Bats Are Carriers of Antimicrobial-Resistant Staphylococcaceae in Their Skin
Abstract
1. Introduction
2. Results
2.1. Number of Bats Captured and Sampled
2.2. Staphylococcaceae Richness
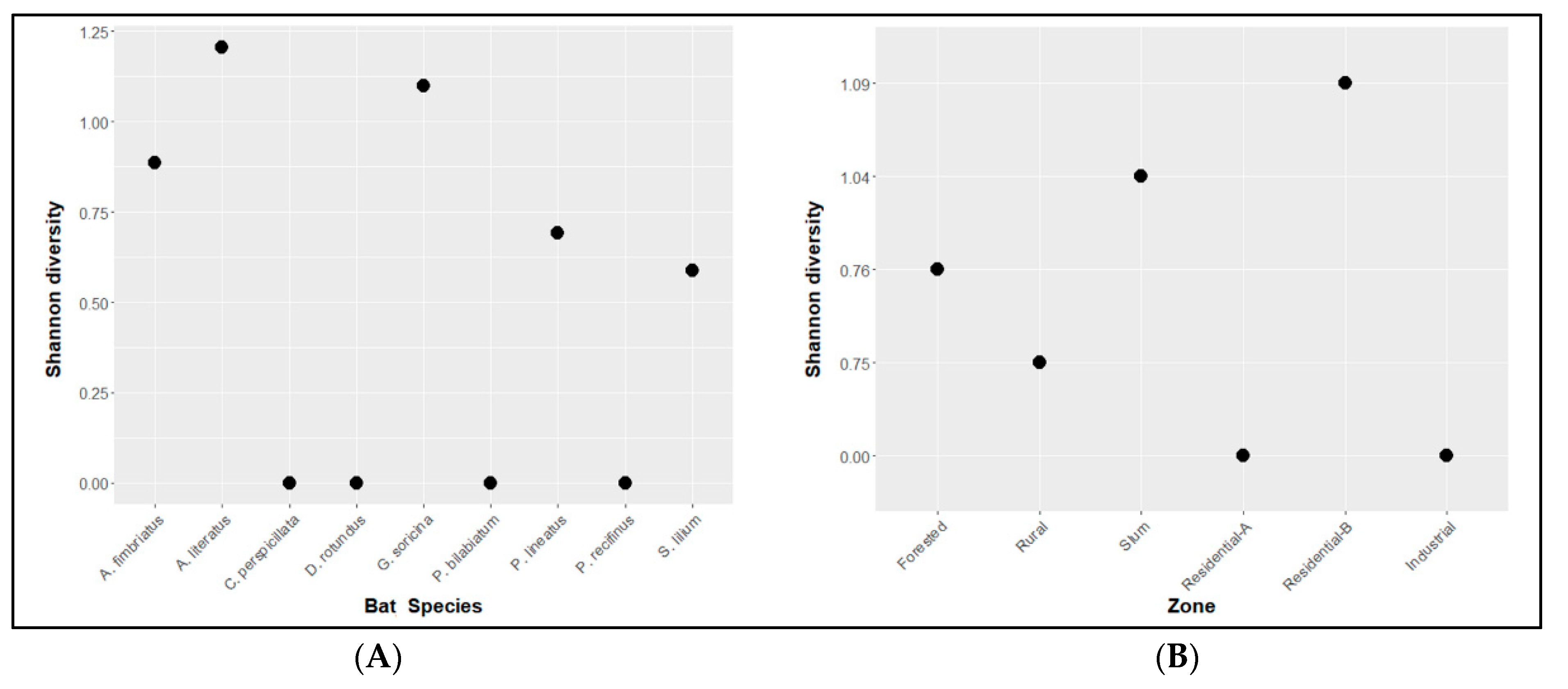
2.3. Diversity Analysis
2.4. Antimicrobial Susceptibility Profile
3. Discussion
4. Material and Methods
4.1. Bat Capture and Sample Collection
4.2. Microbiologic Tests and Antimicrobial Susceptibility
4.3. DNA Extraction
4.4. Spa Typing
4.5. Amplification of Resistance Genes
4.6. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonilla-Aldana, K.; Jimenez-Diaz, D.; Patel, S.K.; Dhama, K.; Rabaan, A.A.; Sah, R.; Sierra, M.; Zambrano, L.I.; Arteaga-Livias, K.; Rodriguez-Morales, L. Importance of Bats in Wildlife: Not Just Carriers of Pandemic SARS-CoV-2 and Other Viruses. J. Pure Appl. Microbiol. 2020, 14 (Suppl. S1), 709–712. [Google Scholar] [CrossRef]
- Boyles, J.G.; Cryangary, M.; Mccrackenand, F.; Kunz, T.H. Economic Importance of Bats in Agriculture. Science 2011, 332, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.K.; Cunze, S.; Kochmann, J.; Klimpel, S. Bats as putative Zaire ebolavirus reservoir hosts and their habitat suitability in Africa. Sci. Rep. 2020, 10, 14268. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aravena, M.; McKee, C.; Gamble, A.; Lunn, T.; Morris, A.; Snedden, C.E.; Yinda, C.K.; Port, J.R.; Buchholz, D.W.; Yeo, Y.Y.; et al. Ecology, evolution and spillover of coronaviruses from bats. Nat. Rev. Microbiol. 2022, 20, 299–314. [Google Scholar] [CrossRef]
- Veikkolainen, V.; Vesterinen, E.J.; Lilley, T.M.; Pulliainen, A.T. Bats as reservoir hosts of human bacterial pathogen, Bartonella mayotimonensis. Emerg. Infect. Dis. 2014, 20, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.Q.; Dos Reis, E.M.; Bezerra, A.V.A.; Cerva, C.; Rosa, J.; Cibulski, S.P.; Lima, F.E.S.; Pacheco, S.M.; Rodrigues, R.O. Pathogenic Leptospira spp. in bats: Molecular investigation in Southern Brazil. Comp. Immunol. Microbiol. Infect. Dis. 2017, 52, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Silva-Ramos, C.R.; Chala-Quintero, S.M.; Faccini-Martínez, Á.A.; Hidalgo, M.; Pulido-Villamarín, A.D.P.; Pérez-Torres, J.; Cuervo, C. Pathogenic Leptospira Species in Bats: Molecular Detection in a Colombian Cave. Trop. Med. Infect. Dis. 2022, 7, 84. [Google Scholar] [CrossRef]
- Esteves, S.B.; Gaeta, N.C.; Batista, J.M.N.; Dias, R.A.; Heinemann, M.B. Leptospira sp. infection in bats: A systematic review and meta-analysis. Transbound. Emerg. Dis. 2022, 65, e2456–e2473. [Google Scholar] [CrossRef]
- Muñoz-Leal, S.; Faccini-Martínez, Á.A.; Pérez-Torres, J.; Chala-Quintero, S.M.; Herrera-Sepúlveda, M.T.; Cuervo, C.; Labruna, M.B. Novel Borrelia genotypes in bats from the Macaregua Cave, Colombia. Zoonoses Public Health 2021, 68, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Urushadze, L.; Osikowicz, L.; McKee, C.; Kuzmin, I.; Kandaurov, A.; Babuadze, G.; Natradze, I.; Imnadze, P.; Kosoy, M. Molecular Survey of Bacterial Zoonotic Agents in Bats from the Country of Georgia (Caucasus). PLoS ONE 2017, 12, e0171175. [Google Scholar] [CrossRef] [PubMed]
- Banskar, S.; Bhute, S.; Suryavanshi, M.; Punekar, S.; Shouche, Y.S. Microbiome analysis reveals the abundance of bacterial pathogens in Rousettus leschenaultii guano. Sci. Rep. 2016, 6, 36948. [Google Scholar] [CrossRef]
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, P.O.; Gagnaire, J.; Botelho-Nevers, E.; Grattard, F.; Carricajo, A.; Lucht, F.; Pozzetto, B.; Berthelot, P. Detection and clinical relevance of Staphylococcus aureus nasal carriage: An update. Expert Rev. Anti Infect. Ther. 2014, 12, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Wójcik-Bojek, U.; Rozalska, B.; Sadowska, B. Staphylococcus aureus—A Known Opponent against Host Defense Mechanisms and Vaccine Development—Do We Still Have a Chance to Win? Int. J. Mol. Sci. 2022, 23, 948. [Google Scholar] [CrossRef] [PubMed]
- Held, J.; Gmeiner, M.; Mordmüller, B.; Matsiégui, P.B.; Schaer, J.; Eckerle, I.; Weber, N.; Matuschewski, K.; Bletz, S.; Schaumburg, F. Bats are rare reservoirs of Staphylococcus aureus complex in Gabon. Infect. Genet. Evol. 2017, 47, 118–120. [Google Scholar] [CrossRef]
- Olatimehin, A.; Shittu, A.O.; Onwugamba, F.C.; Mellmann, A.; Becker, K.; Schaumburg, F. Staphylococcus aureus Complex in the Straw-Colored Fruit Bat (Eidolon helvum) in Nigeria. Front. Microbiol. 2018, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Fountain, K.; Barbon, A.; Gibbon, M.J.; Lloyd, D.H.; Loeffler, A.; Feil, E.J. Staphylococcus aureus lineages associated with a free-ranging population of the fruit bat Pteropus livingstonii retained over 25 years in captivity. Sci. Rep. 2022, 12, 13457. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, A.; Dai, W.; Leng, H.; Liu, S.; Jin, L.; Sun, K.; Feng, J. Skin Microbiota Variation Among Bat Species in China and Their Potential Defense Against Pathogens. Front. Microbiol. 2022, 13, 808788. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Wirth, J.S.; Saravanan, V.S. Phylogenomic analyses of the Staphylococcaceae family suggest the reclassification of five species within the genus Staphylococcus as heterotypic synonyms, the promotion of five subspecies to novel species, the taxonomic reassignment of five Staphylococcus species to Mammaliicoccus gen. nov., and the formal assignment of Nosocomiicoccus to the family Staphylococcaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 5926–5936. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.; Tackling drug-resistant infections globally: Final report and recommendations. Rev. Antim. Res. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 5 November 2022).
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Nowakiewicz, A.; Zięba, P.; Gnat, S.; Trościańczyk, A.; Osińska, M.; Łagowski, D.; Kosior-Korzecka, U.; Puzio, I. Bats as a reservoir of resistant Escherichia coli: A methodical view. Can we fully estimate the scale of resistance in the reservoirs of free-living animals? Res. Vet. Sci. 2020, 128, 49–58. [Google Scholar] [CrossRef]
- Sens-Junior, H.; Trindade, W.A.; Oliveira, A.F.; Zaniolo, M.M.; Serenini, G.F.; Araujo-Ceranto, J.B.; Gonçalves, D.D.; Germano, R.M. Bacterial resistance in bats from the Phyllostomidae family and its relationship with unique health. Pesq. Vet. Bras. 2018, 38, 1207–1216. [Google Scholar] [CrossRef]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Prim. 2018, 4, 18033. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S. Methicillin-resistant Staphylococcus aureus in animals. ILAR J. 2010, 51, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Peacock, S.J.; Paterson, G.K. Mechanisms of Methicillin Resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef]
- Katayama, Y.; Ito, T.; Hiramatsu, K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2000, 44, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Hartman, B.J.; Tomasz, A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in. Staphylococcus aureus. J. Bacteriol. 1984, 158, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; van Alen, S.; Idelevich, E.A.; Schleimer, N.; Seggewiß, J.; Mellmann, A.; Kaspar, U.; Peters, G. Plasmid-encoded transferable mecB-mediated methicillin resistance in Staphylococcus aureus. Emerg. Infect. Dis. 2018, 24, 242–248. [Google Scholar] [CrossRef]
- Walther, B.; Wieler, L.H.; Friedrich, A.W.; Hanssen, A.-M.; Kohn, B.; Brunnberg, L.; Lubke-Becker, A. Methicillin-resistant Staphylococcus aureus (MRSA) isolated from small and exotic animals at a university hospital during routine microbiological examinations. Vet. Microbiol. 2008, 127, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Fountain, K.; Roberts, L.; Young, V.; Barbon, A.; Frosini, S.M.; Lloyd, D.H.; Loeffler, A. Diversity of Staphylococcal species cultured from captive livingstone’s fruit bats (Pteropus livingstonii) and their environment. J. Zoo Wildl. Med. 2019, 50, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Vittecoq, M.; Godreuil, S.; Prugnolle, F.; Durand, P.; Brazier, L.; Renaud, N.; Arnal, A.; Aberkane, S.; Jean-Pierre, H.; Gauthier-Clerc, M.; et al. Antimicrobial resistance in wildlife. J. Appl. Ecol. 2016, 53, 519–529. [Google Scholar] [CrossRef]
- Van de Vuurst, P.; Díaz, M.M.; Pedro, R.-S.; Allendes, J.L.; Brown, N.; Gutiérrez, J.D.; Zarza, H.; de Oliveira, S.V.; Cárdenas-Canales, E.; Barquez, R.M. A database of common vampire bat reports. Sci. Data 2022, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Devnath, P.; Karah, N.; Graham, J.P.; Rose, E.S.; Asaduzzaman, M. Evidence of Antimicrobial Resistance in Bats and Its Planetary Health Impact for Surveillance of Zoonotic Spillover Events: A Scoping Review. Int. J. Environ. Res. Public Health 2023, 20, 243. [Google Scholar] [CrossRef] [PubMed]
- Furness, L.E.; Campbell, A.; Zhang, L.; Gaze, W.H.; McDonald, R.A. Wild small mammals as sentinels for the environmental transmission of antimicrobial resistance. Environ. Res. 2017, 154, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Beltz, L.A. Bats and Human Health: Ebola, SARS, Rabies and Beyond, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Oh, W.T.; Jun, J.W.; Giri, S.S.; Yun, S.; Kim, H.J.; Kim, S.G.; Han, S.J.; Kwon, J.; Park, S.C. Staphylococcus xylosus infection in rainbow trout (Oncorhynchus mykiss) as a primary pathogenic cause of eye protrusion and mortality. Microorganisms 2019, 7, 330. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, K. Isolation and characterization of Staphylococcus hominis JX961712 from oil contaminated soil. J. Pharm. Res. 2013, 7, 252–256. [Google Scholar] [CrossRef]
- Silva, V.; Ferreira, E.; Manageiro, V.; Reis, L.; Tejedor-Junco, M.T.; Sampaio, A.; Capelo, J.L.; Caniça, M.; Igrejas, G.; Poeta, P. Distribution and Clonal Diversity of Staphylococcus aureus and Other Staphylococci in Surface Waters: Detection of ST425-t742 and ST130-t843 mecC-Positive MRSA Strains. Antibiotics 2021, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- Kozajda, A.; Jeżak, K.; Kapsa, A. Airborne Staphylococcus aureus in different environments—A review. Environ. Sci. Pollut. Res. 2019, 26, 34741–34753. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.A.S.; Pessoa, D.M.A.; Rui, A.M. Habitat use and seasonal activity of insectivorous bats (Mammalia: Chiroptera) in the grasslands of southern Brazil. Zoologia 2014, 31, 153–161. [Google Scholar] [CrossRef]
- Gorman, K.M.; Barr, E.L.; Ries, L.; Nocera, T.; MarkFord, W. Bat activity patterns relative to temporal and weather effects in a temperate coastal environment. Global Ecol. Conserv. 2021, 30, e01769. [Google Scholar] [CrossRef]
- Public Health England. Standards for Microbiology Investigations Identification of Staphylococcus Species, Micrococcus Species and Rothia Species; Public Health England: London, UK, 2020; Volume 4, pp. 1–26. [Google Scholar]
- Kloos, W.E.; Schleifer, K.H.; Smith, R.F. Characterization of Staphylococcus sciuri sp. nov. and its subspecies. Int. J. Syst. Bacteriol. 1976, 26, 22–37. [Google Scholar] [CrossRef]
- Hu, X.; Zheng, B.; Jiang, H.; Kang, Y.; Cao, Q.; Ning, H.; Shang, J. Draft Genome Sequence of Staphylococcus sciuri subsp. sciuri Strain Z8, Isolated from Human Skin. Genome Announc. 2015, 3, e00714-15. [Google Scholar] [CrossRef] [PubMed]
- Meservey, A.; Sullivan, A.; Wu, C.; Lantos, P.M. Staphylococcus sciuri peritonitis in a patient on peritoneal dialysis. Zoonoses Public Health 2019, 67, 93–95. [Google Scholar] [CrossRef]
- Stepanovic, S.; Jezek, P.; Vukovic, D.; Dakic, I.; Patras, P. Isolation of Members of the Staphylococcus sciuri Group from Urine and Their Relationship to Urinary Tract Infections. J. Clin. Microbiol. 2003, 41, 5262–5264. [Google Scholar] [CrossRef]
- Dick, C.W.; Patterson, B.D. Bat flies: Obligate ectoparasites of bats. In Micromammals and macroparasites; Morand, S., Krasnov, B.R., Poulin, R., Eds.; Springer: Tokyo, Japan, 2006; pp. 179–194. [Google Scholar]
- Rayner, C.; Munckhof, W.J. Antibiotics currently used in the treatment of infections caused by Staphylococcus aureus. Int. Med. J. 2005, 35, S3–S16. [Google Scholar] [CrossRef] [PubMed]
- Pantosti, A.; Sanchini, A.; Monaco, M. Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiol. 2007, 2, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Skov, R.; Lonsway, D.R.; Larsen, J.; Larsen, A.R.; Samulioniené, J.; Limbago, B.M. Evaluation of methods for detection of β-lactamase production in MSSA. J. Antimicrob. Chemother. 2021, 76, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Tanaka, T.; Oikawa, K.; Fukano, N.; Goto, M.; Takahashi, T. Prevalence of blaZ Gene and Performance of Phenotypic Tests to Detect Penicillinase in Staphylococcus aureus Isolates from Japan. Ann. Lab. Med. 2018, 38, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Mohammadtaheri, Z.; Pourpaki, M.; Mohammadi, F.; Raeissi, S.; Khodadoust, M.A.; Masjedi, M. Disc diffusion methods versus PCR for mecA gene in detection of Methicillin-Resistant Staphylococcus aureus. Int. J. Infect. Dis. 2010, 14, e351. [Google Scholar] [CrossRef]
- Sacristán, I.; Esperón, F.; Acuña, F.; Aguilar, E.; García, S.; Lopes, M.J.; Cevidanes, A.; Neves, E.; Cabello, J.; Hidalgo-Hermoso, E.; et al. Antibiotic resistance genes as landscape anthropization indicators: Using a wild felid as sentinel in Chile. Sci. Total Environ. 2020, 703, 134900. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, N.C.; Bean, E.; Miles, A.M.; De Carvalho, D.U.O.G.; Alemán, M.A.R.; Carvalho, J.S.; Gregory, L.; Ganda, E. A cross-sectional study of dairy cattle metagenomes reveals increased antimicrobial resistance in animals farmed in a heavy metal contaminated environment. Front. Microbiol. 2020, 11, 590325. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Allocati, N.; Petrucci, A.G.; Giovanni, P.D.; Masulli, M.; Ilio, C.D.; Laurenzi, V.D. Bat-man disease transmission: Zoonotic pathogens from wildlife reservoirs to human populations. Cell Death Discov. 2016, 2, 16048. [Google Scholar] [CrossRef] [PubMed]
- Kunz, T.H.; Parsons, S. Ecological and Behavioral Methods for Study of Bats, 2nd ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2009; p. 901. [Google Scholar]
- CLSI. Supplement M100, 30th ed.; Clinical Laboratory Research Institute: Wayne, PA, USA, 2022. [Google Scholar]
- Gaeta, N.C.; Hellmeister, A.; Possebon, F.S.; Araujo, J.P.; Heinemann, M.B. Genomic analysis of a multidrug methicillin-resistant staphylococcus epidermidis recovered from the urine of a guinea pig (Cavia porcellus) with suspected pyelonephritis. Vet. Res. Commun. 2022. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.H.; Kleven, S.H.; Jackwood, M.W. Application of polymerase chain reaction with arbitrary primers to strain identification of Mycoplasma gallisepticum. Avian Dis. 1995, 39, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, D.; Claus, H.; Witte, W.; Rothgänger, J.; Claus, H.; Turnwald, D.; Vogel, U. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 2003, 41, 5442–5448. [Google Scholar] [CrossRef] [PubMed]
- Martineau, F.; Picard, F.J.; Lansac, N.; Ménard, C.; Roy, P.H.; Ouellette, M.; Bergeron, M.G. Correlation between the resistance genotype determined by multiplex PCR assays and the antibiotic susceptibility patterns of Staphylococcus aureus and Staphylococcus epidermidis. Antim. Agents Chemother. 2000, 44, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, M.; Wang, G.; Johnson, W.M. Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J. Clin. Microbiol. 2000, 38, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Paterson, G.; Larsen, A.R.; Robb, A.; Edwards, G.E.; Pennycott, T.W.; Foster, G.; Mot, D.; Hermans, K.; Baert, K.; Peacock, S.J.; et al. The newly described mecA homologue, mecALGA251, is present in methicillin-resistant Staphylococcus aureus isolates from a diverse range of host species. J. Antimicrob. Chemother. 2012, 67, 2809–2813. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R. RStudio, 2020, PBC, Boston, MA. Available online: http://www.rstudio.com/ (accessed on 12 November 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 13 November 2022).
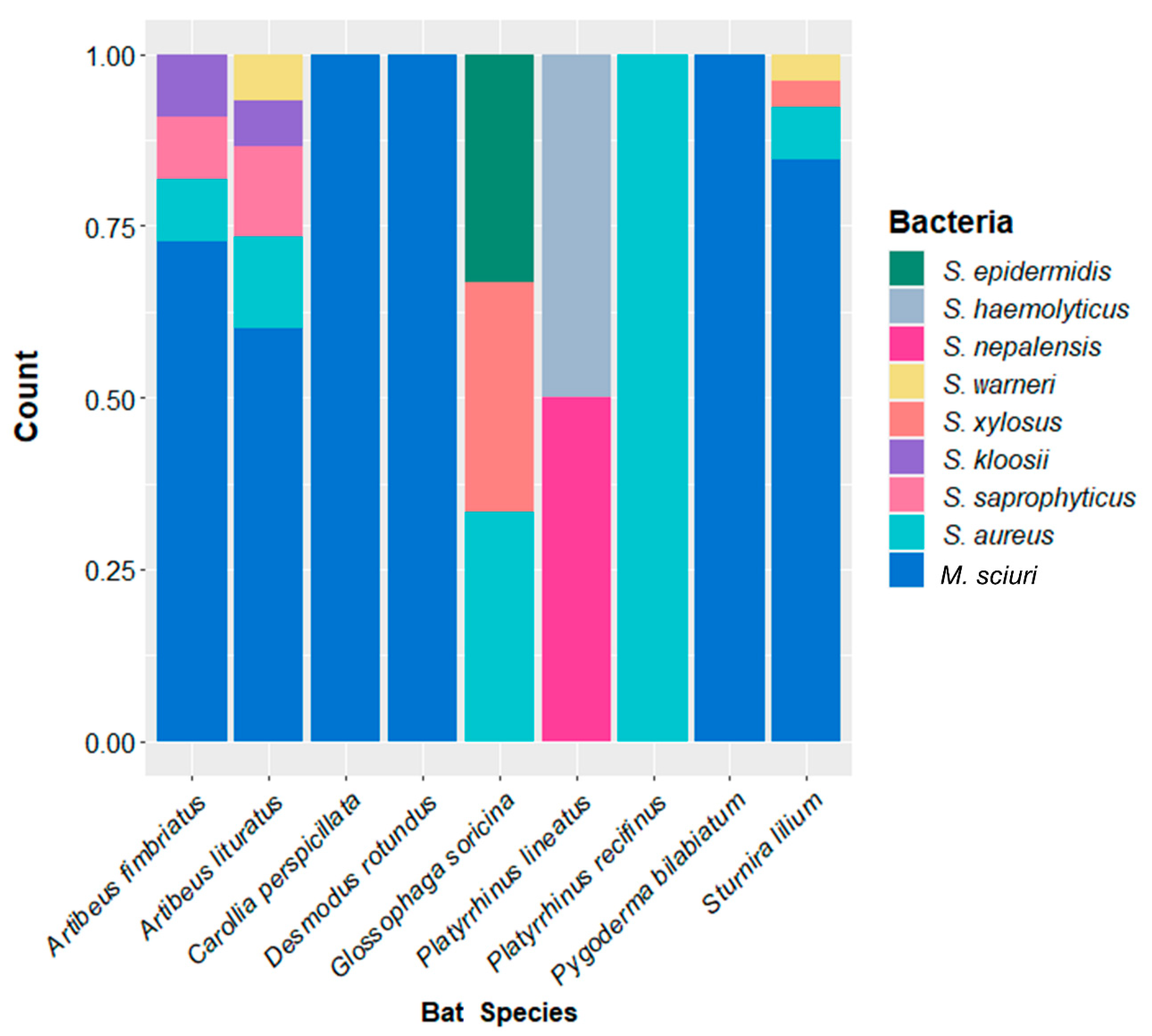

| Bat Species | Staphylococcus spp. | % (N/BSC) | Total Isolates % (N/BSC) |
|---|---|---|---|
| Desmodus rotundus | M. sciuri | 100 (01/01) | 100 (01/01) |
| Pygoderma bilabiatum | M. sciuri | 100 (01/01) | 100 (01/01) |
| Carollia perspicillata | M. sciuri | 75.0 (03/04) | 75.0 (03/04) |
| Platyrrhinus recifinus | S. aureus | 50.0 (01/02) | 50.0 (01/02) |
| Platyrrhinus lineatus | S. nepalensis | 25.0 (01/04) | 50.0 (02/04) |
| S. haemolyticus | 25.0 (01/04) | ||
| Sturnira lilium | M. sciuri | 39.3 (22/56) | 46.4 (26/56) |
| S. aureus | 3.6 (02/56) | ||
| S. warneri | 1.8 (01/56) | ||
| S. xylosus | 1.8 (01/56) | ||
| Artibeus lituratus | M. sciuri | 23.7 (09/38) | 39.5 (15/38) |
| S. aureus | 5.3 (02/38) | ||
| S. saprophyticus | 5.3 (02/38) | ||
| S. warneri | 2.6 (01/38) | ||
| S. kloosii | 2.6 (01/38) | ||
| Artibeus fimbriatus | M. sciuri | 28.6 (08/28) | 39.3 (11/28) |
| S. aureus | 3.6 (01/28) | ||
| S. saprophyticus | 3.6 (01/28) | ||
| S. kloosii | 3.6 (01/28) | ||
| Glossophaga soricina | S. epidermidis | 9.1 (01/11) | 27.3 (03/11) |
| S. xylosus | 9.1 (01/11) | ||
| S. aureus | 9.1 (01/11) |
| Zone | Bat Species | Total Resistant Isolates % (N/T) * | Cefoxitin | Ciprofloxacin | Penicillin | Erythromycin | mecA | blaz |
|---|---|---|---|---|---|---|---|---|
| Forested | Pygoderma bilabiatum | 100 (1/1) | 0 | 0 | 1 | 0 | 0 | 0 |
| Carollia perspicillata | 100 (2/2) | 1 | 0 | 2 | 0 | 0 | 0 | |
| Sturnira lilium | 83.3 (5/6) | 2 | 0 | 4 | 2 | 0 | 1 | |
| Artibeus lituratus | 75.0 (6/8) | 1 | 1 | 4 | 2 | 1 | 0 | |
| Total Area 1 | 77.8 (14/18) | 4 | 1 | 10 | 4 | 1 | 1 | |
| Rural | Platyrrhinus lineatus | 100 (1/1) | 1 | 0 | 1 | 1 | 1 | 1 |
| Platyrrhinus recifinus | 100 (1/1) | 0 | 0 | 1 | 0 | 0 | 1 | |
| Sturnira lilium | 66.7 (12/18) | 1 | 0 | 9 | 3 | 1 | 2 | |
| Artibeus fimbriatus | 44.4 (4/9) | 1 | 0 | 4 | 0 | 1 | 1 | |
| Artibeus lituratus | 50.0 (2/4) | 1 | 0 | 2 | 0 | 1 | 0 | |
| Total Area 2 | 57.1 (20/35) | 4 | 0 | 17 | 4 | 4 | 5 | |
| Residential-A | Platyrrhinus lineatus | 100 (1/1) | 0 | 0 | 1 | 0 | 0 | 0 |
| Glossophaga soricina | 100 (1/1) | 0 | 1 | 0 | 1 | 0 | 0 | |
| Total Area 3 | 66.7 (2/3) | 0 | 1 | 1 | 1 | 0 | 0 | |
| Slum | Artibeus lituratus | 100 (1/1) | 0 | 0 | 1 | 0 | 0 | 0 |
| Glossophaga soricina | 100 (1/1) | 0 | 0 | 1 | 0 | 0 | 1 | |
| Sturnira lilium | 50.0 (1/2) | 0 | 0 | 1 | 0 | 0 | 0 | |
| Total Area 4 | 75.0 (3/4) | 0 | 0 | 3 | 0 | 0 | 1 | |
| Residential-B | Artibeus lituratus | 100 (1/1) | 0 | 0 | 0 | 1 | 0 | 0 |
| Total Area 5 | 100 (1/1) | 0 | 0 | 0 | 1 | 0 | 0 | |
| Industrial | Glossophaga soricina | 100 (1/1) | 0 | 0 | 1 | 0 | 0 | 1 |
| Artibeus lituratus | 100 (1/1) | 0 | 0 | 1 | 0 | 0 | 1 | |
| Total Area 6 | 100 (2/2) | 0 | 0 | 2 | 0 | 0 | 2 |
| Zone | Species (N) | CFO-Resistant/MRS | mecA | PEN-Resistant | blaZ |
|---|---|---|---|---|---|
| Forested | S. aureus (3) | 0 | - | 100.0 (3/3) | 100.0 (3/3) |
| S. haemolyticus (1) | 100.0 (1/1) | 100.0 (1/1) | 100.0 (1/1) | 100.0 (1/1) | |
| S. saprophyticus (1) | 0 | - | 100.0 (1/1) | 0 | |
| M. sciuri (28) | 10.7 (3/28) | 0 | 7.1 (2/28) | 0 | |
| S. warneri (2) | 0 | - | 0 | - | |
| TOTAL (35) | 11.4 (4/35) | 2.9 (1/35) | 20.0 (7/35) | 11.4 (4/35) | |
| Rural | S. aureus (1) | 0 | - | 100.0 (1/1) | 100.0 (1/1) |
| S. kloosii (1) | 0 | - | 100.0 (1/1) | 0 | |
| S. saprophyticus (2) | 0 | - | 0 | - | |
| M. sciuri (14) | 28.6 (4/14) | 0 | 64.3 (9/14) | 0 | |
| TOTAL (18) | 22.2 (4/18) | 0 | 61.1 (11/18) | 5.6 (1/18) | |
| Residential-A | S. kloosii (1) | 0 | - | 0 | - |
| S. nepalensis (1) | 0 | - | 0 | - | |
| S. xylosus (1) | 0 | - | 100.0 (1/1) | 0 | |
| TOTAL (3) | 0 | - | 33.3 (1/3) | 0 | |
| Slum | S. epidermidis (1) | 0 | - | 100.0 (1/1) | 100.0 (1/1) |
| M. sciuri (2) | 0 | - | 50.0 (1/2) | 0 | |
| S. xylosus (1) | 0 | - | 100.0 (1/1) | 0 | |
| TOTAL (4) | 0 | - | 75.0 (3/4) | 25.0 (1/4) | |
| Residential-B | S. aureus (1) | 0 | - | 100.0 (1/1) | 100.0 (1/1) |
| Industrial | S. aureus (2) | 0 | - | 100.0 (2/2) | 100(2/2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo Gaeta, N.; Cavalcante Brito, J.E.; Nunes Batista, J.M.; Gagete Veríssimo de Mello, B.; Dias, R.A.; Heinemann, M.B. Bats Are Carriers of Antimicrobial-Resistant Staphylococcaceae in Their Skin. Antibiotics 2023, 12, 331. https://doi.org/10.3390/antibiotics12020331
Carrillo Gaeta N, Cavalcante Brito JE, Nunes Batista JM, Gagete Veríssimo de Mello B, Dias RA, Heinemann MB. Bats Are Carriers of Antimicrobial-Resistant Staphylococcaceae in Their Skin. Antibiotics. 2023; 12(2):331. https://doi.org/10.3390/antibiotics12020331
Chicago/Turabian StyleCarrillo Gaeta, Natália, João Eduardo Cavalcante Brito, Juliana Maria Nunes Batista, Beatriz Gagete Veríssimo de Mello, Ricardo Augusto Dias, and Marcos B. Heinemann. 2023. "Bats Are Carriers of Antimicrobial-Resistant Staphylococcaceae in Their Skin" Antibiotics 12, no. 2: 331. https://doi.org/10.3390/antibiotics12020331
APA StyleCarrillo Gaeta, N., Cavalcante Brito, J. E., Nunes Batista, J. M., Gagete Veríssimo de Mello, B., Dias, R. A., & Heinemann, M. B. (2023). Bats Are Carriers of Antimicrobial-Resistant Staphylococcaceae in Their Skin. Antibiotics, 12(2), 331. https://doi.org/10.3390/antibiotics12020331








