The Clash of the Titans: COVID-19, Carbapenem-Resistant Enterobacterales, and First mcr-1-Mediated Colistin Resistance in Humans in Romania
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Resistance Patterns According to COVID-19
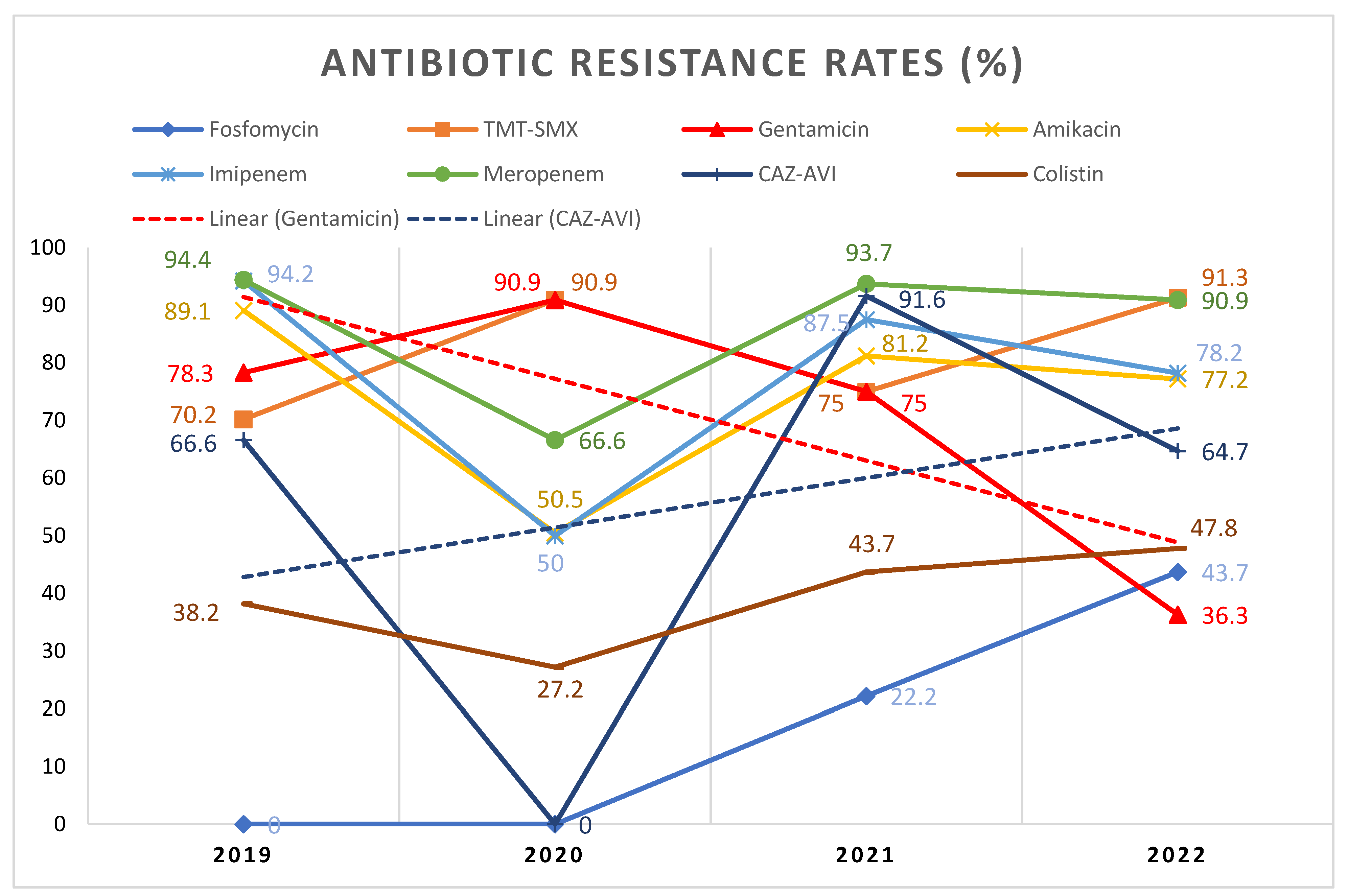
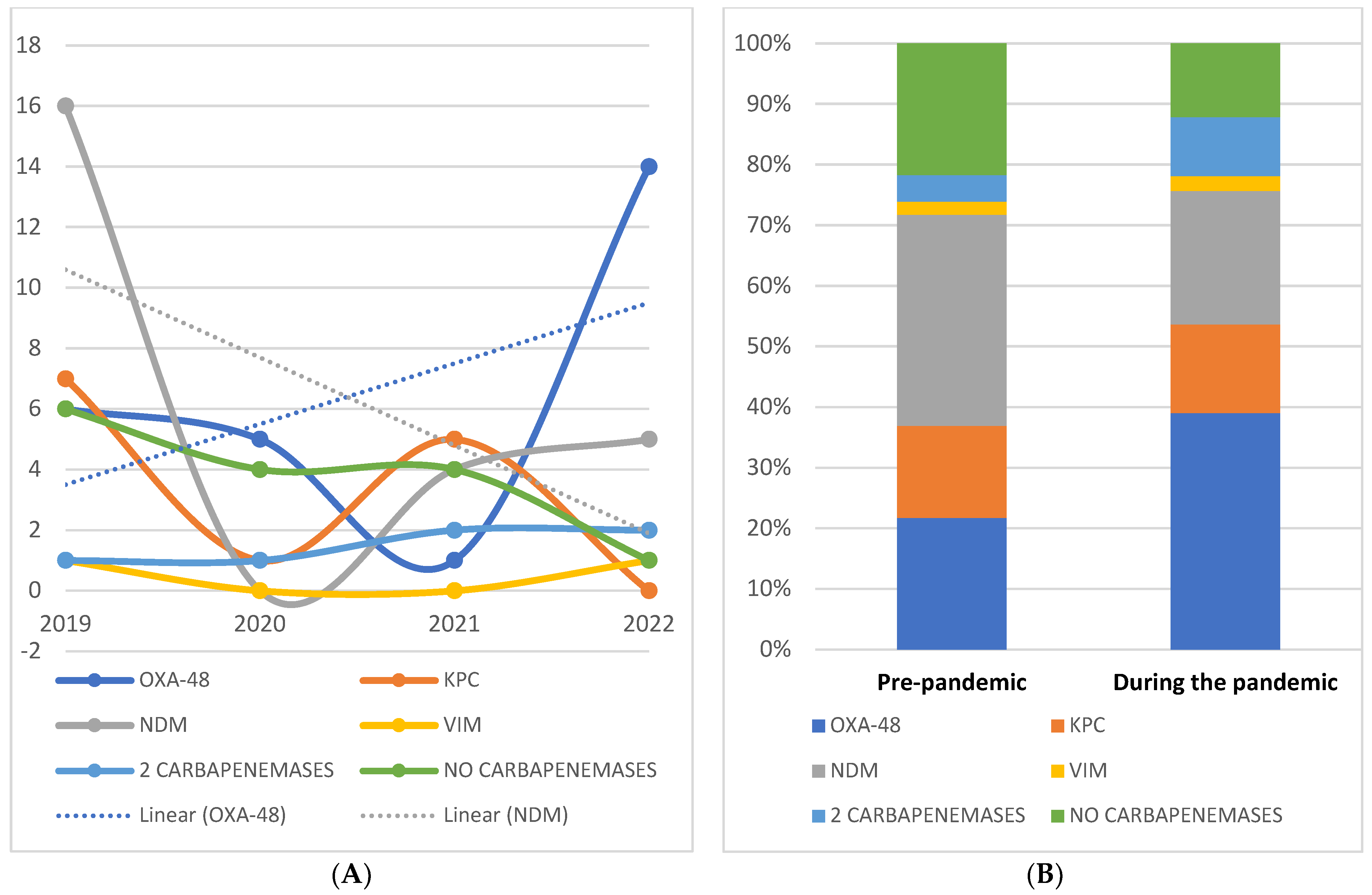
2.3. Specific Resistance Profiles
2.4. Risk Factors
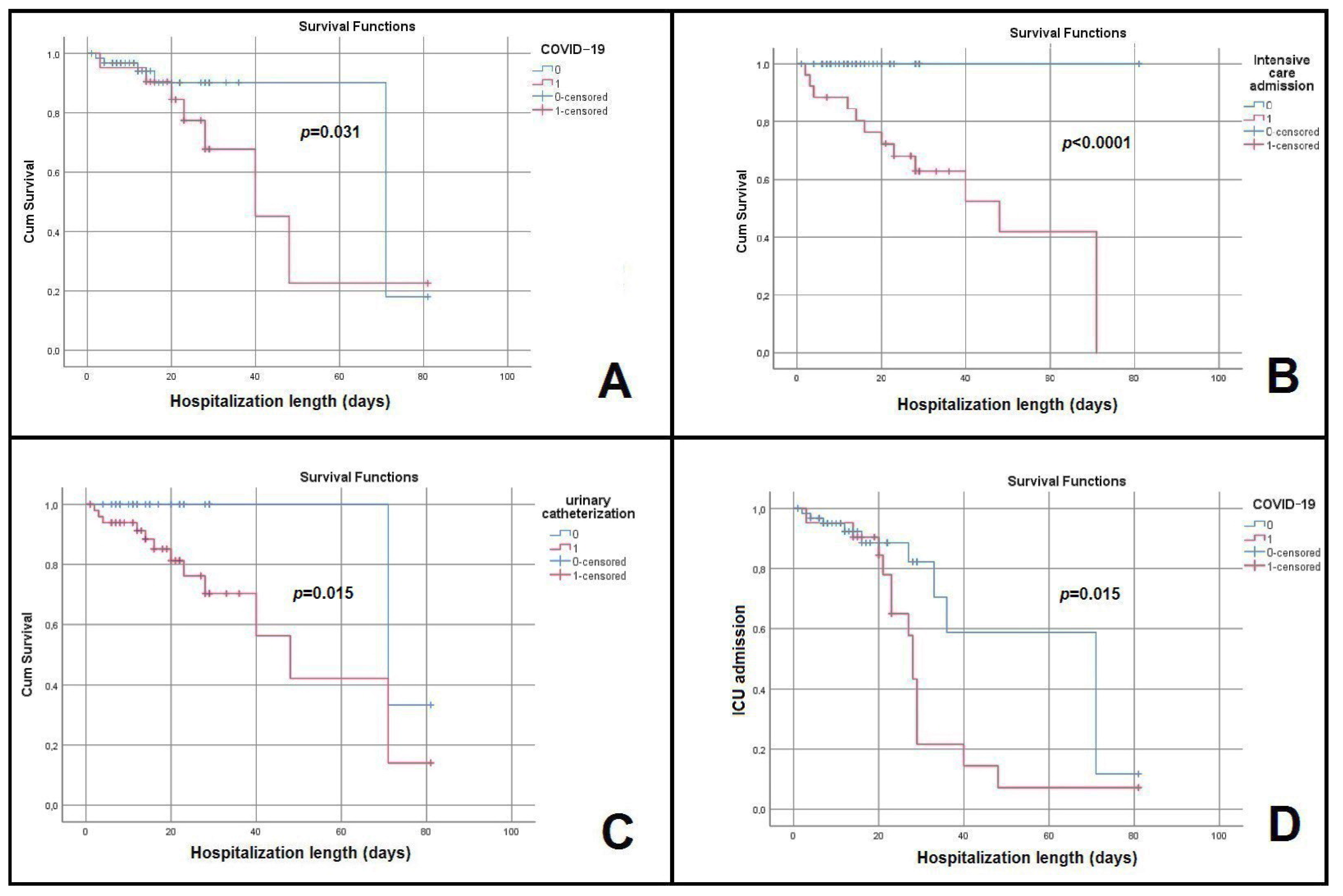
2.5. Evolution, Prognosis, and Predictors of Poor Outcome
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Statistical Analysis
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 18 November 2022).
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Said, K.B.; Alsolami, A.; Moussa, S.; Alfouzan, F.; Bashir, A.I.; Rashidi, M.; Aborans, R.; Taha, T.E.; Almansour, H.; Alazmi, M.; et al. COVID-19 Clinical Profiles and Fatality Rates in Hospitalized Patients Reveal Case Aggravation and Selective Co-Infection by Limited Gram-Negative Bacteria. Int. J. Environ. Res. Public Health 2022, 19, 5270. [Google Scholar] [CrossRef] [PubMed]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Contou, D.; Claudinon, A.; Pajot, O.; Micaëlo, M.; Longuet Flandre, P.; Dubert, M.; Cally, R.; Logre, E.; Fraissé, M.; Mentec, H.; et al. Bacterial and Viral Co-Infections in Patients with Severe SARS-CoV-2 Pneumonia Admitted to a French ICU. Ann. Intensive Care 2020, 10, 119. [Google Scholar] [CrossRef]
- Shafran, N.; Shafran, I.; Ben-Zvi, H.; Sofer, S.; Sheena, L.; Krause, I.; Shlomai, A.; Goldberg, E.; Sklan, E.H. Secondary bacterial infection in COVID-19 patients is a stronger predictor for death compared to influenza patients. Sci. Rep. 2021, 11, 12703. [Google Scholar] [CrossRef]
- Lai, C.C.; Wang, C.Y.; Hsueh, P.R. Co-Infections among Patients with COVID-19: The Need for Combination Therapy with Non-Anti-SARS-CoV-2 Agents? J. Microbiol. Immunol. Infect. 2020, 53, 505–512. [Google Scholar] [CrossRef]
- Greco, R.; Panetta, V.; Della Rocca, M.T.; Durante, A.; Di Caprio, G.; Maggi, P. Profile of Co-Infection Prevalence and Antibiotics Use among COVID-19 Patients. Pathogens 2022, 11, 1250. [Google Scholar] [CrossRef]
- Sulayyim, H.J.A.; Ismail, R.; Hamid, A.A.; Ghafar, N.A. Antibiotic Resistance during COVID-19: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 11931. [Google Scholar] [CrossRef]
- Bentivegna, E.; Luciani, M.; Arcari, L.; Santino, I.; Simmaco, M.; Martelletti, P. Reduction of multidrug-resistant (MDR) bacterial infections during the COVID-19 pandemic: A retrospective study. Int. J. Environ. Res. Public Health 2021, 18, 1003. [Google Scholar] [CrossRef]
- El-Sokkary, R.; Erdem, H.; Kullar, R.; Pekok, A.U.; Amer, F.; Grgić, S.; Carevic, B.; El-Kholy, A.; Liskova, A.; Özdemir, A.; et al. Self-reported antibiotic stewardship and infection control measures from 57 intensive care units: An international ID-IRI survey. J. Infect. Public Health 2022, 15, 950–954. [Google Scholar] [CrossRef]
- Torjesen, I. COVID-19: Omicron May Be More Transmissible than Other Variants and Partly Resistant to Existing Vaccines, Scientists Fear. BMJ 2021, 375, n2943. [Google Scholar] [CrossRef]
- O’Toole, R.F. The interface between COVID-19 and bacterial healthcare-associated infections. Clin. Microbiol. Infect. 2021, 27, 1772–1776. [Google Scholar] [CrossRef]
- El-Sokkary, R.; Uysal, S.; Erdem, H.; Kullar, R.; Pekok, A.U.; Amer, F.; Grgi’c, S.; Carevic, B.; El-Kholy, A.; Liskova, A.; et al. Profiles of multidrug-resistant organisms among patients with bacteremia in intensive care units: An international ID-IRI survey. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2323–2334. [Google Scholar] [CrossRef]
- Bader, M.S.; Loeb, M.; Leto, D.; Brooks, A.A. Treatment of urinary tract infections in the era of antimicrobial resistance and new antimicrobial agents. Postgrad. Med. 2020, 132, 234–250. [Google Scholar] [CrossRef]
- Miftode, I.-L.; Nastase, E.V.; Miftode, R.; Miftode, E.G.; Iancu, L.S.; Luncă, C.; Păduraru, D.-T.A.; Costache, I.-I.; Stafie, C.S.; Dorneanu, O.-S. Insights into multidrug-resistant K. pneumoniae urinary tract infections: From susceptibility to mortality. Exp. Ther. Med. 2021, 22, 1086. [Google Scholar] [CrossRef]
- Miftode, I.-L.; Pasare, M.-A.; Miftode, R.-S.; Nastase, E.; Plesca, C.E.; Lunca, C.; Miftode, E.-G.; Timpau, A.-S.; Iancu, L.S.; Dorneanu, O.S. What Doesn’t Kill Them Makes Them Stronger: The Impact of the Resistance Patterns of Urinary Enterobacterales Isolates in Patients from a Tertiary Hospital in Eastern Europe. Antibiotics 2022, 11, 548. [Google Scholar] [CrossRef]
- Ginsburg, A.S.; Klugman, K.P. COVID-19 pneumonia and the appropriate use of antibiotics. Lancet Glob. Health 2020, 8, e1453–e1454. [Google Scholar] [CrossRef]
- Ghosh, S.; Bornman, C.; Zafer, M.M. Antimicrobial Resistance Threats in the emerging COVID-19 pandemic: Where do we stand? J. Infect. Public Health 2021, 14, 555–560. [Google Scholar] [CrossRef]
- Miftode, E.; Luca, C.; Manciuc, C.; Vâtă, A.; Hunea, I.; Miftode, L.; Bădescu, A.; Dorneanu, O. COVID-19: A Course Through Stormy Waters. Med. Surg. J. Rev. Med. Chir. 2020, 124, 351–362. [Google Scholar]
- Timpau, A.S.; Miftode, R.S.; Petris, A.O.; Costache, I.I.; Miftode, I.L.; Rosu, F.M.; Anton-Paduraru, D.T.; Leca, D.; Miftode, E.G. Mortality Predictors in Severe COVID-19 Patients from an East European Tertiary Center: A Never-Ending Challenge for a No Happy Ending Pandemic. J. Clin. Med. 2022, 11, 58. [Google Scholar] [CrossRef]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.W.; Wu, X.X.; Jiang, X.G.; Xu, K.J.; Ying, L.J.; Ma, C.L.; Li, S.B.; Wang, H.Y.; Zhang, S.; Gao, H.N.; et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-CoV-2) outside of Wuhan, China: Retrospective case series. BMJ 2020, 368, m606. [Google Scholar] [CrossRef] [PubMed]
- Lakbar, I.; Delamarre, L.; Curtel, F.; Duclos, G.; Bezulier, K.; Gragueb-Chatti, I.; Martin-Loeches, I.; Forel, J.-M.; Leone, M. Antimicrobial Stewardship during COVID-19 Outbreak: A Retrospective Analysis of Antibiotic Prescriptions in the ICU across COVID-19 Waves. Antibiotics 2022, 11, 1517. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Lucien, M.A.B.; Canarie, M.F.; Kilgore, P.E.; Jean-Denis, G.; Fénélon, N.; Pierre, M.; Cerpa, M.; Joseph, G.A.; Maki, G.; Zervos, M.J.; et al. Antibiotics and antimicrobial resistance in the COVID-19 era: Perspective from resource-limited settings. Int. J. Infect. Dis. 2021, 104, 250–254. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M. Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Rawson, T.M.; Wilson, R.C.; Holmes, A. Understanding the role of bacterial and fungal infection in COVID-19. Clin. Microbiol. Infect. 2021, 27, 9–11. [Google Scholar] [CrossRef]
- Lasko, M.J.; Nicolau, D.P. Carbapenem-Resistant Enterobacterales: Considerations for Treatment in the Era of New Antimicrobials and Evolving Enzymology. Curr. Infect. Dis. Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Thaden, J.T.; Pogue, J.M.; Kaye, K.S. Role of newer and re-emerging older agents in the treatment of infections caused by carbapenem-resistant Enterobacteriaceae. Virulence 2017, 8, 403–416. [Google Scholar] [CrossRef]
- Trecarichi, E.M.; Pagano, L.; Martino, B.; Candoni, A.; Di Blasi, R.; Nadali, G.; Fianchi, L.; Delia, M.; Sica, S.; Perriello, V.; et al. Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: Clinical impact of carbapenem resistance in a multicentre prospective survey. Am. J. Hematol. 2016, 91, 1076–1081. [Google Scholar] [CrossRef]
- Livermore, D.M.; Warner, M.; Mushtaq, S.; Doumith, M.; Zhang, J.; Woodford, N. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int. J. Antimicrob. Agents 2011, 37, 415–419. [Google Scholar] [CrossRef]
- Livermore, D.M.; Nicolau, D.P.; Hopkins, K.L.; Meunier, D. Carbapenem-Resistant Enterobacterales, Carbapenem Resistant Organisms, Carbapenemase-Producing Enterobacterales, and Carbapenemase-Producing Organisms: Terminology Past its “Sell-By Date” in an Era of New Antibiotics and Regional Carbapenemase Epidemiology. Clin. Infect. Dis. 2020, 71, 1776–1782. [Google Scholar] [CrossRef]
- van Duin, D.; Lok, J.J.; Earley, M.; Cober, E.; Richter, S.S.; Perez, F.; Salata, R.A.; Kalayjian, R.C.; Watkins, R.R.; Doi, Y.; et al. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin. Infect. Dis. 2018, 66, 163–171. [Google Scholar] [CrossRef]
- Wunderink, R.G.; Giamarellos-Bourboulis, E.J.; Rahav, G.; Mathers, A.J.; Bassetti, M.; Vazquez, J.; Cornely, O.A.; Solomkin, J.; Bhowmick, T.; Bishara, J.; et al. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: The TANGO II randomized clinical trial. Infect. Dis. Ther. 2018, 7, 439–455. [Google Scholar] [CrossRef]
- Livermore, D.M.; Mushtaq, S.; Warner, M.; Zhang, J.; Maharjan, S.; Doumith, M.; Woodford, N. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2011, 55, 390–394. [Google Scholar] [CrossRef]
- Sousa, A.; Pérez-Rodríguez, M.T.; Soto, A.; Rodríguez, L.; Pérez-Landeiro, A.; Martínez-Lamas, L.; Nodar, A.; Crespo, M. Effectiveness of ceftazidime/avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 3170–3175. [Google Scholar] [CrossRef]
- Di Bella, S.; Giacobbe, D.R.; Maraolo, A.E.; Viaggi, V.; Luzzati, R.; Bassetti, M.; Luzzaro, F.; Principe, L. Resistance to ceftazidime/avibactam in infections and colonisations by KPC-producing Enterobacterales: A systematic review of observational clinical studies. J. Glob. Antimicrob. Resist. 2021, 25, 268–281. [Google Scholar] [CrossRef]
- Tiri, B.; Sensi, E.; Marsiliani, V.; Cantarini, M.; Priante, G.; Vernelli, C.; Martella, L.A.; Costantini, M.; Mariottini, A.; Andreani, P.; et al. Antimicrobial Stewardship Program, COVID-19, and Infection Control: Spread of Carbapenem-Resistant Klebsiella Pneumoniae Colonization in ICU COVID-19 Patients. What Did Not Work? J. Clin. Med. 2020, 9, 2744. [Google Scholar] [CrossRef]
- Jeon, K.; Jeong, S.; Lee, N.; Park, M.-J.; Song, W.; Kim, H.-S.; Kim, H.S.; Kim, J.-S. Impact of COVID-19 on Antimicrobial Consumption and Spread of Multidrug-Resistance in Bacterial Infections. Antibiotics 2022, 11, 535. [Google Scholar] [CrossRef]
- Wielders, C.C.H.; Schouls, L.M.; Woudt, S.H.S.; Notermans, D.W.; Hendrickx, A.P.A.; Bakker, J.; Kuijper, E.J.; Schoffelen, A.F.; de Greeff, S.C.; Infectious Diseases Surveillance Information System-Antimicrobial Resistance (ISIS-AR) Study Group; et al. Epidemiology of carbapenem-resistant and carbapenemase-producing Enterobacterales in the Netherlands 2017–2019. Antimicrob. Resist. Infect. Control. 2022, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Kostyanev, T.; Vilken, T.; Lammens, C.; Timbermont, L.; Van’t Veen, A.; Goossens, H. Detection and prevalence of carbapenem-resistant Gram-negative bacteria among European laboratories in the COMBACTE network: A COMBACTE LAB-Net survey. Int. J. Antimicrob. Agents 2019, 53, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Cogliati Dezza, F.; Arcari, G.; Alessi, F.; Valeri, S.; Curtolo, A.; Sacco, F.; Ceccarelli, G.; Raponi, G.; Alessandri, F.; Mastroianni, C.M.; et al. Clinical Impact of COVID-19 on Multi-Drug-Resistant Gram-Negative Bacilli Bloodstream Infections in an Intensive Care Unit Setting: Two Pandemics Compared. Antibiotics 2022, 11, 926. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Fang, X.; Zhang, J.; Zheng, X.; Shangguan, S.; Chen, S.; Shen, Y.; Liu, Z.; Li, J.; Zhang, R.; et al. Impact of carbapenem resistance on mortality in patients infected with Enterobacteriaceae: A systematic review and meta-analysis. BMJ Open 2021, 11, e054971. [Google Scholar] [CrossRef]
- Paño Pardo, J.R.; Serrano Villar, S.; Ramos Ramos, J.C.; Pintado, V. Infections caused by carbapenemase-producing Enterobacteriaceae: Risk factors, clinical features and prognosis. Enferm. Infecc. Microbiol. Clin. 2014, 32, 41–48. [Google Scholar] [CrossRef]
- Fattorini, L.; Creti, R.; Palma, C.; Pantosti, A.; Unit of Antibiotic Resistance and Special Pathogens; Unit of Antibiotic Resistance and Special Pathogens of the Department of Infectious Diseases; Istituto Superiore di Sanità, Rome. Bacterial coinfections in COVID-19: An underestimated adversary. Ann. Dell’istituto Super. Sanita 2020, 56, 359–364. [Google Scholar]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism mcr-1 in animals and human beings in China:A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Fenwick, A.J.; Bergman, Y.; Lewis, S.; Yee, R.; Uhlemann, A.C.; Cole, N.; Kohner, P.; Ordak, C.; Green, D.A.; Schuetz, A.N.; et al. Evaluation of the NG-Test MCR-1 Lateral Flow Assay and EDTA-Colistin Broth Disk Elution Methods to Detect Plasmid-Mediated Colistin Resistance among Gram-Negative Bacterial Isolates. J. Clin. Microbiol. 2020, 58, e01823-19. [Google Scholar] [CrossRef]
- Maciuca, I.E.; Cummins, M.L.; Cozma, A.P.; Rimbu, C.M.; Guguianu, E.; Panzaru, C.; Licker, M.; Szekely, E.; Flonta, M.; Djordjevic, S.P.; et al. Genetic Features of mcr-1 Mediated Colistin Resistance in CMY-2-Producing Escherichia coli From Romanian Poultry. Front. Microbiol. 2019, 10, 2267. [Google Scholar] [CrossRef]
- Gagliotti, C.; Bolzoni, L.; Carretto, E.; Sarti, M.; Ricchizzi, E.; Ambretti, S.; Barozzi, A.; Bracchi, C.; Confalonieri, M.; Menozzi, I.; et al. Reduction trend of mcr-1 circulation in Emilia-Romagna Region, Italy. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2585–2592. [Google Scholar] [CrossRef]
- Stefaniuk, E.M.; Kozińska, A.; Waśko, I.; Baraniak, A.; Tyski, S. Occurrence of Beta-Lactamases in Colistin-Resistant Enterobacterales Strains in Poland—A Pilot Study. Pol. J. Microbiol. 2021, 70, 283–288. [Google Scholar] [CrossRef]
- Vijay, S.; Bansal, N.; Rao, B.K.; Veeraraghavan, B.; Rodrigues, C.; Wattal, C.; Goyal, J.P.; Tadepalli, K.; Mathur, P.; Venkateswaran, R.; et al. Secondary Infections in Hospitalized COVID-19 Patients: Indian Experience. Infect. Drug Resist. 2021, 14, 1893–1903. [Google Scholar] [CrossRef]
- Montrucchio, G.; Costamagna, A.; Pierani, T.; Petitti, A.; Sales, G.; Pivetta, E.; Corcione, S.; Curtoni, A.; Cavallo, R.; De Rosa, F.G.; et al. Bloodstream Infections Caused by Carbapenem-Resistant Pathogens in Intensive Care Units: Risk Factors Analysis and Proposal of a Prognostic Score. Pathogens 2022, 11, 718. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, M.; Sun, F.; Zhou, J.; Wang, Y.; Zhu, D.; Chen, Z.; Chen, Q.; Chang, Q.; Liu, H.; et al. Epidemiology, mortality and risk factors for patients with K. pneumoniae bloodstream infections: Clinical impact of carbapenem resistance in a tertiary university teaching hospital of Beijing. J. Infect. Public Health 2020, 13, 1710–1714. [Google Scholar] [CrossRef]
- Fraenkel-Wandel, Y.; Raveh-Brawer, D.; Wiener-Well, Y.; Yinnon, A.M.; Assous, M.V. Mortality due to blaKPC Klebsiella pneumoniae bacteraemia. J. Antimicrob. Chemother. 2016, 71, 1083–1087. [Google Scholar] [CrossRef]
- Miftode, E.; Miftode, L.; Coman, I.; Prepeliuc, C.; Obreja, M.; Stămăteanu, O.; Părângă, T.G.; Leca, D.; Pleşca, C.E. Diabetes Mellitus-A Risk Factor for Unfavourable Outcome in COVID-19 Patients-The Experience of an Infectious Diseases Regional Hospital. Healthcare 2021, 9, 788. [Google Scholar] [CrossRef]
- Soontaros, S.; Leelakanok, N. Association between carbapenem-resistant Enterobacteriaceae and death: A systematic review and meta-analysis. Am. J. Infect. Control 2019, 47, 1200–1212. [Google Scholar] [CrossRef]
- Miftode, R.-S.; Costache, I.-I.; Cianga, P.; Petris, A.O.; Cianga, C.-M.; Maranduca, M.-A.; Miftode, I.-L.; Constantinescu, D.; Timpau, A.-S.; Crisan, A.; et al. The Influence of Socioeconomic Status on the Prognosis and Profile of Patients Admitted for Acute Heart Failure during COVID-19 Pandemic: Overestimated Aspects or a Multifaceted Hydra of Cardiovascular Risk Factors? Healthcare 2021, 9, 1700. [Google Scholar] [CrossRef]
- Richards, F.; Kodjamanova, P.; Chen, X.; Li, N.; Atanasov, P.; Bennetts, L.; Patterson, B.J.; Yektashenas, B.; Mesa-Frias, M.; Tronczynski, K.; et al. Economic Burden of COVID-19: A Systematic Review. Clinicoecon. Outcomes Res. 2022, 14, 293–307. [Google Scholar] [CrossRef]
- Kanahashi, T.; Matsumura, Y.; Yamamoto, M.; Tanaka, M.; Nagao, M. Comparison of the Xpert Carba-R and NG-Test CARBA5 for the detection of carbapenemases in an IMP-type carbapenemase endemic region in Japan. J. Infect. Chemother. 2021, 27, 503–506. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, L.; Liu, J.; Lei, J.; Gao, X.; Tenover, F.C.; Lei, K.; Tang, Y.W.; Geng, Y.; He, A. Parallel Validation of the NG-Test Carba 5 and the Xpert Carba-R for Detection and Characterization of Carbapenem-Resistant Enterobacterales Causing Bloodstream Infections. J. Mol. Diagn. 2021, 23, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Kuo, Y.W.; Lee, N.Y.; Tien, N.; Liao, C.H.; Teng, L.J.; Ko, W.C.; Hsueh, P.R.; SMART study group. Evaluating NG-Test CARBA 5 Multiplex Immunochromatographic and Cepheid Xpert CARBA-R Assays among Carbapenem-Resistant Enterobacterales Isolates Associated with Bloodstream Infection. Microbiol. Spectr. 2022, 10, e0172821. [Google Scholar] [CrossRef] [PubMed]
- Volland, H.; Dortet, L.; Bernabeu, S.; Boutal, H.; Haenni, M.; Madec, J.Y.; Robin, F.; Beyrouthy, R.; Naas, T.; Simon, S. Development and Multicentric Validation of a Lateral Flow Immunoassay for Rapid Detection of MCR-1-Producing Enterobacteriaceae. J. Clin. Microbiol. 2019, 57, e01454-18. [Google Scholar] [CrossRef] [PubMed]
| COVID-19 Patients (N = 21) | Non-COVID-19 Patients (N = 66) | p-Value | OR (CI 95%) | |
|---|---|---|---|---|
| Mean age (years) | 70 ± 12.2 | 68.9 ± 12.5 | 0.745 | - |
| Male gender | 6 (28.5%) | 37 (56%) | 0.02 | 0.31 (0.10–0.90) |
| Urinary catheterization | 17 (80.9%) | 34 (51.5%) | 0.017 | 4.00 (1.21–13.16) |
| Previous hospitalizations | 15 (71.4%) | 47 (71.2%) | 0.985 | 1.01 (0.34–2.99) |
| Previous antibiotic therapy | 13 (61.9%) | 33 (50%) | 0.453 | 1.62 (0.59–4.43) |
| Rural area of residence | 9 (42.9%) | 31 (47%) | 0.805 | 0.84 (0.31–2.27) |
| Inappropriate empirical therapy | 19 (90.5%) | 28 (43.1%) | <0.001 | 12.55 (2.69–58.41) |
| Associated pathologies | ||||
| Respiratory | 19 (90.5%) | 12 (18.2%) | <0.001 | 42.75 (8.75–208.71) |
| Cardio-vascular | 14 (66.7%) | 51(77.3%) | 0.390 | 0.58 (0.20–1.72) |
| Neurological | 9 (42.9%) | 29 (43.9%) | 0.998 | 0.95 (0.33–2.57) |
| Oncological | 2 (9.5%) | 20 (30.3%) | 0.083 | 0.24 (0.05–1.13) |
| Digestive | 11 (52.4%) | 40 (60.6%) | 0.613 | 0.71 (0.26- 1.91) |
| Diabetes mellitus | 10 (47.6%) | 19 (28.8%) | 0.121 | 2.24 (0.82–6.16) |
| Chronic kidney disease | 5 (23.8%) | 22 (33.3%) | 0.589 | 0.62 (0.202–1.92) |
| Hydronephrosis | 0 (0%) | 8 (12.1%) | 0.094 | 0.73 (0.64–0.83) |
| rUTIs | 1 (4.8%) | 18 (27.3%) | 0.034 | 0.13 (0.01–1.06) |
| Obesity | 4 (19%) | 8 (12.1%) | 0.473 | 1.70 (0.45–6.36) |
| Chronic alcohol consumption | 0 (0%) | 14 (21.2%) | 0.018 | 0.71 (0.61–0.82) |
| Smoking | 0 (0%) | 9 (13.6%) | 0.106 | 0.73 (0.63–0.83) |
| Outcome | ||||
| ICU transfer | 14 (66.7%) | 12 (18.2%) | <0.001 | 9.00 (2.99–27.02) |
| Death | 7 (33.3%) | 8 (12.1%) | 0.043 | 3.62 (1.12–11.68) |
| Hospitalization length | 26.1 ± 15.7 | 18.1 ± 17.9 | 0.014 | - |
| Antibiotic | Non COVID-19 (N = 66) | COVID-19 (N = 21) | p-Value | χ2 | OR (CI 95%) |
|---|---|---|---|---|---|
| Nitrofurantoin | 8/10 (80%) | 6/6 (100%) | 0.187 | 1.37 | UND |
| Fosfomycin | 4/18 (22.2%) | 5/10 (50%) | 0.015 | 2.27 | 0.28 (0.05–1.50) |
| Trimethoprim–sulfamethoxazole | 53/66 (80.3%) | 16/21 (76.1%) | 0.341 | 0.16 | 1.27 (0.39–4.11) |
| Cefepime | 63/65 (96.9%) | 21/21 (100%) | 0.248 | 0.06 | UND |
| Cefoxitine | 31/33 (93.9%) | 21/21 (100%) | 0.184 | 1.32 | UND |
| Gentamicin | 50/65 (76.9%) | 9/21 (42.8%) | 0.002 | 8.55 | 4.44 (1.57–15.56) |
| Tobramycin | 35/37 (94.5%) | 21/21 (100%) | 0.201 | 1.17 | UND |
| Amikacin | 51/65 (78.4%) | 18/21 (85.7%) | 0.250 | 0.52 | 0.60 (0.15–2.36) |
| Ciprofloxacin | 65/66 (98.4%) | 20/21 (95.2%) | 0.241 | 0.74 | 3.25 (0.19–54.35) |
| Ertapenem | 66/66 (100%) | 21/21 (100%) | 1 | 3.13 | UND |
| Imipenem | 53/63 (84.1%) | 17/21 (80.9%) | 0.363 | 0.11 | 1.24 (0.34–4.49) |
| Meropenem | 56/63 (88.8%) | 19/20 (95%) | 0.238 | 0.65 | 0.42 (0.04–3.64) |
| Piperacillin + tazobactam | 66/66 (100%) | 20/21 (95.2%) | 0.136 | 3.17 | UND |
| Ceftazidime + avibactam | 23/37 (62.1%) | 13/18 (72.2%) | 0.242 | 0.54 | 0.63 (0.18–2.15) |
| Colistin | 22/63 (34.9%) | 12/21 (57.1%) | 0.041 | 3.22 | 0.40 (0.14–1.10) |
| Antibiotic | Pre-Pandemic (N = 46) | During the Pandemic (N = 41) | p-Value | χ2 | OR (CI 95%) |
|---|---|---|---|---|---|
| Nitrofurantoin | 0/1 (0%) | 14/15 (93.3%) | 0.062 | 7.46 | UND |
| Fosfomycin | 0/3 (0%) | 9/25 (36%) | 0.147 | 1.59 | UND |
| Trimethoprim– sulfamethoxazole | 35/46 (76%) | 34/41 (82.9%) | 0.224 | 0.61 | 0.65 (0.22–1.88) |
| Cefepime | 46/46 (100%) | 39/41 (95.1%) | 0.109 | 2.29 | UND |
| Cefoxitine | 46/46 (100%) | 31/33 (93.9%) | 0.085 | 2.86 | UND |
| Gentamicin | 38/46 (82.6%) | 21/40 (52.5%) | 0.001 | 9.00 | 4.29 (1.60–11.48) |
| Tobramycin | 46/46 (100%) | 36/38 (94.7%) | 0.100 | 2.48 | UND |
| Amikacin | 38/46 (82.6%) | 31/40 (77.5%) | 0.283 | 0.35 | 1.37 (0.47–3.99) |
| Ciprofloxacin | 45/46 (97.8%) | 40/41 (97.5%) | 0.471 | 0.006 | 1.12 (0.06–18.58) |
| Ertapenem | 46/46 (100%) | 41/41 (100%) | 0.115 | 2.18 | UND |
| Imipenem | 37/43 (86%) | 33/41 (80.4%) | 0.256 | 0.46 | 1.49 (0.46–4.75) |
| Meropenem | 38/43 (88.3%) | 37/40 (92.5%) | 0.277 | 0.40 | 0.61 (0.13–2.76) |
| Piperacillin + tazobactam | 46/46 (100%) | 39/40 (97.5%) | 0.232 | 1.16 | UND |
| Ceftazidime + avibactam | 14/26 (53.8%) | 22/29 (75.8%) | 0.049 | 2.93 | 0.37 (0.11–1.17) |
| Colistin | 15/43 (34.8%) | 19/41 (46.3%) | 0.148 | 1.14 | 0.62 (0.25–1.49) |
| Parameter | Deceased (N = 15) | Survivors (N = 72) | χ2 | p-Value | RR | CI 95% |
|---|---|---|---|---|---|---|
| Mean age (years) | 66.6 ± 12.075 | 69.7 ± 12.436 | - | 0.368 | - | - |
| Male gender | 6 (40%) | 37 (51.3%) | 0.64 | 0.422 | 0.77 | 0.40–1.50 |
| Rural area of residence | 8 (53.3%) | 32 (44.4%) | 0.39 | 0.529 | 1.20 | 0.69–2.05 |
| Urinary catheterization | 13 (86.7%) | 38 (52.8%) | 5.87 | 0.015 | 1.64 | 1.22–2.20 |
| K. pneumoniae sepsis | 5 (33.3%) | 0 | 25.46 | <0.0001 | - | - |
| Associated respiratory pathologies | 10 (66.6%) | 21 (21.1%) | 7.61 | 0.005 | 2.28 | 1.37–3.79 |
| Diabetes mellitus | 1 (6.6%) | 28 (38.8%) | 5.80 | 0.016 | 0.17 | 0.02–1.16 |
| Previous hospitalization | 11 (73.3%) | 51 (70.83%) | 0.03 | 0.845 | 1.03 | 0.73–1.45 |
| Previous antibiotic treatment | 8 (53.3%) | 38 (52.7%) | 0.001 | 0.968 | 1.01 | 0.60–1.70 |
| CKD | 4 (26.6%) | 23 (31.9%) | 0.16 | 0.687 | 0.83 | 0.33–2.06 |
| rUTIs | 5 (33.3%) | 14 (19.4%) | 1.40 | 0.236 | 1.71 | 0.72–4.03 |
| Urea (mg/dL—mean value) | 148.4 ± 118.006 | 68.9 ± 58.071 | - | 0.0001 | - | - |
| Creatinine (mg/dL—mean value) | 2.2 ± 2.164 | 1.4 ± 1.033 | - | 0.047 | - | - |
| ASAT (UI/L—mean value) | 1021.6 ± 2474.638 | 37.6 ± 57.283 | - | 0.0008 | - | - |
| GGT (UI/L—mean value) | 214.5 ± 227.158 | 82.5 ± 118.862 | - | 0.001 | - | - |
| Length of hospital stay | 32.9 ± 26.908 | 17.3 ± 13.908 | - | 0.001 | - | - |
| ICU transfer | 15 (100%) | 11 (15.2%) | 42.52 | <0.0001 | 6.54 | 3.79–11.27 |
| Inadequate empirical therapy | 13 (86.6%) | 34 (47.2%) | 7.77 | 0.005 | 1.83 | 1.33–2.51 |
| ICU Transfer | Death | |||||
|---|---|---|---|---|---|---|
| UTIs with an OXA-48-producing strain (N = 26) | 11 (42.3%) | r | 0.177 | 5 (19.2%) | r | 0.034 |
| p | 0.101 | p | 0.752 | |||
| UTIs with a KPC-producing strain (N = 13) | 5 (38.4%) | r | 0.079 | 3 (23%) | r | 0.065 |
| p | 0.470 | p | 0.551 | |||
| UTIs with a NDM-producing strain (N = 25) | 5 (20%) | r | −0.137 | 4 (16%) | r | −0.021 |
| p | 0.205 | p | 0.848 | |||
| UTIs with two carbapenemases- producing strain (N = 6) | 2 (33.3%) | r | 0.021 | 2 (33.3%) | r | 0.116 |
| p | 0.850 | p | 0.285 | |||
| UTIs with a non-carbapenemase-producing strain (N = 15) | 3 (20%) | r | −0.099 | 1 (6.6%) | r | −0.128 |
| p | 0.364 | p | 0.238 | |||
| COVID-19 patients (N = 21) | 14 (66.6%) | r | 0.453 | 7 (33.3%) | r | 0.240 |
| p | <0.0001 | p | 0.025 | |||
| Variables in the Equation | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. for EXP(B) | ||
| Lower | Upper | |||||||
| COVID 19 | 1.288 | 0.597 | 4.652 | 1 | 0.031 | 3.625 | 1.125 | 11.683 |
| a. Variable(s) entered: COVID 19. | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miftode, I.-L.; Leca, D.; Miftode, R.-S.; Roşu, F.; Plesca, C.; Loghin, I.; Timpau, A.S.; Mitu, I.; Mititiuc, I.; Dorneanu, O.; et al. The Clash of the Titans: COVID-19, Carbapenem-Resistant Enterobacterales, and First mcr-1-Mediated Colistin Resistance in Humans in Romania. Antibiotics 2023, 12, 324. https://doi.org/10.3390/antibiotics12020324
Miftode I-L, Leca D, Miftode R-S, Roşu F, Plesca C, Loghin I, Timpau AS, Mitu I, Mititiuc I, Dorneanu O, et al. The Clash of the Titans: COVID-19, Carbapenem-Resistant Enterobacterales, and First mcr-1-Mediated Colistin Resistance in Humans in Romania. Antibiotics. 2023; 12(2):324. https://doi.org/10.3390/antibiotics12020324
Chicago/Turabian StyleMiftode, Ionela-Larisa, Daniela Leca, Radu-Stefan Miftode, Florin Roşu, Claudia Plesca, Isabela Loghin, Amalia Stefana Timpau, Ivona Mitu, Irina Mititiuc, Olivia Dorneanu, and et al. 2023. "The Clash of the Titans: COVID-19, Carbapenem-Resistant Enterobacterales, and First mcr-1-Mediated Colistin Resistance in Humans in Romania" Antibiotics 12, no. 2: 324. https://doi.org/10.3390/antibiotics12020324
APA StyleMiftode, I.-L., Leca, D., Miftode, R.-S., Roşu, F., Plesca, C., Loghin, I., Timpau, A. S., Mitu, I., Mititiuc, I., Dorneanu, O., & Miftode, E. (2023). The Clash of the Titans: COVID-19, Carbapenem-Resistant Enterobacterales, and First mcr-1-Mediated Colistin Resistance in Humans in Romania. Antibiotics, 12(2), 324. https://doi.org/10.3390/antibiotics12020324











