Abstract
Topical cariostatic agents have become a reasonable alternative for managing dental caries in young children. Silver diamine fluoride (SDF) is a practical topical approach to arrest caries and avoid extensive and risky dental treatment. However, the literature demonstrates a parental hesitation towards accepting SDF because of black unaesthetic tooth discolouration following application. The rapid oxidation of ionic silver darkens demineralised tooth structure permanently. In this regard, nano-metallic antimicrobials could augment or substitute for silver, and thereby enhance SDF aesthetic performance. Recently, biomedical research has drawn attention to selenium nanoparticles (SeNPs) due to their antimicrobial, antioxidant, and antiviral potencies. Various in vitro studies have examined the effect of SeNPs on the virulence of bacteria. This narrative review explores practical issues when using SDF and suggests future directions to develop it, focusing on antimicrobial metals. Several methods are described that could be followed to reduce the discolouration concern, including the use of nanoparticles of silver, of silver fluoride, or of selenium or other metals with antimicrobial actions. There could also be value in using remineralising agents other than fluoride, such as NPs of hydroxyapatite. There could be variations made to formulations in order to lower the levels of silver and fluoride in the SDF or even to replace one or both of the silver and fluoride components completely. Moreover, since oxidation processes appear central to the chemistry of the staining, adding SeNPs which have antioxidant actions could have an anti-staining benefit; SeNPs could be used for their antimicrobial actions as well. Future research should address the topic of selenium chemistry to optimise how SeNPs would be used with or in place of ionic silver. Incorporating other antimicrobial metals as nanoparticles should also be explored, taking into account the optimal physicochemical parameters for each of these.
Keywords:
early childhood caries; ECC; silver diamine fluoride; SDF; nanotechnology; nanoparticles; selenium 1. Introduction
Globally, one of the most widespread childhood illnesses is Early Childhood Caries (ECC). Statistically, this represents some 48% of chronic childhood diseases [1], and is the major reason for preventable hospital admissions in children. Given that it affects children who are too young to cooperate with conventional dental treatment, ECC is typically treated invasively under general anaesthesia (GA). From an economic point of view, untreated ECC is a significant burden to the healthcare system. According to a recent study, a single dental GA session costs over USD 1000 (AUD 1793.23 with SD 803.45) [2], and there is often a long GA waitlist for public sector dental treatment. Private treatment under GA is not affordable for many parents. Hence, there is great value in alternative management options to treat and arrest ECC in young pre-cooperative children that can be quick, simple, painless, affordable, effective, and easy to apply.
In recent years, silver diamine fluoride (SDF) has emerged as a popular topical treatment for achieving caries arrest in young children, thereby reducing the need for invasive and costly restorative or surgical dental treatment [1]. SDF is composed of silver, ammonia, fluoride, and water. Silver ions inhibit bacterial growth by reacting with the bacterial cell wall and with intracellular contents, causing reproductive and metabolic disturbances [2,3]. Fluoride at high concentrations also exerts antibacterial actions, and it remineralises tooth structure. Ammonia elevates the pH and acts as a stabiliser [3].
Studies have shown that when SDF at a concentration of 38% is applied semi-annually, an arrest of 81% of carious lesions in the dentine will predictably occur [4,5]. Furthermore, application does not lead to acute complications such as systemic diseases or toxicity [6]. However, there is still practitioner and parental reluctance towards the use of SDF for caries arrest in children [7,8]. The most common reason cited for avoidance is black discolouration of carious lesions following its application, which is unaesthetic [6]. In a systematic review, parents’ decision whether to accept or reject SDF treatment was linked to tooth position; and a low acceptance rate was reported if SDF treatment was proposed for anterior teeth [9]. Dental aesthetic issues are unlikely to have effects on preschool child social interactions or self-esteem [10,11]. Despite this, parental acceptance of SDF is low because of concerns regarding the black appearance of treated sites on the teeth [8,12]. A survey of 920 school children revealed that, unlike their preschool counterparts, those with abnormalities in tooth shape or colour were more likely to experience bullying, with its attendant psychological and emotional impacts [13].
SDF discolouration is caused by oxidation of ionic silver to metallic silver and silver oxide, with subsequent precipitation of silver–protein and silver phosphate complexes on tooth structure. Given this issue, there is a need to find additional antimicrobial agents to augment or replace silver, reducing the need for high concentrations of ionic silver. Any such replacements should reduce the tendency to darken carious lesions. Nanomaterial-based antimicrobial agents have become more widely used for a range of medical applications. Nanoparticles have distinctive characteristics such as a large surface area and enhanced reactivity [14]. In dentistry, nanoparticles have been loaded into various dental materials to provide antibacterial actions [15]. The antimicrobial potential of metallic nanoparticles can be controlled by altering various physio-chemical parameters, such as particle size, shape, and zeta potential, and by altering the method of synthesis or by applying capping agents [16]. Each physio-chemical property is potentially important when evaluating the biological behaviour and impacts of nanoparticles. For instance, nano morphology plays an important role in the fate and performance of nanoparticles. Different nanoparticle shapes give different diffusion rates. A further point is that shape affects steric hindrance when nanoparticles collide with and interact intimately with surfaces [17]. An additional example of nanoscale parameters influencing behaviour is agglomeration, where many nanoparticles come together into clusters due to attraction forces. Agglomerations cause large clusters to form; these have a smaller surface area and so are not as biologically active as separate individual nanoparticles. A range of important nano specifications are illustrated in Figure 1.
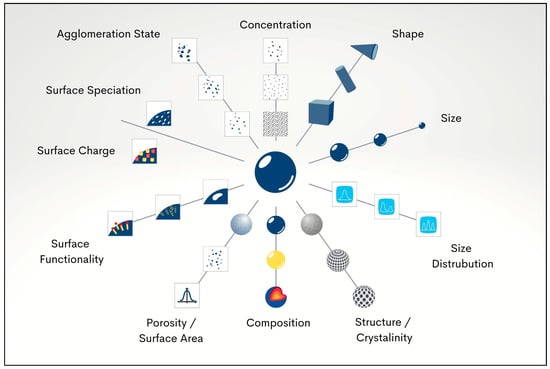
Figure 1.
Physio-chemical parameters which determine the biological impact of nanoparticles.
Lately, selenium nanoparticles (SeNPs) have been attracting interest in biomedical research owing to their suitable biocompatibility as well as their antibacterial, antifungal, antiviral, and antioxidant effects [18]. SeNPs have been shown to exert antimicrobial actions against multiple species of bacteria [19]. The antibacterial and antioxidant effects of SeNPs in topical anticaries agents has not been investigated thoroughly [19]. The specific aims of this review are to (1) explore how the chemical composition of SDF could be altered to reduce staining and (2) explore the possibility of using alternative nanomaterial-based antimicrobial agents to augment or replace ionic silver, thus lowering the potential to cause black discolouration of teeth.
2. Early Childhood Caries: The Ripple Effect
ECC is one of the most common chronic disorders in the world [20]. ECC is defined as “the presence of one or more decayed (non-cavitated or cavitated lesions), missing (due to caries), or filled tooth surfaces in any primary tooth in a child under the age of six” [21]. It is challenging to provide restorative treatment for younger children because of their immaturity as well as their inability and unwillingness to cooperate for treatment by dentists and therapists provided in the dental chair. Recently, the COVID-19 pandemic crisis has worsened the situation regarding caries in children because parents have postponed their children’s dental appointments [22]. Moreover, in communities that are facing major public health issues because of social disadvantage, high rates of caries in the primary dentition may not be a high priority compared with other health needs [23,24].
ECC affects a child’s life in multiple ways, causing pain, infection, nutrition difficulties, interrupted sleep, self-esteem concerns, and aesthetic problems [25,26]. Advanced cases of ECC can cause further complications such as impaired dietary intake, school absence, impaired growth and development, and repeated emergency admissions and hospitalisations for severe dental infections. Due to the cognitive and communication problems of treating preschool and very young children, dental treatment under GA is often needed. In Australia, there is a growing need for paediatric dental GA, and the waitlist in some public healthcare facilities may extend up to two years [27]. Children who undergo dental GA for the treatment of ECC have a reported relapse rate of up to 79%, as they continue to develop new carious lesions and symptoms requiring repeated treatment [28]. Dental GA sessions are a major financial burden for the health care system. According to the National Independent Hospital Pricing Authority, the average direct cost for a typical ECC treatment episode (including extractions and restorations) under GA was AUD 3029 in 2012–2013. Additionally, a retrospective study (from 2018–2019) reported that dental extraction under GA was the most frequent treatment choice when young children presented to emergency departments with severe dental infections [29].
Dental caries is a complex disease that involves multiple microbes (both bacteria and fungi) which emerge due to ecological changes in the dental plaque biofilm. This dysbiosis is driven by diet and other lifestyle factors [30]. There is an imbalance between acidogenic and aciduric microbes on one hand, and health-associated commensal bacteria on the other [31]. Even though dental caries is polymicrobial in nature, Streptococcus mutans (S. mutans) serves as a key pathogen by initiating the formation of a dense extracellular polymer matrix composed of glucan-rich exopolysaccharides. S. mutans and Streptococcus sobrinus (S. sobrinus) can both produce large quantities of these insoluble glucans [32], which give the biofilm enhanced bulk and adhesiveness and serve as a source of fermentable carbohydrates [33,34]. Therefore, impairing the growth and metabolism of these bacteria is desirable.
3. Silver Diamine Fluoride SDF: Sharpening an Anticaries Agent
As traditional approaches to the management of caries in young children are challenging, expensive, and often risky, there has recently been a marked shift towards minimally invasive approaches. SDF, as a topically applied antimicrobial agent, leverages the antimicrobial actions of several components: the alkaline pH (pH range from 9–13), the silver ions (25% by wt), fluoride ions (5% by wt), and ammonia (8% by wt). The balance of 62% is water, which acts as the solvent [35].
SDF was first introduced in Japan in the 1960s by Nishino, who added ammonia to improve on existing silver fluoride formulations [36]. While marketed with regulatory approval for treating dentinal hypersensitivity, SDF is used widely off-label for arresting dental caries in both deciduous and permanent teeth [37]. As a topical agent, it is more effective than fluoride varnishes [3]. SDF has demonstrated greater efficacy for halting the progress of lesions and gives increased fluoride absorption into tooth structure when compared with fluoride varnishes and topical fluoride gels, in both in vitro and in vivo studies [38,39].
The effectiveness of SDF in causing caries arrest is attributed to the combined actions of the high concentrations of fluoride and silver ions as well as the alkaline pH [40]. Ionic silver attacks bacterial microbes via multiple approaches, as explained in detail below. Moreover, the silver ions in SDF form a layer of silver phosphate that provides resistance to further decay, while fluoride ions convert hydroxyapatite to less soluble fluorapatite [41]. Together, these actions inhibit the further progression of demineralisation and help to preserve dentine collagen, protecting it from further degradation [42].
SDF has been produced commercially in various concentrations (12%, 30%, 38%, and 40%), with the 38% versions being in widespread use [4]. These formulations contain 44,800 ppm of fluoride, which is 8 times above the threshold needed for antimicrobial actions on bacteria [4]. When applied to primary teeth, 38% SDF has greater potency than 12% SDF for caries arrest [5,43]. Worldwide, multiple SDF brands are manufactured. Advantage ArrestTM has a pH of 10 and contains 24.4–28.8% silver (w/v) and 5.0–5.9% fluoride. SDI Riva StarTM has a pH of 13 and contains 35–40% (w/v) silver fluoride (AgF) and 15–20% (w/v) ammonia. The manufacturer also provides a solution of potassium iodide (KI) to be applied immediately onto the surface to scavenge precipitated silver [44]. The same manufacturer also has an ammonia-free neutral formulation (pH 7.4), SDI Riva Star Aqua™, with 40% AgF. Removing the ammonia was intended to prevent transient gingival/mucosal burns. Another notable product, CSDS, is made by Whiteley. This is also ammonia-free, and contains 40% AgF and 10% stannous fluoride (SnF2). The SnF2 acts as a reducer for excessive silver ions [45].
The use of silver fluoride in various forms can exert potent effects on microbial growth. A significant reduction in levels of S. mutans has been seen following the application of SDF onto an infected dentine surface in vitro [46]. A similar beneficial effect on impairing the growth of Lactobacilli [47] has also been seen in an in vitro study. The high concentration of fluoride ions (44,800 ppm) exerts a two-level action on bacteria. Firstly, it disrupts bacterial enzymes that regulate carbohydrate uptake and metabolism. Secondly, it impairs biofilm formation [47].
With regard to the interaction between SDF and tooth structure, there is some evidence that SDF remineralises dentine [38]. This occurs through the creation of silver phosphate and the deposition of calcium fluoride, both of which contribute to an increase in pH (from 5.5 to 9–13). The calcium fluoride, which is believed to remain on tooth surfaces via protein-based globules, can discharge fluoride ions in acidic mediums (cariogenic conditions) and serves as an effective medium-term fluoride reservoir [48]. The formation of fluorapatite with its reduced acid solubility lessens the impact of acids produced by the dental plaque biofilm [35]. Silver ions that react and bind with hydroxyapatite form a type of protection shield against future cariogenic attacks [42]. Additionally, hydrolytic collapse of dentine collagen is impaired via inhibition of proteolytic enzymes, including cathepsins (or cysteine cathepsins) and matrix metalloproteinases (MMPs) [49]. Thus, the effectiveness of SDF results from multiple pathways, a point that is very relevant when considering how to enhance the formulation or substitute other components for the silver.
The ionic silver in SDF creates several issues. Due to the alkaline pH of SDF, during application, the solution must be applied in very small amounts, and some manufacturers recommend that gingival protection be applied before SDF. Proper cotton roll isolation is important because of the risk of causing burns to skin or mucosa [50]. The fate of silver applied to deep lesions that are close to the dental pulp is a further point. In the 1990s, Gotjamanos et al. [51] reported the possibility of silver ions penetrating through the tooth structure and reaching the pulp chamber. This situation would occur when SDF was applied to very deep carious lesions that were close to the dental pulp [52]. A further point is that SDF has a noticeable, odd metallic taste that may cause momentary nausea [7].
Going beyond these short-term issues, the most significant drawback of using SDF, especially in high concentrations, is that it creates long-lasting black stains on the treated surfaces due to the formation of silver compounds, especially silver phosphate and silver sulphide [53]. Within two minutes of SDF application, the treated dentine surface darkens noticeably and irreversibly. This is followed by a gradual increase in the intensity of staining from 5 min to 5 h post-application. The maximum colour change following SDF application occurs at 12 h. The intensity of the stains varies according to the frequency of application [6]. Paradoxically, the discolouration has been considered an SDF success indicator, since it represents a zone high in calcium, phosphorus, fluoride, and silver ions [38].
While parents might accept SDF staining on primary posterior teeth, they have lower acceptance of its application to primary anterior teeth, as these are within the aesthetic zone [9]. They may accede to its use on anterior teeth as a fall-back measure when the child displays an uncooperative attitude during regular dental visits [54,55]. In contrast, parents of a cooperative child are more inclined to prefer aesthetic tooth-coloured restorations for the anterior teeth of their child in order to alleviate the sense of guilt from not having managed their child’s oral hygiene, and because reliable restorative techniques and procedures exist [56].
The black discolouration caused by SDF is an issue that could be addressed by using less silver or by using a scavenging agent. As mentioned previously, one manufacturer includes a topical application of saturated potassium iodide [57]. The rationale behind this is that the reaction between available silver ions and KI results in the precipitation of silver iodide (a bright yellow chemical compound) [58]. Silver iodide formation should minimise the concentration of free silver ions that eventually discolour the tooth surface. Despite this approach having been rated with “insufficient clinical evidence” in systematic reviews [59], in vitro studies have reported that the use of the SDF followed by KI application may lead to better aesthetic outcomes [60]. However, these outcomes appear to be temporary. A randomised clinical trial (RCT) showed no significant difference between the SDF/KI group and the SDF-only group at 30 months follow up [61]. They concluded that the further decomposition of silver iodide due to its photosensitivity leads to further release of silver and iodine. There is a concern that immediate application of KI following SDF may stain demineralised dentin [62]. This has prompted the search for a future antimicrobial agent that can achieve a balance between providing an adequate antibacterial effect and not significantly staining the tooth surface. Alternative potential antimicrobial agents include silver nanoparticles (AgNPs), selenium nanoparticles, and copper nanoparticles.
Nanoparticles (NPs) have diameters ranging from 1 to 100 nm. Their large surface area and strong chemical reactivity are desirable features [63]. Several metal-based nanomaterials are being actively considered for inclusion in dental materials and therapeutic products [14]. Furthermore, combining NPs with polymers and coating them with other nanocomposites provides an opportunity for multiple types of physio-chemical modification [64]. Each modified form can have unique chemical and antibacterial properties. A high level of antibacterial activity is an anticipated result of the strong interactions between certain metal NPs and the negatively charged surface of bacterial cells. These interactions occur because of the vast surface area and high charge density of NPs [65].
As the size of NPs reduces, their antibacterial properties improve. For instance, 10 nm AgNPs are small enough to penetrate the bacterial matrix and can cause an imbalance in vital cellular functions like DNA replication, particularly in Gram-negative bacteria [2]. Moreover, by triggering oxygen radical production, they cause lipid peroxidation, which disrupts bacterial cell membranes and decreases bacterial metabolism [66]. There have been some preliminary studies of AgNPs. Tirupathi and others assessed the anticariogenic capacity of AgNPs loaded into a sodium fluoride (NaF) varnish and compared this with 38% SDF [67]. It proved to be as effective as the SDF but without causing undesirable postoperative staining [67]. Additionally, the AgNPs did not generate silver oxide when exposed to oxygen in the medium; hence, the demineralised enamel did not stain black [68]. Furthermore, in an in vitro study, Targino and others compared 38% SDF and a nano form of silver fluoride, finding that AgNPs exert greater antimicrobial actions than silver ions [69]. This provides some support for the extension of this line of work. Likewise, the further development of hydroxyapatite NPs might result in the replacement of fluoride in topical anticaries agents, thusavoiding issues with fluoride in young patients [2].
4. Silver: The Old New
For thousands of years, the antimicrobial actions of silver have been recognised. In 335 BC, Alexander the Great used silver vessels for storing water and silver jugs for drinking [70]. The action of silver in keeping water from fouling was recognised before the advent of the “Germ Theory of Disease” [71]. Silver compounds were used in wound healing remedies by ancient Greeks due to their antimicrobial properties [72], and this idea now extends into the modern age to dressings for burns. Prior to the advent of antibiotics, silver was a widely used antibacterial agent [73].
In dentistry, silver nitrate (AgNO3) was used as a caries-arresting solution and an antibacterial agent since the 18th century [74]. In the 1840s, AgNO3 was used to treat caries in primary teeth and for control of gingival disease, marking the first documented use of silver compounds in dentistry. Thereafter, it was further used as a dentine desensitiser, cavity disinfectant, and caries preventive treatment for permanent molars [75,76,77]. In 1908, GV Black described using AgNO3 to treat caries in children as “the first measure against the disease” [78]. Then, in the 1960s, it was proposed that silver and fluoride should work together to prevent cavities; however, because of the discolouration of caries lesions, therapeutic use of silver fluoride compounds was limited [3].
Several modes of action of silver explain its antibacterial actions. Firstly, it attacks bacterial cell walls and changes their permeability, which leads to the leakage of cellular contents and impaired transport activity across the membrane [79]. Silver also generates reactive oxygen species (ROS) and hydroxyl radicals, which damage microbial DNA and cause lipid destruction through oxidation [80]. Ionic silver also inhibits the proton motive force, leading to apoptosis [81]. Moreover, AgNPs deactivate respiratory chain dehydrogenases, which inhibits cell respiration and growth. Once combined with halides, AgNPs release silver ions and produce more ROS [82]. A recent study by Hovhannisyan and colleagues pointed out that the effect of green synthesised AgNPs on bacterial membrane permeability was due to effects on formate hydrogenlyase proton–potassium transporters, energy-dependent H+-fluxes, and H+-translocating ATPase activity [83]. In addition, by specifically disturbing FOF1-ATPase activity, these biogenic AgNPs can affect bacteria that have no respiratory chain such as the Enterococcaceae species. The antimicrobial mechanisms of action of AgNPs are demonstrated in Figure 2.
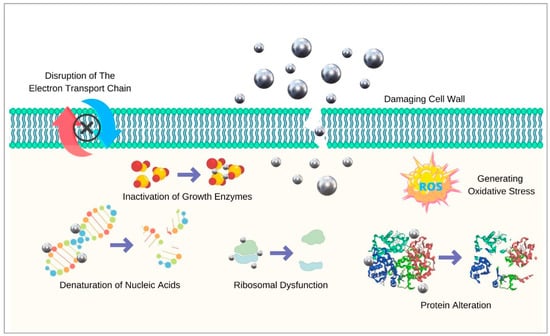
Figure 2.
Antimicrobial mechanisms of silver nanoparticles.
5. SDF Re-Composition: Nanometals vs. Ionic Silver
Although the individual chemical ingredients of SDF work in cooperation to halt caries, the question arises as to whether the same or better clinical actions would be achieved with less staining by reformulation of the product. Hence, several research groups have evaluated the use of nano formulated approaches instead of traditional silver-based topical cariostatic agents [63,68,84,85,86]. Nano-silver fluoride is one of the suggested substitutes [63]. A study conducted by Nagireddy et al. used chemically synthesised AgNPs added to 0.05 ppm NaF and tested this formula for anticaries effects on 100 primary teeth. The results of their study showed 78% caries arrest within seven days. However, an issue with the design of their study was that normal saline was used as a control treatment, and there was no direct comparison to SDF [63].
In another study, SDF was modified by adding different concentrations of copper-doped bioglass nanoparticles (CuBGNPs), and the mixtures were assessed for their viscosity and antibacterial actions [84]. This modified form of SDF showed improved ion release and decreased cytotoxicity; in addition, a cumulative increase in the antimicrobial effect was observed as the concentration of CuBGNPs rose. Discolouration was not investigated.
The combination of fluoride ions and silver NPs has also been compared with SDF [86]; the two provide similar bactericidal effects, but the version with AgNPs does not cause noticeable tooth discolouration. Taken together, these results suggest that the use of nanoparticles of silver or of silver fluoride could be one way to solve the problem of discolouration.
6. Selenium: A Plausible Substitute
Selenium (Se) was identified as a natural element by the Swedish chemist Jon Jakob Berzeliusin 1817 [87]. It occurs naturally in limited amounts. Most commercial Se is refined from other metals such as copper (Cu) using electrolysis [88]. Se can be organic (selenocysteine and selenothionine) or inorganic (selenate and selenite). Based on its configuration, it can exist either in a crystal form (monoclinic and trigonal) or in an amorphous polymorphic form [89]. Se is one of the group-6 elements in the periodic table and is situated between non-metallic sulphur (S) and metallic tellurium (Te) in the oxygen group [90]. Thus, the chemistry of Se is somewhat similar to that of sulphur, although the two have different oxidation capacities [91]. Se shows multiple oxidation states: Se2+, Se4+, Se6+, and Se2–18.
6.1. Selenium and Human Health
Selenium is an essential micronutrient in the human diet. Generally, Se has a valuable impact on human health at different levels. It plays a vital role in several intracellular activities [92], and its sufficient intake is linked to protection against various diseases, including hypercholesterolemia, some malignancies, and cardiovascular illnesses [93]. Se has unique growth-regulating capabilities and fulfils a role as a cofactor for essential enzymes such as thioredoxin reductases (which control the redox state of the cell by inhibiting thioredoxin) and glutathione peroxidases (which prevent cellular destruction caused by oxygen-free radicals) [94]. Other health-related effects of Se include its antibacterial and antiviral actions [95]. As a trace element in the diet, Se is essential for human health [96]. Decreased Se levels have been correlated to impaired cognitive ability, weak immunity, and increased mortality risk [97].
6.2. Selenium Nanoparticle Properties: Size, Shape, and Synthesis
SeNPs have a core of inorganic Se (0). They can exert their own actions or can be loaded with additional therapeutic agents [98]. SeNPs have superior biosafety and biocompatibility to organic or inorganic Se molecules [99]. In addition, they exhibit distinctive chemical and physical characteristics owing to their large surface-to-volume ratio and high surface energy [100]. SeNPs have high bioavailability but low toxicity [101].
Additionally, the parameters of SeNPs can be adjusted to optimise their physical and chemical features, including through altering the synthesis process or by using capping agents [102]. SeNPs have been synthesised in a range of configurations, including spheres [103], cubes [104], ribbons [105], flowers [106], nanorods [107], and nanoneedles [108]. Generally, the most frequently used shape for therapeutic purposes is spherical [109]. Wire-shaped SeNPs have shown greater photoconductivity, while spherical-shaped SeNPs have demonstrated greater biocompatibility [110,111]. Furthermore, certain forms of SeNPs are generated by specific synthetic processes. For instance, green synthesis can result in SeNPs with a hexagonal ring form [112,113,114].
The adjustability of SeNPs extends to their charge. SeNPs are normally negatively charged, but this can be altered to positive by using surface modifiers such as chitosan [115,116]. This type of tuning is important in terms of binding to different types of microorganisms. In terms of the antimicrobial actions of SeNPs, one study reported that both Gram-positive and Gram-negative bacteria were inhibited to a similar extent by SeNPs [117]. In contrast, another study by Wang and others found that SeNPs were less effective against Gram-negative bacteria (P. aeruginosa and E. coli) after 72 h of treatment [118]. It is possible that SeNPs could be less active against Gram-negative bacteria due to electrostatic repulsion between negatively charged lipopolysaccharide in the bacterial cell membrane and SeNPs, unlike the situation with Gram-positive bacteria, which have a slight positive or neutral surface charge [119]. Although the antimicrobial impact of SeNPs has not yet been fully explored [120], this parameter has been demonstrated in several studies, and these are summarised in Table 1.
6.3. Selenium Nanoparticles and Toxicity Concerns
Although selenium is a trace element and is essential for human body, there is a fine line between healthy therapeutic levels of selenium and a harmful toxic concentration. As a supplement, the World Health Organisation recommends 55 µg of selenium per day for healthy individuals [121]. Selenium compounds, especially at high concentrations, exhibit noticeable toxicity. On the contrary, SeNPs have better safety use and cause less cytotoxicity [110]. In vivo evidence has shown that the SeNP lethal dose is 4–6 times less than other formulations of organic and inorganic selenium [122]. In general, the cytotoxic actions of SeNPs are closely associated with their ability to damage cell membranes and to generate oxidative stresses [123]. Furthermore, stabilisation of SeNPs could play a part in further reducing their toxicity [107]. For example, polysaccharide-stabilised SeNPs show less toxicity and enhanced bioavailability [110].
It has been reported that if human selenium consumption surpasses 400 µg/day, symptoms of selenosis (selenium intoxication) might be observed. Selenosis causes symptoms that range from hair and nail damage to severe neuromotor disturbances. However, other studies argued that a much higher intake (e.g., 3000 µg/day) is needed to cause symptoms of selenosis [123]. The median lethal dose of SeNPs is 92.1 mg/kg, while it is only 14.6 mg/kg for seleno-methylselenocysteine [101]. The cytotoxicity of SeNPs varied according to their synthesis method. Biogenic (green) synthesis results in less toxic forms of nano selenium compared with chemically synthesised SeNPs [124]. Lastly, in terms of genotoxic impacts of SeNPs, some studies have found that bare (uncoated) SeNPs cause a dose-dependent genotoxic action; however, this problem can be reduced markedly by capping or coating SeNPs with surfactants [107]. Overall, in terms of clinical use under controlled conditions where little or no ingestion is likely to occur, concerns over the cytotoxic effects of SeNPs are likely to be low. Moreover, this concern can be minimised by using green synthesis and by applying suitable capping agents.
6.4. SeNPs and the Role of Capping Agents
Stabilisers/capping agents can be added to chemically synthesised SeNPs to enhance their physio-chemical characteristics and maintain their bioactivity and size by enhancing their stability and preventing them from agglomerating [125]. Furthermore, such stabilisation influences the ultimate size of SeNPs and, thus, is strongly related to antioxidant capacity, cytotoxicity, and antimicrobial actions [93]. Multiple agents have been used to coat SeNPs. Chitosan is a type of chitin that can be extracted from insects and crustaceans. Chitosan-capped SeNPs have reasonable bioavailability, a positive charge, and minimal toxicity; thus, they have been used in several therapeutic applications [126]. Various polymers have also been used to stabilise SeNPs, including polyvinyl alcohol, polyethylenimine, and polyvinylpyrrolidone [110]. Plant-derived polysaccharides have been used to cap SeNPs, and this enhances their antioxidant actions [127,128]. Proper selection of capping agents can be used to optimise the chemical, physical, and biological properties of selenium nanoparticles.

Table 1.
Summary of published research on antimicrobial action of selenium nanoparticles.
Table 1.
Summary of published research on antimicrobial action of selenium nanoparticles.
| STUDY | METHODS | KEY FINDINGS |
|---|---|---|
| [103] | Synthesis: chemically—Na2SeO3 reduction; Stabiliser: BSA Characterisation: TEM and DLS Tested microbes: S. aureus SeNPs tested concentrations: 7.8, 15.5, 31 μg/mL | -SeNPs shape/size: spherical/40–60 nm -SeNPs inhibited S. aureus growth compared with no treatment. -SeNPs killed approx. 40% of S. aureus after 3, 4, and 5 h |
| [129] | Synthesis: chemically—chitosan dissolution Characterisation: DLS Sample: skin infection swabs (n = 25) Tested microbes: 49 various bacterial strains Antibacterial tests: agar diffusion assay, growth curves SeNPs tested concentrations: up to 100 μg/mL | * Size and shape of SeNPs: not revealed -Bacterial growth curves were inhibited by low concentration (1 μg/mL) in all tested bacterial isolates -64 μg/mL of SeNPs shows complete inhibition when applied on E. fergusonii, P. aeruginosa, and S. agalactiae assays |
| [130] | Synthesis: biosynthesis—R. Alstonia eutropha bacterium-based Na2SeO3 reduction Characterisation: TEM, SEM, XRD, and SAED Tested microbes: E. coli, P. aeruginosa, S. aureus, S. pyogenes, and A. clavatus | -SeNPs shape/size: spherical 40–120 nm hexagonal crystalline -MIC: E. coli 125 μg/mL, P. aeruginosa 100 μg/mL, S. aureus 100 μg/mL, S. pyogenes 250 μg/mL and A. clavatus 500 μg/mL |
| [131] | Synthesis: chemically—Na2SeO3 reduction using glutathione Stabiliser: BSA Characterisation: FTIR, UV–Vis, DLS, SEM, and TEM Tested microbes: E. coli, S. aureus, Salmonella, and Listeria | -SeNPs shape/size: spherical ~79 nm -SeNPs show dose-dependent antimicrobial action against S. aureus but not the other three pathogens -SeNPs exerts cytotoxicity on cancer cells Caco-2 after 24 h exposure |
| [132] | Synthesis: (1) biosynthesised SeNPs (B. mycoides); (2) chemically synthesised SeNPs Characterisation: TEM, EDX, and DLS Tested microbes: P. aeruginosa and S. aureus Antibacterial tests: biofilms on hydroxyapatite discs | -SeNPs shape: spherical -Biosynthesized SeNPs had greater antibacterial action than chemically synthesized SeNPs |
| [133] | Synthesis: chemical method (using SeO2 as a precursor) Stabiliser: polyvinyl alcohol Characterisation: TEM, EDS, and FTIR Tested microbes: S. aureus Cytotoxicity: human dermal fibroblasts | -SeNPs shape/size: amorphous and spherical 43–205 nm -SeNPs antimicrobial; action is size dependent -greatest antibacterial action was observed in 81 nm SeNPs |
| [134] | Synthesis: chemical method (using SeO2 and ascorbic acid) Stabiliser: egg white lysozyme (EWL) Characterisation: UV–Vis, FTIR, TEM, and XRD Tested microbes: E. coli, S. pneumoniae, B. cereus, K. pneumoniae, P. mirabilis, and B. subtilis | -SeNPs shape/size: spherical (crystalline structure)/40–60 nm -MIC: 10.0 μg mL−1 -ZOI: 19 mm (B. subtilis), 15 mm (E. coli), 14 mm (B. cereus), and 13 mm (K. pneumoniae) -SeNPs exhibits prolonged stability (minimum of 12 months) |
| [135] | Synthesis: chemical method (using dissolution of Na2SeO3, L-ascorbic acid, and polyvinyl alcohol PVA in purified water) Characterisation: TEM, XPS, and SEM (Ti implants coated with SeNPs 30–70 nm) Tested microbes: S. aureus | -SeNPs shape/size: spherical (crystalline structure)/50–200 nm -Oxidation state: zero -SeNPs showed great antibacterial activity even at 0.5 ppm |
| [136] | Synthesis: chemical method (using Na2SeO3 as a precursor) Stabiliser: BSA, D-glucose, and soluble starch Characterisation: UV–Vis, FTIR, SEM, and EDXTested microbes: B. subtilis (mid-log) Antimicrobial test: SEM | -SeNPs shape/size: (BSA): rod shaped/200–250 nm (D-Glucose): spherical/200 nm (starch): cubes/250–300 nm -Antioxidation capacity: starch > D-Glucose > BSA -Antibacterial effect seen in all samples but not quantified |
| [137] | Synthesis: green synthesis—ascorbic acid as reductant Stabiliser: BSA Tested microbes: S. aureus and E. coli | -SeNPs shape/size: spherical/10–100 nm Proliferation of fibroblasts was promoted by SeNPs, whereas growth of S. aureus was suppressed |
| [138] | Synthesis: green method (Na2SeO3 as a precursor, using bovine urine) Characterisation: UV–Vis, SEM, TEM, DLS, AFM, and EDX Tested microbes: E. coli, K. pneumoniae, P. aeruginosa, Serratia, Proteus, and S. aureus | -SeNPs shape/size: spherical/110 nm -SeNPs effective against all species especially against Klebsiella species. |
| [139] | Synthesis: chemically—using Na2SeO3 Characterisation: UV–Vis Tested microbes: P. aeruginosa, Salmonella typhimurium, E.coli, S. sanguinis, S. aureus, and E. faecalis | MIC: S. sanguinis, 68 μg/mL S. aureus, 137 μg/mL E. faecalis 274 μg/mL |
| [140] | Synthesis: chemically—Na2SeO3 as a precursor Stabiliser: (i) BSA + ascorbic acid, (ii) Chitosan + ascorbic acid, and (iii) glucose Characterisation: FTIR, XRD, DLS, TEM, DLS, and UV–Vis Tested microbes: mixed biofilm S. aureus and C. albicans Cytotoxicity: human dermal fibroblasts | -SeNPs shape/size: spherical/70–300 nm -MIC: The lowest MIC was against C. albicans (25 μg/mL) -Zeta potential: SeNPs-Chit < SeNPs-BSA < SeNPs-Gluc * SeNPs-BSA was less cytotoxic than the other two formulations |
| [141] | Synthesis: chemical method (using Na2SeO3 as a precursor) Characterisation: UV–Vis, XRD, and SEM Tested microbes: S. aureus isolated from public water Antibacterial tests: disk diffusion assay, microdilution assay | -SeNPs shape/size: rod/85–275 nm -MIC: 50 μg/mL -synthetic SeNPs contributed to the breakdown of S. aureus biofilms |
| [109] | Synthesis: chemically—reduction of Na2SeO3 Characterisation: TEM, DLS, and XRD Tested microbes: E. faecalis Cytotoxicity: human fibroblasts | -SeNPs shape/size: spherical/77 + 27 nm -CFU: significantly reduced compared with control -Methylene Blue-induced Photodynamic Antimicrobial Chemotherapy SeNPs-MB-PACT showed the greatest antibiofilm impact -At 128 μg/mL SeNPs, 50% of human fibroblasts survived |
| [142] | Synthesis: biosynthesis—guava leaves (Psidium guajava) Characterisation: UV–Vis, DLS, TEM, and XRD Tested microbes: E. faecalis Group I: Distilled water (control), Group II: SeNPs (1 mg/mL), Group III: Calcium hydroxide (1 mg/mL), Group IV: 2% CHX, and Group V: 5.25% NaOCl Antibacterial tests: agar diffusion method, microdilution, viable cell count, antibiofilm assay, and Anthrone and Bradford’s tests | -SeNPs shape/size: spherical/30–50 nm -Zeta potential: −60 mV. -MIC: 25 mg/mL -ZOI: SeNPs 11.33–28.50 mm (based on the concentration) -SeNPs are the most active against E. faecalis biofilm, followed by NaOCl, CHX and Ca(OH)2 |
| [19] | Synthesis: green method (using Brassica Oleracea (Broccoli)) Characterisation: UV–Vis, TEM, FTIR, and EDX Tested microbes: S. mutans, S. aureus, E. faecalis, Lactobacillus, and C. albicans | -SeNPs shape/size: spherical/10–25 nm -greatest ZOI reported against S. mutans |
| [143] | Synthesis: biosynthesis—Na2SeO3 with citrus fruit extracts (lemons and grapefruits) Characterisation: UV–Vis, TEM, FTIR, and DLS Tested microbes: E. coli, M. luteus, K. pneumoniae, and B. subtilis Antimicrobial tests: agar diffusion assay | -SeNPs shape/size: not determined -SeNPs ZOI: 20 ± 1.646 mm against K. pneumoniae -A noticeable antimicrobial effect was detected after testing Citrus SeNPs -SeNPs could replace traditional antibiotics |
| [144] | Synthesis: biosynthesis—Na2SeO3 using Rosmarinus officinalis extract Characterisation: UV–Vis, DLS, TEM, XRD, and FTIR Tested microbes: M. tuberculosis, S. aureus, S. mutans, E. coli, and P. aeruginosa | -SeNPs shape/size: Spherical 20 to 40 nm -MIC in µg/mL: (M. tuberculosis), 256; (S. aureus), 16; (S. mutans), 32; (E. coli), 128; and (P. aeruginosa), 64 |
| [145] | Synthesis: chemical reduction Bacterial tests: MHB microdilution to assess MIC and MBC | SeNPs size: 81.4 nm MIC in µg/mL: S. mutans 68, L. acidophilus 137 and C. albicans 274 MBC in µg/mL: S. mutans 274 after 1–2 h and 137 after 6–24 h |
| [120] | Synthesis: biosynthesis—Na2SeO3 (precursor) using Calendula officinalis L. flowers as a (capping agent) Characterisation: SEM, TEM, FTIR, and EDX Tested microbes: Serratia marcescens, Enterobacter cloacae, and Alcaligenes faecalis bacteria Antimicrobial tests: disc diffusion | -SeNPs shape/size: spherical/40–60 nm -SeNPs demonstrated superior antibacterial effect, at various incubation times compared with the antibiotic ciprofloxacin CIP -SeNPs had greater antioxidant activity than methanolic extracts of flowers Cof-Met extract and Na2SeO3 |
| [146] | Synthesis: chemically—concentration was 128 and 64 μg/mL Characterisation: chemical reduction method Cytotoxicity: MTT test on human gingival fibroblast Tested Microbes: S. mutans | SeNPs enhanced photodynamic therapy (PDT) activity and exhibited significant antibiofilm action against S. mutans |
| [15] | Tested Microbes: S. salivarius, S. mutans, and S. sanguinis Control: untreated sealant Analysis: SEM, CLSM | Organo-selenium dental sealant was able to inhibit the growth of all species individually and in a mixed biofilm. |
Abbreviations: AFM: Atomic force microscopy; BSA: Bovine serum albumin; CHX: Chlorhexidine gluconate; CLSM: Confocal laser scanning microscopy; DLS: Dynamic light scattering; EDX: Energy dispersive X-ray analysis; EWL: Egg white lysozyme; FTIR: Fourier transform infrared; Na2SeO3: Sodium selenite; MBC: Minimum bactericidal concentration; MB-PACT: Methylene Blue-induced photodynamic antimicrobial chemotherapy; MHB: Mueller Hinton broth; MIC: Minimum inhibitory concentration; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide assay; NaOCl: Sodium hypochlorite; SAED: Selected area (electron) diffraction; SEM: Scanning electron microscope; SeO2: Selenium dioxide; TEM: Transmission electron microscope; UV–Vis: Ultraviolet–visible; XPS: X-ray photoelectron spectroscopy; XRD: X-ray diffraction analysis. ZOI: Zone of inhibition.
As shown in Table 1, SeNPs possess microbial inhibition action against a variety of bacterial strains as well as against important fungi (C. albicans), and this action varies according to the synthesis method and the SeNP properties. Some studies have adopted eco-friendly green synthesis using plants, fruit, vegetables, and natural substances. Others have followed traditional chemical synthesis approaches and then used capping agents. Most studies of SeNPs began with Na2SeO3 as the precursor molecule. A noteworthy pattern across these studies was that, regardless of the variables being examined, all the microbes used were affected by exposure to SeNPs to some extent. A limitation of the work to date is that most studies used organisms in the planktonic state rather than in biofilms; moreover, biofilm studies were single species rather than multispecies. Hence, additional work is needed to determine the optimum profile of SeNPs for achieving caries-arresting actions.
7. Conclusions and Future Directions
SDF has become a popular treatment choice for halting dental caries in young children, especially children with minimal cooperation for dental procedures. However, it darkens hard tooth structure permanently, which compromises aesthetics, particularly in the anterior smile zone. This review identifies several methods that could be followed to reduce this concern, including the use of AgNPs and NPs of silver fluoride. There could also be value in using remineralising agents other than fluoride, such as NPs of hydroxyapatite. There could be variations made to formulations in order to lower the levels of silver and fluoride in the SDF or even to replace one or both of the silver and fluoride components completely. Moreover, since oxidation processes appear central to the chemistry of the staining, adding SeNPs which have antioxidant actions could have an anti-staining benefit; SeNPs could be used for their antimicrobial actions as well. Future research should address the topic of selenium chemistry to optimise how SeNPs could be used with or in place of ionic silver. Incorporating other antimicrobial metals as nanoparticles such as copper, zinc oxide, and cerium oxide should also be deeply explored in the context of topical anticaries agents, taking into account the optimal physicochemical parameters for each of these.
Author Contributions
Conceptualization, A.A., L.J.W. and S.Z.; methodology, A.A.; writing—original draft preparation, A.A. and L.J.W.; writing—review and editing, I.P.K., C.J.S. and S.Z.; visualization, A.A.; supervision, I.P.K., L.J.W., C.J.S. and S.Z.; project administration, S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nguyen, T.M.; Tonmukayakul, U.; Hall, M.; Calache, H. Cost-effectiveness analysis of silver diamine fluoride to divert dental general anaesthesia compared to standard care. Aust. Dent. J. 2022, 67, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.O.; Vaghela, P.M. Silver diamine fluoride: A successful anticarious solution with limits. Adv. Dent. Res. 2018, 29, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, A.; Stamford, T.C.; Niederman, R. Silver diamine fluoride: A caries “silver-fluoride bullet”. J. Dent. Res. 2009, 88, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Zhao, I.S.; Hiraishi, N.; Duangthip, D.; Mei, M.L.; Lo, E.C.M.; Chu, C.H. Clinical trials of silver diamine fluoride in arresting caries among children: A systematic review. JDR Clin. Transl. Res. 2016, 1, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Fung, M.H.T.; Duangthip, D.; Wong, M.C.M.; Lo, E.C.M.; Chu, C.H. Arresting dentine caries with different concentration and periodicity of silver diamine fluoride. JDR Clin. Transl. Res. 2016, 1, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Duangthip, D.; Fung, M.H.T.; Wong, M.C.M.; Chu, C.H.; Lo, E.C.M. Adverse effects of silver diamine fluoride treatment among preschool children. J. Dent. Res. 2018, 97, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Crystal, Y.O.; Niederman, R. Evidence-based dentistry update on silver diamine fluoride. Dent. Clin. North Am. 2019, 63, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.; Scott, J.M.; Crystal, Y.O.; Berg, J.H.; Milgrom, P. Silver diamine fluoride in pediatric dentistry training programs: Survey of graduate program directors. Pediatr. Dent. 2016, 38, 212–217. [Google Scholar]
- Sabbagh, H.; Othman, M.; Khogeer, L.; Al-Harbi, H.; Al Harthi, A.; Abdulgader Yaseen Abdulgader, A. Parental acceptance of silver diamine fluoride application on primary dentition: A systematic review and meta-analysis. BMC Oral Health 2020, 20, 227. [Google Scholar] [CrossRef]
- Duangthip, D.; Gao, S.S.; Chen, K.J.; Lo, E.C.M.; Chu, C.H. Oral health-related quality of life of preschool children receiving silver diamine fluoride therapy: A prospective 6-month study. J. Dent. 2019, 81, 27–32. [Google Scholar] [CrossRef]
- Gomes, M.C.; Perazzo, M.F.; Neves, E.T.; Martins, C.C.; Paiva, S.M.; Granville-Garcia, A.F. Oral problems and self-confidence in preschool children. Braz. Dent. J. 2017, 28, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Seifo, N.; Cassie, H.; Radford, J.R.; Innes, N.P.T. “I guess it looks worse to me, it doesn’t look like there’s been a problem solved but obviously there is”: A qualitative exploration of children’s and their parents’ views of silver diamine fluoride for the management of carious lesions in children. BMC Oral Health 2021, 21, 367. [Google Scholar] [CrossRef]
- Al-Bitar, Z.B.; Al-Omari, I.K.; Sonbol, H.N.; Al-Ahmad, H.T.; Cunningham, S.J. Bullying among Jordanian schoolchildren, its effects on school performance, and the contribution of general physical and dentofacial features. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Saafan, A.; Zaazou, M.H.; Sallam, M.K.; Mosallam, O.; El Danaf, H.A. Assessment of photodynamic therapy and nanoparticles effects on caries models. Open Access Maced. J. Med. Sci. 2018, 6, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Kopel, J.; Ray, C.; Reed, J.; Reid, T.W. Organo-selenium containing dental sealant inhibits biofilm formation by oral bacteria. Dent. Mater. 2022, 38, 848–857. [Google Scholar] [CrossRef]
- Bisht, N.; Phalswal, P.; Khanna, P.K. Selenium nanoparticles: A review on synthesis and biomedical applications. Mater. Adv. 2022, 3, 1415–1431. [Google Scholar] [CrossRef]
- Handy, R.D.; Owen, R.; Valsami-Jones, E. The ecotoxicology of nanoparticles and nanomaterials: Current status, knowledge gaps, challenges, and future needs. Ecotoxicology 2008, 17, 315–325. [Google Scholar] [CrossRef]
- Jolly, J.; Mohd Ahmar, R.; Zeeshan, A. Selenium nanoparticles: Small is the new big: Mini review. Open J. Chem. 2020, 6, 13–16. [Google Scholar] [CrossRef]
- Dhanraj, G.; Rajeshkumar, S. Anticariogenic effect of selenium nanoparticles synthesized using Brassica oleracea. J. Nanomater. 2021, 2021, 8115585. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabe, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of untreated caries: A systematic review and metaregression. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef]
- AAPD. Policy on Early Childhood Caries (ECC): Classifications, Consequences, and Preventive Strategies. The Reference Manual of Pediatric Dentistry; American Academy of Pediatric Dentistry: Chicago, IL, USA, 2022; pp. 90–93. [Google Scholar]
- Paolone, G.; Mazzitelli, C.; Formiga, S.; Kaitsas, F.; Breschi, L.; Mazzoni, A.; Tete, G.; Polizzi, E.; Gherlone, E.; Cantatore, G. One-year impact of COVID-19 pandemic on Italian dental professionals: A cross-sectional survey. Minerva Dent. Oral Sci. 2022, 71, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.H.; Ho, P.-L.; Lo, E.C. Oral health status and behaviours of preschool children in Hong Kong. BMC Public Health 2012, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Duangthip, D.; Gao, S.S.; Lo, E.C.; Chu, C.H. Early childhood caries among 5- to 6-year-old children in Southeast Asia. Int. Dent. J. 2017, 67, 98–106. [Google Scholar] [CrossRef]
- Casamassimo, P.S.; Thikkurissy, S.; Edelstein, B.L.; Maiorini, E. Beyond the dmft: The human and economic cost of early childhood caries. J. Am. Dent. Assoc. 2009, 140, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Ladewig, N.M.; Camargo, L.B.; Tedesco, T.K.; Floriano, I.; Gimenez, T.; Imparato, J.C.P.; Mendes, F.M.; Braga, M.M.; Raggio, D.P. Management of dental caries among children: A look at the cost-effectiveness. Expert Rev. Pharm. Outcomes Res. 2018, 18, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Alcaino, E.; KIilpatrick, N.M.; Smith, E.D.K. Utilization of day stay general anaesthesia for the provision ofdental treatment to children in New South Wales, Australia. Int. J. Paediatr. Dent. 2000, 10, 206–212. [Google Scholar] [CrossRef]
- Almeida, A.G.; Roseman, M.M.; Sheff, M.; Huntington, N.; Hughes, C.V. Future caries susceptibility in children with early childhood caries following treatment under general anesthesia. Pediatr. Dent. 2000, 22, 302–306. [Google Scholar]
- Alshehri YF, A.; Nicholls, W.; Mai, N.Q.; Park, J.S.; Kruger, E. Cross-sectional analysis of dental treatment under general anaesthesia in hospitalised Western Australian children in 2018–19. Aust. Health Rev. 2021. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Prim. 2017, 3, 17030. [Google Scholar] [CrossRef]
- Marsh, P.D. Are dental diseases examples of ecological catastrophes? Microbiology 2003, 149 Pt. 2, 279–294. [Google Scholar] [CrossRef]
- Bowen, W.; Koo, H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Paes Leme, A.F.; Koo, H.; Bellato, C.M.; Bedi, G.; Cury, J.A. The role of sucrose in cariogenic dental biofilm formation—New insight. J. Dent. Res. 2006, 85, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, E.; Parsaei, Y.; Klein, M.I.; Koo, H. Advances in the microbial etiology and pathogenesis of early childhood caries. Mol. Oral Microbiol. 2017, 32, 24–34. [Google Scholar] [CrossRef]
- Mei, M.L.; Nudelman, F.; Marzec, B.; Walker, J.M.; Lo, E.C.M.; Walls, A.W.; Chu, C.H. Formation of fluorohydroxyapatite with silver diamine fluoride. J. Dent. Res. 2017, 96, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Yoshida, S.; Sobue, S.; Kato, J.; Nishida, M. Effect of topically applied ammoniacal silver fluoride on dental caries in children. J. Osaka Univ. Dent. Sch. 1969, 9, 149–155. [Google Scholar]
- Horst, J.; Ellenikiotis, H.; Milgrom, P. UCSF Protocol for caries arrest using silver diamine fluoride: Rationale, indications, and consent. J. Calif. Dent. Assoc. 2016, 44, 16–28. [Google Scholar]
- Mei, M.L.; Ito, L.; Cao, Y.; Lo, E.C.; Li, Q.; Chu, C. An ex vivo study of arrested primary teeth caries with silver diamine fluoride therapy. J. Dent. 2014, 42, 395–402. [Google Scholar] [CrossRef]
- Shah, S.; Bhaskar, V.; Chawla, S.; Venkataraghavan, K.; Choudhary, P.; Ganesh, M.; Trivedi, K. Efficacy of silver diamine fluoride as a topical fluoride agent compared to fluoride varnish and acidulated phosphate fluoride gel: An in vivo study. Indian J. Dent. Res. 2013, 24, 575–581. [Google Scholar] [CrossRef]
- Greenwall-Cohen, J.; Greenwall, L.; Barry, S. Silver diamine fluoride—An overview of the literature and current clinical techniques. Br. Dent. J. 2020, 228, 831–838. [Google Scholar] [CrossRef]
- Rajendra, A.; Veitz-Keenan, A.; Oliveira, B.H.; Ruff, R.R.; Wong, M.C.M.; Innes, N.P.T.; Radford, J.; Seifo, N.; Niederman, R. Topical silver diamine fluoride for managing dental caries in children and adults. Cochrane Database Syst. Reviews. 2017, CD012718. [Google Scholar] [CrossRef]
- Mei, M.L.; Ito, L.; Cao, Y.; Li, Q.L.; Lo, E.C.; Chu, C.H. Inhibitory effect of silver diamine fluoride on dentine demineralisation and collagen degradation. J. Dent. 2013, 41, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Fung, M.H.T.; Duangthip, D.; Wong, M.C.M.; Lo, E.C.M.; Chu, C.H. Randomized clinical trial of 12% and 38% silver diamine fluoride treatment. J. Dent. Res. 2018, 97, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Craig, G.G.; Knight, G.M.; McIntyre, J.M. Clinical evaluation of diamine silver fluoride/potassium iodide as a dentine desensitizing agent. A pilot study. Aust. Dent. J. 2012, 57, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, A. An alternate technique of care using silver fluoride followed by stannous fluoride in the management of root caries in aged care. Spec. Care Dent. 2016, 36, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Knight, G.M.; McIntyre, J.M.; Craig, G.G.; Mulyani; Zilm, P.S.; Gully, N. An in vitro model to measure the effect of a silver fluoride and potassium. Aust. Dent. J. 2005, 50, 242–245. [Google Scholar] [CrossRef]
- Mei, M.L.; Chu, C.H.; Low, K.H.; Che, C.M.; Lo, E.C. Caries arresting effect of silver diamine fluoride on dentine carious lesion with S. mutans and L. acidophilus dual-species cariogenic biofilm. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e824–e831. [Google Scholar] [CrossRef]
- Mei, M.L.; Lo, E.C.M.; Chu, C.H. Arresting dentine caries with silver diamine fluoride: What’s behind it? J. Dent. Res. 2018, 97, 751–758. [Google Scholar] [CrossRef]
- Mei, M.L.; Li, Q.L.; Chu, C.H.; Yiu, C.K.; Lo, E.C. The inhibitory effects of silver diamine fluoride at different concentrations on matrix metalloproteinases. Dent. Mater. 2012, 28, 903–908. [Google Scholar] [CrossRef]
- Llodra, J.C.; Rodriguez, A.; Ferrer, B.; Menardia, V.; Ramos, T.; Morato, M. Efficacy of silver diamine fluoride for caries reduction in primary teeth and first permanent molars of schoolchildren: 36-month clinical trial. J. Dent. Res. 2005, 84, 721–724. [Google Scholar] [CrossRef]
- Gotjamanos, T. Pulp response in primary teeth with deep residual caries treated with silver fluoride and glass ionorner cement (‘atraumatic’ technique). Aust. Dent. J. 1996, 41, 328–362. [Google Scholar] [CrossRef]
- Hu, S.; Meyer, B.; Duggal, M. A silver renaissance in dentistry. Eur. Arch. Paediatr. Dent. 2018, 19, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Duangthip, D.; Wong, M.C.M.; Chu, C.H.; Lo, E.C.M. Caries arrest by topical fluorides in preschool children: 30-month results. J. Dent. 2018, 70, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Bagher, S.M.; Sabbagh, H.J.; AlJohani, S.M.; Alharbi, G.; Aldajani, M.; Elkhodary, H. Parental acceptance of the utilization of silver diamine fluoride on their child’s primary and permanent teeth. Patient Prefer. Adherence 2019, 13, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Crystal, Y.O.; Janal, M.N.; Hamilton, D.S.; Niederman, R. Parental perceptions and acceptance of silver diamine fluoride staining. J. Am. Dent. Assoc. 2017, 148, 510–518.e4. [Google Scholar] [CrossRef]
- Paolone, G.; Scolavino, S.; Gherlone, E.; Spagnuolo, G. Direct esthetic composite restorations in anterior teeth: Managing symmetry strategies. Symmetry 2021, 13, 797. [Google Scholar] [CrossRef]
- Knight, G.M.; McIntyre, J.M.; Craig, G.G.; Mulyani; Zilm, P.S.; Gully, N.J. Inability to form a biofilm of Streptococcus mutans on silver fluoride- and potassium iodide-treated demineralized dentin. Quintessence Int. 2009, 40, 155–161. [Google Scholar]
- Zhao, I.S.; Chu, S.; Yu, O.Y.; Mei, M.L.; Chu, C.H.; Lo, E.C.M. Effect of silver diamine fluoride and potassium iodide on shear bond strength of glass ionomer cements to caries-affected dentine. Int. Dent. J. 2019, 69, 341–347. [Google Scholar] [CrossRef]
- Roberts, A.; Bradley, J.; Merkley, S.; Pachal, T.; Gopal, J.V.; Sharma, D. Does potassium iodide application following silver diamine fluoride reduce staining of tooth? A systematic review. Aust. Dent. J. 2020, 65, 109–117. [Google Scholar] [CrossRef]
- Haiat, A.; Ngo, H.C.; Samaranayake, L.P.; Fakhruddin, K.S. The effect of the combined use of silver diamine fluoride and potassium iodide in disrupting the plaque biofilm microbiome and alleviating tooth discoloration: A systematic review. PLoS ONE 2021, 16, e0252734. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lo, E.C.M.; Liu, B.Y.; Wong, M.C.M.; Chu, C.H. Randomized clinical trial on arresting dental root caries through silver diammine fluoride applications in community-dwelling elders. J. Dent. 2016, 51, 15–20. [Google Scholar] [CrossRef]
- Fröhlich, T.T.; Gindri, L.D.; Pedrotti, D.; Cavalheiro, C.P.; Soares, F.; Rocha, R.O. Evaluation of the use of potassium iodide application on stained demineralized dentin under resin composite following silver diamine fluoride application. Pediatr. Dent. 2021, 43, 57–61. [Google Scholar]
- Nagireddy, V.R.; Reddy, D.; Kondamadugu, S.; Puppala, N.; Mareddy, A.A.C. Nanosilver fluoride—A paradigm shift for arrest in dental caries in primary teeth of schoolchildren: A randomized controlled clinical trial. Int. J. Clin. Pediatr. Dent. 2019, 12, 484–490. [Google Scholar] [PubMed]
- Song, H.; Ahmad Nor, Y.; Yu, M.; Yang, Y.; Zhang, J.; Zhang, H.; Xu, C.; Mitter, N.; Yu, C. Silica nanopollens enhance adhesion for long-term bacterial inhibition. J. Am. Chem. Soc. 2016, 138, 6455–6462. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, Y.; Wang, X.; Li, Q.; Xiao, Y.; Li, P.; Wang, L.; Ye, Z.; Xing, X. Novel resin-based dental material with anti-biofilm activity and improved mechanical property by incorporating hydrophilic cationic copolymer functionalized nanodiamond. J. Mater. Sci. Mater. Med. 2018, 29, 162. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Snigdha, S.; Bhavitha, K.B.; Babu, S.; Ajith, A.; Radhakrishnan, E.K. Biofabricated silver nanoparticles incorporated polymethyl methacrylate as a dental adhesive material with antibacterial and antibiofilm activity against Streptococcus mutans. 3 Biotech 2018, 8, 404. [Google Scholar] [CrossRef]
- Tirupathi, S.; Svsg, N.; Rajasekhar, S.; Nuvvula, S. Comparative cariostatic efficacy of a novel nano-silver fluoride varnish with 38% silver diamine fluoride varnish a double-blind randomized clinical trial. J. Clin. Exp. Dent. 2019, 11, e105-12. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.E., Jr.; Vasconcelos Filho, A.; Targino, A.G.; Flores, M.A.; Galembeck, A.; Caldas, A.F., Jr.; Rosenblatt, A. A new “silver-bullet” to treat caries in children—Nano silver fluoride: A randomised clinical trial. J. Dent. 2014, 42, 945–951. [Google Scholar] [CrossRef]
- Targino, A.G.; Flores, M.A.; dos Santos Junior, V.E.; de Godoy Bene Bezerra, F.; de Luna Freire, H.; Galembeck, A.; Rosenblatt, A. An innovative approach to treating dental decay in children. A new anti-caries agent. J. Mater. Sci. Mater. Med. 2014, 25, 2041–2047. [Google Scholar] [CrossRef]
- Melaiye, A.; Youngs, W.J. Silver and its application as an antimicrobial agent. Expert Opin. Ther. Pat. 2005, 15, 125–130. [Google Scholar] [CrossRef]
- White, R.J. An historical overview of the use of silver in wound management. Br. J. Community Nurs. 2001, 6 (Suppl. S1), 3–8. [Google Scholar] [CrossRef]
- Barras, F.; Aussel, L.; Ezraty, B. Silver and antibiotic, new facts to an old story. Antibiotics 2018, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Mijnendonckx, K.; Leys, N.; Mahillon, J.; Silver, S.; Van Houdt, R. Antimicrobial silver: Uses, toxicity and potential for resistance. BioMetals 2013, 26, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.J.-Y.; Botelho, M.G.; Matinlinna, J.P. Silver compounds used in dentistry for caries management: A review. J. Dent. 2012, 40, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, E. What value has argenti nitras as a therapeutic agent in dentistry? Int. Dent. J. 1891, 12, 661–670. [Google Scholar]
- Seltzer, S. Effective duration of some agents used for dentin sterilization. J. Dent. Res. 1942, 21, 115–123. [Google Scholar] [CrossRef]
- James, P.; Parfitt, G. A clinical note on the use of silver nitrate in the prevention of fissure caries in newly erupted first permanant molars. Britsh Dent. J. 1954, 96, 35–36. [Google Scholar]
- Kidd, E. Caries removal and the pulpo-dentinal complex. Dent. Update 2000, 27, 476–482. [Google Scholar] [CrossRef]
- Silver, S.; Phung le, T.; Silver, G. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J. Ind. Microbiol. Biotechnol. 2006, 33, 627–634. [Google Scholar] [CrossRef]
- Maillard, J.Y.; Hartemann, P. Silver as an antimicrobial: Facts and gaps in knowledge. Crit. Rev. Microbiol. 2013, 39, 373–383. [Google Scholar] [CrossRef]
- Edwards-Jones, V. The benefits of silver in hygiene, personal care and healthcare. Lett. Appl. Microbiol. 2009, 49, 147–152. [Google Scholar] [CrossRef]
- Wijnhoven, S.W.P.; Peijnenburg, W.J.G.M.; Herberts, C.A.; Hagens, W.I.; Oomen, A.G.; Heugens, E.H.W.; Roszek, B.; Bisschops, J.; Gosens, I.; Van De Meent, D.; et al. Nano-silver—A review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
- Hovhannisyan, Z.; Timotina, M.; Manoyan, J.; Gabrielyan, L.; Petrosyan, M.; Kusznierewicz, B.; Bartoszek, A.; Jacob, C.; Ginovyan, M.; Trchounian, K.; et al. Extract-mediated green synthesis and antibacterial action mechanisms of silver nanoparticles. Antibiotics 2022, 11, 1415. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.J.; Jun, S.K.; Kim, Y.J.; Ahn, J.Y.; Vu, H.T.; Mandakhbayar, N.; Han, M.R.; Lee, J.H.; Kim, J.B.; Kim, J.S.; et al. Characterization of physical and biological properties of a caries-arresting liquid containing copper doped bioglass nanoparticles. Pharmaceutics 2022, 14, 1137. [Google Scholar] [CrossRef]
- Favaro, J.C.; de Mello Peixoto, Y.C.T.; Geha, O.; Dias, F.A.; Guiraldo, R.D.; Lopes, M.B.; Berger, S.B. Can silver diamine fluoride or silver nanoparticle-based anticaries agents to affect enamel bond strength? Restor. Dent. Endod. 2021, 46, e7. [Google Scholar] [CrossRef]
- Favaro, J.C.; Detomini, T.R.; Maia, L.P.; Poli, R.C.; Guiraldo, R.D.; Lopes, M.B.; Berger, S.B. Anticaries agent based on silver nanoparticles and fluoride: Characterization and biological and remineralizing effects-an in vitro study. Int. J. Dent. 2022, 2022, 9483589. [Google Scholar] [CrossRef] [PubMed]
- Drabowicz, J.; Mikołajczyk, M. Selenium at higher oxidation state. In Organoselenium Chemistry. Topics in Current Chemistry; Wirth, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; Volume 208, pp. 143–176. [Google Scholar]
- Pasdar, H.; Hedayati Saghavaz, B.; Masoumi, M. A simple method for the recovery of selenium from copper anode slime sample using alkaline roasting process. Int. J. New Chem. 2019, 6, 143–150. [Google Scholar]
- Zhu, M.; Niu, G.; Tang, J. Elemental Se: Fundamentals and its optoelectronic applications. J. Mater. Chem. C 2019, 7, 2199–2206. [Google Scholar] [CrossRef]
- McNeal, J.M.; Balistrieri, L.S. Geochemistry and occurrence of selenium: An overview. Selenium Agric. Environ. 1989, 23, 1–13. [Google Scholar]
- Lakin, H.W. Selenium in Our Enviroment. Adv. Chem. 1973, 123, 96–111. [Google Scholar]
- Wallenberg, M.; Misra, S.; Wasik, A.M.; Marzano, C.; Björnstedt, M.; Gandin, V.; Fernandes, A.P. Selenium induces a multi-targeted cell death process in addition to ROS formation. J. Cell. Mol. Med. 2014, 18, 671–684. [Google Scholar] [CrossRef]
- Bai, K.; Hong, B.; He, J.; Hong, Z.; Tan, R. Preparation and antioxidant properties of selenium nanoparticles-loaded chitosan microspheres. Int. J. Nanomed. 2017, 12, 4527. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, J.K.; Power, R.; Toborek, M. Biological activity of selenium: Revisited. IUBMB Life 2016, 68, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Majeed, W.; Zafar, M.; Bhatti, A.; John, P. Therapeutic potential of selenium nanoparticles. J. Nanomed. Nanotechnol. 2018, 9, 1000487. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 2555–2566. [Google Scholar] [CrossRef]
- Stroyuk, A.L.; Raevskaya, A.E.; Kuchmiy, S.Y.; Dzhagan, V.M.; Zahn, D.R.; Schulze, S. Structural and optical characterization of colloidal Se nanoparticles prepared via the acidic decomposition of sodium selenosulfate. Colloids Surf. A Physicochem. Eng. Asp. 2008, 320, 169–174. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Yu, H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free. Radic. Biol. Med. 2007, 42, 1524–1533. [Google Scholar] [CrossRef]
- Kumar, A.; Sevonkaev, I.; Goia, D.V. Synthesis of selenium particles with various morphologies. J. Colloid Interface Sci. 2014, 416, 119–123. [Google Scholar] [CrossRef]
- Tran, P.A.; Webster, T.J. Selenium nanoparticles inhibit Staphylococcus aureus growth. Int. J. Nanomed. 2011, 6, 1553–1558. [Google Scholar]
- Luesakul, U.; Komenek, S.; Puthong, S.; Muangsin, N. Shape-controlled synthesis of cubic-like selenium nanoparticles via the self-assembly method. Carbohydr. Polym. 2016, 153, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, H.; Cao, W.; Dong, Y.; Zou, C.; Yang, Y.; Huang, S.; Dai, N.; Zhu, D.-M. Antimony doped cadmium selenium nanobelts with enhanced electrical and optoelectrical properties. Appl. Surf. Sci. 2014, 307, 608–614. [Google Scholar] [CrossRef]
- Nonsuwan, P.; Puthong, S.; Palaga, T.; Muangsin, N. Novel organic/inorganic hybrid flower-like structure of selenium nanoparticles stabilized by pullulan derivatives. Carbohydr. Polym. 2018, 184, 9–19. [Google Scholar] [CrossRef]
- Chaudhary, S.; Chauhan, P.; Kumar, R.; Bhasin, K.K. Toxicological responses of surfactant functionalized selenium nanoparticles: A quantitative multi-assay approach. Sci. Total Environ. 2018, 643, 1265–1277. [Google Scholar] [CrossRef]
- Sarkar, J.; Mridha, D.; Davoodbasha, M.A.; Banerjee, J.; Chanda, S.; Ray, K.; Roychowdhury, T.; Acharya, K.; Sarkar, J. A state-of-the-art systemic review on selenium nanoparticles: Mechanisms and factors influencing biogenesis and its potential applications. Biol. Trace Elem. Res. 2023. [Google Scholar] [CrossRef]
- Shahmoradi, S.; Shariati, A.; Zargar, N.; Yadegari, Z.; Asnaashari, M.; Amini, S.M.; Darban-Sarokhalil, D. Antimicrobial effects of selenium nanoparticles in combination with photodynamic therapy against Enterococcus faecalis biofilm. Photodiagnosis Photodyn. Ther. 2021, 35, 102398. [Google Scholar] [CrossRef]
- Ferro, C.; Florindo, H.F.; Santos, H.A. Selenium nanoparticles for biomedical applications: From development and characterization to therapeutics. Adv. Healthc. Mater. 2021, 10, e2100598. [Google Scholar] [CrossRef]
- Cavalu, S.; Antoniac, I.V.; Fritea, L.; Mates, I.M.; Milea, C.; Laslo, V.; Vicas, S.; Mohan, A. Surface modifications of the titanium mesh for cranioplasty using selenium nanoparticles coating. J. Adhes. Sci. Technol. 2018, 32, 2509–2522. [Google Scholar] [CrossRef]
- Srivastava, P.; Braganca, J.M.; Kowshik, M. In vivo synthesis of selenium nanoparticles by Halococcus salifodinae BK18 and their anti-proliferative properties against HeLa cell line. Biotechnol. Prog. 2014, 30, 1480–1487. [Google Scholar] [CrossRef]
- Liao, G.; Tang, J.; Wang, D.; Zuo, H.; Zhang, Q.; Liu, Y.; Xiong, H. Selenium nanoparticles (SeNPs) have potent antitumor activity against prostate cancer cells through the upregulation of miR-16. World J. Surg. Oncol. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Galić, E.; Radić, K.; Golub, N.; Vitali Čepo, D.; Kalčec, N.; Vrček, E.; Vinković, T. Utilization of olive pomace in green synthesis of selenium nanoparticles: Physico-chemical characterization, bioaccessibility and biocompatibility. Int. J. Mol. Sci. 2022, 23, 9128. [Google Scholar] [CrossRef] [PubMed]
- Selmani, A.; Ulm, L.; Kasemets, K.; Kurvet, I.; Erceg, I.; Barbir, R.; Pem, B.; Santini, P.; Marion, I.D.; Vinković, T. Stability and toxicity of differently coated selenium nanoparticles under model environmental exposure settings. Chemosphere 2020, 250, 126265. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Fu, Y.; Li, M.; Jiang, D.; Kutyreff, C.J.; Engle, J.W.; Lan, X.; Cai, W.; Chen, T. Chirality-driven transportation and oxidation prevention by chiral selenium nanoparticles. Angew. Chem. 2020, 132, 4436–4444. [Google Scholar] [CrossRef]
- Hariharan, H.; Al-Harbi, N.; Karuppiah, P.; Rajaram, S. Microbial synthesis of selenium nanocomposite using Saccharomyces cerevisiae and its antimicrobial activity against pathogens causing nosocomial infection. Chalcogenide Lett. 2012, 9, 509–515. [Google Scholar]
- Wang, Q.; Larese-Casanova, P.; Webster, T.J. Inhibition of various gram-positive and gram-negative bacteria growth on selenium nanoparticle coated paper towels. Int. J. Nanomed. 2015, 10, 2885. [Google Scholar]
- Tran, P.A.; O’Brien-Simpson, N.; Reynolds, E.C.; Pantarat, N.; Biswas, D.P.; O’Connor, A.J. Low cytotoxic trace element selenium nanoparticles and their differential antimicrobial properties against S. aureus and E. coli. Nanotechnology 2016, 27, 045101. [Google Scholar] [CrossRef]
- Hernandez-Diaz, J.A.; Garza-Garcia, J.J.; Leon-Morales, J.M.; Zamudio-Ojeda, A.; Arratia-Quijada, J.; Velazquez-Juarez, G.; Lopez-Velazquez, J.C.; Garcia-Morales, S. Antibacterial activity of biosynthesized selenium nanoparticles using extracts of calendula officinalis against potentially clinical bacterial strains. Molecules 2021, 26, 5929. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, X.; Ning, Z.; Kwon, S.Y.; Li, M.-L.; Tack, F.M.G.; Kwon, E.E.; Rinklebe, J.; Yin, R. The beneficial and hazardous effects of selenium on the health of the soil-plant-human system: An overview. J. Hazard. Mater. 2022, 422, 126876. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Ochi, A.; Mihara, H.; Ogra, Y. Comparison of nutritional availability of biogenic selenium nanoparticles and chemically synthesized selenium nanoparticles. Biol. Trace Elem. Res. 2023. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107. [Google Scholar] [CrossRef] [PubMed]
- Mal, J.; Veneman, W.J.; Nancharaiah, Y.V.; van Hullebusch, E.D.; Peijnenburg, W.J.; Vijver, M.G.; Lens, P.N. A comparison of fate and toxicity of selenite, biogenically, and chemically synthesized selenium nanoparticles to zebrafish (Danio rerio) embryogenesis. Nanotoxicology 2017, 11, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Yang, J.; Zhang, Y.; Fang, Y.; Nishinari, K.; Phillips, G.O. Synthesis and antioxidant properties of gum arabic-stabilized selenium nanoparticles. Int. J. Biol. Macromol. 2014, 65, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhai, X.; Zhao, G.; Ren, F.; Leng, X. Synthesis, characterization, and controlled release of selenium nanoparticles stabilized by chitosan of different molecular weights. Carbohydr. Polym. 2015, 134, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Liu, Z.; He, L.; Luk, K.H.; Cheung, S.T.; Wong, K.H.; Chen, T. Autophagy is an important action mode for functionalized selenium nanoparticles to exhibit anti-colorectal cancer activity. Biomater. Sci. 2018, 6, 2508–2517. [Google Scholar] [CrossRef]
- Cai, W.; Hu, T.; Bakry, A.M.; Zheng, Z.; Xiao, Y.; Huang, Q. Effect of ultrasound on size, morphology, stability and antioxidant activity of selenium nanoparticles dispersed by a hyperbranched polysaccharide from Lignosus rhinocerotis. Ultrason. Sonochemistry 2018, 42, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Dagmar, H.; Kristyna, C.; Pavel, K.; Vojtech, A.; Rene, K. Selenium nanoparticles and evaluation of their antimicrobial activity on bacterial isolates obtained from clinical specimens. In Proceedings of the Nanocon, Brno, Czech Republic, 14–16 October 2015. [Google Scholar]
- Srivastava, N.; Mukhopadhyay, M. Green synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial property. Bioprocess Biosyst. Eng. 2015, 38, 1723–1730. [Google Scholar] [CrossRef]
- Nguyen, T.H.D.; Vardhanabhuti, B.; Lin, M.; Mustapha, A. Antibacterial properties of selenium nanoparticles and their toxicity to Caco-2 cells. Food Control 2017, 77, 17–24. [Google Scholar] [CrossRef]
- Piacenza, E.; Presentato, A.; Zonaro, E.; Lemire, J.A.; Demeter, M.; Vallini, G.; Turner, R.J.; Lampis, S. Antimicrobial activity of biogenically produced spherical Se-nanomaterials embedded in organic material against Pseudomonas aeruginosa and Staphylococcus aureus strains on hydroxyapatite-coated surfaces. Microb. Biotechnol. 2017, 10, 804–818. [Google Scholar] [CrossRef]
- Huang, T.; Holden, J.A.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. Engineering highly effective antimicrobial selenium nanoparticles through control of particle size. Nanoscale 2019, 11, 14937–14951. [Google Scholar] [CrossRef]
- Muthu, S.; Raju, V.; Gopal, V.B.; Gunasekaran, A.; Narayan, K.S.; Malairaj, S.; Lakshmikanthan, M.; Duraisamy, N.; Krishnan, K.; Perumal, P. A rapid synthesis and antibacterial property of selenium nanoparticles using egg white lysozyme as a stabilizing agent. SN Appl. Sci. 2019, 1, 1543. [Google Scholar] [CrossRef]
- Tran, P.A.; O’Brien-Simpson, N.; Palmer, J.A.; Bock, N.; Reynolds, E.C.; Webster, T.J.; Deva, A.; Morrison, W.A.; O’Connor, A.J. Selenium nanoparticles as anti-infective implant coatings for trauma orthopedics against methicillin-resistant Staphylococcus aureus and epidermidis: In vitro and in vivo assessment. Int. J. Nanomed. 2019, 14, 4613–4624. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, S.; Sundar, K.; Muthukumaran, A. Reducing agents influence the shapes of selenium nanoparticles (SeNPs) and subsequently their antibacterial and antioxidant activity. Mater. Res. Express 2019, 6, 0850i2. [Google Scholar] [CrossRef]
- Chung, S.; Zhou, R.; Webster, T.J. Green synthesized bsa-coated selenium nanoparticles inhibit bacterial growth while promoting mammalian cell growth. Int. J. Nanomed. 2020, 15, 115–124. [Google Scholar] [CrossRef]
- Menon, S.; Agarwal, H.; Rajeshkumar, S.; Jacquline Rosy, P.; Shanmugam, V.K. Investigating the antimicrobial activities of the biosynthesized selenium nanoparticles and its statistical analysis. BioNanoScience 2020, 10, 122–135. [Google Scholar] [CrossRef]
- Rangrazi, A.; Bagheri, H.; Ghazvini, K.; Boruziniat, A.; Darroudi, M. Synthesis and antibacterial activity of colloidal selenium nanoparticles in chitosan solution: A new antibacterial agent. Mater. Res. Express 2020, 6, 1250h3. [Google Scholar] [CrossRef]
- Filipović, N.; Ušjak, D.; Milenković, M.T.; Zheng, K.; Liverani, L.; Boccaccini, A.R.; Stevanović, M.M. Comparative study of the antimicrobial activity of selenium nanoparticles with different surface chemistry and structure. Front. Bioeng. Biotechnol. 2021, 8, 624621. [Google Scholar] [CrossRef]
- Hasani, S.; Khare, T.; Oak, U. Antibiofilm activity of selenium nanorods against multidrug-resistant staphylococcus aureus. MGM J. Med. Sci. 2021, 8, 415–421. [Google Scholar]
- Miglani, S.; Tani-Ishii, N. Biosynthesized selenium nanoparticles: Characterization, antimicrobial, and antibiofilm activity against Enterococcus faecalis. PeerJ 2021, 9, e11653. [Google Scholar] [CrossRef] [PubMed]
- Alvi, G.B.; Iqbal, M.S.; Ghaith, M.M.S.; Haseeb, A.; Ahmed, B.; Qadir, M.I. Biogenic selenium nanoparticles (SeNPs) from citrus fruit have anti-bacterial activities. Sci. Rep. 2021, 11, 4811. [Google Scholar] [CrossRef]
- Adibian, F.; Ghaderi, R.S.; Sabouri, Z.; Davoodi, J.; Kazemi, M.; Ghazvini, K.; Youssefi, M.; Soleimanpour, S.; Darroudi, M. Green synthesis of selenium nanoparticles using Rosmarinus officinalis and investigated their antimicrobial activity. BioMetals 2022, 35, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Darroudi, M.; Rangrazi, A.; Ghazvini, K.; Bagheri, H.; Boruziniat, A. Antimicrobial activity of colloidal selenium nanoparticles in chitosan solution against streptococcus mutans, lactobacillus acidophilus, and candida albicans. Pesqui. Bras. Em Odontopediatria E Clínica Integr. 2021, 21, e0121. [Google Scholar] [CrossRef]
- Shahmoradi, S.; Shariati, A.; Amini, S.M.; Zargar, N.; Yadegari, Z.; Darban-Sarokhalil, D. The application of selenium nanoparticles for enhancing the efficacy of photodynamic inactivation of planktonic communities and the biofilm of Streptococcus mutans. BMC Res. Notes 2022, 15, 84. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).