Evaluation of Piperacillin/Sulbactam, Piperacillin/Tazobactam and Cefoperazone/Sulbactam Dosages in Gram-Negative Bacterial Bloodstream Infections by Monte Carlo Simulation
Abstract
:1. Introduction
2. Results
2.1. In Vitro Activity of Antibacterial Agents
2.2. Monte Carlo Simulation Analysis
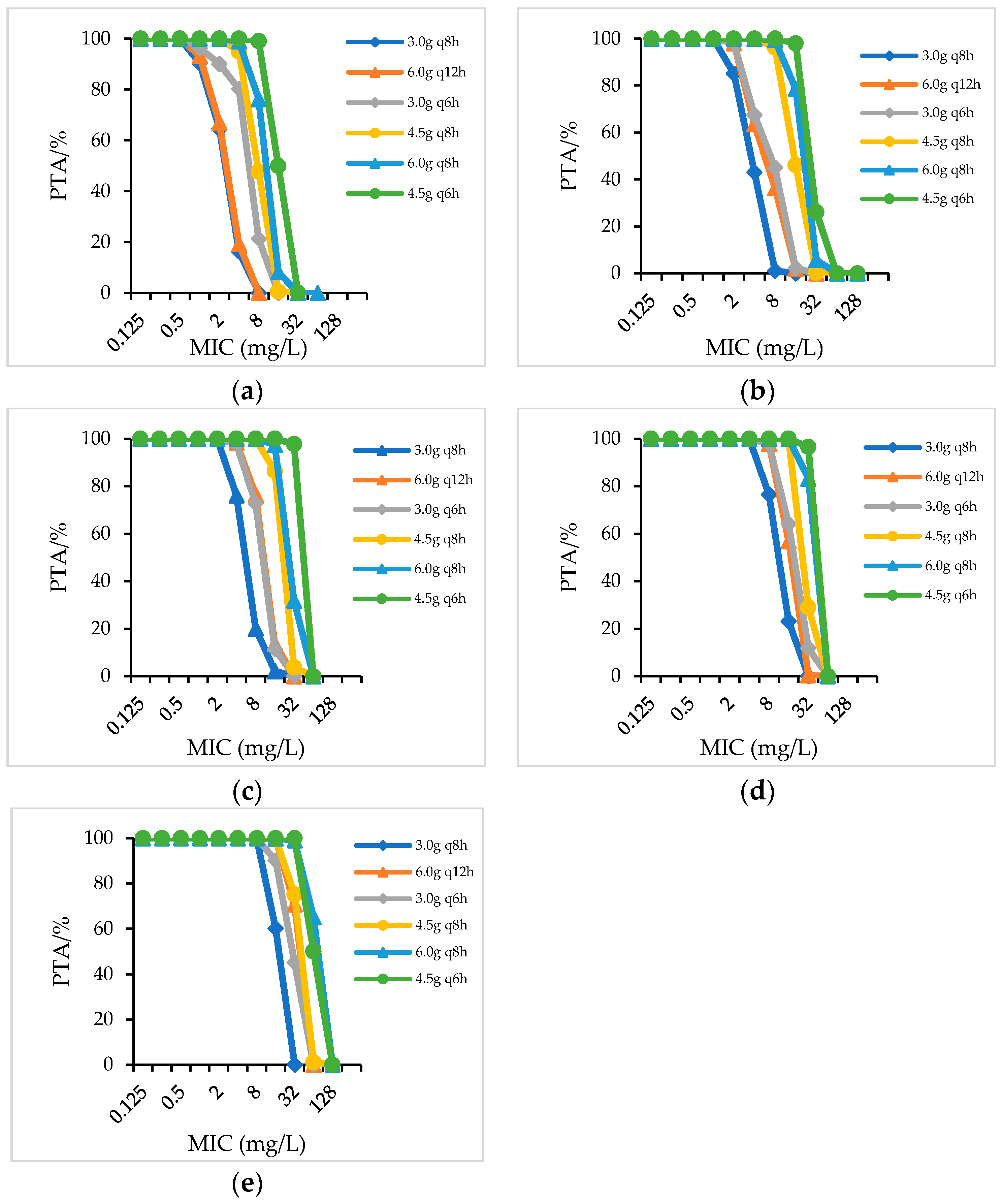
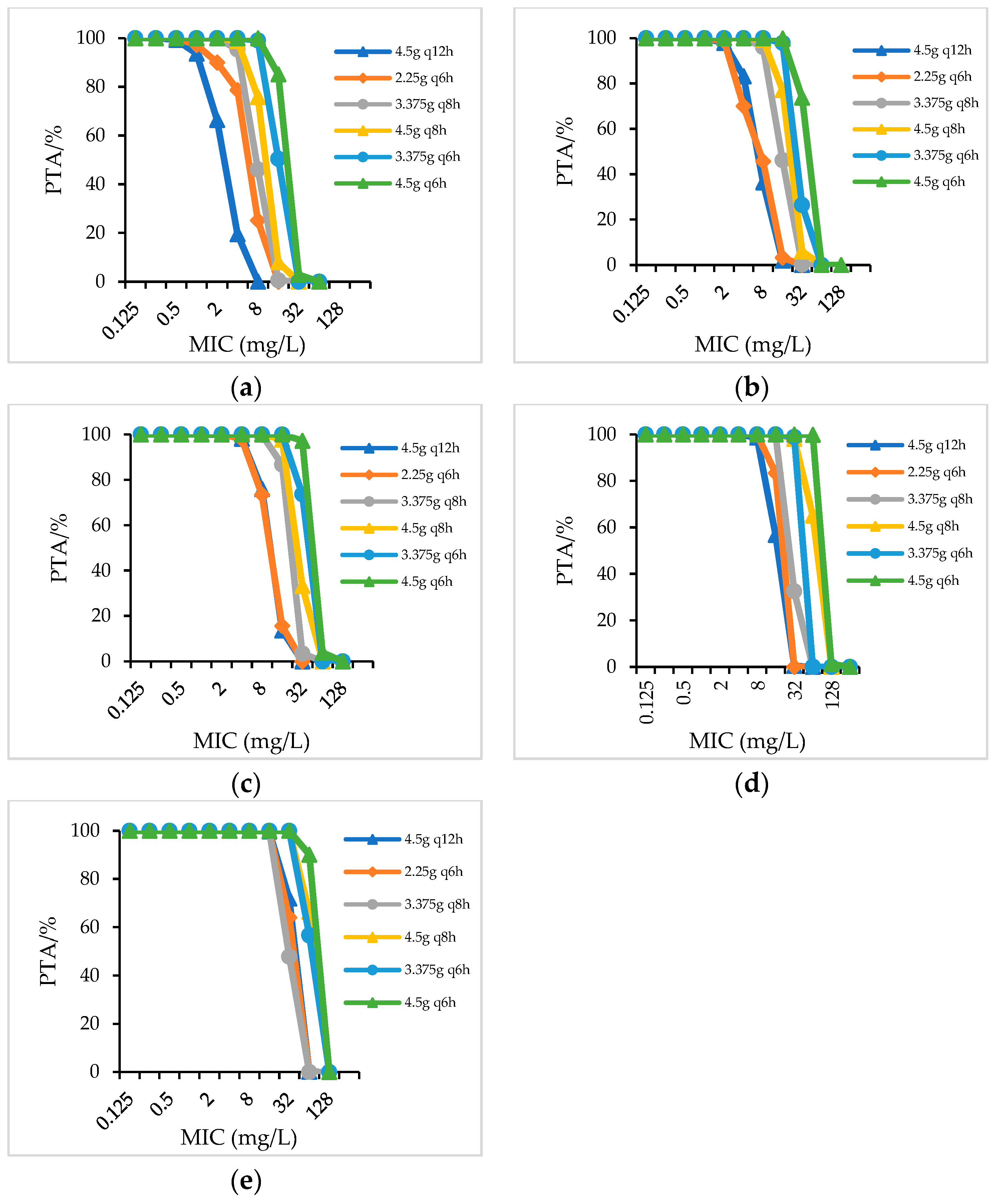
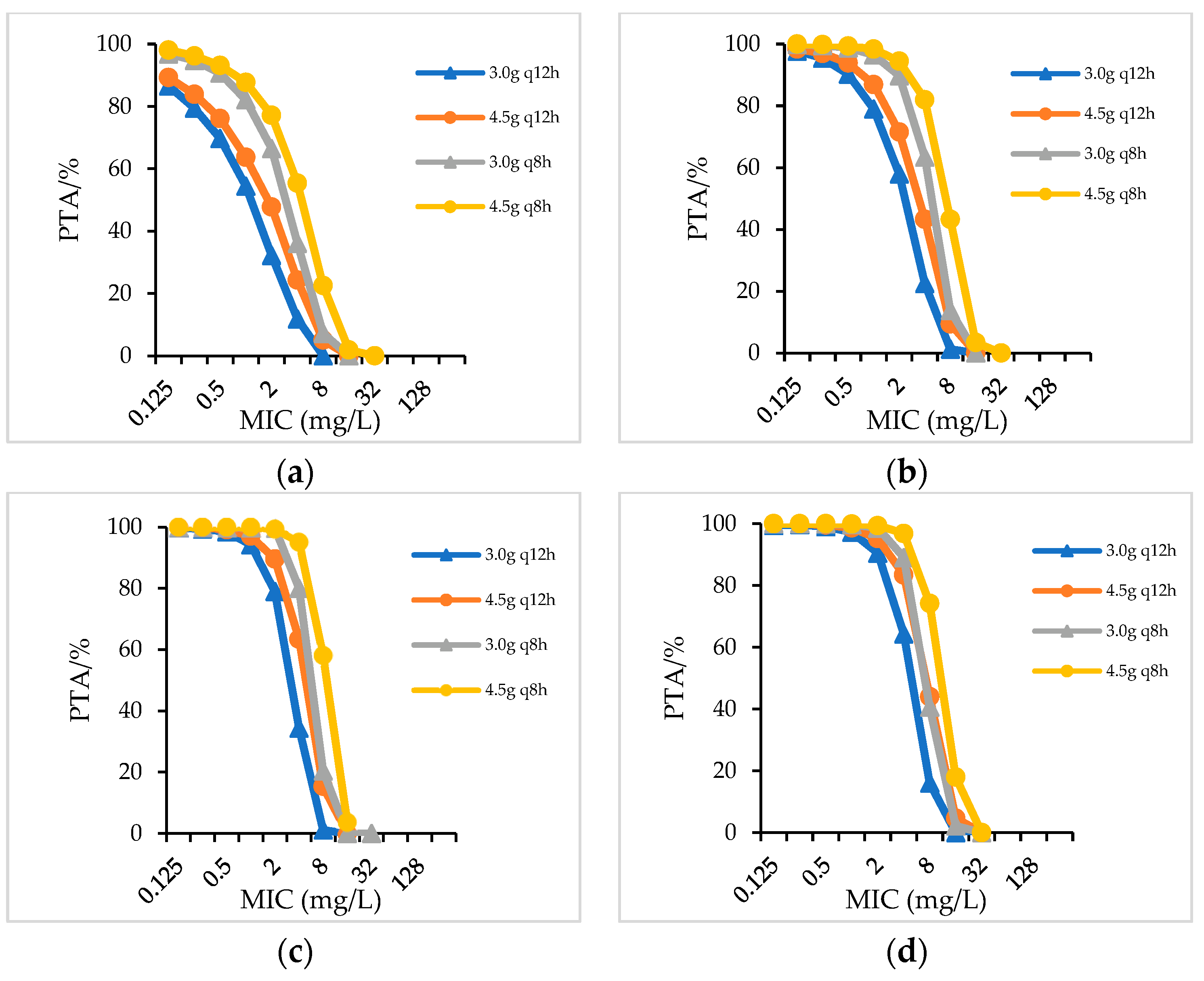
2.2.1. PTA
2.2.2. CFR
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates
4.2. Antimicrobial Agents and Culture Media
4.3. Antimicrobial Susceptibility Testing
4.4. Pharmacokinetics (PK) Parameters Used for Simulations
4.5. Monte Carlo Simulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paterson, D.L. Resistance in gram-negative bacteria: Enterobacteriaceae. Am. J. Med. 2006, 119 (Suppl. 1), S20–S70. [Google Scholar] [CrossRef] [PubMed]
- Pitout, J.D.; Laupland, K.B. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: An emerging public-health concern. Lancet Infect. Dis. 2008, 8, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kidd, J.M.; Stein, G.E.; Nicolau, D.P.; Kuti, J.L. Monte Carlo Simulation Methodologies for β-Lactam/β-Lactamase Inhibitor Combinations: Effect on Probability of Target Attainment Assessments. J. Clin. Pharmacol. 2020, 60, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Trang, M.; Dudley, M.N.; Bhavnani, S.M. Use of Monte Carlo simulation and considerations for PK-PD targets to support antibacterial dose selection. Curr. Opin. Pharmacol. 2017, 36, 107–113. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.; Advincula, M.R.; Malczynski, M.; Qi, C.; Bolon, M.; Scheetz, M.H. Correlations of antibiotic use and carbapenem resistance in Enterobacteriaceae. Antimicrob. Agents Chemother. 2013, 57, 5131–5133. [Google Scholar] [CrossRef]
- Rodríguez-Baño, J.; Navarro, M.D.; Retamar, P.; Picón, E.; Pascual, Á. Extended Spectrum Beta-Lactamases–Red Española de Investigación en Patología Infecciosa/Grupo de Estudio de Infección Hospitalaria Group. β-Lactam/β-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum β lactamase-producing Escherichia coli: A post hoc analysis of prospective cohorts. Clin. Infect. Dis. 2012, 54, 167–174. [Google Scholar]
- Chaubey, V.P.; Pitout, J.D.; Dalton, B.; Ross, T.; Church, D.L.; Gregson, D.B.; Laupland, K.B. Clinical outcome of empiric antimicrobial therapy of bacteremia due to extended-spectrum beta-lactamase producing Escherichia coli and Klebsiella pneumoniae. BMC Res. Notes 2010, 3, 116. [Google Scholar] [CrossRef]
- Harris, P.N.; Tambyah, P.A.; Paterson, D.L. β-lactam and β-lactamase inhibitor combinations in the treatment of extended-spectrum β-lactamase producing Enterobacteriaceae: Time for a reappraisal in the era of few antibiotic options? Lancet Infect. Dis. 2015, 15, 475–485. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, B.; Wang, J.; Cai, Y. Non-carbapenem β-lactam/β-lactamase inhibitors versus carbapenems for urinary tract infections caused by extended spectrum β-lactamase-producing Enterobacteriaceae: A systematic review. Int. J. Antimicrob. Agents. 2021, 58, 106410. [Google Scholar] [CrossRef]
- Zong, Z.; Lü, X.; Liu, Y.; Yang, Y.; Yu, R.; Fu, X.; Huang, W.; Cai, S.; Yu, Z.; Zeng, X.; et al. Piperacillin-sulbactam versus piperacillin-tazobactam: A multicenter, randomized, single-blind, controlled clinical trial. Int. J. Antimicrob. Agents. 2005, 26, 22–27. [Google Scholar] [CrossRef]
- Fleischhack, G.; Schmidt-Niemann, M.; Wulff, B.; Havers, W.; Marklein, G.; Hasan, C.; Bode, U. Piperacillin, beta-lactam inhibitor plus gentamicin as empirical therapy of a sequential regimen in febrile neutropenia of pediatric cancer patients. Support Care Cancer. 2001, 9, 372–379. [Google Scholar]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2022 Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacter ales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2022, 75, 187–212. [Google Scholar]
- Henderson, A.; Paterson, D.L.; Chatfield, M.D.; Tambyah, P.A.; Lye, D.C.; De, P.P.; Lin, R.T.P.; Chew, K.L.; Yin, M.; Lee, T.H.; et al. Association Between Minimum Inhibitory Concentration, Beta-lactamase Genes and Mortality for Patients Treated with Piperacillin/Tazobactam or Meropenem from the MERINO Study. Clin. Infect. Dis. 2021, 73, e3842–e3850. [Google Scholar] [CrossRef]
- Penwell, W.F.; Shapiro, A.B.; Giacobbe, R.A.; Gu, R.-F.; Gao, N.; Thresher, J.; McLaughlin, R.E.; Huband, M.D.; DeJonge, B.L.M.; Ehmann, D.E.; et al. Molecular mechanisms of sulbactam antibacterial activity and resistance determinants in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2015, 59, 1680–1689. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Matsumoto, K.; Ikawa, K.; Watanabe, E.; Shigemi, A.; Umezaki, Y.; Nakamura, K.; Ueno, K.; Morikawa, N.; Takeda, Y. Pharmacokinetic/pharmacodynamic evaluation of sulbactam against Acinetobacter baumannii in in vitro and murine thigh and lung infection models. Int. J. Antimicrob. Agents 2014, 43, 547–552. [Google Scholar] [CrossRef]
- Fishbain, J.; Peleg, A.Y. Treatment of Acinetobacter infections. Clin. Infect. Dis. 2010, 51, 79–84. [Google Scholar] [CrossRef]
- Crass, R.L.; Pai, M.P. Pharmacokinetics and Pharmacodynamics of β-Lactamase Inhibitors. Pharmacotherapy 2019, 39, 182–195. [Google Scholar] [CrossRef]
- Ambrose, P.G.; Bhavnani, S.M.; Jones, R.N. Pharmacokinetics-pharmacodynamics of cefepime and piperacillin-tazobactam against Escherichia coli and Klebsiella pneumoniae strains producing extended-spectrum beta-lactamases: Report from the ARREST program. Antimicrob. Agents Chemother. 2003, 47, 1643–1646. [Google Scholar] [CrossRef]
- Tamma, P.D.; Harris, P.N.A.; Mathers, A.J.; Wenzler, E.; Humphries, R.M. Breaking Down the Breakpoints: Rationale for the 2022 Clinical and Laboratory Standards Institute Revised Piperacillin-Tazobactam Breakpoints Against Enterobacterales. Clin. Infect. Dis. 2022, ciac688. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. CLSI supplement M100. 32th Edition. 2022. Available online: http://www.clsi.org/ (accessed on 5 September 2022).
- Rho, J.P.; Castle, S.; Smith, K.; Bawdon, R.E.; Norman, D.C. Effect of impaired renal function on the pharmacokinetics of coadministered cefoperazone and sulbactam. J. Antimicrob. Chemother. 1992, 29, 701–709. [Google Scholar] [CrossRef]
- Johnson, C.; Halstenson, C.; Kelloway, J.S.; Shapiro, B.E.; Zimmerman, S.W.; Tonelli, A.; Faulkner, R.; Dutta, A.; Haynes, J.; Greene, D.S.; et al. Single-dose pharmacokinetics of piperacillin and tazobactam in patients with renal disease. Clin. Pharmacol. Ther. 1992, 51, 32–41. [Google Scholar] [CrossRef] [PubMed]
| Organism, Phenotype/Genotype (No. of Isolates) | Antimicrobial Agent | MIC (mg/L) | % Susceptible | % Resistant | ||
|---|---|---|---|---|---|---|
| MIC Range | MIC50 | MIC90 | ||||
| Enterobacteriaceae (5692) | PIS | 0.5/0.25–56/128 | 8/4 | 64/32 | 62.9 | 20.9 |
| PTZ | 0.125/4–256/4 | 4/4 | 64/4 | 76.2 | 16.2 | |
| CSL | 0.125/0.06–128/64 | 1/0.5 | 32/16 | 85.9 | 7.0 | |
| Enterobacteriaceae, ESBL+ (2510) | PIS | 0.5/0.25–256/128 | 16/8 | 256/128 | 30.52 | 46.1 |
| PTZ | 0.125/4–256/4 | 8/4 | 128/4 | 54.15 | 37.81 | |
| CSL | 0.25/0.125–128/64 | 16/8 | 128/64 | 59.53 | 26.54 | |
| Enterobacteriaceae, ESBL− (3182) | PIS | 0.5/0.25–256/128 | 4/2 | 16/8 | 81.64 | 9.81 |
| PTZ | 0.125/4–128/4 | 4/4 | 16/4 | 83.87 | 8.85 | |
| CSL | 0.25/0.125–128/64 | 0.25/0.125 | 2/1 | 97.02 | 2.2 | |
| A. baumannii (116) | PIS | 0.5/0.25–256/128 | 2/1 | 16/8 | 83.6 | 9.4 |
| PTZ | 1/4–128/4 | 4/4 | 128/4 | 56.8 | 33.6 | |
| CSL | 1/0.5–128/64 | 4/2 | 16/8 | 76.7 | 7.7 | |
| P. aeruginosa (280) | PIS | 0.5/0.25–256/128 | 2/1 | 16/8 | 93.9 | 2.5 |
| PTZ | 0.25/4–128/4 | 2/4 | 4/4 | 95.0 | 3.9 | |
| CSL | 4/2–128/64 | 4/2 | 16/8 | 91.8 | 1.1 | |
| Species/CrCL(mL/min) | Dosage Regimens (CFR/%) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PIS | PTZ | CSL a | |||||||||||||||
| 3.0 g q8h | 6.0 g q12h | 3.0 g q6h | 4.5 g q8h | 6.0 g q8h | 4.5 g q6h | 4.5 gq12h | 3.375 g q8h | 2.25 g q6h | 4.5 g q8h | 3.375 g q6h | 4.5 g q6h | 3.0 gq12h | 4.5 gq12h | 3.0 g q8h | 4.5 g q8h | ||
| Gram-negative bacteria | >90 | 58.39 | 35.43 | 72.61 | 64.21 | 76.18 | 78.71 | 67.87 | 69.38 | 76.18 | 77.96 | 80.19 | 82.56 | - | - | - | - |
| 60–90 | 61.67 | 68.75 | 76.42 | 79.7 | 76 | 81.34 | 78.99 | 80.09 | 81.47 | 83 | 85.11 | 87 | 40.21 | 46.19 | 53.77 | 58.77 | |
| 40–59 | 68.92 | 72.11 | 79.26 | 80.25 | 81.82 | 86.24 | 81.23 | 83.54 | 83.78 | 85.65 | 87.25 | 87.49 | 51.03 | 57 | 62.01 | 68.65 | |
| 20–39 | 78.68 | 79.87 | 82.41 | 81.95 | 87.42 | 88.67 | 83.25 | 84.86 | 84.97 | 87.22 | 87.99 | 89.09 | 56.63 | 61.68 | 65.49 | 70.84 | |
| <20 | 84.77 | 85.71 | 89.34 | 88.49 | 90.22 | 92 | 86.85 | 88.23 | 87.69 | 90.1 | 90.35 | 91.78 | 62.03 | 68.27 | 68.5 | 75.96 | |
| Enterobacteriaceae | >90 | 50.99 | 55.32 | 71.12 | 62.37 | 70.29 | 78.83 | 67.05 | 69.05 | 74.84 | 74.1 | 78.48 | 82.61 | - | - | - | - |
| 60–90 | 60.03 | 65.78 | 75.44 | 79.28 | 75.77 | 81.2 | 78.55 | 79.59 | 82.41 | 82.7 | 85.1 | 86.59 | 42.03 | 46.88 | 55.68 | 60.08 | |
| 40–59 | 68.26 | 73.85 | 79.43 | 81.69 | 82.12 | 86.97 | 82.09 | 83.2 | 83.6 | 85.23 | 87.21 | 87.99 | 53.29 | 57.65 | 63.36 | 68.18 | |
| 20–39 | 76.07 | 80.97 | 82.74 | 82.04 | 87.45 | 89.13 | 83.46 | 85.16 | 84.28 | 87.04 | 88.25 | 89.22 | 58.61 | 62.94 | 66.22 | 70.93 | |
| <20 | 83.53 | 85.8 | 88.86 | 89.23 | 91.8 | 91.81 | 86.63 | 89.82 | 87.97 | 91.7 | 90.22 | 91.8 | 64.38 | 69.23 | 69.01 | 75.17 | |
| Enterobacteriaceae, ESBL+ | >90 | 42.78 | 21.78 | 49.24 | 46.43 | 48.02 | 52.61 | 43.89 | 46.87 | 52.83 | 51.19 | 57.38 | 62.43 | - | - | - | - |
| 60–90 | 47.94 | 34.67 | 58.11 | 52.16 | 59.47 | 60.71 | 55.01 | 56.55 | 60.99 | 61 | 63.89 | 66.23 | 5.91 | 9.4 | 13.37 | 19.66 | |
| Enterobacteriaceae, ESBL+ | 40–59 | 57.78 | 45.33 | 63.43 | 61.45 | 60.01 | 64.77 | 60.24 | 61.12 | 62.14 | 62.99 | 66.33 | 68.44 | 9.27 | 15.28 | 20.19 | 28.22 |
| 20–39 | 60.49 | 53.78 | 66.56 | 69.03 | 68.96 | 69.12 | 62.35 | 64.07 | 63.58 | 67.35 | 67.52 | 70.31 | 13.22 | 20.35 | 23.63 | 33.03 | |
| <20 | 61.13 | 63.38 | 69.04 | 69.25 | 74.3 | 74.59 | 65.8 | 67.46 | 68 | 71.41 | 85.34 | 87.27 | 20.67 | 29.47 | 29.23 | 38.93 | |
| Enterobacteriaceae, ESBL− | >90 | 58.19 | 64.65 | 78.98 | 71.28 | 77.9 | 85.23 | 74.78 | 76.07 | 81.73 | 81.32 | 87.54 | 90.25 | - | - | - | - |
| 60–90 | 77.46 | 83.3 | 87.87 | 84.8 | 88.39 | 91.13 | 85.13 | 86.13 | 89.55 | 89.54 | 91.85 | 93.46 | 65.87 | 72.34 | 83.15 | 87.09 | |
| 40–59 | 83.45 | 87.56 | 89.85 | 88.33 | 91.46 | 93.25 | 88.3 | 90.22 | 91.34 | 91.96 | 93.05 | 93.52 | 82.46 | 86.18 | 91.86 | 93.2 | |
| 20–39 | 89.28 | 89.98 | 91.48 | 91.26 | 93.46 | 94.48 | 89.14 | 91.43 | 92.31 | 92.95 | 93.4 | 94.33 | 89.21 | 91.6 | 93.15 | 95.17 | |
| <20 | 92.05 | 92.82 | 94.44 | 94.4 | 95.69 | 95.72 | 92.78 | 93.31 | 93.44 | 94.55 | 94.79 | 95.46 | 92.17 | 93.63 | 94.08 | 95.28 | |
| A.baumannii b | >60 | 22.01 | 26.76 | 35.72 | 29.75 | 35.17 | 43.24 | 52.15 | 55.35 | 54.1 | 57.42 | 57.52 | 60.35 | 3.87 | 6.87 | 11.45 | 19.65 |
| 31–60 | 71.46 | 73.48 | 79.8 | 78.16 | 81.24 | 82.42 | 53.87 | 56.68 | 56.95 | 57.92 | 58.57 | 60.92 | 25.39 | 39.35 | 55.22 | 66.83 | |
| 10–30 | 81.39 | 82.08 | 83.57 | 85.59 | 87.71 | 88.03 | 55.98 | 58.69 | 58.51 | 60.39 | 60.6 | 61.87 | 62.01 | 72.13 | 73.84 | 82.61 | |
| <10 | 87.35 | 87.93 | 89.05 | 90.71 | 90.82 | 92.13 | 58.76 | 60.21 | 61.59 | 63.21 | 63.5 | 65.75 | 81.96 | 90.68 | 86.43 | 92.14 | |
| P. aeruginosa | >90 | 71.75 | 76.28 | 81.9 | 88.47 | 86.48 | 91.16 | 91 | 92.79 | 94.21 | 93.68 | 94.95 | 94.94 | - | - | - | - |
| Species/CrCL(mL/min) | Dosage Regimens (CFR/%) | ||||||||||||||||
| PIS | PTZ | CSL | |||||||||||||||
| 3.0 g q8h | 6.0 g q12h | 3.0 g q6h | 4.5 g q8h | 6.0 g q8h | 4.5 g q6h | 4.5 gq12h | 3.375 g q8h | 2.25 g q6h | 4.5 g q8h | 3.375 g q6h | 4.5 g q6h | 3.0 gq12h | 4.5 gq12h | 3.0 g q8h | 4.5 g q8h | ||
| P. aeruginosa | 60–90 | 87.14 | 89.35 | 91.57 | 92.82 | 92.77 | 94.52 | 92.35 | 94.52 | 95.11 | 94.83 | 95.28 | 95.54 | 7.15 | 15.52 | 23.79 | 38.44 |
| 40–59 | 90.8 | 91.28 | 94.02 | 93.61 | 94.56 | 95.93 | 93.46 | 95.16 | 95.17 | 95.44 | 95.35 | 95.76 | 13.22 | 27.76 | 41.96 | 59.01 | |
| 20–39 | 93.58 | 94.12 | 94.9 | 94.47 | 96.25 | 96.18 | 94.11 | 95.22 | 95.25 | 95.51 | 95.38 | 95.85 | 20.81 | 41.54 | 52.89 | 69.97 | |
| <20 | 95.12 | 95.32 | 96.79 | 96.85 | 96.86 | 97.22 | 96.33 | 95.32 | 95.83 | 96.01 | 95.54 | 96.32 | 42.4 | 60.99 | 63.39 | 76.17 | |
| CrCL (mL/min) | No.of Participants | Vd (L) | t1/2 (h) | CL (L/h) | CLR (L/h) | PB (%) | |
|---|---|---|---|---|---|---|---|
| Piperacillin | >90 | 8 | 14.9 ± 1.6 | 0.95 ± 5.6 | 13.5 ± 1.32 | 10.08 ± 1.2 | 30 |
| 60–90 | 8 | 13.0 ± 1.4 | 1.10 ± 7.3 | 9.54 ± 1.14 | 4.64 ± 0.72 | 30 | |
| 40–59 | 9 | 12.5 ± 1.2 | 1.26 ± 8.5 | 8.04 ± 0.9 | 3.48 ± 0.54 | 30 | |
| 20–39 | 13 | 12.4 ± 1.0 | 1.43 ± 8.4 | 6.84 ± 0.66 | 2.28 ± 0.36 | 30 | |
| <20 | 12 | 13.1 ± 1.1 | 1.92 ± 15.0 | 4.98 ± 0.6 | 0.96 ± 0.18 | 30 | |
| Cefoperazone | >60 | 6 | 11.6 ± 4.8 | 1.4 ± 0.2 | 5.66 ± 2.39 | 0.8 ± 0.08 | 82–93 |
| 31–60 | 6 | 11.1 ± 4.3 | 1.9 ± 0.9 | 4.06 ± 1.17 | 0.82 ± 0.26 | 82–93 | |
| 10–30 | 6 | 12.9 ± 4.6 | 2.5 ± 0.8 | 3.65 ± 0.81 | 0.46 ± 0.44 | 82–93 | |
| <10 | 6 | 15.4 ± 5.8 | 4.0 ± 1.9 | 2.95 ± 1.2 | NA | 82–93 | |
| Sulbactam | >60 | 6 | 30.5 ± 14.5 | 0.7 ± 0.2 | 35.57 ± 20.75 | 10.34 ± 1.94 | 38 |
| 31–60 | 6 | 24.8 ± 8.4 | 1.6 ± 0.8 | 11.93 ± 2.75 | 6.22 ± 1.96 | 38 | |
| 10–30 | 6 | 28.5 ± 8.0 | 4.1 ± 2.9 | 7.0 ± 4.2 | 3.97 ± 3.97 | 38 | |
| <10 | 6 | 27.6 ± 8.8 | 8.4 ± 3.9 | 2.68 ± 0.98 | NA | 38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Xiong, L.; Yu, W.; Huang, C.; Ji, J.; Ying, C.; Liu, Z.; Chen, Y.; Xiao, Y. Evaluation of Piperacillin/Sulbactam, Piperacillin/Tazobactam and Cefoperazone/Sulbactam Dosages in Gram-Negative Bacterial Bloodstream Infections by Monte Carlo Simulation. Antibiotics 2023, 12, 363. https://doi.org/10.3390/antibiotics12020363
Wang X, Xiong L, Yu W, Huang C, Ji J, Ying C, Liu Z, Chen Y, Xiao Y. Evaluation of Piperacillin/Sulbactam, Piperacillin/Tazobactam and Cefoperazone/Sulbactam Dosages in Gram-Negative Bacterial Bloodstream Infections by Monte Carlo Simulation. Antibiotics. 2023; 12(2):363. https://doi.org/10.3390/antibiotics12020363
Chicago/Turabian StyleWang, Xueting, Luying Xiong, Wei Yu, Chen Huang, Jinru Ji, Chaoqun Ying, Zhiying Liu, Yunbo Chen, and Yonghong Xiao. 2023. "Evaluation of Piperacillin/Sulbactam, Piperacillin/Tazobactam and Cefoperazone/Sulbactam Dosages in Gram-Negative Bacterial Bloodstream Infections by Monte Carlo Simulation" Antibiotics 12, no. 2: 363. https://doi.org/10.3390/antibiotics12020363
APA StyleWang, X., Xiong, L., Yu, W., Huang, C., Ji, J., Ying, C., Liu, Z., Chen, Y., & Xiao, Y. (2023). Evaluation of Piperacillin/Sulbactam, Piperacillin/Tazobactam and Cefoperazone/Sulbactam Dosages in Gram-Negative Bacterial Bloodstream Infections by Monte Carlo Simulation. Antibiotics, 12(2), 363. https://doi.org/10.3390/antibiotics12020363







