trans-Cinnamaldehyde as a Novel Candidate to Overcome Bacterial Resistance: An Overview of In Vitro Studies
Abstract
1. Introduction
2. Methodology
3. Results
3.1. Potentiating Effects of Cinnamaldehyde in Gram-Positive Bacteria
| Bacteria | Strain | Antibiotic | MIC [µg/mL] | Antibiotic and trans- Cinnamaldehyde Combination | FICI/Type of Interaction | Ref. | |
|---|---|---|---|---|---|---|---|
| Cinnamaldehyde Concentration [µg/mL] | MIC [µg/mL] (RR) | ||||||
| Gram-positive | |||||||
| Listeria monocytogenes | ATCC 15313 | Nisin | 125 | 16.25 | 62.5 (4) | 0.50/Synergism | [58] |
| Staphylococcus aureus | JL10001 | Nisin | 16 | 50 | 2 (8) | 0.32/Synergism | [59] |
| JL10002, JL10006, JL10008, JL10011 | 16 | 62.5 | 1 (16) | 0.31/Synergism | |||
| JL1000, JL10005, JL10009, JL10013 | 32 | 125 | 2 (16) | 0.31/Synergism | |||
| JL10004 | 16 | 125 | 2 (8) | 0.37/Synergism | |||
| JL10007 JL10012 | 16 | 62.5 | 2 (8) | 0.37/Synergism | |||
| JL10010 | 32 | 62.5 | 4 (8) | 0.37/Synergism | |||
| ATCC 29213 | 32 | 50 | 2 (16) | 0.26/Synergism | |||
| ATCC 25923 | 110 | 25 | 27.5 (4) | 0.50/Synergism | [58] | ||
| bla Z | Ampicillin | 32 | 41.3 | 4 (8) | 0.25/Synergism | [60] | |
| Bacitracin | 32 | 41.3 | 4 (8) | 0.24/Synergism | |||
| Piperacillin | 128 | 0.37/Synergism | |||||
| Methicillin-resistant Staphylococcus aureus (MRSA) | ATCC 33571 | Amikacin | 31.2 | 31.25 | 7.8 (4) | 0.38/Synergism | [61] |
| Dps-1 | 31.2 | 31.25 | 3.9 (8) | 0.25/Synergism | |||
| Dps-3 | 62.5 | 31.25 | 3.9 (16) | 0.19/Synergism | |||
| ATCC 33571 | Amoxicillin | 62.5 | 125 | 7.8 (8) | 0.63/Additive effect | ||
| Dps-1 | 125 | 62.5 | 31.25 (4) | 0.5/Synergism | |||
| Dps-3 | 125 | 31.25 | 15.6 (8) | 0.25/Synergism | |||
| ATCC 33571 | Ampicillin | 62.5 | 125 | 31.25 (2) | 1.00/Additive effect | ||
| Dps-1 | 31.3 | 125 | 7.8 (4) | 0.75/Additive effect | |||
| Dps-3 | 62.5 | 125 | 15.6 (4) | 0.75/Additive effect | |||
| ATCC 33571 | Cefoxitin | 31.2 | 125 | 7.8 (4) | 0.75/Additive effect | ||
| Dps-1 | 62.5 | 125 | 7.8 (8) | 0.62/Additive effect | |||
| Dps-3 | 250 | 31.25 | 31.25 (4) | 0.50/Synergism | |||
| ATCC 33571 | Ceftazidime | 125 | 125 | 62.5 (2) | 1.00/Additive effect | ||
| Dps-1 | 125 | 125 | 62.5 (2) | 1.00/Additive effect | |||
| Dps-3 | 250 | 125 | 62.5 (4) | 0.75/Additive effect | |||
| ATCC 33571 | Gentamicin | 3.9 | 125 | 0.97 (4) | 0.75/Additive effect | ||
| Dps-1 | 125 | 31.25 | 31.25 (4) | 0.37/Synergism | |||
| Dps-3 | 250 | 62.5 | 62.5 (4) | 0.50/Synergism | |||
| ATCC 33571 | Oxacillin | 62.5 | 125 | 15.6 (4) | 0.75/Additive effect | ||
| Dps-1 | 500 | 125 | 250 (2) | 1.00/Additive effect | |||
| Dps-3 | 500 | 31.25 | 62.5 (8) | 0.25/Synergism | |||
| ATCC 33571 | Vancomycin | 250 | 31.25 | 31.25 (8) | 0.25/Synergism | ||
| Dps-1 | 250 | 125 | 125 (2) | 1.00/Additive effect | |||
| Dps-3 | 500 | 125 | 250 (2) | 1.00/Additive effect | |||
| Streptococcus pyogenes | erm B | Erythromycin | >512 | 41.6 | >256 (8) | 1.00/Additive effect | [60] |
| Nitrofurantoin | 0.13/Synergism | ||||||
| Gram-negative | |||||||
| Escherichia coli | 28 clinically isolated strains | Cefotaxime | 512 | 0.22 | 1 (512) | 0.07–0.30/75% synergism | [62] |
| Ciprofloxacin | 512 | 0.11 | 8 (64) | 0.07–0.50/39.6% synergism | |||
| ATCC 11775 | Erythromycin | 16 | 100 | 4 (4) | 0.50/Synergism | [63] | |
| ATCC 23739 | 32 | - | - | 0.30/Synergism | |||
| 8WT | 64 | 100 | 16 (4) | 0.50/Synergism | |||
| 02:0627 | 16 | 100 | 4 (4) | 0.50/Synergism | |||
| ATCC 23739 | Tetracycline | 32 | - | - | 0.30/Synergism | ||
| ATCC 23739 | Novobiocin | 128 | - | - | 0.20/Synergism | ||
| 8WT | 64 | 32 | 32 (2) | 1.00/Additive effect | |||
| 02:0627 | 128 | 100 | 32 (4) | 0.50/Synergism | |||
| ATCC 11775 | Bacitracin | >512 | - | - | >1.00/Lacking effect | ||
| ATCC 23739 | >512 | - | - | >1.00/Lacking effect | |||
| 8WT | >512 | - | - | >1.00/Lacking effect | |||
| 02:0627 | >512 | - | - | >1.00/Lacking effect | |||
| N00 666 | Ampicillin | >512 | 0.37/Synergism | [60] | |||
| Bacitracin | >512 | 165.2 | >64 (8) | 0.63/Additive effect | |||
| Erythromycin | 512 | 41.3 | 64 (8) | 0.24/Synergism | |||
| Novobiocin | 64 | 41.3 | 8 (8) | 0.24/Synergism | |||
| Piperacillin | >512 | 41.3 | >64 (8) | 0.24/Synergism | |||
| Tetracycline | 128 | 0.37/Synergism | |||||
| Klebsiella sp. | 33 clinically isolated strains | Cefotaxime | 512 | 0.05 | 0.5 (1024) | 0.10–0.50/42.4% synergism | [62] |
| Ciprofloxacin | 512 | 0.03 | 2 (256) | 0.07–0.50/60.6% synergism | |||
| Pseudomonas aeruginosa | PAO1 | Carbenicillin | 128 | 396.5 | 64 (2) | 0.75/Additive effect | [64] |
| Colistin | 7.86 | 396.5 | 1.96 (4) | 0.50/Synergism | |||
| Erythromycin | 256 | 396.5 | 128 (2) | 0.75/Additive effect | |||
| Tobramycin | 1443.8 | 396.5 | 721.9 (2) | 0.75/Additive effect | |||
| Gentamicin | 4.0 | 7.5 | 0.25 (16) | 0.37/Synergism | [65] | ||
| Salmonella typhimurium | SGI 1 | Ampicillin | >512 | 41.3 | >64 (8) | 0.25/Synergism | [60] |
| Bacitracin | >512 | 41.3 | >64 (8) | 0.24/Synergism | |||
| Erythromycin | 1024 | 41.3 | 128 (8) | 0.24/Synergism | |||
| Novobiocin | 256 | 41.3 | 32 (8) | 0.24/Synergism | |||
| Piperacillin | >512 | 165.2 | >64 (8) | 0.63/Additive effect | |||
| Tetracycline | 64 | 0.37/Synergism | |||||
| Fungi | |||||||
| Aspergillus fumigatus | MTCC 2550 | Fluconazole | 200 | 5 | 25 (8) | 0.19/Synergism | [66] |
| Malassezia pachydermatis | 30 isolated strains | Clotrimazole | 0.03–64 (GM 4.5) | 1.25–40 (GM 3.15) | 0.063–8 (GM 0.52) | 0.064–2.125 (GM: 0.52)/40% synergism 60% null effect | [67] |
| Fluconazole | 1–64 (GM 9.4) | 1.25–40 (GM 6.64) | 0.25–16 GM 0.7 (4) | 0.066–12 (GM 0.73)/ 26.6% synergism 70% antagonism | |||
| Ketoconazole | 0.015–4 (GM 0.08) | 1.25–160 (GM 5.48) | 0.016–0.062 (GM 0.02) | 0.093–6.006 (GM 1.55)/ 23.3% synergism 30% null effect 46,6% antagonism | |||
| Itraconazole | 0.0039–1 (GM 0.02) | 1.25–160 (GM 4.66) | 0.016–0.125 (GM 0.02) | 0.007–16.52 (GM: 0.85)/ 30.0% synergism 56.6% null effect 13.3% antagonism | |||
| Miconazole | 0.03–64 (GM 8.96) | 1.25–40 (GM 2.17) | 0.016–8 (GM 0.72) | 0.039–2.003 (GM: 0.31)/ 66.6% synergism 33.3% null | |||
| Nystatin | 4–64 (GM 41.96) | 1.25–20 (GM 2.22) | 0.25–64 (GM 29.2) | 0.062–1.25 (GM 0.31)/ 70% synergism 30% null effect | |||
| Terbinafine | 0.03–64 (GM 2.57) | 1.25–40 (GM 8.31) | 0.125–8 (GM 0.29) | 0.046–4.5 (GM: 0.97)/ 16.6% synergism 70% null effect 13.3% antagonism | |||
| Trichophyton rubrum | IO A-9 | Fluconazole | 200 | 1.25 | 25 (8) | 0.16/Synergism | [66] |
3.2. Potentiating Effects of Cinnamaldehyde in Gram-Negative Bacteria
3.3. Potentiating Effects of Cinnamaldehyde in Fungi
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, D.; Veeraraghavan, B.; Elangovan, R.; Vivekanandan, P. Antibiotic resistance and epigenetics: More to it than meets the eye. Antimicrob. Agents Chemother. 2020, 64, e02225-19. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, G.S.; Tapsall, J.W.; Allegranzi, B.; Talbot, E.A.; Lazzari, S. The antimicrobial resistance containment and surveillance approach--a public health tool. Bull. World Health Organ. 2004, 82, 928–934. [Google Scholar] [PubMed]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.; Murphy, C.; Wolcott, R. Biofilm exacerbates antibiotic resistance: Is this a current oversight in antimicrobial stewardship? Antimicrob. Resist. Infect. Control 2020, 9, 162. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as promoters of bacterial antibiotic resistance and tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Parrino, B.; Carbone, D.; Cirrincione, G.; Diana, P.; Cascioferro, S. Inhibitors of antibiotic resistance mechanisms: Clinical applications and future perspectives. Future Med. Chem. 2020, 12, 357–359. [Google Scholar] [CrossRef]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef]
- Yang, C.; Chowdhury, M.A.K.; Huo, Y.; Gong, J. Phytogenic compounds as alternatives to in-feed antibiotics: Potentials and challenges in application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef]
- Stan, D.; Enciu, A.M.; Mateescu, A.L.; Ion, A.C.; Brezeanu, A.C.; Stan, D.; Tanase, C. Natural compounds with antimicrobial and antiviral effect and nanocarriers used for their transportation. Front. Pharmacol. 2021, 12, 723233. [Google Scholar] [CrossRef]
- Brochot, A.; Guilbot, A.; Haddioui, L.; Roques, C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. Microbiologyopen 2017, 6, e00459. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic analyses, in vitro biological activities, and cytotoxicity of Cannabis sativa L. essential oil: A multidisciplinary study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef] [PubMed]
- Garzoli, S.; Pirolli, A.; Vavala, E.; Di Sotto, A.; Sartorelli, G.; Božović, M.; Angiolella, L.; Mazzanti, G.; Pepi, F.; Ragno, R. Multidisciplinary approach to determine the optimal time and period for extracting the essential oil from Mentha suaveolens Ehrh. Molecules 2015, 20, 9640–9655. [Google Scholar] [CrossRef]
- Menghini, L.; Ferrante, C.; Carradori, S.; D’Antonio, M.; Orlando, G.; Cairone, F.; Cesa, S.; Filippi, A.; Fraschetti, C.; Zengin, G.; et al. Chemical and bioinformatics analyses of the anti-leishmanial and anti-oxidant activities of hemp essential oil. Biomolecules 2021, 11, 272. [Google Scholar] [CrossRef]
- Di Sotto, A.; Gullì, M.; Acquaviva, A.; Tacchini, M.; Di Simone, S.C.; Chiavaroli, A.; Recinella, L.; Leone, S.; Brunetti, L.; Orlando, G.; et al. Phytochemical and pharmacological profiles of the essential oil from the inflorescences of the Cannabis sativa L. Ind. Crops Prod. 2022, 183, 114980. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, L.; Wang, S.; Gao, Y.; Jin, F. Molecular mechanism of the anti-inflammatory effects of plant essential oils: A systematic review. J. Ethnopharmacol. 2023, 301, 115829. [Google Scholar] [CrossRef]
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential oils as anticancer agents: Potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed. Pharmacother. 2022, 146, 112514. [Google Scholar] [CrossRef]
- Shala, A.; Singh, S.; Hameed, S.; Khurana, S.M.P. Essential oils as alternative promising anti-candidal agents: Progress and prospects. Curr. Pharm. Des. 2022, 28, 58–70. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Kedia, A.; Das, S.; Dubey, N.K. Essential oils and their bioactive compounds as eco-friendly novel green pesticides for management of storage insect pests: Prospects and retrospects. Environ. Sci. Pollut. Res. Int. 2021, 28, 18918–18940. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential oils: Sources of antimicrobials and food preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Khorshidian, N.; Yousefi, M.; Khanniri, E.; Mortazavian, A.M. Potential application of essential oils as antimicrobial preservatives in cheese. Innov. Food Sci. Emerg. Technol. 2018, 45, 62–72. [Google Scholar] [CrossRef]
- Qu, S.; Yang, K.; Chen, L.; Liu, M.; Geng, Q.; He, X.; Li, Y.; Liu, Y.; Tian, J. Cinnamaldehyde, a promising natural preservative against Aspergillus flavus. Front. Microbiol. 2019, 10, 2895. [Google Scholar] [CrossRef] [PubMed]
- Yanakiev, S. Effects of Cinnamon (Cinnamomum spp.) in dentistry: A review. Molecules 2020, 25, 4184. [Google Scholar] [CrossRef]
- Didehdar, M.; Chegini, Z.; Tabaeian, S.P.; Razavi, S.; Shariati, A. Cinnamomum: The new therapeutic agents for inhibition of bacterial and fungal biofilm-associated infection. Front. Cell. Infect. Microbiol. 2022, 12, 930624. [Google Scholar] [CrossRef]
- Chalchat, J.C.; Valade, I. Chemical composition of leaf oils of Cinnamomum from Madagascar: C. zeylanicum Blume, C. camphora L., C. fragrans Baillon and C. angustifolium. J. Essent. Oil Res. 2000, 12, 537–540. [Google Scholar] [CrossRef]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef]
- Khallouki, F.; Hmamouchi, M.; Younos, C.; Soulimani, R.; Bessiere, J.M.; Essassi, E.M. Antibacterial and molluscicidal activities of the essential oil of Chrysanthemum viscidehirtum. Fitoterapia 2000, 71, 544–546. [Google Scholar] [CrossRef]
- Ali, N.A.M.; Mohtar, M.; Shaari, K.; Rahmanii, M.; Ali, A.M.; Jantan, I.B. Chemical composition and antimicrobial activities of the essential oils of Cinnamomum aureofulvum Gamb. J. Essent. Oil Res. 2002, 14, 135–138. [Google Scholar] [CrossRef]
- Salleh, W.M.N.H.; Ahmad, F.; Yen, K.H.; Zulkifli, R.M. Essential oil compositions of Malaysian Lauraceae: A mini review. Pharm. Sci. 2016, 22, 60–67. [Google Scholar] [CrossRef]
- Al-Dhubiab, B.E. Pharmaceutical applications and phytochemical profile of Cinnamomum burmannii. Pharmacogn. Rev. 2012, 6, 125. [Google Scholar] [CrossRef] [PubMed]
- Ooi, L.S.; Li, Y.; Kam, S.L.; Wang, H.; Wong, E.Y.; Ooi, V.E. Antimicrobial activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia Blume. Am. J. Chin. Med. 2006, 34, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Firmino, D.F.; Cavalcante, T.T.A.; Gomes, G.A.; Firmino, N.C.S.; Rosa, L.D.; de Carvalho, M.G.; Catunda Jr, F.E.A. Antibacterial and antibiofilm activities of Cinnamomum sp. essential oil and cinnamaldehyde: Antimicrobial activities. Sci. World J. 2018, 2018, 7405736. [Google Scholar] [CrossRef]
- Dai, D.N.; Lam, N.T.; Chuong, N.T.; Ngan, T.Q.; Truong, N.C.; Ogunwande, I.A. Essential oils of Cinnamomum curvifolium (Lour.) Nees and Cinnamomum mairei H. Lev. Am. J. Essent. Oil. Nat. Prod. 2019, 7, 11–14. [Google Scholar]
- Son, L.C.; Dai, D.N.; Thai, T.H.; Huyen, D.D.; Thang, T.D.; Ogunwande, I.A. The leaf essential oils of four Vietnamese species of Cinnamomum (Lauraceae). J. Essent. Oil Res. 2013, 25, 267–271. [Google Scholar] [CrossRef]
- Li, Y.; Tan, B.; Cen, Z.; Fu, Y.; Zhu, X.; He, H.; Kong, D.; Wu, H. The variation in essential oils composition, phenolic acids and flavonoids is correlated with changes in antioxidant activity during Cinnamomum loureirii bark growth. Arab. J. Chem. 2021, 14, 103249. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Jiang, Z.T.; Jiang, S. Chemical composition of the essential oils of Cinnamomum loureirii Nees from China obtained by hydrodistillation and microwave-assisted hydrodistillation. J. Essent. Oil Res. 2010, 22, 129–131. [Google Scholar] [CrossRef]
- Cheng, S.S.; Liu, J.Y.; Tsai, K.H.; Chen, W.J.; Chang, S.T. Chemical composition and mosquito larvicidal activity of essential oils from leaves of different Cinnamomum osmophloeum provenances. J. Agric. Food Chem. 2004, 52, 4395–4400. [Google Scholar] [CrossRef]
- Islam, R.; Khan, R.I.; Al-Reza, S.M.; Jeong, Y.T.; Song, C.H.; Khalequzzaman, M. Chemical composition and insecticidal properties of Cinnamomum aromaticum (Nees) essential oil against the stored product beetle Callosobruchus maculatus (F.). J. Sci. Food Agric. 2009, 89, 1241–1246. [Google Scholar] [CrossRef]
- Abdelwahab, S.I.; Zaman, F.Q.; Mariod, A.A.; Yaacob, M.; Ahmed Abdelmageed, A.H.; Khamis, S. Chemical composition, antioxidant and antibacterial properties of the essential oils of Etlingera elatior and Cinnamomum pubescens Kochummen. J. Sci. Food Agric. 2010, 90, 2682–2688. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.Z.; Lohani, H.; Bhandari, U.; Naik, G.G.; Chauhan, N.K. Nutritional Value and Volatile Composition of Leaf and Bark of Cinnamomum tamala from Uttarakhand (India). J. Essent. Oil-Bear. Plants 2018, 21, 732–740. [Google Scholar] [CrossRef]
- Sharma, V.; Rao, L.J.M. An overview on chemical composition, bioactivity and processing of leaves of Cinnamomum tamala. Crit. Rev. Food Sci. Nutr. 2014, 54, 433–448. [Google Scholar] [CrossRef]
- Farias, A.P.P.; Monteiro, O.S.; da Silva, J.K.R.; Figueiredo, P.L.B.; Rodrigues, A.A.C.; Monteiro, I.N.; Maia, J.G.S. Chemical composition and biological activities of two chemotype-oils from Cinnamomum verum J. Presl growing in North Brazil. J. Food Sci. Technol. 2020, 57, 3176–3183. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.K.S.; Kulshrestha, M.; Sahu, U.; Karbhal, K.S.; Inchulkar, S.R.; Chauhan, N.S. Quality control of Dalchini (Cinnamomum zeylanicum): A review. Adv. Tradit. Med. 2021. [Google Scholar] [CrossRef]
- Gursale, A.; Dighe, V.; Parekh, G. Simultaneous quantitative determination of cinnamaldehyde and methyl eugenol from stem bark of Cinnamomum zeylanicum Blume using RP-HPLC. J. Chromatogr. Sci. 2010, 48, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Javidnia, K.; Miri, R.; Soltani, M.; Khosravi, A.R. Constituents of the essential oil of Marrubium astracanicum Jacq. from Iran. J. Essent. Oil Res. 2007, 19, 559–561. [Google Scholar] [CrossRef]
- Vernin, G.; Vernin, C.; Pieribattesti, J.C.; Roque, C. Analysis of the volatile compounds of Psidium cattleianum sabine fruit from Reunion Island. J. Essent. Oil Res. 1998, 10, 353–362. [Google Scholar] [CrossRef]
- Bang, H.B.; Lee, Y.H.; Kim, S.C.; Sung, C.K.; Jeong, K.J. Metabolic engineering of Escherichia coli for the production of cinnamaldehyde. Microb. Cell Fact. 2016, 15, 16. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Dong, L.; Wen, Y.; Zheng, X.; Zhang, C.; Chen, R.; Zhang, Y.; Li, Y.; He, T.; et al. Cinnamaldehyde inhibits inflammation and brain damage in a mouse model of permanent cerebral ischaemia. Br. J. Pharmacol. 2015, 172, 5009–5023. [Google Scholar] [CrossRef] [PubMed]
- Hajinejad, M.; Ghaddaripouri, M.; Dabzadeh, M.; Forouzanfar, F.; Sahab-Negah, S. Natural cinnamaldehyde and its derivatives ameliorate neuroinflammatory pathways in neurodegenerative diseases. BioMed Res. Int. 2020, 2020, 1034325. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Ismail, I.A.; Kang, S.M.; Han, D.C.; Kwon, B.M. Cinnamaldehydes in cancer chemotherapy. Phytother. Res. 2016, 30, 754–767. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Mazzanti, G.; Sarpietro, M.G.; Di Sotto, A. α-Hexylcinnamaldehyde inhibits the genotoxicity of environmental pollutants in the bacterial reverse mutation assay. J. Nat. Prod. 2014, 77, 2664–2670. [Google Scholar] [CrossRef] [PubMed]
- Sarpietro, M.G.; Di Sotto, A.; Accolla, M.L.; Castelli, F. Interaction of α-Hexylcinnamaldehyde with a biomembrane model: A possible MDR reversal mechanism. J. Nat. Prod. 2015, 78, 1154–1159. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Di Sotto, A.; El-Readi, M.Z.; Mazzanti, G.; Wink, M. α-Hexylcinnamaldehyde synergistically increases doxorubicin cytotoxicity towards human cancer cell lines. Anticancer Res. 2016, 36, 3347–3351. [Google Scholar] [PubMed]
- Welch, V.; Petticrew, M.; Petkovic, J.; Moher, D.; Waters, E.; White, H.; Tugwell, P. Extending the PRISMA statement to equity-focused systematic reviews (PRISMA-E 2012): Explanation and elaboration. Int. J. Equity Health 2015, 14, 92. [Google Scholar] [CrossRef]
- Alves, F.C.B.; Barbosa, L.N.; Andrade, B.F.M.T.; Albano, M.; Furtado, F.B.; Marques Pereira, A.F.; Rall, V.L.M.; Fernandes Júnior, A. Short communication: Inhibitory activities of the antibiotic nisin combined with phenolic compounds against Staphylococcus aureus and Listeria monocytogenes in cow milk. J. Dairy Sci. 2016, 99, 1831–1836. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, X.; Zhao, X.; Meng, R.; Liu, Z.; Chen, X.; Guo, N. Synergistic interactions of nisin in combination with cinnamaldehyde against Staphylococcus aureus in pasteurized milk. Food Control 2017, 71, 10–16. [Google Scholar] [CrossRef]
- Palaniappan, K.; Holley, R.A. Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int. J. Food Microbiol. 2010, 140, 164–168. [Google Scholar] [CrossRef]
- Wang, S.; Kang, O.H.; Kwon, D.Y. Trans-Cinnamaldehyde exhibits synergy with conventional antibiotic against methicillin-resistant Staphylococcus aureus. Int. J. Mol. Sci. 2021, 22, 2752. [Google Scholar] [CrossRef]
- Dhara, L.; Tripathi, A. Cinnamaldehyde: A compound with antimicrobial and synergistic activity against ESBL-producing quinolone-resistant pathogenic Enterobacteriaceae. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Visvalingam, J.; Palaniappan, K.; Holley, R.A. In vitro enhancement of antibiotic susceptibility of drug resistant Escherichia coli by cinnamaldehyde. Food Control 2017, 79, 288–291. [Google Scholar] [CrossRef]
- Topa, S.H.; Palombo, E.A.; Kingshott, P.; Blackall, L.L. Activity of cinnamaldehyde on quorum sensing and biofilm susceptibility to antibiotics in Pseudomonas aeruginosa. Microorganisms 2020, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Chadha, J.; Ravi; Singh, J.; Chhibber, S.; Harjai, K. Gentamicin augments the quorum quenching potential of cinnamaldehyde in vitro and protects Caenorhabditis elegans from Pseudomonas aeruginosa infection. Front. Cell. Infect. Microbiol. 2022, 12, 899566. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, M.; Khan, A.; Ahmad, I. Antifungal activity of essential oils and their synergy with fluconazole against drug-resistant strains of Aspergillus fumigatus and Trichophyton rubrum. Appl. Microbiol. Biotechnol. 2011, 90, 1083–1094. [Google Scholar]
- Schlemmer, K.B.; Jesus, F.P.K.; Tondolo, J.S.M.; Weiblen, C.; Azevedo, M.I.; Machado, V.S.; Botton, S.A.; Alves, S.H.; Santurio, J.M. In vitro activity of carvacrol, cinnamaldehyde and thymol combined with antifungals against Malassezia pachydermatis. J. Mycol. Med. 2019, 29, 375–377. [Google Scholar] [CrossRef]
- Gómara, M.; Ramón-García, S. The FICI paradigm: Correcting flaws in antimicrobial in vitro synergy screens at their inception. Biochem. Pharmacol. 2019, 163, 299–307. [Google Scholar] [CrossRef]
- Farber, J.M.; Peterkin, P.I. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 1991, 55, 476–511. [Google Scholar] [CrossRef]
- Lomonaco, S.; Nucera, D.; Filipello, V. The evolution and epidemiology of Listeria monocytogenes in Europe and the United States. Infect. Genet. Evol. 2015, 35, 172–183. [Google Scholar] [CrossRef]
- Baquero, F.; Lanza, V.F.; Duval, M.; Coque, T.M. Ecogenetics of antibiotic resistance in Listeria monocytogenes. Mol. Microbiol. 2020, 113, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Lister, J.L.; Horswill, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol. 2014, 4, 178. [Google Scholar] [CrossRef] [PubMed]
- Jenul, C.; Horswill, A.R. Regulation of Staphylococcus aureus virulence. Microbiol Spectr. 2019, 7, 1–21. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular characterization, evolution, and epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, T.; Köller, T.; Kreikemeyer, B. Streptococcus pyogenes biofilms formation, biology, and clinical relevance. Front. Cell. Infect. Microbiol. 2015, 5, 15. [Google Scholar] [CrossRef]
- Rood, I.G.H.; Li, Q. Review: Molecular detection of extended spectrum-β-lactamase- and carbapenemase-producing Enterobacteriaceae in a clinical setting. Diagn. Microbiol. Infect. Dis. 2017, 89, 245–250. [Google Scholar] [CrossRef]
- Jang, J.; Hur, H.G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and public health implications-a review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef]
- Herridge, W.P.; Shibu, P.; O’Shea, J.; Brook, T.C.; Hoyles, L. Bacteriophages of Klebsiella spp., their diversity and potential therapeutic uses. J. Med. Microbiol. 2020, 69, 176–194. [Google Scholar]
- Mielko, K.A.; Jabłoński, S.J.; Milczewska, J.; Sands, D.; Łukaszewicz, M.; Młynarz, P. Metabolomic studies of Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2019, 35, 178. [Google Scholar] [CrossRef]
- Yang, C.; Li, H.; Zhang, T.; Chu, Y.; Zuo, J.; Chen, D. Study on antibiotic susceptibility of Salmonella typhimurium L forms to the third and fourth generation cephalosporins. Sci Rep. 2020, 10, 3042. [Google Scholar] [CrossRef]
- Tetard, A.; Zedet, A.; Girard, C.; Plésiat, P.; Llanesa, C. Cinnamaldehyde induces expression of efflux pumps and multidrug resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 63, e01081-19. [Google Scholar] [CrossRef] [PubMed]
- Tetard, A.; Gaillot, S.; Dubois, E.; Aarras, S.; Valot, B.; Phan, G.; Plésiat, P.; Llanes, C. Exposure of Pseudomonas aeruginosa to Cinnamaldehyde Selects Multidrug Resistant Mutants. Antibiotics 2022, 11, 1790. [Google Scholar] [CrossRef] [PubMed]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta. 2009, 1794, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, O.H. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Epstein, A.K.; Wong, T.S.; Belisle, R.A.; Boggs, E.M.; Aizenberg, J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc. Natl. Acad. Sci. USA 2012, 109, 13182–13187. [Google Scholar] [CrossRef]
- Solano, C.; Echeverz, M.; Lasa, I. Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 2014, 18, 96–104. [Google Scholar] [CrossRef]
- Yang, D.; Wang, H.; Yuan, H.; Li, S. Quantitative structure activity relationship of cinnamaldehyde compounds against wood decaying fungi. Molecules 2016, 21, 1563. [Google Scholar] [CrossRef]
- Thirapanmethee, K.; Kanathum, P.; Khuntayaporn, P.; Huayhongthong, S.; Surassmo, S.; Chomnawang, M.T. Cinnamaldehyde: A plant-derived antimicrobial for overcoming multidrug-resistant Acinetobacter baumannii infection. Eur. J. Integr. Med. 2021, 48, 101376. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, M.; Caillet, S.; Lacroix, M. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157:H7 and Listeria monocytogenes. J. Food Prot. 2006, 69, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Nowotarska, S.W.; Nowotarski, K.; Grant, I.R.; Elliott, C.T.; Friedman, M.; Situ, C. Mechanisms of antimicrobial action of cinnamon and oregano oils, cinnamaldehyde, carvacrol, 2,5-dihydroxybenzaldehyde, and 2-hydroxy-5-methoxybenzaldehyde against Mycobacterium avium subsp. paratuberculosis (Map). Foods 2017, 6, 72. [Google Scholar] [CrossRef] [PubMed]
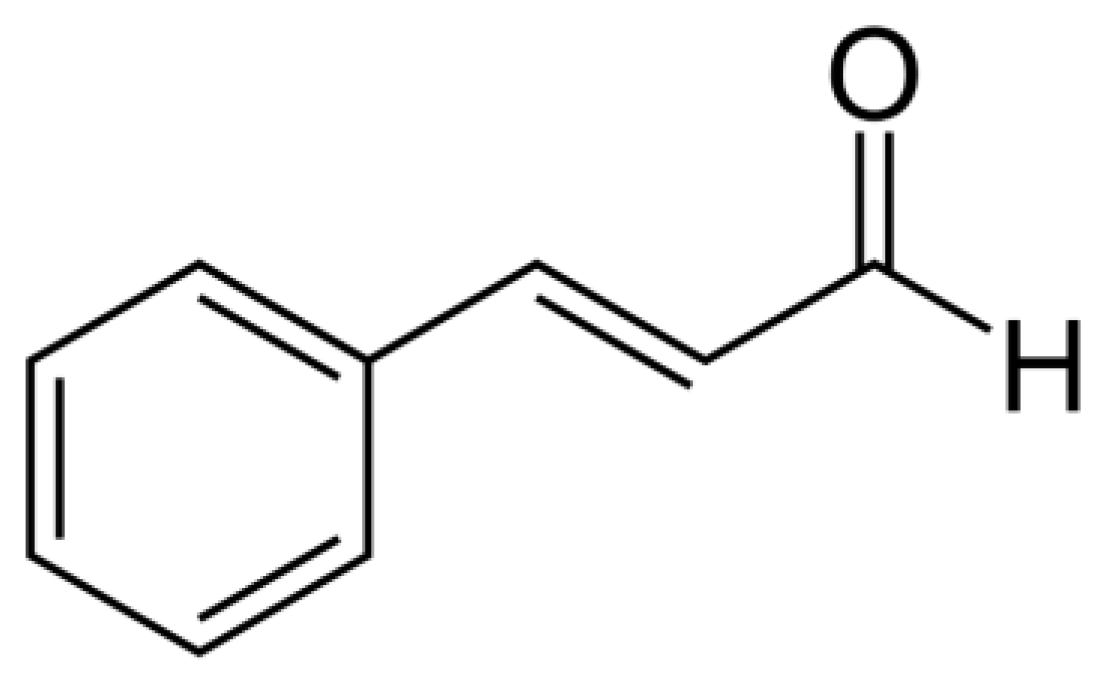



| Plant Species/Family | Plant Part | trans-Cinnamaldehyde (%) | Ref. |
|---|---|---|---|
| Chrysanthemum viscidehirtum Schott Tell/Lauraceae | Leaf | 2.1 | [30] |
| Aerial parts | 0.7 | ||
| Cinnamomum angustifolium Lukman/Lauraceae | Leaf and bark | 0.2 | [28] |
| Cinnamomum aureofulvum Gamble/Lauraceae | Bark | 46.6 | [31,32] |
| Cinnamomum burmannii Nees & T. Nees/Lauraceae | Leaf | 45–62 | [33] |
| Bark | 17–32 | ||
| Cinnamomum cassia Nees/Lauraceae | Bark | 85 | [28,34,35] |
| Cinnamomum curvifolium Nees/Lauraceae | Leaf | 8.9 | [36] |
| Steam bark | 1.2 | ||
| Cinnamomum durifolium Kosterm/Lamiaceae | Aerial parts | 0.6 | [37] |
| Cinnamomum loureirii Nees/Lauraceae | Bark | 50.2–92.9 | [38,39] |
| Cinnamomum mairei H. Léveillé/Lauraceae | Leaf | 1.9 | [36] |
| Steam bark | 6.5 | ||
| Cinnamomum osmophloeum Kaneh/Lauraceae | Leaf | 79.8 | [40,41] |
| Cinnamomum pubescens Kochummen/Lauraceae | Leaf | 56.1 | [42] |
| Cinnamomum sericans Hance/Lauraceae | Leaf | 0.6 | [37] |
| Cinnamomum tamala Nees Eberm/Lauraceae | Leaf Bark | 68.7–79.4 64.8 | [43,44] [43] |
| Cinnamomum verum J. Presl/Lauraceae | Leaf | 0.6 | [28,45] |
| Bark | 89.3 | ||
| Cinnamomum zeylanicum Blume/Lauraceae | Bark | 44.2–68.7 | [32,35,46,47] |
| Leaf | 1–5 | [46] | |
| Marrubium astracanicum Jacq./Lauraceae | Aerial parts | 2.2 | [48] |
| Psidium cattleianum Sabine/Lamiaceae | Aerial parts | 2.2 | [49] |
| Fruit | 0.6 | ||
| Teucrium persicum Boiss/Myrtaceae | Aerial parts | 0.4 | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usai, F.; Di Sotto, A. trans-Cinnamaldehyde as a Novel Candidate to Overcome Bacterial Resistance: An Overview of In Vitro Studies. Antibiotics 2023, 12, 254. https://doi.org/10.3390/antibiotics12020254
Usai F, Di Sotto A. trans-Cinnamaldehyde as a Novel Candidate to Overcome Bacterial Resistance: An Overview of In Vitro Studies. Antibiotics. 2023; 12(2):254. https://doi.org/10.3390/antibiotics12020254
Chicago/Turabian StyleUsai, Federica, and Antonella Di Sotto. 2023. "trans-Cinnamaldehyde as a Novel Candidate to Overcome Bacterial Resistance: An Overview of In Vitro Studies" Antibiotics 12, no. 2: 254. https://doi.org/10.3390/antibiotics12020254
APA StyleUsai, F., & Di Sotto, A. (2023). trans-Cinnamaldehyde as a Novel Candidate to Overcome Bacterial Resistance: An Overview of In Vitro Studies. Antibiotics, 12(2), 254. https://doi.org/10.3390/antibiotics12020254







