A European International Multicentre Survey on the Current Practice of Perioperative Antibiotic Prophylaxis for Paediatric Liver Transplantations
Abstract
1. Introduction
2. Results
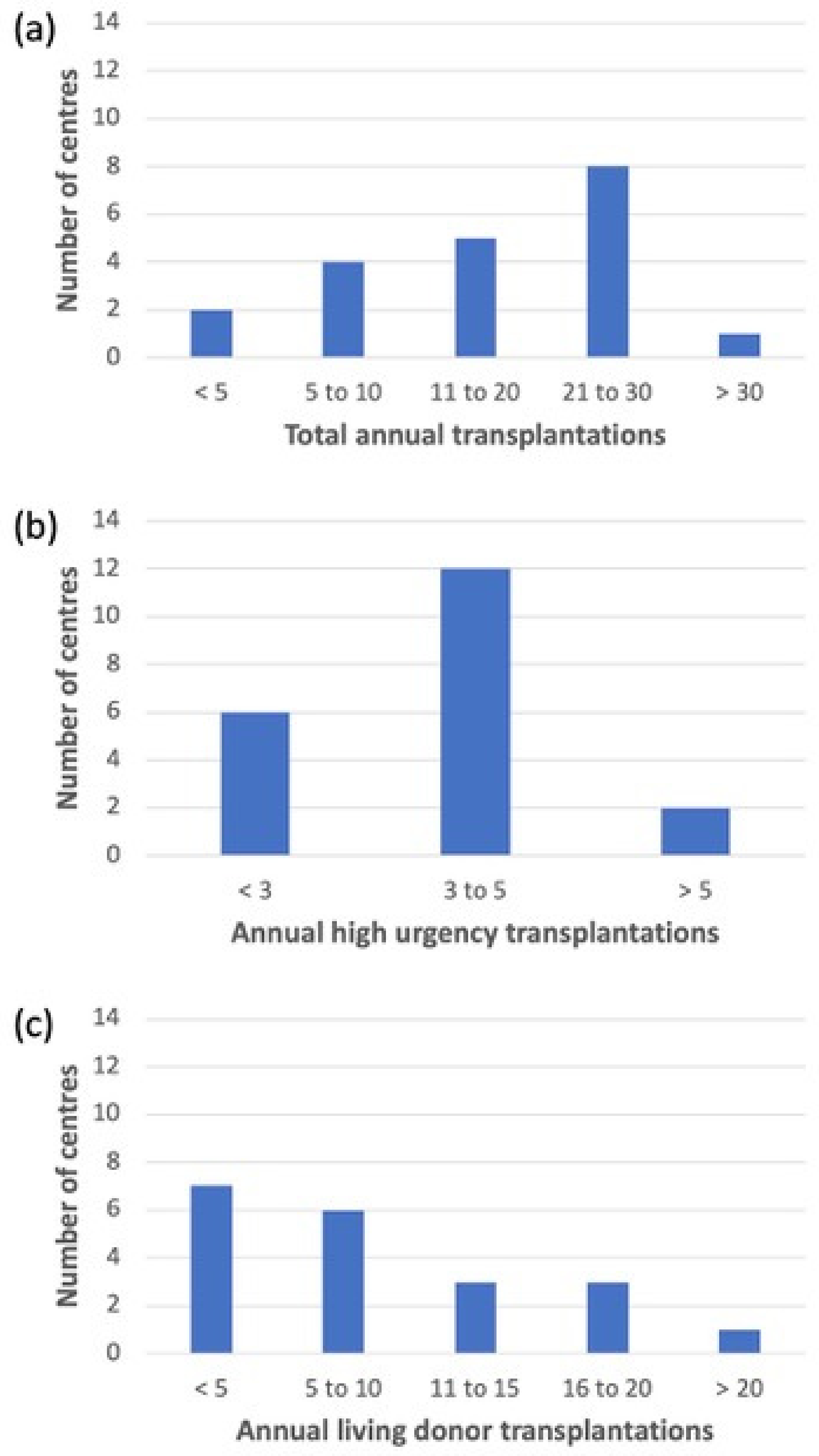
2.1. Demographics of Participating Centres
2.2. Immunosuppression
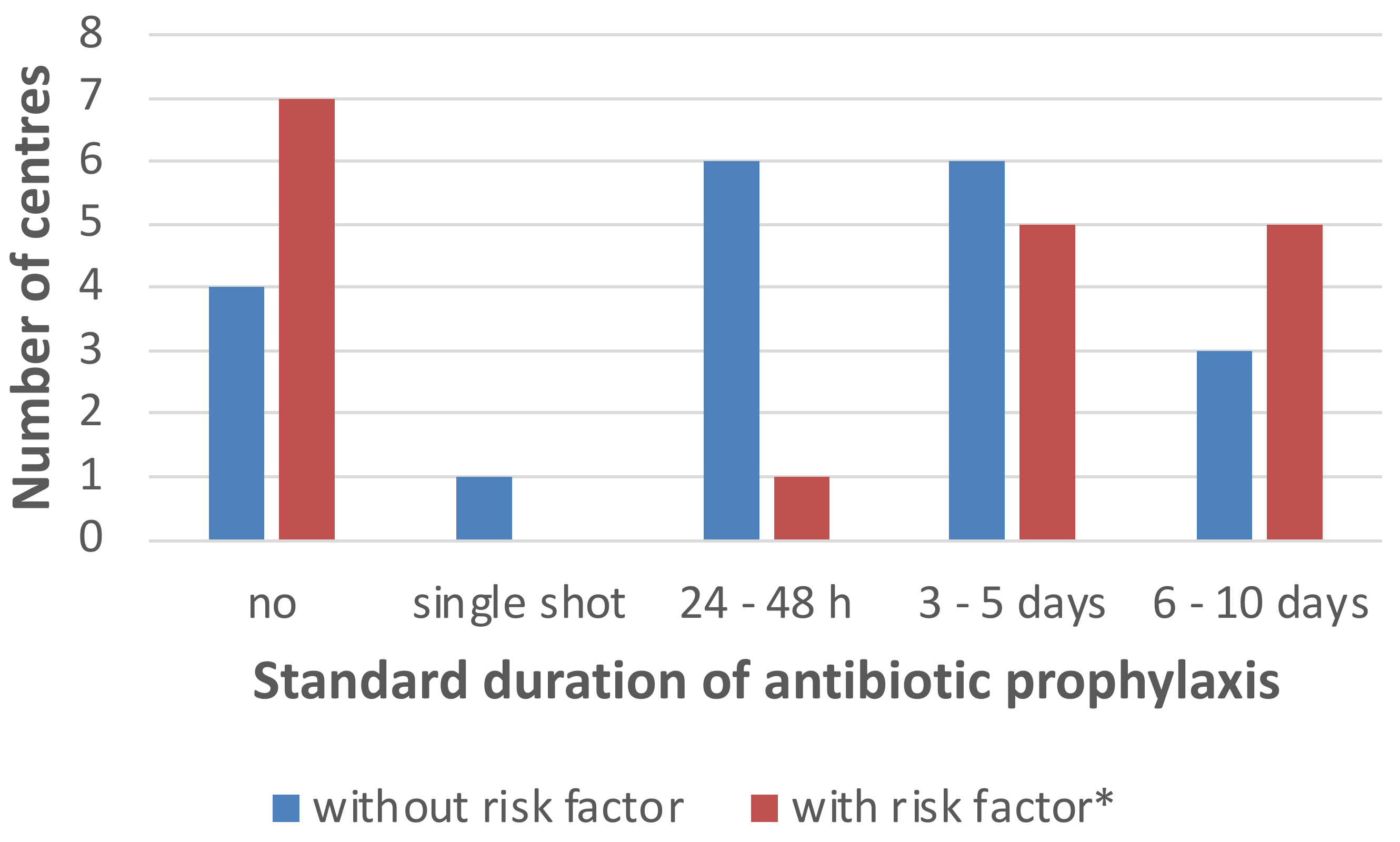
2.3. Antimicrobial Strategies
2.4. MDR Prevalence and Perioperative Prophylaxis
2.5. Availability of a (Paediatric) Infectious Disease Specialist and Perioperative Prophylaxis
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Data Analysis
4.3. Ethics Approval and Support
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Starzl, T.E.; Groth, C.G.; Brettschneider, L.; Penn, I.; Fulginiti, V.A.; Moon, J.B.; Blanchard, H.; Martin, A.J.; Porter, K.A. Orthotopic Homotransplantation of the Human Liver. Ann. Surg. 1968, 168, 392–415. [Google Scholar] [CrossRef]
- Basturk, A.; Yılmaz, A.; Sayar, E.; Dinçkan, A.; Aliosmanoğlu, İ.; Erbiş, H.; Aydınlı, B.; Artan, R. Pediatric Liver Transplantation: Our Experiences. Eurasian J. Med. 2016, 48, 209–212. [Google Scholar] [CrossRef]
- Pfister, E.-D. 40 Jahre Lebertransplantation im Kindes- und Jugendalter. Mon. Kinderheilkd. 2016, 164, 455–464. [Google Scholar] [CrossRef]
- Sundaram, S.S.; Alonso, E.M.; Anand, R.; Study of Pediatric Liver Transplantation Research Group. Outcomes after liver transplantation in young infants. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 486–492. [Google Scholar] [CrossRef]
- Cacciarelli, T.V.; Dvorchik, I.; Mazariegos, G.V.; Gerber, D.; Jain, A.B.; Fung, J.J.; Reyes, J. An analysis of pretransplantation variables associated with long-term allograft outcome in pediatric liver transplant recipients receiving primary tacrolimus (FK506) therapy. Transplantation 1999, 68, 650–655. [Google Scholar] [CrossRef]
- Saint-Vil, D.; Luks, F.I.; Lebel, P.; Brandt, M.L.; Paradis, K.; Weber, A.; Guay, J.; Guttman, F.M.; Bensoussan, A.; Laberge, J.-M.; et al. Infectious complications of pediatric liver transplantation. J. Pediatr. Surg. 1991, 26, 908–913. [Google Scholar] [CrossRef]
- Van Heerden, Y.; Maher, H.; Etheredge, H.; Fabian, J.; Grieve, A.; Loveland, J.; Botha, J. Outcomes of paediatric liver transplant for biliary atresia. S. Afr. J. Surg. 2019, 57, 17–23. [Google Scholar] [CrossRef]
- Alcamo, A.M.; Alessi, L.J.; Vehovic, S.N.; Bansal, N.; Bond, G.J.; Carcillo, J.A.; Green, M.; Michaels, M.G.; Aneja, R.K. Severe Sepsis in Pediatric Liver Transplant Patients: The Emergence of Multidrug-Resistant Organisms. Pediatr. Crit. Care Med. 2019, 20, e326–e332. [Google Scholar] [CrossRef]
- Ashkenazi-Hoffnung, L.; Mozer-Glassberg, Y.; Bilavsky, E.; Yassin, R.; Shamir, R.; Amir, J. Children Post Liver Trans-plantation Hospitalized with Fever Are at a High Risk for Bacterial Infections. Transpl. Infect. Dis. 2016, 18, 333–340. [Google Scholar] [CrossRef]
- Bouchut, J.-C.; Stamm, D.; Boillot, O.; Lepape, A.; Floret, D. Postoperative infectious complications in paediatric liver transplantation: A study of 48 transplants. Pediatr. Anesth. 2001, 11, 93–98. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, J.K.; Yang, S.I.; Park, S.H.; Yoon, K.W.; Lee, H.; Yi, N.J.; Suh, K.S.; Choi, E.H.; Lee, H.J. Incidence and Risk Factors for Infections After Liver Transplant in Children: Single-Center Experience for 15 Years. Open Forum Infect. Dis. 2017, 4, S704. [Google Scholar] [CrossRef]
- Béranger, A.; Capito, C.; Lacaille, F.; Ferroni, A.; Bouazza, N.; Girard, M.; Oualha, M.; Renolleau, S.; Debray, D.; Chardot, C.; et al. Early Bacterial Infections after Pediatric Liver Transplantation in the Era of Multidrug-Resistant Bacteria: Nine-Year Single-Center Retrospective Experience. Pediatr. Infect. Dis. J. 2020, 39, e169–e175. [Google Scholar] [CrossRef]
- Schwake, C.D.; Guiddir, T.; Cuzon, G.; Benissa, M.-R.; Dubois, C.; Miatello, J.; Merchaoui, Z.; Durand, P.; Tissieres, P. For the Bicêtre Pediatric Liver Transplant Group Bacterial infections in children after liver transplantation: A single-center surveillance study of 345 consecutive transplantations. Transpl. Infect. Dis. 2019, 22, e13208. [Google Scholar] [CrossRef]
- Shepherd, R.W.; Turmelle, Y.; Nadler, M.; Lowell, J.A.; Narkewicz, M.R.; McDiarmid, S.V.; Anand, R.; Song, C. Risk Factors for Rejection and Infection in Pediatric Liver Transplantation. Am. J. Transplant. 2007, 8, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Ganschow, R.; Nolkemper, D.; Helmke, K.; Harps, E.; Commentz, J.C.; Broering, D.C.; Pothmann, W.; Rogiers, X.; Hellwege, H.H.; Burdelski, M. Intensive Care Management after Pediatric Liver Transplantation: A Single-Center Expe-rience. Pediatr. Transplant. 2000, 4, 273–279. [Google Scholar] [CrossRef]
- Phichaphop, C.; Apiwattanakul, N.; Techasaensiri, C.; Lertudomphonwanit, C.; Treepongkaruna, S.; Thirapattaraphan, C.; Boonsathorn, S. High prevalence of multidrug-resistant gram-negative bacterial infection following pediatric liver transplantation. Medicine 2020, 99, e23169. [Google Scholar] [CrossRef]
- Uemoto, S.; Tanaka, K.; Fujita, S.; Sano, K.; Shirahase, I.; Kato, H.; Yamamoto, E.; Inomata, Y.; Ozawa, K. Infectious complications in living related liver transplantation. J. Pediatr. Surg. 1994, 29, 514–517. [Google Scholar] [CrossRef]
- Bio, L.L.; Schwenk, H.T.; Chen, S.F.; Conlon, S.; Gallo, A.; Bonham, C.A.; Gans, H.A. Standardization of Post-Operative Antimicrobials Reduced Exposure While Maintaining Good Outcomes in Pediatric Liver Transplant Recipients. Transpl. Infect. Dis. 2021, 23, e13538. [Google Scholar] [CrossRef]
- Kohli, R.; Cortes, M.; Heaton, N.D.; Dhawan, A. Liver transplantation in children: State of the art and future persoectives. Arch. Dis. Child. 2018, 103, 192–198. [Google Scholar] [CrossRef]
- Pham, Y.H.; Miloh, T. Liver transplantation in children. Clin. Liver. Dis. 2018, 22, 807–821. [Google Scholar] [CrossRef]
- Rock, N.M.; McLin, V.A. Listing for Transplantation; Postoperative Management and Long-Term Follow-Up. In Pediatric Hepatology and Liver Transplantation; D’Antiga, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 515–534. [Google Scholar]
- Noël, K.C.; Papenburg, J.; Lacroix, J.; Quach, C.; O’Donnell, S.; Gonzales, M.; Willson, D.F.; Gilfoyle, E.; McNally, J.D.; Reynolds, S.; et al. International Survey on Determinants of Antibiotic Duration and Discontinuation in Pediatric Critically Ill Patients. Pediatr. Crit. Care Med. 2020, 21, e696–e706. [Google Scholar] [CrossRef]
- Versporten, A.; Bielicki, J.; Drapier, N.; Sharland, M.; Goossens, H.; ARPEC Project Group; Calle, G.M.; Garrahan, J.P.; Clark, J.; Cooper, C.; et al. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: Developing hospital-quality indicators of antibiotic prescribing for children. J. Antimicrob. Chemother. 2016, 71, 1106–1117. [Google Scholar] [CrossRef]
- Bruns, N.; Dohna-Schwake, C. Antibiotics in critically ill children—A narrative review on different aspects of a rational approach. Pediatr. Res. 2021, 91, 440–446. [Google Scholar] [CrossRef]
- Hollenbeak, C.S.; Alfrey, E.J.; Sheridan, K.; Burger, T.L.; Dillon, P.W. Surgical site infections following pediatric liver transplantation: Risks and costs. Transpl. Infect. Dis. 2003, 5, 72–78. [Google Scholar] [CrossRef]
- Antimicrobial Rsistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Vandecasteele, E.; De Waele, J.; Vandijck, D.; Blot, S.; Vogelaers, D.; Rogiers, X.; Van Vlierberghe, H.; Decruyenaere, J.; Hoste, E. Antimicrobial prophylaxis in liver transplant patients—A multicenter survey endorsed by the European Liver and Intestine Transplant Association. Transpl. Int. 2009, 23, 182–190. [Google Scholar] [CrossRef]
| n (%) | Steroid | Tacrolimus | CSA | MMF |
|---|---|---|---|---|
| 10 (50%) | ||||
| 6 (30%) | ||||
| 2 (10%) | ||||
| 1 (5%) | ||||
| 1 (5%) |
| Narrow Spectrum | Broad Spectrum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | 1st gen. Cephalosporin | Amino-Penicillin | Amino-Penicillin + BLI | 3rd gen. Cephalosporin | 4th gen. Cephalosporin | Ureidopenicillin + BLI | Carbapenem | Fluorquinolone | Glycopeptide | Amino-Glycoside | Colistin | Tigecycline |
| 6 (30%) | ||||||||||||
| 3 (15%) | ||||||||||||
| 2 (10%) | ||||||||||||
| 1 (5%) | ||||||||||||
| 1 (5%) | ||||||||||||
| 2 (10%) | ||||||||||||
| 1 (5%) | ||||||||||||
| 1 (5%) | ||||||||||||
| 1 (5%) | ||||||||||||
| 17 (85%) | ||||||||||||
| 7 (35%) | ||||||||||||
| 5 (25%) | ||||||||||||
| 1 (5%) | ||||||||||||
| 2 (10%) | ||||||||||||
| 2 (10%) | ||||||||||||
| 1 (5%) | ||||||||||||
| 1 (5%) | ||||||||||||
| 1 (5%) | ||||||||||||
| n (%) | Narrow-Spectrum Prophylaxis n (%) | Broad-Spectrum Prophylaxis n (%) | ||
|---|---|---|---|---|
| (Paediatric) infectious disease specialist | Yes | 16 (80%) | 9 (56%) | 7 (44%) |
| No | 4 (20%) | 1 (25%) | 3 (75%) | |
| MRSA prevalence * | Low (<5%) | 11 (69%) | 4 (36%) | 7 (64%) |
| High (≥5%) | 5 (31%) | 5 (100%) | 0 (0%) | |
| ESBL prevalence * | Low (<20%) | 10 (63%) | 8 (80%) | 2 (20%) |
| High (≥20%) | 6 (38%) | 1 (17%) | 5 (63%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hauschild, J.; Bruns, N.; Lainka, E.; Dohna-Schwake, C. A European International Multicentre Survey on the Current Practice of Perioperative Antibiotic Prophylaxis for Paediatric Liver Transplantations. Antibiotics 2023, 12, 292. https://doi.org/10.3390/antibiotics12020292
Hauschild J, Bruns N, Lainka E, Dohna-Schwake C. A European International Multicentre Survey on the Current Practice of Perioperative Antibiotic Prophylaxis for Paediatric Liver Transplantations. Antibiotics. 2023; 12(2):292. https://doi.org/10.3390/antibiotics12020292
Chicago/Turabian StyleHauschild, Juliane, Nora Bruns, Elke Lainka, and Christian Dohna-Schwake. 2023. "A European International Multicentre Survey on the Current Practice of Perioperative Antibiotic Prophylaxis for Paediatric Liver Transplantations" Antibiotics 12, no. 2: 292. https://doi.org/10.3390/antibiotics12020292
APA StyleHauschild, J., Bruns, N., Lainka, E., & Dohna-Schwake, C. (2023). A European International Multicentre Survey on the Current Practice of Perioperative Antibiotic Prophylaxis for Paediatric Liver Transplantations. Antibiotics, 12(2), 292. https://doi.org/10.3390/antibiotics12020292






