Induction of Endogenous Antimicrobial Peptides to Prevent or Treat Oral Infection and Inflammation
Abstract
1. Introduction
2. AMPs in the Oral Cavity
2.1. AMPs Expressed in Oral Tissues
2.2. AMPs in Oral Secretions
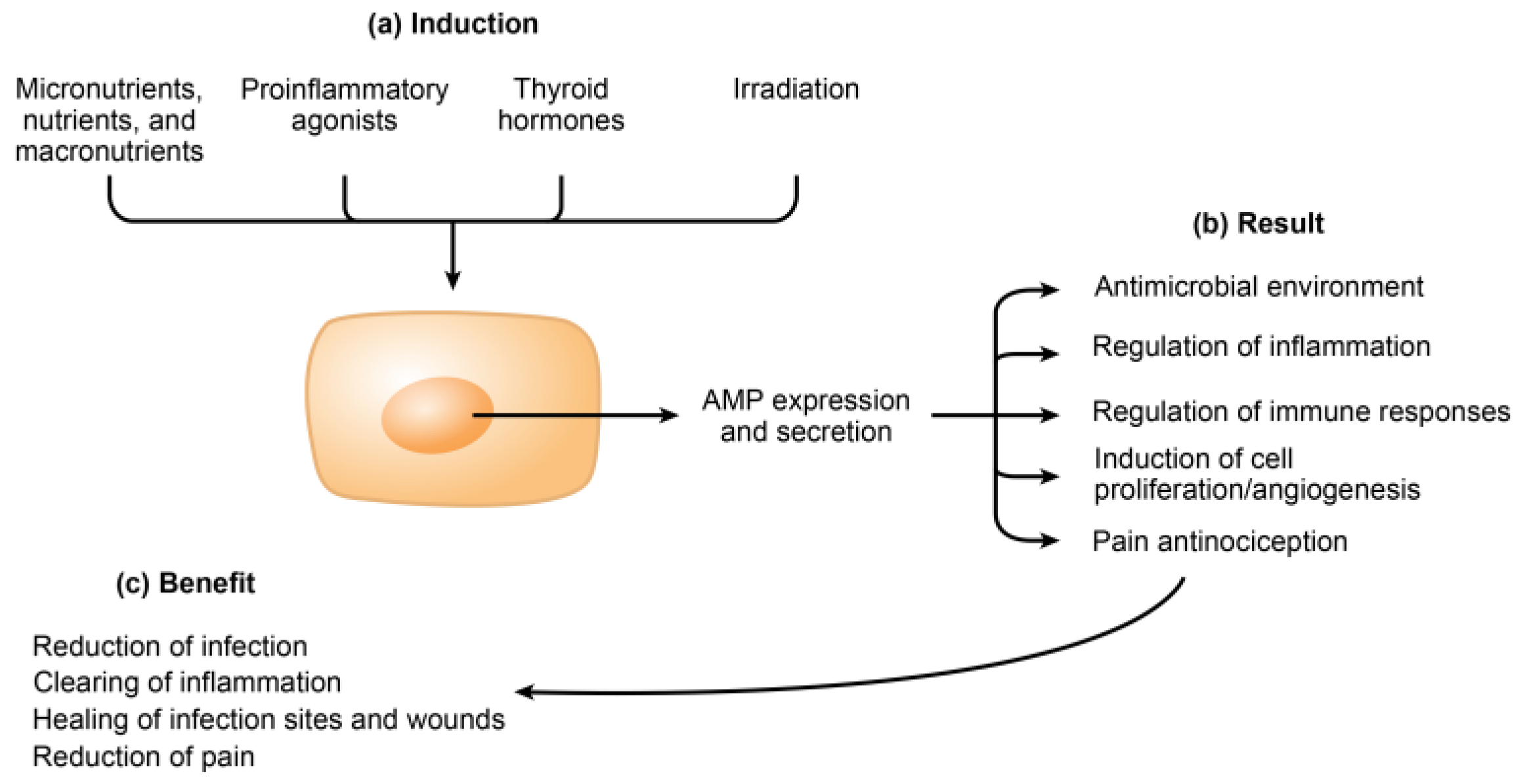
3. Induction of Endogenous AMPs
3.1. Micronutrients
3.1.1. Trace Elements
| Family of Inducers | Inducer of AMP Transcription or Expression | References |
|---|---|---|
| Micronutrients | ||
| Trace elements | Selenium | [46] |
| Zinc | [47] | |
| Copper | [48,49] | |
| Iron | [50] | |
| Elements | Calcium | [51] |
| Vitamins | A (retinoic acid) | [52,53] |
| B3 (niacin) | [54,55] | |
| C (ascorbic acid) | [56] | |
| D, D3, and calcitriol (1,25(OH)2D3) | [57,58,59,60,61,62,63,64,65,66,67,68] | |
| E (alpha-tocopherol) | [69] | |
| Nutrients and macronutrients | ||
| Mono-, di-, and polysaccharides | Glucose | [56,70,71] |
| Lactose | [72] | |
| β-glucans | [73] | |
| Amino acids, pyroglutamyl peptides, and proteins | Arginine | [74] |
| Isoleucine | [74,75,76,77] | |
| Pyroglutamyl peptides, pyroglutamyl dipeptides, and pyroglutamyl polypeptides | [78,79] | |
| Bovine serum albumin (BSA) | [74] | |
| Free fatty acids (FFA) and histone deacetylase (HDAC) inhibitors | Short FFA (≤5 carbons) including butyrate and phenylbutyrate | [15,57,72,80,81,82,83,84,85,86,87,88,89,90,91,92] |
| Medium FFA (6–11 carbons) including hexanoate and heptanoate | [15] | |
| Long FFA (≥12 carbons) including laurate, palmitate, and oleate | [15,93] | |
| Proinflammatory agonists | ||
| Pam3csk4 peptide (Toll-like receptor (TLR) 2) | [61] | |
| Lipopolysaccharide (LPS) (TLR4) | [23,26,94,95,96] | |
| CpG (TLR9) | [97] | |
| IL-1β | [23,98] | |
| TNF-α | [95,96,98] | |
| IFN-γ | [98] | |
| Hormones | ||
| Triiodothyronine (T3) | [86] | |
| Thyroxine (T4) | [86] | |
| Irradiation | ||
| Ultraviolet | Ultraviolet C, 100–280 nm | [56] |
| Ultraviolet B, 280–315 nm | [94,95,96,99,100,101,102] | |
| Ultraviolet A, 340–400 nm | [56] | |
| Red light | Laser, 625 nm | [103] |
| Near infrared | Laser, 810 nm | [104] |
3.1.2. Elements
3.1.3. Vitamins
3.2. Nutrients and Macronutrients
3.2.1. Mono-, Di-, and Polysaccharides
3.2.2. Amino Acids, Pyroglutamyl Peptides, and Proteins
3.2.3. Free Fatty Acids
3.2.4. Foodstuffs
3.3. Proinflammatory Agonists
3.4. Thyroid Hormones
3.5. Irradiation
3.5.1. UVC
3.5.2. UVB
3.5.3. UVA
3.5.4. Red Light
3.5.5. Near-Infrared Irradiation (NIR)
3.6. Synergy among Inducers
4. Roles of AMPs in Inflammation, Immunity, Healing, and Pain
4.1. Roles of AMPs in Inflammation
4.2. Roles of AMPs in Immunity
4.3. Roles of AMPs in Angiogenesis, Vasculogenesis, and Wound Healing
4.4. Roles of AMPs in Pain Nociception
5. Potential Applications of Inducing Endogenous AMPs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmadi, H.; Ebrahimi, A.; Ahmadi, F. Antibiotic therapy in dentistry. Int. J. Dent. 2021, 2021, 6667624. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Peralvo, A.O.; Pena-Cardelles, J.F.; Kewalramani, N.; Garcia-Sanchez, A.; Mateos-Moreno, M.V.; Velasco-Ortega, E.; Ortiz-Garcia, I.; Jimenez-Guerra, A.; Vegh, D.; Pedrinaci, I.; et al. Is antibiotic prophylaxis necessary before dental implant procedures in patients with orthopaedic prostheses? a systematic review. Antibiotics 2022, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chhabra, K.G.; Reche, A.; Madhu, P.P.; Kunghadkar, A.; Kalmegh, S. Antibiotic stewardship program in dentistry: Challenges and opportunities. J. Family Med. Prim. Care 2021, 10, 3951–3955. [Google Scholar] [CrossRef] [PubMed]
- Griffith, A.; Mateen, A.; Markowitz, K.; Singer, S.R.; Cugini, C.; Shimizu, E.; Wiedman, G.R.; Kumar, V. Alternative antibiotics in dentistry: Antimicrobial peptides. Pharmaceutics 2022, 14, 1679. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, T.K. Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef]
- Prado Montes de Oca, E. Antimicrobial peptide elicitors: New hope for the post-antibiotic era. Innate Immun. 2013, 19, 227–241. [Google Scholar] [CrossRef]
- Ramazi, S.; Mohammadi, N.; Allahverdi, A.; Khalili, E.; Abdolmaleki, P. A review on antimicrobial peptides databases and the computational tools. Database 2022, 2022. [Google Scholar] [CrossRef]
- Bowdish, D.M.; Davidson, D.J.; Hancock, R.E. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr. Protein Pept. Sci. 2005, 6, 35–51. [Google Scholar] [CrossRef]
- Alford, M.A.; Baquir, B.; Santana, F.L.; Haney, E.F.; Hancock, R.E.W. Cathelicidin host defense peptides and inflammatory signaling: Striking a balance. Front. Microbiol. 2020, 11, 1902. [Google Scholar] [CrossRef]
- Bergman, P.; Raqib, R.; Rekha, R.S.; Agerberth, B.; Gudmundsson, G.H. Host directed therapy against infection by boosting innate immunity. Front. Immunol. 2020, 11, 1209. [Google Scholar] [CrossRef]
- Wu, J.; Ma, N.; Johnston, L.J.; Ma, X. Dietary nutrients mediate intestinal host defense peptide expression. Adv. Nutr. 2020, 11, 92–102. [Google Scholar] [CrossRef]
- Nishimura, E.; Kato, M.; Hashizume, S. Human beta-defensin-2 induction in human foreskin keratinocyte by synergetic stimulation with foods and Escherichia coli. Cytotechnology 2003, 43, 135–144. [Google Scholar] [CrossRef]
- Campbell, Y.; Fantacone, M.L.; Gombart, A.F. Regulation of antimicrobial peptide gene expression by nutrients and by-products of microbial metabolism. Eur. J. Nutr. 2012, 51, 899–907. [Google Scholar] [CrossRef]
- van der Does, A.M.; Bergman, P.; Agerberth, B.; Lindbom, L. Induction of the human cathelicidin LL-37 as a novel treatment against bacterial infections. J. Leukoc. Biol. 2012, 92, 735–742. [Google Scholar] [CrossRef]
- Jiang, W.; Sunkara, L.T.; Zeng, X.; Deng, Z.; Myers, S.M.; Zhang, G. Differential regulation of human cathelicidin LL-37 by free fatty acids and their analogs. Peptides 2013, 50, 129–138. [Google Scholar] [CrossRef]
- Wang, G. Human antimicrobial peptides and proteins. Pharmaceuticals 2014, 7, 545–594. [Google Scholar] [CrossRef]
- Chen, J.; Zhai, Z.; Long, H.; Yang, G.; Deng, B.; Deng, J. Inducible expression of defensins and cathelicidins by nutrients and associated regulatory mechanisms. Peptides 2020, 123, 170177. [Google Scholar] [CrossRef]
- Morio, K.A.; Sternowski, R.H.; Zeng, E.; Brogden, K.A. Antimicrobial peptides and biomarkers induced by ultraviolet irradiation have the potential to reduce endodontic inflammation and facilitate tissue healing. Pharmaceutics 2022, 14, 1979. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Lu, W. a-Defensins in human innate immunity. Immunol. Rev. 2012, 245, 84–112. [Google Scholar] [CrossRef]
- Niu, J.Y.; Yin, I.X.; Mei, M.L.; Wu, W.K.K.; Li, Q.L.; Chu, C.H. The multifaceted roles of antimicrobial peptides in oral diseases. Mol. Oral Microbiol. 2021, 36, 159–171. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed]
- Gorr, S.U. Antimicrobial peptides of the oral cavity. Periodontol. 2000 2009, 51, 152–180. [Google Scholar] [CrossRef] [PubMed]
- Mathews, M.; Jia, H.P.; Guthmiller, J.M.; Losh, G.; Graham, S.; Johnson, G.K.; Tack, B.F.; McCray, P.B., Jr. Production of beta-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect. Immun. 1999, 67, 2740–2745. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.E.; Harder, J.; Sipos, B.; Maune, S.; Kloppel, G.; Bartels, J.; Schroder, J.M.; Glaser, R. Psoriasin (S100A7) is a principal antimicrobial peptide of the human tongue. Mucosal Immunol. 2008, 1, 239–243. [Google Scholar] [CrossRef]
- Lundy, F.T.; Orr, D.F.; Gallagher, J.R.; Maxwell, P.; Shaw, C.; Napier, S.S.; Gerald Cowan, C.; Lamey, P.J.; Marley, J.J. Identification and overexpression of human neutrophil alpha-defensins (human neutrophil peptides 1, 2 and 3) in squamous cell carcinomas of the human tongue. Oral Oncol. 2004, 40, 139–144. [Google Scholar] [CrossRef]
- Kamekura, R.; Imai, R.; Takano, K.; Yamashita, K.; Jitsukawa, S.; Nagaya, T.; Ito, F.; Hirao, M.; Tsubota, H.; Himi, T. Expression and localization of human efensins in palatine tonsils. Adv. Otorhinolaryngol. 2016, 77, 112–118. [Google Scholar] [CrossRef]
- Sahasrabudhe, K.S.; Kimball, J.R.; Morton, T.H.; Weinberg, A.; Dale, B.A. Expression of the antimicrobial peptide, human beta-defensin 1, in duct cells of minor salivary glands and detection in saliva. J. Dent. Res. 2000, 79, 1669–1674. [Google Scholar] [CrossRef]
- Dunsche, A.; Acil, Y.; Dommisch, H.; Siebert, R.; Schroder, J.M.; Jepsen, S. The novel human beta-defensin-3 is widely expressed in oral tissues. Eur. J. Oral Sci. 2002, 110, 121–124. [Google Scholar] [CrossRef]
- Woo, J.S.; Jeong, J.Y.; Hwang, Y.J.; Chae, S.W.; Hwang, S.J.; Lee, H.M. Expression of cathelicidin in human salivary glands. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 211–214. [Google Scholar] [CrossRef]
- Dale, B.A.; Kimball, J.R.; Krisanaprakornkit, S.; Roberts, F.; Robinovitch, M.; O’Neal, R.; Valore, E.V.; Ganz, T.; Anderson, G.M.; Weinberg, A. Localized antimicrobial peptide expression in human gingiva. J. Periodontal Res. 2001, 36, 285–294. [Google Scholar] [CrossRef]
- Dommisch, H.; Winter, J.; Acil, Y.; Dunsche, A.; Tiemann, M.; Jepsen, S. Human beta-defensin (hBD-1, -2) expression in dental pulp. Oral Microbiol. Immunol. 2005, 20, 163–166. [Google Scholar] [CrossRef]
- Ghattas Ayoub, C.; Aminoshariae, A.; Bakkar, M.; Ghosh, S.; Bonfield, T.; Demko, C.; Montagnese, T.A.; Mickel, A.K. Comparison of IL-1beta, TNF-alpha, hBD-2, and hBD-3 expression in the dental pulp of smokers versus nonsmokers. J. Endod. 2017, 43, 2009–2013. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, Y.; Rao, N.; Li, J.; Li, X.; Fang, T.; Zhao, Y.; Ge, L. Activation and biological properties of human beta defensin 4 in stem cells derived From human exfoliated deciduous teeth. Front. Physiol. 2019, 10, 1304. [Google Scholar] [CrossRef]
- Loureiro, C.; Buzalaf, M.A.R.; Pessan, J.P.; Ventura, T.M.O.; Pela, V.T.; Ribeiro, A.P.F.; Jacinto, R.C. Proteomic analysis of infected root canals with apical periodontitis in patients with type 2 diabetes mellitus: A cross-sectional study. Int. Endod. J. 2022, 55, 910–922. [Google Scholar] [CrossRef]
- Loo, J.A.; Yan, W.; Ramachandran, P.; Wong, D.T. Comparative human salivary and plasma proteomes. J. Dent. Res. 2010, 89, 1016–1023. [Google Scholar] [CrossRef]
- Lau, W.W.; Hardt, M.; Zhang, Y.H.; Freire, M.; Ruhl, S. The human salivary proteome wiki: A community-driven research platform. J. Dent. Res. 2021, 100, 1510–1519. [Google Scholar] [CrossRef]
- Waniczek, D.; Swietochowska, E.; Snietura, M.; Kiczmer, P.; Lorenc, Z.; Muc-Wierzgon, M. Salivary concentrations of chemerin, alpha-defensin 1, and TNF-alpha as potential biomarkers in the early diagnosis of colorectal cancer. Metabolites 2022, 12, 704. [Google Scholar] [CrossRef]
- Pei, F.; Wang, M.; Wang, Y.; Pan, X.; Cen, X.; Huang, X.; Jin, Y.; Zhao, Z. Quantitative proteomic analysis of gingival crevicular fluids to identify novel biomarkers of gingival recession in orthodontic patients. J. Proteom. 2022, 266, 104647. [Google Scholar] [CrossRef]
- Kido, J.; Bando, M.; Hiroshima, Y.; Iwasaka, H.; Yamada, K.; Ohgami, N.; Nambu, T.; Kataoka, M.; Yamamoto, T.; Shinohara, Y.; et al. Analysis of proteins in human gingival crevicular fluid by mass spectrometry. J. Periodontal Res. 2012, 47, 488–499. [Google Scholar] [CrossRef]
- Tao, R.; Jurevic, R.J.; Coulton, K.K.; Tsutsui, M.T.; Roberts, M.C.; Kimball, J.R.; Wells, N.; Berndt, J.; Dale, B.A. Salivary antimicrobial peptide expression and dental caries experience in children. Antimicrob. Agents Chemother. 2005, 49, 3883–3888. [Google Scholar] [CrossRef]
- Dale, B.A.; Tao, R.; Kimball, J.R.; Jurevic, R.J. Oral antimicrobial peptides and biological control of caries. BMC Oral Health 2006, 6, S13. [Google Scholar] [CrossRef] [PubMed]
- Maggini, S.; Wintergerst, E.S.; Beveridge, S.; Hornig, D.H. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br. J. Nutr. 2007, 98 (Suppl. S1), S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Contribution of selected vitamins and trace elements to immune function. Ann. Nutr. Metab. 2007, 51, 301–323. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, H. Mini-review on the roles of vitamin C, vitamin D, and selenium in the immune system against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef]
- Haase, H.; Rink, L. Signal transduction in monocytes: The role of zinc ions. Biometals 2007, 20, 579–585. [Google Scholar] [CrossRef]
- Xu, S.Z.; Lee, S.H.; Lillehoj, H.S.; Bravo, D. Dietary sodium selenite affects host intestinal and systemic immune response and disease susceptibility to necrotic enteritis in commercial broilers. Br. Poult. Sci. 2015, 56, 103–112. [Google Scholar] [CrossRef]
- Talukder, P.; Satho, T.; Irie, K.; Sharmin, T.; Hamady, D.; Nakashima, Y.; Kashige, N.; Miake, F. Trace metal zinc stimulates secretion of antimicrobial peptide LL-37 from Caco-2 cells through ERK and p38 MAP kinase. Int. Immunopharmacol. 2011, 11, 141–144. [Google Scholar] [CrossRef]
- Portelinha, J.; Duay, S.S.; Yu, S.I.; Heilemann, K.; Libardo, M.D.J.; Juliano, S.A.; Klassen, J.L.; Angeles-Boza, A.M. Antimicrobial peptides and copper(II) ions: Novel therapeutic opportunities. Chem. Rev. 2021, 121, 2648–2712. [Google Scholar] [CrossRef]
- Agbale, C.M.; Sarfo, J.K.; Galyuon, I.K.; Juliano, S.A.; Silva, G.G.O.; Buccini, D.F.; Cardoso, M.H.; Torres, M.D.T.; Angeles-Boza, A.M.; de la Fuente-Nunez, C.; et al. Antimicrobial and antibiofilm activities of helical antimicrobial peptide sequences incorporating metal-binding motifs. Biochemistry 2019, 58, 3802–3812. [Google Scholar] [CrossRef]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef]
- Pernet, I.; Reymermier, C.; Guezennec, A.; Branka, J.E.; Guesnet, J.; Perrier, E.; Dezutter-Dambuyant, C.; Schmitt, D.; Viac, J. Calcium triggers beta-defensin (hBD-2 and hBD-3) and chemokine macrophage inflammatory protein-3 alpha (MIP-3alpha/CCL20) expression in monolayers of activated human keratinocytes. Exp. Dermatol. 2003, 12, 755–760. [Google Scholar] [CrossRef]
- Jacobo-Delgado, Y.M.; Torres-Juarez, F.; Rodriguez-Carlos, A.; Santos-Mena, A.; Enciso-Moreno, J.E.; Rivas-Santiago, C.; Diamond, G.; Rivas-Santiago, B. Retinoic acid induces antimicrobial peptides and cytokines leading to Mycobacterium tuberculosis elimination in airway epithelial cells. Peptides 2021, 142, 170580. [Google Scholar] [CrossRef]
- Lee, S.E.; Lee, J.S.; Kim, M.R.; Kim, M.Y.; Kim, S.C. Topical retinoids induce beta-defensin 3 expression in mouse skin. Int. J. Dermatol. 2010, 49, 1082–1084. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Zhen, R.; Wang, L.; Feng, J.; Xie, Y.; Yang, B.; Xiong, Y.; Niu, J.; Wu, Q.; et al. Effects of niacin on intestinal epithelial barrier, intestinal immunity, and microbial community in weaned piglets challenged by PDCoV. Int. Immunopharmacol. 2022, 111, 109054. [Google Scholar] [CrossRef]
- Zhen, R.; Feng, J.; He, D.; Chen, Y.; Chen, T.; Cai, W.; Xiong, Y.; Qiu, Y.; Jiang, Z.; Wang, L.; et al. Effects of niacin on resistance to enterotoxigenic Escherichia coli infection in weaned piglets. Front. Nutr. 2022, 9, 865311. [Google Scholar] [CrossRef]
- Cruz Diaz, L.A.; Flores Miramontes, M.G.; Chavez Hurtado, P.; Allen, K.; Gonzalez Avila, M.; Prado Montes de Oca, E. Ascorbic acid, ultraviolet C rays, and glucose but not hyperthermia are elicitors of human beta-defensin 1 mRNA in normal keratinocytes. Biomed. Res. Int. 2015, 2015, 714580. [Google Scholar] [CrossRef]
- Robinson, K.; Ma, X.; Liu, Y.; Qiao, S.; Hou, Y.; Zhang, G. Dietary modulation of endogenous host defense peptide synthesis as an alternative approach to in-feed antibiotics. Anim. Nutr. 2018, 4, 160–169. [Google Scholar] [CrossRef]
- Strandberg, K.L.; Richards, S.M.; Gunn, J.S. Cathelicidin antimicrobial peptide expression is not induced or required for bacterial clearance during Salmonella enterica infection of human monocyte-derived macrophages. Infect. Immun. 2012, 80, 3930–3938. [Google Scholar] [CrossRef]
- Martineau, A.R.; Wilkinson, K.A.; Newton, S.M.; Floto, R.A.; Norman, A.W.; Skolimowska, K.; Davidson, R.N.; Sorensen, O.E.; Kampmann, B.; Griffiths, C.J.; et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: The role of cathelicidin LL-37. J. Immunol. 2007, 178, 7190–7198. [Google Scholar] [CrossRef]
- Misawa, Y.; Baba, A.; Ito, S.; Tanaka, M.; Shiohara, M. Vitamin D(3) induces expression of human cathelicidin antimicrobial peptide 18 in newborns. Int. J. Hematol. 2009, 90, 561–570. [Google Scholar] [CrossRef]
- Chen, S.; Ge, L.; Gombart, A.F.; Shuler, F.D.; Carlson, M.A.; Reilly, D.A.; Xie, J. Nanofiber-based sutures induce endogenous antimicrobial peptide. Nanomedicine 2017, 12, 2597–2609. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Carlsson, G.; Larne, O.; Andersson, M.; Putsep, K. Vitamin D3 induces pro-LL-37 expression in myeloid precursors from patients with severe congenital neutropenia. J. Leukoc. Biol. 2008, 84, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, Y.; Indra, A.K.; Ganguli-Indra, G.; Le, M.N.; Wang, H.; Hollins, R.R.; Reilly, D.A.; Carlson, M.A.; Gallo, R.L.; et al. 1alpha,25-dihydroxyvitamin D3-eluting nanofibrous dressings induce endogenous antimicrobial peptide expression. Nanomedicine 2018, 13, 1417–1432. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Ganguli-Indra, G.; Bhattacharya, N.; Logan, I.E.; Indra, A.K.; Gombart, A.F.; Wong, S.L.; Xie, J. Codelivery of 1alpha,25-dihydroxyvitamin D3 and CYP24A1 inhibitor VID400 by nanofiber dressings promotes endogenous antimicrobial peptide LL-37 induction. Mol. Pharm. 2022, 19, 974–984. [Google Scholar] [CrossRef]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef]
- White, J.H. Emerging roles of vitamin D-induced antimicrobial peptides in antiviral innate immunity. Nutrients 2022, 14, 284. [Google Scholar] [CrossRef]
- Subramanian, K.; Bergman, P.; Henriques-Normark, B. Vitamin D promotes pneumococcal killing and modulates inflammatory responses in primary human neutrophils. J. Innate Immun. 2017, 9, 375–386. [Google Scholar] [CrossRef]
- Derradjia, A.; Alanazi, H.; Park, H.J.; Djeribi, R.; Semlali, A.; Rouabhia, M. Alpha-tocopherol decreases interleukin-1beta and -6 and increases human beta-defensin-1 and -2 secretion in human gingival fibroblasts stimulated with Porphyromonas gingivalis lipopolysaccharide. J. Periodontal Res. 2016, 51, 295–303. [Google Scholar] [CrossRef]
- Malik, A.N.; Al-Kafaji, G. Glucose regulation of beta-defensin-1 mRNA in human renal cells. Biochem. Biophys. Res. Commun. 2007, 353, 318–323. [Google Scholar] [CrossRef]
- Barnea, M.; Madar, Z.; Froy, O. Glucose and insulin are needed for optimal defensin expression in human cell lines. Biochem. Biophys. Res. Commun. 2008, 367, 452–456. [Google Scholar] [CrossRef]
- Cederlund, A.; Kai-Larsen, Y.; Printz, G.; Yoshio, H.; Alvelius, G.; Lagercrantz, H.; Stromberg, R.; Jornvall, H.; Gudmundsson, G.H.; Agerberth, B. Lactose in human breast milk an inducer of innate immunity with implications for a role in intestinal homeostasis. PLoS ONE 2013, 8, e53876. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Z.; Tian, X.; Guo, Y.; Zhang, H. Yeast β-D-glucans induced antimicrobial peptide expressions against Salmonella infection in broiler chickens. Int. J. Biol. Macromol. 2016, 85, 573–584. [Google Scholar] [CrossRef]
- Sherman, H.; Chapnik, N.; Froy, O. Albumin and amino acids upregulate the expression of human beta-defensin 1. Mol. Immunol. 2006, 43, 1617–1623. [Google Scholar] [CrossRef]
- Fehlbaum, P.; Rao, M.; Zasloff, M.; Anderson, G.M. An essential amino acid induces epithelial beta-defensin expression. Proc. Natl. Acad. Sci. USA 2000, 97, 12723–12728. [Google Scholar] [CrossRef]
- Rivas-Santiago, C.E.; Rivas-Santiago, B.; Leon, D.A.; Castaneda-Delgado, J.; Hernandez Pando, R. Induction of beta-defensins by l-isoleucine as novel immunotherapy in experimental murine tuberculosis. Clin. Exp. Immunol. 2011, 164, 80–89. [Google Scholar] [CrossRef]
- Konno, Y.; Ashida, T.; Inaba, Y.; Ito, T.; Tanabe, H.; Maemoto, A.; Ayabe, T.; Mizukami, Y.; Fujiya, M.; Kohgo, Y. Isoleucine, an essential amino acid, induces the expression of human β defensin 2 through the activation of the G-protein coupled receptor-ERK pathway in the intestinal epithelia. Food Nutr. Sci. 2012, 3, 548. [Google Scholar] [CrossRef]
- Shirako, S.; Kojima, Y.; Tomari, N.; Nakamura, Y.; Matsumura, Y.; Ikeda, K.; Inagaki, N.; Sato, K. Pyroglutamyl leucine, a peptide in fermented foods, attenuates dysbiosis by increasing host antimicrobial peptide. NPJ Sci. Food 2019, 3, 18. [Google Scholar] [CrossRef]
- Kiyono, T.; Wada, S.; Ohta, R.; Wada, E.; Takagi, T.; Naito, Y.; Yoshikawa, T.; Sato, K. Identification of pyroglutamyl peptides with anti-colitic activity in Japanese rice wine, sake, by oral administration in a mouse model. J. Funct. Foods 2016, 27, 612–621. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.; Roschmann, K.I.L.; van Egmond, D.; Golebski, K.; Fokkens, W.J.; Wang, D.; van Drunen, C.M. Histone deacetylase inhibitors up-regulate LL-37 expression independent of toll-like receptor mediated signalling in airway epithelial cells. J. Inflamm. 2013, 10, 15. [Google Scholar] [CrossRef]
- Sunkara, L.T.; Jiang, W.; Zhang, G. Modulation of antimicrobial host defense peptide gene expression by free fatty acids. PLoS ONE 2012, 7, e49558. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, L.T.; Zeng, X.; Curtis, A.R.; Zhang, G. Cyclic AMP synergizes with butyrate in promoting beta-defensin 9 expression in chickens. Mol. Immunol. 2014, 57, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Sunkara, L.T.; Jiang, W.; Bible, M.; Carter, S.; Ma, X.; Qiao, S.; Zhang, G. Induction of porcine host defense peptide gene expression by short-chain fatty acids and their analogs. PLoS ONE 2013, 8, e72922. [Google Scholar] [CrossRef] [PubMed]
- Schauber, J.; Iffland, K.; Frisch, S.; Kudlich, T.; Schmausser, B.; Eck, M.; Menzel, T.; Gostner, A.; Luhrs, H.; Scheppach, W. Histone-deacetylase inhibitors induce the cathelicidin LL-37 in gastrointestinal cells. Mol. Immunol. 2004, 41, 847–854. [Google Scholar] [CrossRef]
- Raqib, R.; Sarker, P.; Bergman, P.; Ara, G.; Lindh, M.; Sack, D.A.; Nasirul Islam, K.M.; Gudmundsson, G.H.; Andersson, J.; Agerberth, B. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc. Natl. Acad. Sci. USA 2006, 103, 9178–9183. [Google Scholar] [CrossRef]
- Cederlund, A.; Nylen, F.; Miraglia, E.; Bergman, P.; Gudmundsson, G.H.; Agerberth, B. Label-free quantitative mass spectrometry reveals novel pathways involved in LL-37 expression. J. Innate Immun. 2014, 6, 365–376. [Google Scholar] [CrossRef]
- Steinmann, J.; Halldorsson, S.; Agerberth, B.; Gudmundsson, G.H. Phenylbutyrate induces antimicrobial peptide expression. Antimicrob. Agents Chemother. 2009, 53, 5127–5133. [Google Scholar] [CrossRef]
- Xiong, H.; Guo, B.; Gan, Z.; Song, D.; Lu, Z.; Yi, H.; Wu, Y.; Wang, Y.; Du, H. Butyrate upregulates endogenous host defense peptides to enhance disease resistance in piglets via histone deacetylase inhibition. Sci. Rep. 2016, 6, 27070. [Google Scholar] [CrossRef]
- Schauber, J.; Svanholm, C.; Termen, S.; Iffland, K.; Menzel, T.; Scheppach, W.; Melcher, R.; Agerberth, B.; Luhrs, H.; Gudmundsson, G.H. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: Relevance of signalling pathways. Gut 2003, 52, 735–741. [Google Scholar] [CrossRef]
- Schwab, M.; Reynders, V.; Shastri, Y.; Loitsch, S.; Stein, J.; Schroder, O. Role of nuclear hormone receptors in butyrate-mediated up-regulation of the antimicrobial peptide cathelicidin in epithelial colorectal cells. Mol. Immunol. 2007, 44, 2107–2114. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, F.; Wu, W.; Sun, M.; Bilotta, A.J.; Yao, S.; Xiao, Y.; Huang, X.; Eaves-Pyles, T.D.; Golovko, G.; et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal. Immunol. 2018, 11, 752–762. [Google Scholar] [CrossRef]
- Kida, Y.; Shimizu, T.; Kuwano, K. Sodium butyrate up-regulates cathelicidin gene expression via activator protein-1 and histone acetylation at the promoter region in a human lung epithelial cell line, EBC-1. Mol. Immunol. 2006, 43, 1972–1981. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Kao, M.C.; Zhang, L.; Zouboulis, C.C.; Gallo, R.L.; Huang, C.M. Sebum free fatty acids enhance the innate immune defense of human sebocytes by upregulating beta-defensin-2 expression. J. Investig. Dermatol. 2010, 130, 985–994. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, B.J.; Jeong, M.S.; Seo, S.J.; Kim, M.N.; Hong, C.K.; Ro, B.I. Expression and modulation of LL-37 in normal human keratinocytes, HaCaT cells, and inflammatory skin diseases. J. Korean Med. Sci. 2005, 20, 649–654. [Google Scholar] [CrossRef]
- Kim, B.J.; Rho, Y.K.; Lee, H.I.; Jeong, M.S.; Li, K.; Seo, S.J.; Kim, M.N.; Hong, C.K. The effect of calcipotriol on the expression of human beta defensin-2 and LL-37 in cultured human keratinocytes. Clin. Dev. Immunol. 2009, 2009, 645898. [Google Scholar] [CrossRef]
- Seo, S.J.; Ahn, S.W.; Hong, C.K.; Ro, B.I. Expressions of beta-defensins in human keratinocyte cell lines. J. Dermatol. Sci. 2001, 27, 183–191. [Google Scholar] [CrossRef]
- Santamaria, M.H.; Perez Caballero, E.; Corral, R.S. Unmethylated CpG motifs in Toxoplasma gondii DNA induce TLR9- and IFN-beta-dependent expression of alpha-defensin-5 in intestinal epithelial cells. Parasitology 2016, 143, 60–68. [Google Scholar] [CrossRef]
- Joly, S.; Organ, C.C.; Johnson, G.K.; McCray, P.B., Jr.; Guthmiller, J.M. Correlation between beta-defensin expression and induction profiles in gingival keratinocytes. Mol. Immunol. 2005, 42, 1073–1084. [Google Scholar] [CrossRef]
- Yin, Q.; Xu, X.; Lin, Y.; Lv, J.; Zhao, L.; He, R. Ultraviolet B irradiation induces skin accumulation of plasmacytoid dendritic cells: A possible role for chemerin. Autoimmunity 2014, 47, 185–192. [Google Scholar] [CrossRef]
- Glaser, R.; Navid, F.; Schuller, W.; Jantschitsch, C.; Harder, J.; Schroder, J.M.; Schwarz, A.; Schwarz, T. UV-B radiation induces the expression of antimicrobial peptides in human keratinocytes in vitro and in vivo. J. Allergy Clin. Immunol. 2009, 123, 1117–1123. [Google Scholar] [CrossRef]
- Di Nuzzo, S.; Sylva-Steenland, R.M.; Koomen, C.W.; de Rie, M.A.; Das, P.K.; Bos, J.D.; Teunissen, M.B. Exposure to UVB induces accumulation of LFA-1+ T cells and enhanced expression of the chemokine psoriasin in normal human skin. Photochem. Photobiol. 2000, 72, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Mallbris, L.; Edstrom, D.W.; Sundblad, L.; Granath, F.; Stahle, M. UVB upregulates the antimicrobial protein hCAP18 mRNA in human skin. J. Investig. Dermatol. 2005, 125, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.; Lim, W.; Choi, H.; Kim, O. Effects of the antimicrobial peptide cathelicidin (LL-37) on immortalized gingival fibroblasts infected with Porphyromonas gingivalis and irradiated with 625-nm LED light. Lasers Med. Sci. 2015, 30, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.; Khan, I.; Andreana, S.; Arany, P.R. Laser-activated transforming growth factor-beta1 induces human beta-defensin 2: Implications for laser therapies for periodontitis and peri-implantitis. J. Periodontal Res. 2017, 52, 360–367. [Google Scholar] [CrossRef]
- Fedrizzi, L.; Lim, D.; Carafoli, E. Calcium and signal transduction. Biochem. Mol. Biol. Educ. 2008, 36, 175–180. [Google Scholar] [CrossRef]
- KEGG. Calcium Signaling Pathway—Homo Sapiens (Human). Available online: https://www.genome.jp/pathway/hsa04020 (accessed on 2 January 2023).
- Harris, T.A.; Gattu, S.; Propheter, D.C.; Kuang, Z.; Bel, S.; Ruhn, K.A.; Chara, A.L.; Edwards, M.; Zhang, C.; Jo, J.H.; et al. Resistin-like molecule alpha provides vitamin-A-dependent antimicrobial protection in the skin. Cell Host Microbe 2019, 25, 777–788.e778. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Pilz, S.; Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; Keppel, M.H.; Grubler, M.R.; Marz, W.; Pandis, M. Vitamin D testing and treatment: A narrative review of current evidence. Endocr. Connect. 2019, 8, R27–R43. [Google Scholar] [CrossRef]
- Chen, J.; Tang, Z.; Slominski, A.T.; Li, W.; Zmijewski, M.A.; Liu, Y.; Chen, J. Vitamin D and its analogs as anticancer and anti-inflammatory agents. Eur. J. Med. Chem. 2020, 207, 112738. [Google Scholar] [CrossRef]
- Keane, J.T.; Elangovan, H.; Stokes, R.A.; Gunton, J.E. Vitamin D and the liver-correlation or cause? Nutrients 2018, 10, 496. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Edfeldt, K.; Liu, P.T.; Chun, R.; Fabri, M.; Schenk, M.; Wheelwright, M.; Keegan, C.; Krutzik, S.R.; Adams, J.S.; Hewison, M.; et al. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc. Natl. Acad. Sci. USA 2010, 107, 22593–22598. [Google Scholar] [CrossRef]
- Geller, A.I.; Shehab, N.; Weidle, N.J.; Lovegrove, M.C.; Wolpert, B.J.; Timbo, B.B.; Mozersky, R.P.; Budnitz, D.S. Emergency Department Visits for Adverse Events Related to Dietary Supplements. N. Engl. J. Med. 2015, 373, 1531–1540. [Google Scholar] [CrossRef]
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various aspects of calcium signaling in the regulation of apoptosis, autophagy, cell proliferation, and cancer. Int. J. Mol. Sci. 2020, 21, 8323. [Google Scholar] [CrossRef]
- Ronis, M.J.J.; Pedersen, K.B.; Watt, J. Adverse Effects of Nutraceuticals and Dietary Supplements. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 583–601. [Google Scholar] [CrossRef]
- De Marco Castro, E.; Calder, P.C.; Roche, H.M. Beta-1,3/1,6-glucans and immunity: State of the art and future directions. Mol. Nutr. Food Res. 2021, 65, e1901071. [Google Scholar] [CrossRef]
- Paudel, S.; Wu, G.; Wang, X. Amino acids in cell signaling: Regulation and function. Adv. Exp. Med. Biol. 2021, 1332, 17–33. [Google Scholar] [CrossRef]
- Topala, T.; Bodoki, A.; Oprean, L.; Oprean, R. Bovine serum albumin interactions with metal complexes. Clujul. Med. 2014, 87, 215–219. [Google Scholar] [CrossRef]
- Bergsson, G.; Hilmarsson, H.; Thormar, H. Antibacterial, antiviral, and antifungal activities of lipids. In Lipids and Essential Oils as Antimicrobial Agents; Thormar, H., Thormar, H., Eds.; John Wiley & Sons: Chichester, UK, 2011; p. 34. [Google Scholar]
- Gursoy, U.K.; Salli, K.; Soderling, E.; Gursoy, M.; Hirvonen, J.; Ouwehand, A.C. Regulation of hBD-2, hBD-3, hCAP18/LL37, and proinflammatory cytokine secretion by human milk oligosaccharides in an organotypic oral mucosal model. Pathogens 2021, 10, 739. [Google Scholar] [CrossRef]
- Paoletti, I.; Buommino, E.; Tudisco, L.; Baudouin, C.; Msika, P.; Tufano, M.A.; Baroni, A.; Donnarumma, G. Patented natural avocado sugars modulate the HBD-2 expression in human keratinocytes through the involvement of protein kinase C and protein tyrosine kinases. Arch. Dermatol. Res. 2010, 302, 201–209. [Google Scholar] [CrossRef]
- Paoletti, I.; Buommino, E.; Fusco, A.; Baudouin, C.; Msika, P.; Tufano, M.A.; Baroni, A.; Donnarumma, G. Patented natural avocado sugar modulates the HBD-2 and HBD-3 expression in human keratinocytes through toll-like receptor-2 and ERK/MAPK activation. Arch. Dermatol. Res. 2012, 304, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Promsong, A.; Chung, W.O.; Satthakarn, S.; Nittayananta, W. Ellagic acid modulates the expression of oral innate immune mediators: Potential role in mucosal protection. J. Oral Pathol. Med. 2015, 44, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.; Ling, K.H.; Wang, M.F.; El-Nezami, H. Green tea polyphenol epigallocatechin-3-gallate improves epithelial barrier function by inducing the production of antimicrobial peptide pBD-1 and pBD-2 in monolayers of porcine intestinal epithelial IPEC-J2 cells. Mol. Nutr. Food Res. 2016, 60, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Lombardo Bedran, T.B.; Feghali, K.; Zhao, L.; Palomari Spolidorio, D.M.; Grenier, D. Green tea extract and its major constituent, epigallocatechin-3-gallate, induce epithelial beta-defensin secretion and prevent beta-defensin degradation by Porphyromonas gingivalis. J. Periodontal Res. 2014, 49, 615–623. [Google Scholar] [CrossRef]
- Lombardo Bedran, T.B.; Morin, M.P.; Palomari Spolidorio, D.; Grenier, D. Black tea extract and Its theaflavin derivatives inhibit the growth of periodontopathogens and modulate interleukin-8 and beta-defensin secretion in oral epithelial cells. PLoS ONE 2015, 10, e0143158. [Google Scholar] [CrossRef]
- Gan, Y.; Cui, X.; Ma, T.; Liu, Y.; Li, A.; Huang, M. Paeoniflorin upregulates beta-defensin-2 expression in human bronchial epithelial cell through the p38 MAPK, ERK, and NF-kappaB signaling pathways. Inflammation 2014, 37, 1468–1475. [Google Scholar] [CrossRef]
- Holecek, M. Side effects of amino acid supplements. Physiol Res 2022, 71, 29–45. [Google Scholar] [CrossRef]
- Jang, J.H.; Shin, H.W.; Lee, J.M.; Lee, H.W.; Kim, E.C.; Park, S.H. An overview of pathogen recognition receptors for innate immunity in dental pulp. Mediators Inflamm. 2015, 2015, 794143. [Google Scholar] [CrossRef]
- Froy, O. Regulation of mammalian defensin expression by Toll-like receptor-dependent and independent signalling pathways. Cell Microbiol 2005, 7, 1387–1397. [Google Scholar] [CrossRef]
- Gaddis, D.E.; Michalek, S.M.; Katz, J. TLR4 signaling via MyD88 and TRIF differentially shape the CD4+ T cell response to Porphyromonas gingivalis hemagglutinin B. J. Immunol. 2011, 186, 5772–5783. [Google Scholar] [CrossRef]
- Fields, J.K.; Gunther, S.; Sundberg, E.J. Structural basis of IL-1 family cytokine signaling. Front. Immunol. 2019, 10, 1412. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Wassenaar, T.M.; Zimmermann, K. Lipopolysaccharides in Food, Food Supplements, and Probiotics: Should We be Worried? Eur. J. Microbiol. Immunol. 2018, 8, 63–69. [Google Scholar] [CrossRef]
- De Vito, P.; Incerpi, S.; Pedersen, J.Z.; Luly, P.; Davis, F.B.; Davis, P.J. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid 2011, 21, 879–890. [Google Scholar] [CrossRef]
- Chandna, S.; Bathla, M. Oral manifestations of thyroid disorders and its management. Indian J. Endocrinol. Metab. 2011, 15, S113–S116. [Google Scholar] [CrossRef]
- Tangestani, H.; Boroujeni, H.K.; Djafarian, K.; Emamat, H.; Shab-Bidar, S. Vitamin D and the gut microbiota: A narrative literature review. Clin. Nutr. Res. 2021, 10, 181–191. [Google Scholar] [CrossRef]
- Kennedy Crispin, M.; Fuentes-Duculan, J.; Gulati, N.; Johnson-Huang, L.M.; Lentini, T.; Sullivan-Whalen, M.; Gilleaudeau, P.; Cueto, I.; Suarez-Farinas, M.; Lowes, M.A.; et al. Gene profiling of narrowband UVB-induced skin injury defines cellular and molecular innate immune responses. J. Investig. Dermatol. 2013, 133, 692–701. [Google Scholar] [CrossRef]
- Ou, Y.; Petersen, P.M. Application of ultraviolet light sources for in vivo disinfection. Jpn. J. Appl. Phys. 2021, 60, 100501. [Google Scholar] [CrossRef]
- Vieyra-Garcia, P.A.; Wolf, P. A deep dive into UV-based phototherapy: Mechanisms of action and emerging molecular targets in inflammation and cancer. Pharmacol. Ther. 2021, 222, 107784. [Google Scholar] [CrossRef]
- Morio, K.A.; Sternowski, R.H.; Brogden, K.A. Using ultraviolet (UV) light emitting diodes (LED) to create sterile root canals and to treat endodontic infections. Curr. Opin. Biomed. Eng. 2022, 23, 100397. [Google Scholar] [CrossRef]
- Morio, K.A.; Thayer, E.L.; Bates, A.M.; Brogden, K.A. 255-nm light-emitting diode kills Enterococcus faecalis and induces the production of cellular biomarkers in human embryonic palatal mesenchyme cells and gingival fibroblasts. J. Endod. 2019, 45, 774–783.e776. [Google Scholar] [CrossRef] [PubMed]
- Morio, K.A.; Sternowski, R.H.; Brogden, K.A. Dataset of endodontic microorganisms killed at 265 nm wavelength by an ultraviolet C light emitting diode in root canals of extracted, instrumented teeth. Data Brief 2022, 40, 107750. [Google Scholar] [CrossRef] [PubMed]
- Karampela, I.; Christodoulatos, G.S.; Vallianou, N.; Tsilingiris, D.; Chrysanthopoulou, E.; Skyllas, G.; Antonakos, G.; Marinou, I.; Vogiatzakis, E.; Armaganidis, A.; et al. Circulating chemerin and Its kinetics may be a useful diagnostic and prognostic biomarker in critically ill patients with sepsis: A prospective study. Biomolecules 2022, 12, 301. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Skrygan, M.; Tomi, N.S.; Altmeyer, P.; Kreuter, A. Changes of antimicrobial peptide mRNA expression in atopic eczema following phototherapy. Br. J. Dermatol. 2006, 155, 1275–1278. [Google Scholar] [CrossRef]
- Kreuter, A.; Hyun, J.; Skrygan, M.; Sommer, A.; Bastian, A.; Altmeyer, P.; Gambichler, T. Ultraviolet A1-induced downregulation of human beta-defensins and interleukin-6 and interleukin-8 correlates with clinical improvement in localized scleroderma. Br. J. Dermatol. 2006, 155, 600–607. [Google Scholar] [CrossRef]
- Brogden, N.K.; Mehalick, L.; Fischer, C.L.; Wertz, P.W.; Brogden, K.A. The emerging role of peptides and lipids as antimicrobial epidermal barriers and modulators of local inflammation. Skin Pharmacol. Physiol. 2012, 25, 167–181. [Google Scholar] [CrossRef]
- Chaly, Y.V.; Paleolog, E.M.; Kolesnikova, T.S.; Tikhonov, I.I.; Petratchenko, E.V.; Voitenok, N.N. Neutrophil alpha-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur. Cytokine Netw. 2000, 11, 257–266. [Google Scholar]
- Barabas, N.; Rohrl, J.; Holler, E.; Hehlgans, T. Beta-defensins activate macrophages and synergize in pro-inflammatory cytokine expression induced by TLR ligands. Immunobiology 2013, 218, 1005–1011. [Google Scholar] [CrossRef]
- Van Wetering, S.; MannesseLazeroms, S.P.G.; Dijkman, J.H.; Hiemstra, P.S. Effect of neutrophil serine proteinases and defensins on lung epithelial cells: Modulation of cytotoxicity and IL-8 production. J. Leukoc. Biol. 1997, 62, 217–226. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Ushio, H.; Nakano, N.; Ng, W.; Sayama, K.; Hashimoto, K.; Nagaoka, I.; Okumura, K.; Ogawa, H. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J. Investig. Dermatol. 2007, 127, 594–604. [Google Scholar] [CrossRef]
- Petrov, V.; Funderburg, N.; Weinberg, A.; Sieg, S. Human beta defensin-3 induces chemokines from monocytes and macrophages: Diminished activity in cells from HIV-infected persons. Immunology 2013, 140, 413–420. [Google Scholar] [CrossRef]
- Scott, M.G.; Davidson, D.J.; Gold, M.R.; Bowdish, D.; Hancock, R.E. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 2002, 169, 3883–3891. [Google Scholar] [CrossRef]
- Boniotto, M.; Jordan, W.J.; Eskdale, J.; Tossi, A.; Antcheva, N.; Crovella, S.; Connell, N.D.; Gallagher, G. Human beta-defensin 2 induces a vigorous cytokine response in peripheral blood mononuclear cells. Antimicrob. Agents Chemother. 2006, 50, 1433–1441. [Google Scholar] [CrossRef]
- Presicce, P.; Giannelli, S.; Taddeo, A.; Villa, M.L.; Della Bella, S. Human defensins activate monocyte-derived dendritic cells, promote the production of proinflammatory cytokines, and up-regulate the surface expression of CD91. J. Leukoc. Biol. 2009, 86, 941–948. [Google Scholar] [CrossRef]
- Jin, G.; Kawsar, H.I.; Hirsch, S.A.; Zeng, C.; Jia, X.; Feng, Z.; Ghosh, S.K.; Zheng, Q.Y.; Zhou, A.; McIntyre, T.M.; et al. An antimicrobial peptide regulates tumor-associated macrophage trafficking via the chemokine receptor CCR2, a model for tumorigenesis. PLoS ONE 2010, 5, e10993. [Google Scholar] [CrossRef]
- Semple, F.; MacPherson, H.; Webb, S.; Cox, S.L.; Mallin, L.J.; Tyrrell, C.; Grimes, G.R.; Semple, C.A.; Nix, M.A.; Millhauser, G.L.; et al. Human beta-defensin 3 affects the activity of pro-inflammatory pathways associated with MyD88 and TRIF. Eur. J. Immunol. 2011, 41, 3291–3300. [Google Scholar] [CrossRef]
- Semple, F.; Webb, S.; Li, H.N.; Patel, H.B.; Perretti, M.; Jackson, I.J.; Gray, M.; Davidson, D.J.; Dorin, J.R. Human beta-defensin 3 has immunosuppressive activity in vitro and in vivo. Eur. J. Immunol. 2010, 40, 1073–1078. [Google Scholar] [CrossRef]
- Dietrich, D.E.; Xiao, X.; Dawson, D.V.; Belanger, M.; Xie, H.; Progulske-Fox, A.; Brogden, K.A. Human alpha- and beta-defensins bind to immobilized adhesins from Porphyromonas gingivalis. Infect. Immun. 2008, 76, 5714–5720. [Google Scholar] [CrossRef]
- Gallo, S.A.; Wang, W.; Rawat, S.S.; Jung, G.; Waring, A.J.; Cole, A.M.; Lu, H.; Yan, X.; Daly, N.L.; Craik, D.J.; et al. Theta-defensins prevent HIV-1 Env-mediated fusion by binding gp41 and blocking 6-helix bundle formation. J. Biol. Chem. 2006, 281, 18787–18792. [Google Scholar] [CrossRef]
- Wei, G.; de Leeuw, E.; Pazgier, M.; Yuan, W.; Zou, G.; Wang, J.; Ericksen, B.; Lu, W.Y.; Lehrer, R.I.; Lu, W. Through the looking glass, mechanistic insights from enantiomeric human defensins. J. Biol. Chem. 2009, 284, 29180–29192. [Google Scholar] [CrossRef]
- Pingel, L.C.; Kohlgraf, K.G.; Hansen, C.J.; Eastman, C.G.; Dietrich, D.E.; Burnell, K.K.; Srikantha, R.N.; Xiao, X.; Belanger, M.; Progulske-Fox, A.; et al. Human beta-defensin 3 binds to hemagglutinin B (rHagB), a non-fimbrial adhesin from Porphyromonas gingivalis, and attenuates a pro-inflammatory cytokine response. Immunol. Cell Biol. 2008, 86, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Borgwardt, D.S.; Martin, A.D.; Van Hemert, J.R.; Yang, J.; Fischer, C.L.; Recker, E.N.; Nair, P.R.; Vidva, R.; Chandrashekaraiah, S.; Progulske-Fox, A.; et al. Histatin 5 binds to Porphyromonas gingivalis hemagglutinin B (HagB) and alters HagB-induced chemokine responses. Sci. Rep. 2014, 4, 3904. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Weldon, S.; Buchanan, P.J.; Schock, B.; Ernst, R.K.; McAuley, D.F.; Tunney, M.M.; Irwin, C.R.; Elborn, J.S.; Taggart, C.C. Evaluation of the ability of LL-37 to neutralise LPS in vitro and ex vivo. PLoS ONE 2011, 6, e26525. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.D.; Ramos, R.A.; Tobias, P.S.; Ulevitch, R.J.; Mathison, J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990, 249, 1431–1433. [Google Scholar] [CrossRef]
- Gallo, R.L.; Hooper, L.V. Epithelial antimicrobial defence of the skin and intestine. Nat. Rev. Immunol. 2012, 12, 503–516. [Google Scholar] [CrossRef]
- Van Hemert, J.R.; Recker, E.N.; Dietrich, D.; Progulske-Fox, A.; Kurago, Z.B.; Walters, K.S.; Cavanaugh, J.E.; Brogden, K.A. Human beta-defensin-3 alters, but does not inhibit, the binding of Porphyromonas gingivalis haemagglutinin B to the surface of human dendritic cells. Int. J. Antimicrob. Agents 2012, 40, 75–79. [Google Scholar] [CrossRef]
- Nagaoka, I.; Hirota, S.; Niyonsaba, F.; Hirata, M.; Adachi, Y.; Tamura, H.; Heumann, D. Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-alpha by blocking the binding of LPS to CD14(+) cells. J. Immunol. 2001, 167, 3329–3338. [Google Scholar] [CrossRef]
- Miles, K.; Clarke, D.J.; Lu, W.; Sibinska, Z.; Beaumont, P.E.; Davidson, D.J.; Barr, T.A.; Campopiano, D.J.; Gray, M. Dying and necrotic neutrophils are anti-inflammatory secondary to the release of alpha-defensins. J. Immunol. 2009, 183, 2122–2132. [Google Scholar] [CrossRef]
- Shi, J.; Aono, S.; Lu, W.; Ouellette, A.J.; Hu, X.; Ji, Y.; Wang, L.; Lenz, S.; van Ginkel, F.W.; Liles, M.; et al. A novel role for defensins in intestinal homeostasis: Regulation of IL-1beta secretion. J. Immunol. 2007, 179, 1245–1253. [Google Scholar] [CrossRef]
- Mookherjee, N.; Brown, K.L.; Bowdish, D.M.; Doria, S.; Falsafi, R.; Hokamp, K.; Roche, F.M.; Mu, R.; Doho, G.H.; Pistolic, J.; et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 2006, 176, 2455–2464. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Wuerth, K.; Hancock, R.E. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 2013, 9, 761–768. [Google Scholar] [CrossRef]
- Mookherjee, N.; Hamill, P.; Gardy, J.; Blimkie, D.; Falsafi, R.; Chikatamarla, A.; Arenillas, D.J.; Doria, S.; Kollmann, T.R.; Hancock, R.E. Systems biology evaluation of immune responses induced by human host defence peptide LL-37 in mononuclear cells. Mol. Biosyst. 2009, 5, 483–496. [Google Scholar] [CrossRef]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- Brogden, K.A.; Bates, A.M.; Fischer, C.L. Antimicrobial peptides in host defense: Functions beyond antimicrobial activity. In Antimicrobial Peptides—Role in Human Health and Disease; Harder, J., Schroeder, J.M., Kaufmann, S.H., Mercer, A.A., Weber, B., Eds.; Birkhauser Advances in Infectious Diseases; Springer International Publishing: Cham, Switzerland, 2016; pp. 129–146. [Google Scholar] [CrossRef]
- Drayton, M.; Deisinger, J.P.; Ludwig, K.C.; Raheem, N.; Muller, A.; Schneider, T.; Straus, S.K. Host defense peptides: Dual antimicrobial and immunomodulatory action. Int. J. Mol. Sci. 2021, 22, 11172. [Google Scholar] [CrossRef]
- Takahashi, M.; Umehara, Y.; Yue, H.; Trujillo-Paez, J.V.; Peng, G.; Nguyen, H.L.T.; Ikutama, R.; Okumura, K.; Ogawa, H.; Ikeda, S.; et al. The antimicrobial peptide human beta-defensin-3 accelerates wound healing by promoting angiogenesis, cell migration, and proliferation through the FGFR/JAK2/STAT3 signaling pathway. Front. Immunol. 2021, 12, 712781. [Google Scholar] [CrossRef]
- Davidson, D.J.; Currie, A.J.; Reid, G.S.; Bowdish, D.M.; MacDonald, K.L.; Ma, R.C.; Hancock, R.E.; Speert, D.P. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J. Immunol. 2004, 172, 1146–1156. [Google Scholar] [CrossRef]
- Ramos, R.; Silva, J.P.; Rodrigues, A.C.; Costa, R.; Guardao, L.; Schmitt, F.; Soares, R.; Vilanova, M.; Domingues, L.; Gama, M. Wound healing activity of the human antimicrobial peptide LL37. Peptides 2011, 32, 1469–1476. [Google Scholar] [CrossRef]
- Warnke, P.H.; Voss, E.; Russo, P.A.; Stephens, S.; Kleine, M.; Terheyden, H.; Liu, Q. Antimicrobial peptide coating of dental implants: Biocompatibility assessment of recombinant human beta defensin-2 for human cells. Int. J. Oral Maxillofac. Implants 2013, 28, 982–988. [Google Scholar] [CrossRef]
- Heilborn, J.D.; Nilsson, M.F.; Kratz, G.; Weber, G.; Sorensen, O.; Borregaard, N.; Stahle-Backdahl, M. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J. Investig. Dermatol. 2003, 120, 379–389. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Gallo, R.L. Antimicrobial peptides: Old molecules with new ideas. J. Investig. Dermatol. 2012, 132, 887–895. [Google Scholar] [CrossRef]
- Hao, J.; Liu, M.G.; Yu, Y.Q.; Cao, F.L.; Li, Z.; Lu, Z.M.; Chen, J. Roles of peripheral mitogen-activated protein kinases in melittin-induced nociception and hyperalgesia. Neuroscience 2008, 152, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Son, D.J.; Lee, J.W.; Lee, Y.H.; Song, H.S.; Lee, C.K.; Hong, J.T. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol. Ther. 2007, 115, 246–270. [Google Scholar] [CrossRef] [PubMed]
- Rykaczewska-Czerwinska, M.; Oles, P.; Oles, M.; Kuczer, M.; Konopinska, D.; Plech, A. Effect of alloferon 1 on central nervous system in rats. Acta Pol. Pharm. 2015, 72, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Merlo, L.A.; Bastos, L.F.; Godin, A.M.; Rocha, L.T.; Nascimento, E.B., Jr.; Paiva, A.L.; Moraes-Santos, T.; Zumpano, A.A.; Bastos, E.M.; Heneine, L.G.; et al. Effects induced by Apis mellifera venom and its components in experimental models of nociceptive and inflammatory pain. Toxicon 2011, 57, 764–771. [Google Scholar] [CrossRef]
- Logashina, Y.A.; Solstad, R.G.; Mineev, K.S.; Korolkova, Y.V.; Mosharova, I.V.; Dyachenko, I.A.; Palikov, V.A.; Palikova, Y.A.; Murashev, A.N.; Arseniev, A.S.; et al. New disulfide-stabilized fold provides sea anemone peptide to exhibit both antimicrobial and TRPA1 potentiating properties. Toxins 2017, 9, 154. [Google Scholar] [CrossRef]
- Chen, W.F.; Huang, S.Y.; Liao, C.Y.; Sung, C.S.; Chen, J.Y.; Wen, Z.H. The use of the antimicrobial peptide piscidin (PCD)-1 as a novel anti-nociceptive agent. Biomaterials 2015, 53, 1–11. [Google Scholar] [CrossRef]
- Edwards, A.B.; Mastaglia, F.L.; Knuckey, N.W.; Meloni, B.P. Neuroprotective cationic arginine-rich peptides (CARPs): An assessment of their clinical safety. Drug Saf. 2020, 43, 957–969. [Google Scholar] [CrossRef]
- Walker, M.C.; Eslami, S.M.; Hetrick, K.J.; Ackenhusen, S.E.; Mitchell, D.A.; van der Donk, W.A. Precursor peptide-targeted mining of more than one hundred thousand genomes expands the lanthipeptide natural product family. BMC Genomics 2020, 21, 387. [Google Scholar] [CrossRef]
- Ghia, J.E.; Crenner, F.; Metz-Boutigue, M.H.; Aunis, D.; Angel, F. Effects of a chromogranin-derived peptide (CgA 47-66) in the writhing nociceptive response induced by acetic acid in rats. Regul. Pept. 2004, 119, 199–207. [Google Scholar] [CrossRef]
- Wood, S.M.; Kennedy, J.S.; Arsenault, J.E.; Thomas, D.L.; Buck, R.H.; Shippee, R.L.; DeMichele, S.J.; Winship, T.R.; Schaller, J.P.; Montain, S.; et al. Novel nutritional immune formula maintains host defense mechanisms. Mil. Med. 2005, 170, 975–985. [Google Scholar] [CrossRef]
- Krisanaprakornkit, S.; Kimball, J.R.; Dale, B.A. Regulation of human beta-defensin-2 in gingival epithelial cells: The involvement of mitogen-activated protein kinase pathways, but not the NF-kappaB transcription factor family. J. Immunol. 2002, 168, 316–324. [Google Scholar] [CrossRef]
- Li, G.; Domenico, J.; Jia, Y.; Lucas, J.J.; Gelfand, E.W. NF-kappaB-dependent induction of cathelicidin-related antimicrobial peptide in murine mast cells by lipopolysaccharide. Int. Arch. Allergy Immunol. 2009, 150, 122–132. [Google Scholar] [CrossRef]
- Steubesand, N.; Kiehne, K.; Brunke, G.; Pahl, R.; Reiss, K.; Herzig, K.H.; Schubert, S.; Schreiber, S.; Folsch, U.R.; Rosenstiel, P.; et al. The expression of the beta-defensins hBD-2 and hBD-3 is differentially regulated by NF-kappaB and MAPK/AP-1 pathways in an in vitro model of Candida esophagitis. BMC Immunol. 2009, 10, 36. [Google Scholar] [CrossRef]
- Wehkamp, K.; Schwichtenberg, L.; Schroder, J.M.; Harder, J. Pseudomonas aeruginosa- and IL-1beta-mediated induction of human beta-defensin-2 in keratinocytes is controlled by NF-kappaB and AP-1. J. Investig. Dermatol. 2006, 126, 121–127. [Google Scholar] [CrossRef]
- Drover, V.A.; Nguyen, D.V.; Bastie, C.C.; Darlington, Y.F.; Abumrad, N.A.; Pessin, J.E.; London, E.; Sahoo, D.; Phillips, M.C. CD36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. J. Biol. Chem. 2008, 283, 13108–13115. [Google Scholar] [CrossRef]
- Ren, Y.; Li, S.; Zhu, R.; Wan, C.; Song, D.; Zhu, J.; Cai, G.; Long, S.; Kong, L.; Yu, W. Discovery of STAT3 and Histone Deacetylase (HDAC) Dual-Pathway Inhibitors for the Treatment of Solid Cancer. J. Med. Chem. 2021, 64, 7468–7482. [Google Scholar] [CrossRef]
- Li, A.; Gan, Y.; Wang, R.; Liu, Y.; Ma, T.; Huang, M.; Cui, X. IL-22 up-regulates beta-defensin-2 expression in human alveolar epithelium via STAT3 but not NF-kappaB signaling pathway. Inflammation 2015, 38, 1191–1200. [Google Scholar] [CrossRef]
- Sinha, R.; Yen, P.M. Cellular action of thyroid hormone. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2018. [Google Scholar]
- Kohlgraf, K.G.; Ackermann, A.; Lu, X.; Burnell, K.; Belanger, M.; Cavanaugh, J.E.; Xie, H.; Progulske-Fox, A.; Brogden, K.A. Defensins attenuate cytokine responses yet enhance antibody responses to Porphyromonas gingivalis adhesins in mice. Future Microbiol. 2010, 5, 115–125. [Google Scholar] [CrossRef]
- Harvey, L.E.; Kohlgraf, K.G.; Mehalick, L.A.; Raina, M.; Recker, E.N.; Radhakrishnan, S.; Prasad, S.A.; Vidva, R.; Progulske-Fox, A.; Cavanaugh, J.E.; et al. Defensin DEFB103 bidirectionally regulates chemokine and cytokine responses to a pro-inflammatory stimulus. Sci. Rep. 2013, 3, 1232. [Google Scholar] [CrossRef]
- Ruan, Y.; Shen, T.; Wang, Y.; Hou, M.; Li, J.; Sun, T. Antimicrobial peptide LL-37 attenuates LTA induced inflammatory effect in macrophages. Int. Immunopharmacol. 2013, 15, 575–580. [Google Scholar] [CrossRef]
- Oudhoff, M.J.; Blaauboer, M.E.; Nazmi, K.; Scheres, N.; Bolscher, J.G.; Veerman, E.C. The role of salivary histatin and the human cathelicidin LL-37 in wound healing and innate immunity. Biol. Chem. 2010, 391, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yu, F.S. LL-37 via EGFR transactivation to promote high glucose-attenuated epithelial wound healing in organ-cultured corneas. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Aarbiou, J.; Verhoosel, R.M.; Van Wetering, S.; De Boer, W.I.; Van Krieken, J.H.; Litvinov, S.V.; Rabe, K.F.; Hiemstra, P.S. Neutrophil defensins enhance lung epithelial wound closure and mucin gene expression in vitro. Am. J. Respir. Cell Mol. Biol. 2004, 30, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Joly, S.; Librant, J.; Brogden, K.A.; Burnell, K.K.; Johnson, G.; Guthmiller, J.M. Beta-defensins differentially induce cytokines in gingival keratinocyte cultures. In Proceedings of the 83rd General Session IADR meeting, Baltimore, MD, USA, 9–12 March 2005. [Google Scholar]
- Niyonsaba, F.; Ushio, H.; Nagaoka, I.; Okumura, K.; Ogawa, H. The human beta-defensins (-1, -2, -3, -4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J. Immunol. 2005, 175, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Khine, A.A.; Del Sorbo, L.; Vaschetto, R.; Voglis, S.; Tullis, E.; Slutsky, A.S.; Downey, G.P.; Zhang, H. Human neutrophil peptides induce interleukin-8 production through the P2Y6 signaling pathway. Blood 2006, 107, 2936–2942. [Google Scholar] [CrossRef]
- van Wetering, S.; Mannesse-Lazeroms, S.P.; van Sterkenburg, M.A.; Hiemstra, P.S. Neutrophil defensins stimulate the release of cytokines by airway epithelial cells: Modulation by dexamethasone. Inflamm. Res. 2002, 51, 8–15. [Google Scholar] [CrossRef]
- Territo, M.C.; Ganz, T.; Selsted, M.E.; Lehrer, R. Monocyte-chemotactic activity of defensins from human neutrophils. J. Clin. Investig. 1989, 84, 2017–2020. [Google Scholar] [CrossRef]
- Shaykhiev, R.; Beisswenger, C.; Kandler, K.; Senske, J.; Puchner, A.; Damm, T.; Behr, J.; Bals, R. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, L842–L848. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Iwabuchi, K.; Someya, A.; Hirata, M.; Matsuda, H.; Ogawa, H.; Nagaoka, I. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology 2002, 106, 20–26. [Google Scholar] [CrossRef]
- Nishimura, M.; Abiko, Y.; Kurashige, Y.; Takeshima, M.; Yamazaki, M.; Kusano, K.; Saitoh, M.; Nakashima, K.; Inoue, T.; Kaku, T. Effect of defensin peptides on eukaryotic cells: Primary epithelial cells, fibroblasts and squamous cell carcinoma cell lines. J. Dermatol. Sci. 2004, 36, 87–95. [Google Scholar] [CrossRef]
- Baroni, A.; Donnarumma, G.; Paoletti, I.; Longanesi-Cattani, I.; Bifulco, K.; Tufano, M.A.; Carriero, M.V. Antimicrobial human beta-defensin-2 stimulates migration, proliferation and tube formation of human umbilical vein endothelial cells. Peptides 2009, 30, 267–272. [Google Scholar] [CrossRef]
- Lillard, J.W., Jr.; Boyaka, P.N.; Chertov, O.; Oppenheim, J.J.; McGhee, J.R. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc. Natl. Acad. Sci. USA 1999, 96, 651–656. [Google Scholar] [CrossRef]
- Aarbiou, J.; Ertmann, M.; van Wetering, S.; van Noort, P.; Rook, D.; Rabe, K.F.; Litvinov, S.V.; van Krieken, J.H.; de Boer, W.I.; Hiemstra, P.S. Human neutrophil defensins induce lung epithelial cell proliferation in vitro. J. Leukoc. Biol. 2002, 72, 167–174. [Google Scholar] [CrossRef]
- Chamorro, C.I.; Weber, G.; Gronberg, A.; Pivarcsi, A.; Stahle, M. The human antimicrobial peptide LL-37 suppresses apoptosis in keratinocytes. J. Investig. Dermatol. 2009, 129, 937–944. [Google Scholar] [CrossRef]
- Brogden, K.A.; Heidari, M.; Sacco, R.E.; Palmquist, D.; Guthmiller, J.M.; Johnson, G.K.; Jia, H.P.; Tack, B.F.; McCray, P.B. Defensin-induced adaptive immunity in mice and its potential in preventing periodontal disease. Oral Microbiol. Immunol. 2003, 18, 95–99. [Google Scholar] [CrossRef]
- Schrumpf, J.A.; van Sterkenburg, M.A.; Verhoosel, R.M.; Zuyderduyn, S.; Hiemstra, P.S. Interleukin 13 exposure enhances vitamin D-mediated expression of the human cathelicidin antimicrobial peptide 18/LL-37 in bronchial epithelial cells. Infect. Immun. 2012, 80, 4485–4494. [Google Scholar] [CrossRef]

| AMP Concentration (μM) | Activity | AMPs Involved (Reported μM Concentrations) |
|---|---|---|
| Roles of AMPs in inflammation | ||
| 0.02–0.30 | Binds microbial antigens | LL-37 (0.02 μM), HBD3 (0.19 μM), HBD1 (0.25 μM), and Histatin 5 (0.30 μM) |
| 1.94 | Attenuated pathway signaling | HBD3 (1.94 μM) |
| 4.45 | Attenuated gene expression | LL-37 (4.45 μM) |
| 0.19–2.50 | Attenuated cytokine response | HBD3 (0.19 μM), LL-37 (0.22–2.50 μM), HBD1 (0.25 μM), and HNP-1,2 (0.29 μM) |
| 1.11 | Activated pathway signaling | LL-37 (1.11 μM) |
| 2.32 | Enhanced gene expression | HNP-1-3 (2.32 μM) |
| 0.03–29.00 | Enhanced cytokine response | HBD1 (0.03–5.08 μM), LL-37 (0.11–11.12 μM), HBD2 (0.46–4.61 μM), HNP-1-3 (0.87–29.0 μM), and HBD3 (0.97–3.87 μM) |
| Roles of AMPs in immunity | ||
| 0.01–29.00 | Chemotactic activity | HNP-1-3 (0.01–29.00 μM) and LL-37 (0.22-4.45 μM) |
| 1.00–2.32 | Promoted cell migration | LL-37 (1.00 μM) and HNP-1-3 (2.32 μM) |
| 11.12 | Increased cell markers | LL-37 (11.12 μM) |
| 0.22-11.12 | Induced proliferation | LL-37 (0.22–2.22 μM), HNP-1-3 (0.29–2.90 μM), HBD3 (0.97–1.55 μM), HBD2 (1.15–2.31 μM), and HBD1 (1.27 μM) |
| Induced Th1 cytokine profile | LL-37 (11.12 μM) | |
| 0.19–1.45 | Enhanced antibody response | HBD3 (0.19 μM), HBD2 (0.23 μM), HBD1 (0.25 μM), and HNP-1-3 (0.29–1.45 μM) |
| 1.00 | Suppressed apoptosis | LL-37 (1.0 μM) |
| 2.54–12.70 | Decreased cell numbers | HBD1 (2.54–12.70 μM) |
| 2.22–14.50 | Cell cytotoxicity | LL-37 (2.2–11.12 μM), HBD2 (6.92 μM), HNP-1-3 (14.50 μM) |
| Roles of AMPs in angiogenesis, vasculogenesis, and wound healing | ||
| 0.12–58.00 | Angiogenesis | HBD2 (0.12 μM) and HBD3 (58.00 μM) |
| 0.11–2.31 | Promoted cell migration | HBD3 (0.11–0.97 μM), HBD2 (0.12–2.31 μM), and HBD4 (2.22 μM) |
| 1.16–58.00 | Enhanced wound closure | LL-37 (0.11–2.50 μM), HBD2 (0.12 μM), HNP-1-3 (1.16–2.32 μM), and HBD3 (58.00 μM) |
| 14.50 | Delayed wound closure | HNP-1-3 (14.50 μM) |
| 58.00 | Enhanced wound healing | HBD3 (58.00 μM) |
| Roles of AMPs in pain nociception | ||
| 0.2–6.0 mg/kg | Pain antinociception | Alloferon (0.10 μM), PCD-1 (3.69 μM), Ueq 12-1 (0.2 mg/kg), CgA (0.5 mg/kg), and AMV (6 mg/kg) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morio, K.A.; Sternowski, R.H.; Brogden, K.A. Induction of Endogenous Antimicrobial Peptides to Prevent or Treat Oral Infection and Inflammation. Antibiotics 2023, 12, 361. https://doi.org/10.3390/antibiotics12020361
Morio KA, Sternowski RH, Brogden KA. Induction of Endogenous Antimicrobial Peptides to Prevent or Treat Oral Infection and Inflammation. Antibiotics. 2023; 12(2):361. https://doi.org/10.3390/antibiotics12020361
Chicago/Turabian StyleMorio, Kimberly A., Robert H. Sternowski, and Kim A. Brogden. 2023. "Induction of Endogenous Antimicrobial Peptides to Prevent or Treat Oral Infection and Inflammation" Antibiotics 12, no. 2: 361. https://doi.org/10.3390/antibiotics12020361
APA StyleMorio, K. A., Sternowski, R. H., & Brogden, K. A. (2023). Induction of Endogenous Antimicrobial Peptides to Prevent or Treat Oral Infection and Inflammation. Antibiotics, 12(2), 361. https://doi.org/10.3390/antibiotics12020361







