.; Ghaffar, A. Selenium-N-heterocyclic carbene (Se-NHC) complexes with higher aromaticity inhibit microbes: Synthesis, structure, and biological potential. J. Coord. Chem. 2022, 75, 1915–1928. [Google Scholar] [CrossRef]
- Ielo, I.; Iacopetta, D.; Saturnino, C.; Longo, P.; Galletta, M.; Drommi, D.; Rosace, G.; Sinicropi, M.S.; Plutino, M.R. Gold derivatives development as prospective anticancer drugs for breast cancer treatment. Appl. Sci. 2021, 11, 2089. [Google Scholar] [CrossRef]
- Ceramella, J.; Mariconda, A.; Sirignano, M.; Iacopetta, D.; Rosano, C.; Catalano, A.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Novel Au carbene complexes as promising multi-target agents in breast cancer treatment. Pharmaceuticals 2022, 15, 507. [Google Scholar] [CrossRef]
- Massai, L.; Messori, L.; Carpentieri, A.; Amoresano, A.; Melchiorre, C.; Fiaschi, T.; Modesti, A.; Gamberi, T.; Magherini, F. The effects of two gold-N-heterocyclic carbene (NHC) complexes in ovarian cancer cells: A redox proteomic study. Cancer Chemother. Pharmacol. 2022, 89, 809–823. [Google Scholar] [CrossRef]
- Md Zin, N.F.H.; Ooi, S.Y.S.; Khor, B.-K.; Chear, N.J.-Y.; Tang, W.K.; Siu, C.-K.; Razali, M.R.; Haque, R.A.; Yam, W. Cytotoxicity of asymmetric mononuclear silver(I)-N-heterocyclic carbene complexes against human cervical cancer: Synthesis, crystal structure, DFT calculations and effect of substituents. J. Organomet. Chem. 2022, 976, 122439. [Google Scholar] [CrossRef]
- Mariconda, A.; Iacopetta, D.; Sirignano, M.; Ceramella, J.; Costabile, C.; Pellegrino, M.; Rosano, C.; Catalano, A.; Saturnino, C.; El-Kashef, H.; et al. N-Heterocyclic carbene (NHC) silver complexes as versatile chemotherapeutic agents targeting human topoisomerases and actin. ChemMedChem 2022, 17, e202200345. [Google Scholar] [CrossRef]
- Johnson, N.A.; Southerland, M.R.; Youngs, W.J. Recent developments in the medicinal applications of silver-NHC complexes and imidazolium salts. Molecules 2017, 22, 1263. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Ceramella, J.; Rosano, C.; Mariconda, A.; Pellegrino, M.; Sirignano, M.; Saturnino, C.; Catalano, A.; Aquaro, S.; Longo, P.; et al. N-Heterocyclic Carbene-Gold(I) Complexes Targeting Actin Polymerization. Appl. Sci. 2021, 11, 5626. [Google Scholar] [CrossRef]
- Iacopetta, D.; Rosano, C.; Sirignano, M.; Mariconda, A.; Ceramella, J.; Ponassi, M.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Is the way to fight cancer paved with gold? Metal-based carbene complexes with multiple and fascinating biological features. Pharmaceuticals 2020, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Mariconda, A.; Saturnino, C.; Caruso, A.; Palma, G.; Ceramella, J.; Muià, N.; Perri, M.; Sinicropi, M.S.; Caroleo, M.C.; et al. Novel gold and silver carbene complexes exert antitumor effects triggering the reactive oxygen species dependent intrinsic apoptotic pathway. ChemMedChem 2017, 12, 2054–2065. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Pellegrino, M.; Mariconda, A.; Longo, P.; Sinicropi, M.S.; Aquaro, S. COVID-19 at a glance: An up-to-date overview on variants, drug design and therapies. Viruses 2022, 14, 573. [Google Scholar] [CrossRef]
- Gil-Moles, M.; Türck, S.; Basu, U.; Pettenuzzo, A.; Bhattacharya, S.; Rajan, A.; Ma, X.; Büssing, R.; Wölker, J.; Burmeister, H.; et al. Metallodrug profiling against SARS-CoV-2 target proteins identifies highly potent inhibitors of the S/ACE2 interaction and the Papain-like Protease PLpro. Chem.—Eur. J. 2021, 27, 17928–17940. [Google Scholar] [CrossRef]
- Aktas, A.; Barut Celepci, D.; Gok, Y.; Taslimi, P.; Akincioglu, H.; Gulcin, İ. A novel Ag-N-heterocyclic carbene complex bearing the hydroxyethyl ligand: Synthesis, characterization, crystal and spectral structures and bioactivity properties. Crystals 2020, 10, 171. [Google Scholar] [CrossRef]
- Grisi, F.; Costabile, C.; Gallo, E.; Mariconda, A.; Tedesco, C.; Longo, P. Ruthenium-based complexes bearing saturated chiral N-heterocyclic carbene ligands: Dynamic behavior and catalysis. Organometallics 2008, 27, 4649–4656. [Google Scholar] [CrossRef]
- Perfetto, A.; Costabile, C.; Longo, P.; Grisi, F. Ruthenium olefin metathesis catalysts with frozen NHC ligand conformations. Organometallics 2014, 33, 2747–2759. [Google Scholar] [CrossRef]
- Lam, N.Y.S.; Truong, D.; Burmeister, H.; Babak, M.V.; Holtkamp, H.U.; Movassaghi, S.; Ayine-Tora, D.M.; Zafar, A.; Kubanik, M.; Oehninger, L.; et al. From catalysis to cancer: Toward structure–activity relationships for benzimidazol-2-ylidene-derived N-heterocyclic-carbene complexes as anticancer agents. Inorg. Chem. 2018, 57, 14427–14434. [Google Scholar] [CrossRef]
- Tabrizi, L.; Olasunkanmi, L.O.; Fadare, O.A. Experimental and theoretical investigations of cyclometalated ruthenium(ii) complex containing CCC-pincer and anti-inflammatory drugs as ligands: Synthesis, characterization, inhibition of cyclooxygenase and in vitro cytotoxicity activities in various cancer cell lines. Dalton Trans. 2019, 48, 728–740. [Google Scholar] [PubMed]
- Rana, B.K.; Roymahapatra, G.; Das, H.S.; Giri, S.; Cardoso, M.H.; Franco, O.L.; Kiran, N.K.; Santra, M.K.; Bag, P.P.; Bertolasi, V.; et al. Pyridine and pyrimidine functionalized half-sandwich Ru(II)-N heterocyclic carbene complexes: Synthesis, structures, spectra, electrochemistry and biological studies. J. Mol. Struct. 2021, 1231, 129822. [Google Scholar] [CrossRef]
- Rana, B.K.; Das, H.S.; Giri, S.; Roymahapatra, G.; Mahapatra, P.K.; Dinda, J.; Cardoso, M.H.; Franco, O.L.; Bag, P.P. A comparative analysis of Ru(II) complexes containing non-annulated and annulated n-heterocyclic carbene ligand towards structure, spectra, electrochemistry and biological activity. J. Indian Chem. Soc. 2020, 97, 2699–2712. [Google Scholar]
- Rodríguez-Prieto, T.; Michlewska, S.; Hołota, M.; Ionov, M.; de la Mata, F.J.; Cano, J.; Bryszewska, M.; Gómez, R. Organometallic dendrimers based on ruthenium(II) N-heterocyclic carbenes and their implication as delivery systems of anticancer small interfering RNA. J. Inorg. Biochem. 2021, 223, 111540. [Google Scholar] [CrossRef]
- Marzano, C.; Sbovata, S.M.; Gandin, V.; Michelin, R.A.; Venzo, A.; Bertani, R.; Seraglia, R. Cytotoxicity of cis-platinum(II) cycloaliphatic amidine complexes: Ring size and solvent effects on the biological activity. J. Inorg. Biochem. 2009, 103, 1113–1119. [Google Scholar] [CrossRef]
- Paşahan, R.; Akkoç, M.; Yaşar, Ş.; Köprülü, T.; Tekin, Ş.; Yaşar, S.; Özdemir, I. Synthesis and investigation of antiproliferative activity of Ru-NHC complexes against C6 and HeLa cancer cells. Turk. J. Chem. 2022, 46, 1097–1109. [Google Scholar] [CrossRef]
- Chen, C.; Xu, C.; Li, T.; Lu, S.; Luo, F.; Wang, H. Novel NHC-coordinated ruthenium (II) arene complexes achieve synergistic efficacy as safe and effective anticancer therapeutics. Eur. J. Med. Chem. 2020, 203, 112605. [Google Scholar] [CrossRef]
- Sarı, Y.; Gürses, C.; Celepci, D.B.; Keleştemur, Ü.; Aktaş, A.; Yüksel, Ş.; Ateş, B.; Gök, Y. 4-Vinylbenzyl and 2-morpholinoethyl substituted ruthenium(II) complexes: Design, synthesis, and biological evaluation. J. Mol. Struct. 2020, 1202, 127355. [Google Scholar] [CrossRef]
- Wilke, N.L.; Burmeister, H.; Frias, C.; Ott, I.; Prokop, A. Ruthenium complex HB324 induces apoptosis via mitochondrial pathway with an upregulation of Harakiri and overcomes cisplatin resistance in neuroblastoma cells in vitro. Int. J. Mol. Sci. 2023, 24, 952. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug resistance (MDR): A widespread phenomenon in pharmacological therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef]
- Li, F.; Collins, J.G.; Keene, F.R. Ruthenium complexes as antimicrobial agents. Chem. Soc. Rev. 2015, 44, 2529–2542. [Google Scholar] [CrossRef]
- Southam, H.M.; Butler, J.A.; Chapman, J.A.; Poole, R.K. Chapter One—The Microbiology of Ruthenium Complexes. In Advances in Microbial Physiology; Poole, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 71, pp. 1–96. [Google Scholar]
- Roymahapatra, G.; Dinda, J.; Mishra, A.; Mahapatra, A.; Hwang, W.S.; Mandal, S.M. Cytotoxic potency of self-assembled Ruthenium(II)-NHC complexes with pincer type 2,6-bis(N-methylimidazolylidene/benzimidazolylidene)pyrazine ligands. J. Cancer Res. Ther. 2015, 11, 105–113. [Google Scholar]
- Streciwilk, W.; Terenzi, A.; Cheng, X.; Hager, L.; Dabiri, Y.; Prochnow, P.; Bandow, J.E.; Wölfl, S.; Keppler, B.K.; Ott, I. Fluorescent organometallic rhodium(I) and ruthenium(II) metallodrugs with 4-ethylthio-1,8-naphthalimide ligands: Antiproliferative effects, cellular uptake and DNA-interaction. Eur. J. Med. Chem. 2018, 156, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, Y.; Schmid, A.; Theobald, J.; Blagojevic, B.; Streciwilk, W.; Ott, I.; Wölfl, S.; Cheng, X. A ruthenium(II) N-heterocyclic carbene (NHC) complex with naphthalimide ligand triggers apoptosis in colorectal cancer cells via activating the ROS-p38 MAPK pathway. Int. J. Mol. Sci. 2018, 19, 3964. [Google Scholar] [CrossRef]
- Streciwilk, W.; Terenzi, A.; Lo Nardo, F.; Prochnow, P.; Bandow, J.E.; Keppler, B.K.; Ott, I. Synthesis and biological evaluation of organometallic complexes bearing bis-1,8-naphthalimide ligands. Eur.J. Inorg. Chem. 2018, 2018, 3104–3112. [Google Scholar] [CrossRef]
- Boubakri, L.; Chakchouk-Mtibaa, A.; Al-Ayed, A.S.; Mansour, L.; Abutaha, N.; Harrath, A.H.; Mellouli, L.; Özdemir, I.; Yasar, S.; Hamdi, N. Ru(II)–N-heterocyclic carbene complexes: Synthesis, characterization, transfer hydrogenation reactions and biological determination. RSC Adv. 2019, 9, 34406–34420. [Google Scholar] [CrossRef] [PubMed]
- Boubakri, L.; Chakchouk-Mtiba, A.; Naouali, O.; Mellouli, L.; Mansour, L.; Özdemir, I.; Yaser, S.; Sauthier, M.; Hamdi, N. Ruthenium(II) complexes bearing benzimidazole-based N-heterocyclic carbene (NHC) ligands as potential antimicrobial, antioxidant, enzyme inhibition, and antiproliferative agents. J. Coord. Chem. 2022, 75, 645–667. [Google Scholar] [CrossRef]
- Onar, G.; Gürses, C.; Karataş, M.O.; Balcıoğlu, S.; Akbay, N.; Özdemir, N.; Ateş, B.; Alıcı, B. Palladium(II) and ruthenium(II) complexes of benzotriazole functionalized N-heterocyclic carbenes: Cytotoxicity, antimicrobial, and DNA interaction studies. J. Organomet. Chem. 2019, 886, 48–56. [Google Scholar] [CrossRef]
- Slimani, I.; Chakchouk-Mtibaa, A.; Mansour, L.; Mellouli, L.; Özdemir, I.; Gürbüzd, N.; Hamdi, N. Synthesis, characterization, biological determination and catalytic evaluation of ruthenium(II) complexes bearing benzimidazole-based NHC ligands in transfer hydrogenation catalysis. New J. Chem. 2020, 44, 5309–5323. [Google Scholar] [CrossRef]
- Burmeister, H.; Dietze, P.; Preu, L.; Bandow, J.E.; Ott, I. Evaluation of ruthenium(II) N-heterocyclic carbene complexes as antibacterial agents and inhibitors of bacterial thioredoxin reductase. Molecules 2021, 26, 4282. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).