Plant-Derived Xanthones against Clostridial Enteric Infections
Abstract
1. Introduction
2. Results
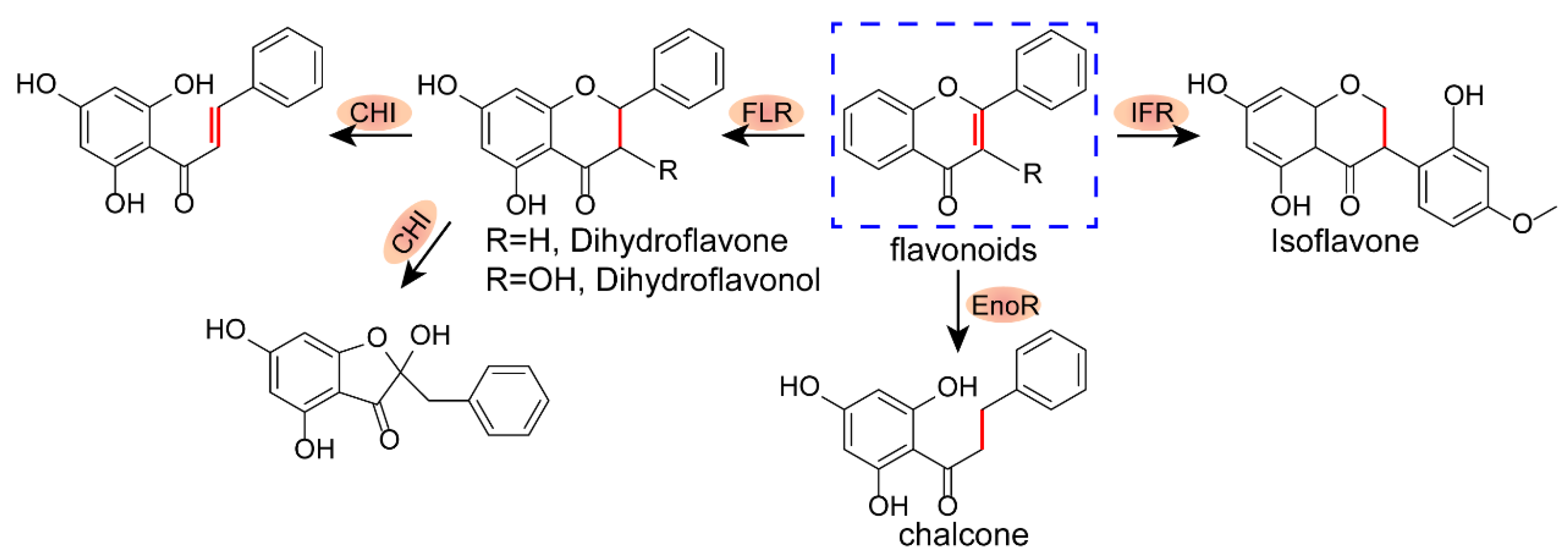
2.1. Structure–Activity Relationship of Xanthones
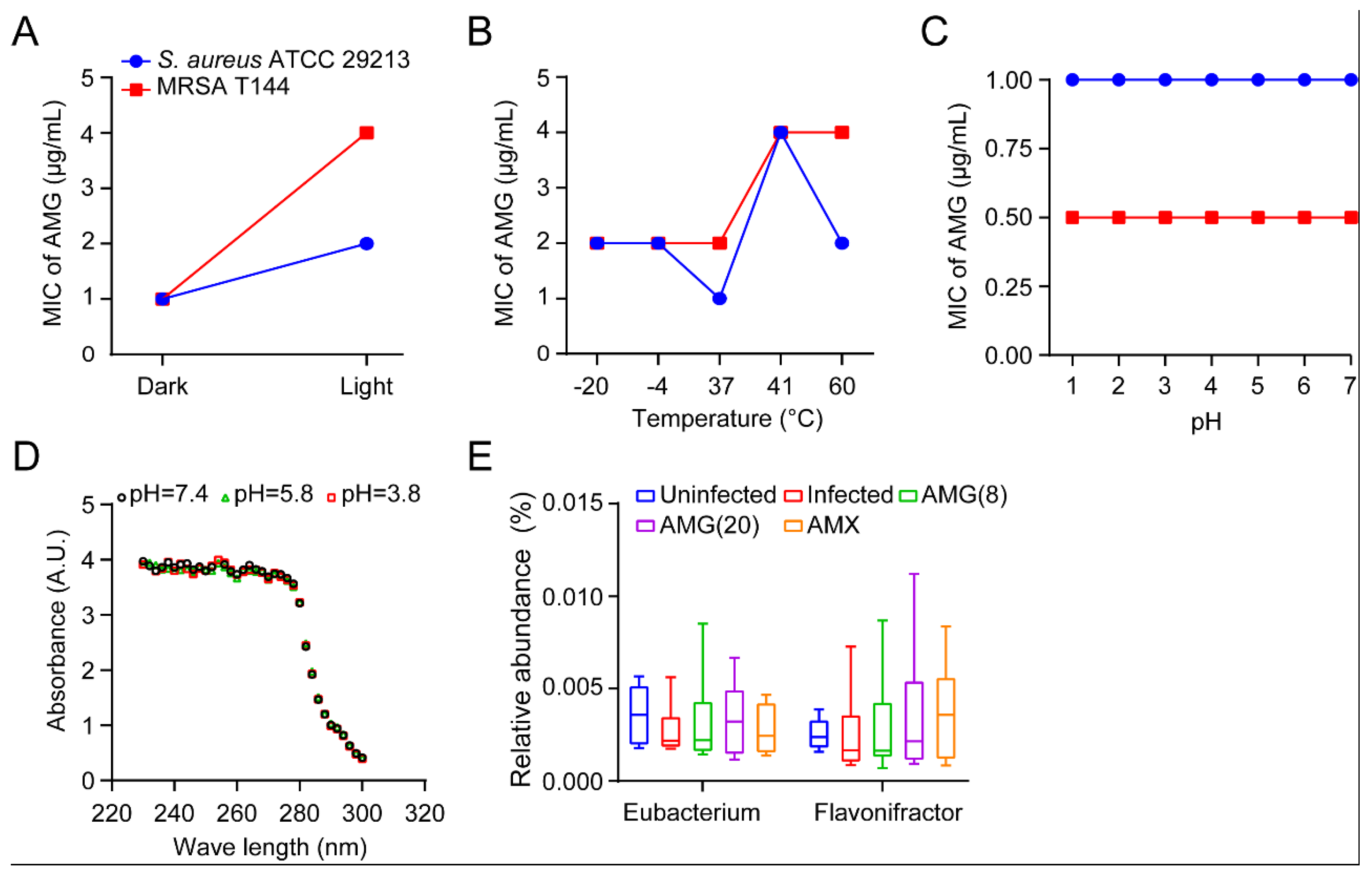
2.2. Stability and Safety Assessment of AMG
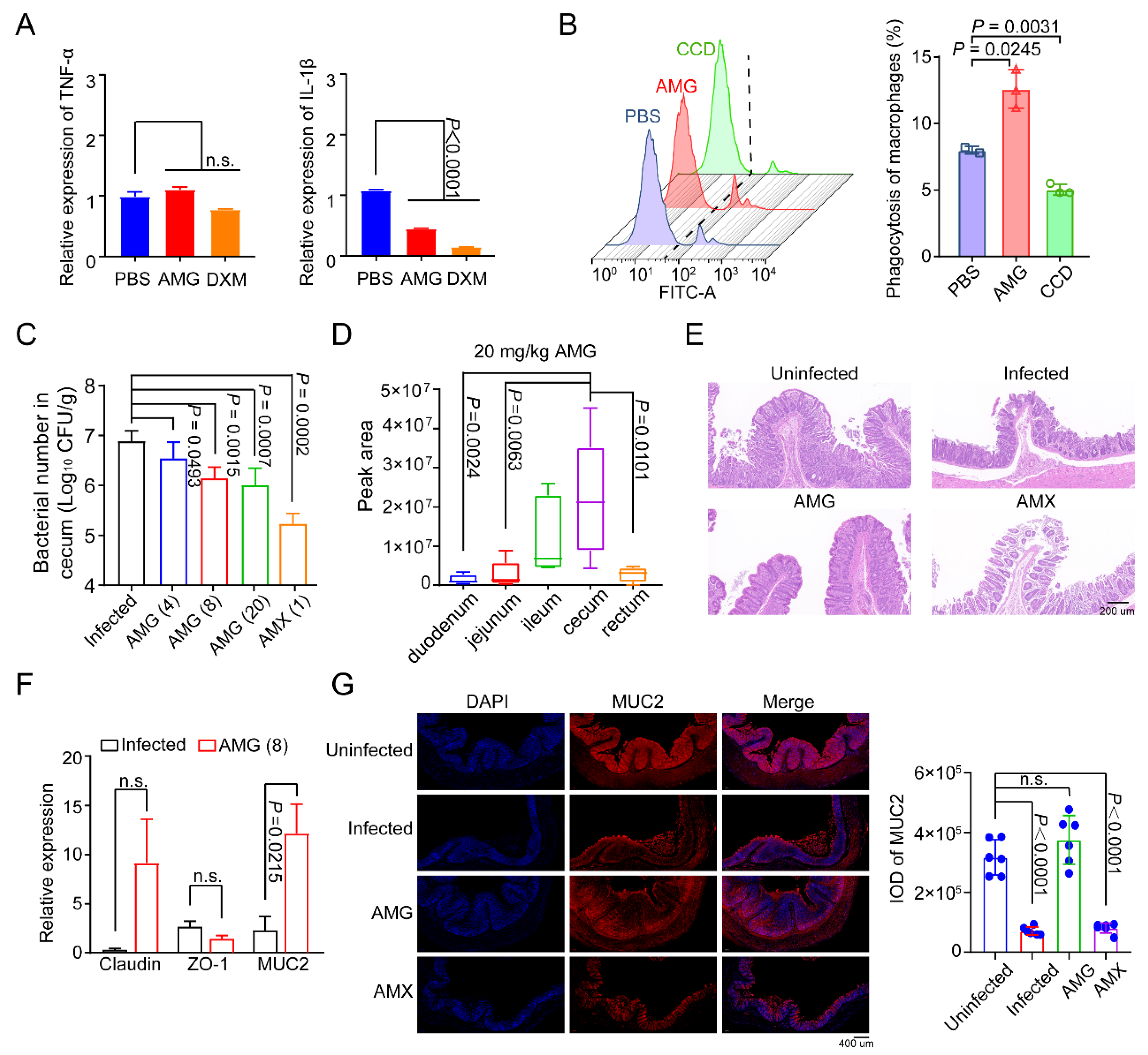
2.3. AMG Modulates the Composition of Intestinal Microbial Community
2.4. AMG Effectively Controls Clostridial Enteric Infections
3. Discussion
4. Materials and Methods
4.1. Chemicals and Bacterial Strains
4.2. Antibacterial Tests
4.3. Stability of AMG
4.4. Antibacterial Activity of AMG in the Simulated Gastrointestinal Model
4.5. In vitro RT-qPCR and ELISA Assays
4.6. Phagocytosis Assay
4.7. Animals and Ethics Statement
4.8. In Vivo Necrotic Enteritis (NE) Disease Model
4.9. Microbiota Analysis
4.10. Tissue Distribution of AMG
4.11. Determination of AMG
4.12. Cecal Epithelial Integrity Assays
4.13. Immunofluorescence Staining
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.C.; Knight, D.R.; Riley, T.V. Clostridium difficile and One Health. Clin. Microbiol. Infect. 2020, 26, 857–863. [Google Scholar] [CrossRef]
- Leffler, D.A.; Lamont, J.T. Clostridium difficile infection. N. Engl. J. Med. 2015, 372, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Buffie, C.G.; Pamer, E.G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Elbestawy, A.R.; El-Shall, N.A.; Saad, A.M.; Salem, H.M.; El-Tahan, A.M.; Khafaga, A.F.; Taha, A.E.; AbuQamar, S.F.; et al. Necrotic enteritis in broiler chickens: Disease characteristics and prevention using organic antibiotic alternatives—A comprehensive review. Poult. Sci. 2022, 101, 101590. [Google Scholar] [CrossRef]
- Imwattana, K.; Knight, D.R.; Kullin, B.; Collins, D.A.; Putsathit, P.; Kiratisin, P.; Riley, T.V. Clostridium difficile ribotype 017—Characterization, evolution and epidemiology of the dominant strain in Asia. Emerg. Microbes Infect. 2019, 8, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.; Malani, P.N. Diagnosis and treatment of Clostridioides (Clostridium) difficile infection in adults in 2020. JAMA 2020, 323, 1403–1404. [Google Scholar] [CrossRef]
- Goldstein, E.J.C.; Johnson, S.J.; Maziade, P.J.; Evans, C.T.; Sniffen, J.C.; Millette, M.; McFarland, L.V. Probiotics and prevention of Clostridium difficile infection. Anaerobe 2017, 45, 114–119. [Google Scholar] [CrossRef]
- Hvas, C.L.; Dahl Jorgensen, S.M.; Jorgensen, S.P.; Storgaard, M.; Lemming, L.; Hansen, M.M.; Erikstrup, C.; Dahlerup, J.F. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent Clostridium difficile infection. Gastroenterology 2019, 156, 1324–1332.e3. [Google Scholar] [CrossRef]
- Wang, J.-W.; Kuo, C.-H.; Kuo, F.-C.; Wang, Y.-K.; Hsu, W.-H.; Yu, F.-J.; Hu, H.-M.; Hsu, P.-I.; Wang, J.-Y.; Wu, D.-C. Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. 2019, 118, S23–S31. [Google Scholar] [CrossRef] [PubMed]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.B.; Hohmann, E.L. Drug-Resistant E. coli bacteremia transmitted by fecal microbiota transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Martlbauer, E.; Dietrich, R.; Luo, H.; Ding, S.; Zhu, K. Multifaceted toxin profile, an approach toward a better understanding of probiotic Bacillus cereus. Crit. Rev. Toxicol. 2019, 49, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, S.; Ding, S.; Shen, J.; Zhu, K. Toxins and mobile antimicrobial resistance genes in Bacillus probiotics constitute a potential risk for One Health. J. Hazard. Mater. 2020, 382, 121266. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Wang, W.; Gan, L.; Li, Z.; Guo, S.; Guo, Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016, 7, 19. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, S.; Shen, J.; Zhu, K. Nonribosomal antibacterial peptides that target multidrug-resistant bacteria. Nat. Prod. Rep. 2019, 36, 573–592. [Google Scholar] [CrossRef]
- Chen, S.; Liu, D.; Zhang, Q.; Guo, P.; Ding, S.; Shen, J.; Zhu, K.; Lin, W. A marine antibiotic kills multidrug-resistant bacteria without detectable high-level resistance. ACS Infect. Dis. 2021, 7, 884–893. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Li, T.; Liu, X.; Hao, Z.; Ding, S.; Panichayupakaranant, P.; Zhu, K.; Shen, J. Plant natural flavonoids against multidrug resistant pathogens. Adv. Sci. 2021, 8, e2100749. [Google Scholar] [CrossRef]
- Maia, M.; Resende, D.; Duraes, F.; Pinto, M.M.M.; Sousa, E. Xanthenes in medicinal chemistry—Synthetic strategies and biological activities. Eur. J. Med. Chem. 2021, 210, 113085. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Barbary, M.A.; El-Ghorab, D.M.; Bohlin, L.; Borg-Karlson, A.K.; Goransson, U.; Verpoorte, R. Recent Insights into the biosynthesis and biological activities of natural xanthones. Curr. Med. Chem. 2010, 17, 854–901. [Google Scholar]
- Soares, J.X.; Loureiro, D.R.P.; Dias, A.L.; Reis, S.; Pinto, M.M.M.; Afonso, C.M.M. Bioactive marine xanthones: A Review. Mar. Drugs 2022, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Hong, S.; Yang, P.; Sun, Y.; Wang, Y.; Zhang, P.; Jiang, W.; Gu, Y. Discovery of an ene-reductase for initiating flavone and flavonol catabolism in gut bacteria. Nat. Commun. 2021, 12, 790. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, Y.; Wu, H.; Yuan, M.; Zheng, C.; Xu, H. Xanthone glucosides: Isolation, bioactivity and synthesis. Molecules 2021, 26, 5575. [Google Scholar] [CrossRef] [PubMed]
- Wezeman, T.; Brase, S.; Masters, K.S. Xanthone dimers: A compound family which is both common and privileged. Nat. Prod. Rep. 2015, 32, 6–28. [Google Scholar] [CrossRef]
- Liu, X.; Shen, J.; Zhu, K. Antibacterial activities of plant-derived xanthones. RSC Med. Chem. 2022, 13, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.J.; Zou, H.; Lin, S.; Lin, H.; Soh, R.T.; Lim, F.H.; Koh, W.L.; Li, J.; Lakshminarayanan, R.; Verma, C.; et al. Nonpeptidic amphiphilic xanthone derivatives: Structure-activity relationship and membrane-targeting properties. J. Med. Chem. 2016, 59, 171–193. [Google Scholar] [CrossRef]
- Lin, S.; Koh, J.J.; Aung, T.T.; Lim, F.; Li, J.; Zou, H.; Wang, L.; Lakshminarayanan, R.; Verma, C.; Wang, Y.; et al. Symmetrically substituted xanthone amphiphiles combat Gram-positive bacterial resistance with enhanced membrane selectivity. J. Med. Chem. 2017, 60, 1362–1378. [Google Scholar] [CrossRef]
- Muñoz, A.; Hergenrother , P.J. Facilitating compound entry as a means to discover antibiotics for Gram-negative bacteria. Acc. Chem. Res. 2021, 54, 1322–1333. [Google Scholar]
- Calixto, J.B. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Braz. J. Med. Biol. Res. 2000, 33, 179–189. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Wu, Z. Drug stability testing and formulation strategies. Pharm. Dev. Technol. 2018, 23, 941. [Google Scholar] [CrossRef] [PubMed]
- Kosem, N.; Ichikawa, K.; Utsumi, H.; Moongkarndi, P. In vivo toxicity and antitumor activity of mangosteen extract. J. Nat. Med. 2013, 67, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Keyburn, A.L.; Boyce, J.D.; Vaz, P.; Bannam, T.L.; Ford, M.E.; Parker, D.; Di Rubbo, A.; Rood, J.I.; Moore, R.J. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008, 4, e26. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef]
- Li, S.; Wu, B.; Fu, W.; Reddivari, L. The anti-inflammatory effects of dietary anthocyanins against ulcerative colitis. Int. J. Mol. Sci. 2019, 20, 2588. [Google Scholar] [CrossRef]
- Theriot, C.; Koenigsknecht, M.; Carlson, P.; Hatton, G.E.; Nelson, A.M.; Li, B.; Huffnagle, G.B.; Young, V.B. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat. Commun. 2014, 5, 3114. [Google Scholar] [CrossRef]
- Liu, D.; Guo, S.; Guo, Y. Xylanase supplementation to a wheat-based diet alleviated the intestinal mucosal barrier impairment of broiler chickens challenged by Clostridium perfringens. Avian Pathol. 2012, 41, 291–298. [Google Scholar] [CrossRef]
- Czepiel, J.; Drozdz, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultanska, D.; Garlicki, A.; Biesiada, G. Clostridium difficile infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1211–1221. [Google Scholar] [CrossRef]
- Wang, Y.H. Sialidases from Clostridium perfringens and their Inhibitors. Front. Cell. Infect. Microbiol. 2019, 9, 462. [Google Scholar] [CrossRef]
- Gharaibeh, S.; Al Rifai, R.; Al-Majali, A. Molecular typing and antimicrobial susceptibility of Clostridium perfringens from broiler chickens. Anaerobe 2010, 16, 586–589. [Google Scholar] [CrossRef]
- Finegold, S.M.; Summanen, P.H.; Corbett, K.; Downes, J.; Henning, S.M.; Li, Z. Pomegranate extract exhibits in vitro activity against Clostridium difficile. Nutrition 2014, 30, 1210–1212. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Antibiotic-resistant bacteria: Prevalence in food and inactivation by food-compatible compounds and plant extracts. J. Agric. Food Chem. 2015, 63, 3805–3822. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Olszewska, M.A.; Diez-Gonzalez, F. Selection and application of natural antimicrobials to control Clostridium perfringens in sous-vide chicken breasts. Int. J. Food Microbiol. 2021, 347, 109193. [Google Scholar] [CrossRef] [PubMed]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the role of plant natural products in antibiotic drug discovery. Chem. Rev. 2021, 121, 3495–3560. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; You, B.H.; Kim, Y.C.; Chin, Y.W.; Choi, Y.H. Dose-independent ADME properties and tentative identification of metabolites of alpha-mangostin from Garcinia mangostana in mice by automated microsampling and UPLC-MS/MS methods. PLoS ONE 2015, 10, e0131587. [Google Scholar]
- Zhao, Y.; Tang, G.; Tang, Q.; Zhang, J.; Hou, Y.; Cai, E.; Liu, S.; Lei, D.; Zhang, L.; Wang, S. A method of effectively improved alpha-mangostin bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 605–613. [Google Scholar] [CrossRef]
- Wang, K.; Bao, L.; Zhou, N.; Zhang, J.; Liao, M.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, J.; Wang, L.; et al. Structural modification of natural product ganomycin I leading to discovery of a alpha-Glucosidase and HMG-CoA reductase dual inhibitor improving obesity and metabolic dysfunction in vivo. J. Med. Chem. 2018, 61, 3609–3625. [Google Scholar] [CrossRef]
- Liu, X.; Diarra, M.S.; Zhang, Y.; Wang, Q.; Yu, H.; Nie, S.P.; Xie, M.Y.; Gong, J. Effect of encapsulated carvacrol on the incidence of necrotic enteritis in broiler chickens. Avian Pathol. 2016, 45, 357–364. [Google Scholar] [CrossRef]
- Han, S.Y.; Chin, Y.-W.; Kim, D.Y.; Choi, Y.H. Simultaneous determination of α- and γ-mangostins in mouse plasma by HPLC–MS/MS method: Application to a pharmacokinetic study of mangosteen extract in mouse. Chromatographia 2013, 76, 643–650. [Google Scholar] [CrossRef]



| C. perfringens | Genotype | MIC (μg/mL) | ||||
|---|---|---|---|---|---|---|
| AMG | Isobavachalcone | Mulberrin | Lupalbigenin | Glabrol | ||
| CVCC2030 | A | 0.5 | 2 | 2 | 0.5 | 1 |
| CVCC60082 | B | 0.5 | 2 | 2 | 0.5 | 1 |
| CVCC60101 | C | 0.5 | 2 | 1 | 0.5 | 1 |
| CVCC60201 | D | 0.5 | 2 | 1 | 1 | 1 |
| 20SX 1RX187 | A | 0.5 | 4 | 1 | 1 | 1 |
| 20SJ BNP12 | A | 0.5 | 4 | 2 | 1 | 1 |
| 19NM 2CM8 | A | 0.5 | 4 | 2 | 0.5 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhu, J.; Qu, S.; Shen, J.; Zhu, K. Plant-Derived Xanthones against Clostridial Enteric Infections. Antibiotics 2023, 12, 232. https://doi.org/10.3390/antibiotics12020232
Liu Y, Zhu J, Qu S, Shen J, Zhu K. Plant-Derived Xanthones against Clostridial Enteric Infections. Antibiotics. 2023; 12(2):232. https://doi.org/10.3390/antibiotics12020232
Chicago/Turabian StyleLiu, Ying, Jianfei Zhu, Shaoqi Qu, Jianzhong Shen, and Kui Zhu. 2023. "Plant-Derived Xanthones against Clostridial Enteric Infections" Antibiotics 12, no. 2: 232. https://doi.org/10.3390/antibiotics12020232
APA StyleLiu, Y., Zhu, J., Qu, S., Shen, J., & Zhu, K. (2023). Plant-Derived Xanthones against Clostridial Enteric Infections. Antibiotics, 12(2), 232. https://doi.org/10.3390/antibiotics12020232






