Abstract
Necrotic enteritis (NE), caused by Clostridium perfringens, is an emerging issue in poultry farming. New approaches, other than antibiotics, are necessary to prevent NE development and the emergence of multidrug-resistant bacteria. Enterococci are commensal microorganisms that can produce enterocins, antimicrobial peptides with activities against pathogens, and could be excellent candidates for protective cultures. This study aimed to screen and characterize Enterococcus strains of poultry origin for their inhibitory activity against C. perfringens. In total, 251 Enterococcus strains of poultry origin plus five bacteriocin-producing (BP+) E. durans strains of other origins were screened for antimicrobial activity against the indicator C. perfringens X2967 strain using the “spot on the lawn” method. We detected thirty-two BP+ strains (eleven Enterococcus faecium, nine E. gallinarum, eight E. faecalis, three E. durans, and one E. casseliflavus). We further studied the antimicrobial activity of the supernatants of these 32 BP+ strains using agar well diffusion and microtitration against a collection of 20 C. perfringens strains. Twelve BP+ enterococci that were found to exhibit antimicrobial activity against C. perfringens were characterized using whole genome sequencing. Among these, E. faecium X2893 and X2906 were the most promising candidates for further studies as protective cultures for poultry farming. Both strains belong to the sequence type ST722, harbor the genes encoding for enterocin A and enterocin B, do not possess acquired resistance genes, do not carry plasmids, and present the acm gene, which is implicated in host colonization. Further research is needed to determine the utility of these strains as protective cultures.
1. Introduction
Antibiotic resistance is a serious public health concern that compromises the treatment of infections in humans and animals and is associated with the unnecessary prescription and/or misuse of antibiotics. Besides their clinical use in humans, antibiotics are also used in veterinary and animal farming. Antibiotics have also been extensively used as growth promoters in food-producing animals; however, even though this practice has been banned in Europe since 2006 [1] and also in several other countries, it is still allowed in some others [2]. This contributes to the increase and spread of antibiotic resistance, not only among pathogenic bacteria but also among commensal bacteria of the intestinal tract of humans and animals, which can lead to contamination via feces. Therefore, resistant bacteria can reach humans via the food chain and water or by contact with animals. For this reason, the World Health Organization (WHO) proposed to address this issue from a “One Health” perspective, establishing new alternatives to the use of antibiotics in livestock and agriculture [3].
Clostridium perfringens is associated with necrotic enteritis (NE) in poultry, and its prevalence has been increasing in countries that no longer use antibiotic growth promoters, which suggests that the same trend could also originate among other relevant pathogens [4]. NE caused by C. perfringens is one of the most common poultry diseases that cause substantial economic losses to the industry [5]. A prominent characteristic of NE is acute death, with mortality rates reaching 50%. Clinical signs include depression, dehydration, somnolence, ruffled feathers, diarrhea, and decreased feed consumption [6]. The subclinical form of this disease causes chronic damage to the intestinal mucosa of the chickens, leading to impaired nutrient absorption, reduced weight gain, and decreased overall performance. Clostridium perfringens is present in the intestines of healthy chickens but in a small proportion (less than 105 CFU/g of the intestinal content); when its count increases, hen birds become susceptible to NE [1].
Antibiotic-resistant bacteria are prevalent in different environments and can be introduced into the food chain at various points. Poultry is a reservoir for antibiotic-resistant bacteria that can be transmitted to humans. The continuous and widespread use of antibiotics in farm animals may lead to changes in the bacterial environment, eliminating susceptible strains and allowing antimicrobial-resistant bacteria to survive and predominate. Furthermore, the continuous administration of antibiotics in feed may cause cross-resistance to therapeutic antimicrobial agents. Antimicrobial resistance and a gradual decrease in antibiotic sensitivity to anticoccidials in some strains of Eimeria spp. (a predisposing factor for NE) can exacerbate the presence of C. perfringens strains [7].
Protective cultures essentially consist of bacteria specifically selected for their ability to inhibit the growth of other pathogenic organisms or microbiological spoilage agents, having the status of GRAS (Generally Recognized as Safe). These bacterial species are entirely natural. Therefore, they provide a useful “green” benefit to food product labeling [8]. Bacteriocin-producing strains have gained considerable interest in recent years. They are considered one of the most promising alternatives to antibiotics for use as protective cultures.
Enterococci are ubiquitous microorganisms found in the gastrointestinal tracts of humans and animals and in water, soil, plants, and food. These microorganisms produce bacteriocins known as enterocins [9], which exhibit an inhibition spectrum against taxonomically close bacteria and even those with a broad spectrum of action, inhibiting a wide range of bacteria, including the emergent C. perfringens [10,11]. Using enterococci as potential probiotic strains or protective cultures can be an excellent alternative to antibiotic use in poultry farming [12].
However, in recent years, the use of enterococci in the food industry has been debated because of their implications for opportunistic infections and their potential acquisition of antimicrobial resistance and virulence genes [9]. Therefore, developing new enterococcal probiotics requires a strict safety assessment to select the truly harmless enterococcal strains for safe applications [13].
This study aimed to isolate and characterize Enterococcus strains of poultry origin that might exhibit antimicrobial activity against C. perfringens and other relevant microorganisms.
2. Results
2.1. Enterococcus Sampling and Identification
Sixty enterococcus strains were isolated from poultry meat samples collected from local markets in La Rioja, Spain. These strains were identified using MALDI-TOF mass spectrometry as E. faecium (n = 33), E. faecalis (n = 19), E. gallinarum (n = 5), E. casseliflavus (n = 1), E. durans (n = 1), and E. avium (n = 1). These isolates were combined with another 191 Enterococcus, previously obtained from poultry (in Spain and Tunisia), and with five bacteriocin-producing (BP+) Enterococcus from other origins, to develop the entire collection of 256 Enterococcus used to detect and characterize the BP+ isolates.
2.2. Screening of Enterococci for Antimicrobial, Specifically Anti-C. perfringens Activity
In total, 32 of the 256 enterococci tested (12.84%) demonstrated antimicrobial activity against C. perfringens X2967 using the “spot on the lawn” method. These strains belonged to the species E. faecium (n = 11), E. gallinarum (n = 9), E. faecalis (n = 8), E. durans (n = 3), and E. casseliflavus (n = 1). Among them, 27 (84,37%) were active against Listeria monocytogenes, Micrococcus luteus, and Streptococcus suis (Table 1). One Enterococcus strain alone showed antimicrobial activity against methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-resistant S. aureus (MRSA). None of the tested strains showed inhibitory activity against gram-negative bacteria (Escherichia coli, Salmonella enterica, Yersinia enterocolitica, and Pseudomonas aeruginosa). Figure 1 shows the inhibition halo against C. perfringens X2967 produced by two of the thirty-two BP+ strains.

Table 1.
Antimicrobial activity of the 32 bacteriocin producer (BP+) enterococci against C. perfringens X2967 and other relevant indicator bacteriaa, as detected by the “spot on the lawn” assay.
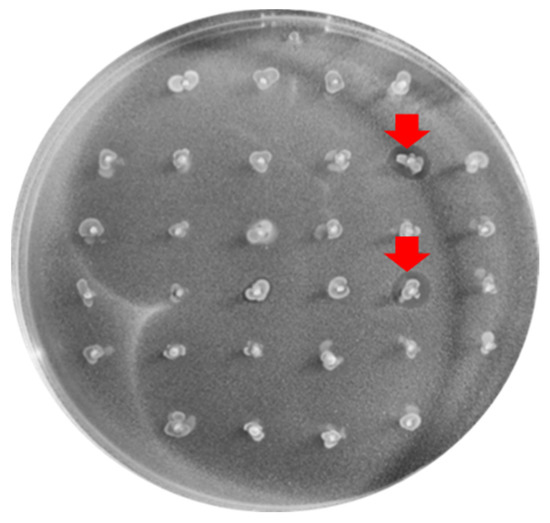
Figure 1.
Inhibition halos (marked with the red arrow) produced by 2 of the BP+ enterococci tested against the C. perfringens X2967 indicator strain.
2.3. Effects of the Supernatants of BP+ Enterococci on C. perfringens Isolates
The supernatants of the 32 BP+ enterococci were tested against a collection of 20 C. perfringens isolates of poultry origin. The antimicrobial activity was detected in 18 concentrated supernatants against at least one of the C. perfringens strains. Nevertheless, antimicrobial activity was observed in six of the heated supernatants (HS) and non-heated supernatants (NHS) (Figure 2, Table 2), corresponding to four E. faecium and two E. durans isolates. In general, the inhibitory activities of the HS and NHS were similar; both inhibited the growth of 2–8 strains of the 20 C. perfringens tested. The concentrated supernatants showed a broad spectrum of inhibition against 2–20 C. perfringens isolates (Table 2). The remaining 14 supernatants, either HS, NHS, or concentrated supernatants, did not show any inhibitory activity.

Figure 2.
Inhibition halo of the E. faecium strain, X3179, against one of the 20 C. perfringens isolates. The bigger halo corresponds to the activity of nisin, used as a control.

Table 2.
The number of C. perfringens isolates to which the supernatants of 18 BP+ enterococci present antimicrobial activity in their supernatants.
Supernatant activity could only be quantified for the E. faecalis X3198 and E. faecium X3179 strains (16 AU/mL).
2.4. Phenotypic and Genotypic Characterization of the Selected BP+ Enterococci
For a complete genome analysis, 12 BP+ enterococci were selected based on their antimicrobial activity detected using the previously described methods. Five E. faecium and two E. faecalis of poultry origin were selected, as well as five E. durans of milk and camel milk origin, chosen as the BP+ controls.
2.4.1. Bacteriocinome
Structural genes encoding for bacteriocins were detected in 12 BP+ strains (Table 3). The structural genes for enterocins P and Enterocin L50 A/B were detected in all five E. durans isolates, and the genes for bac 32 were also observed in three of them. Genes encoding enterocin A and enterocin B were detected in all the E. faecium strains; two of these strains carried the genes encoding enterocin NKR-5-3-A/D/Z. Moreover, the genes encoding enterocin SE-K4 and staphylococcin C55a/b were identified in two E. faecalis strains.

Table 3.
Putative enterocins detected by WGS in the 12 selected BP+ enterococci.
2.4.2. Antibiotic Resistance phenotype and resistome
Five of the twelve selected BP+ enterococci (41.7%) were susceptible to the nine antibiotics tested, all of them from the species E. durans. The remaining strains were resistant to at least one of the antibiotics tested. The most frequent resistance was against ciprofloxacin (58.3%), followed by tetracycline (25.0%), erythromycin (25.0%), penicillin (16.7%), chloramphenicol (8.3%), high-level streptomycin (8.3%), and high-level gentamicin (8.3%). In addition, all the isolates showed susceptibility to vancomycin and linezolid.
Genes encoding antibiotic resistance were detected in all 12 BP+ strains (Table 4), although only five (three E. faecium and two E. faecalis isolates) had genes for acquired-type resistance. The mutations associated with resistance phenotypes for beta-lactams (pbp5) and fluoroquinolones (gyrA and parC) were detected only in E. faecium isolates (Supplementary Material).

Table 4.
Antibiotic resistance phenotype and genotype of the BP+ enterococci.
2.4.3. Virulence
- Gelatinase activity and hemolysis
Among the 12 selected BP+ enterococci, only E. faecalis X3198 was positive for gelatinase activity, and all the strains showed gamma hemolysis.
- Virulome
Among the 12 BP+ enterococcal strains, virulence genes were detected in E. faecium and E. faecalis but not in E. durans (Table 5).

Table 5.
Virulence genes and sequence types detected in the BP+ E. faecalis and E. faecium isolates of poultry origin by WGS.
2.4.4. Plasmidome
The replicon plasmids identified in the selected enterococci are listed in Table 6. All of the E. durans strains carried RepA_N, Inc18, and Rep3 or Rep1 plasmidic replicons. Both E. faecalis strains carried the type Rep trans. Moreover, most of the faecium strains carried at least three different types of plasmidic replicons.

Table 6.
Plasmidome of the 12 BP+ enterococci detected by WGS.
2.4.5. Genetic Lineages
Multi-locus sequence typing (MLST) of the two E. faecalis and five E. faecium strains yielded the following results: (a) the two E. faecalis strains were typed as ST397; (b) the five E. faecium strains showed four different sequence types, with two isolates typed as ST722, one isolate typed as ST784, and the remaining two with an unknown ST (Table 5, Figure 3).
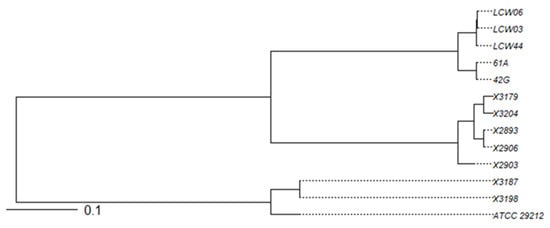
Figure 3.
Phylogenetic tree based on the average nucleotide identity (ANI) of the 12 BP+ enterococci. The reference strain, ATCC 29212, was also included.
3. Discussion
3.1. Screening for BP+ Enterococci
A total of 32 of the 256 enterococci tested (12.84%) showed antimicrobial activity against the C. perfringens X2967 strain, as determined using the “spot on the lawn” method; however, among these, only 18 supernatants of the BP+ strains were active against the collection of 20 C. perfringens isolates used as indicators. The inhibitory activities of these supernatants were attributed to the Enterococcus-derived enterocins [14]. The absence of inhibitory activity in the supernatants obtained from the strains showing inhibition using the spot-on-the-lawn method may be explained by the fact that bacteriocins sometimes remain attached to the cell wall and are not released in the supernatant. Furthermore, the production of bacteriocins is commonly mediated by quorum sensing [15]; hence, we detected 14 strains as BP+ via the spot-on-the-lawn method (in which the producer and the indicator strains are confronted) but without activity in their supernatants (the extract produced without previous exposure to the indicator bacteria) [16].
3.2. Phenotypic and Genotypic Characteristics of the BP+ Enterococci
According to their antimicrobial activity, 12 BP+ enterococci were selected for further characterization.
3.2.1. Bacteriocinome
The structural genes for enterocins P and Enterocin L50A/B were detected in all five E. durans isolates. Enterocin P (entP) was first detected in an E. faecium strain isolated from a dry-fermented sausage [17], showing activity against gram-positive pathogenic bacteria such as C. perfringens, L. monocytogenes, and S. aureus. Enterocin P is chromosomally encoded [18,19]; however, other studies have detected entP genes in the plasmid location [20]. Enterocin P and L50A/B have been detected in different enterococcal species [21]. This study is the first study to detect Enterocin P in E. durans.
Enterocin L50A/B was first detected in an E. faecium L50 strain isolated from Spanish fermented sausage [22]. Enterocin L50A/B consists of two peptides, L50A and L50B, which synergistically promote their antimicrobial activity. The strain E. faecium L50 has also been shown to produce enterocins Q and P at different temperatures [18,23]. Enterocin L50 A/B exhibits a broad spectrum of antimicrobial activities, including inhibition of Enterococcus spp., Lactobacillus spp., Lactococcus lactis, Pediococcus pentosaceus, L. monocytogenes, S. aureus, B. cereus, C. botulinum, Streptococcus pneumoniae, S. mitis, S. oralis, S. parasanguis, S. agalactiae, and C. perfringens. Other enterocins, such as enterocins 7A/7B and MR10A/10B, share a strong homology with enterocin L50 A/B [21].
Enterocin bac 32 was identified in three of our five E. durans strains. This peptide was firstly detected in a vancomycin-resistant clinical E. faecium VRE200 strain, exhibiting activity against Enterococcus spp [24]. Although this bacteriocin has not been extensively studied, it seems to be identical to enterocin IT [25].
The strain E. durans 61A has been previously described, and durancin 61A and enterocins L50A and L50B were identified using mass spectrometry [26,27]. However, the genetic determinants for these bacteriocins were not detected in strain 61A using whole genome sequencing (WGS) in our study; instead, enterocin P was detected. Duracin 61A is not in the anti-SMASH and BAGEL4 databases (we used data from the NCBI and NCBI plus UniProt, respectively), whose genetic determinants have yet to be described. In contrast, enterocin P might not have been detected in other studies, as it is a temperature-regulated bacteriocin that is synthesized optimally at 37–47 °C [23].
Genes encoding enterocin A and enterocin B were detected in all of our five E. faecium strains, two of which also carried the genes encoding Enterocin NKR-5-3-A/D/Z.
Enterocin A was first identified in 1996 [28] and is produced by several strains of E. faecium—CTC492, T136, and P21—isolated from Spanish sausage; BFE900 from black olives; DPC 1146, WHE 81, and EFM01 from dairy products; and the N5 strain of “nuka”, a Japanese rice paste. Enterocin A shows activity against Enterococcus spp., Lactobacillus spp., Pediococcus spp., and L. monocytogenes [10]. However, its activity has not been tested against clostridial species. Enterocin A is usually co-produced with enterocin B, which is produced by E. faecium T136 isolated from Spanish fermented sausages [29]. Enterocin B shows antimicrobial activity against gram-positive bacteria, such as L. monocytogenes, Propionibacterium spp., C. sporogens, and C. tyrobutyricum [29]. When enterocin A and enterocin B are co-produced, they form a heterodimer, and studies have demonstrated its potential anti-bacterial and anti-biofilm activities against S. aureus, Acinetobacter baumannii, L. monocytogenes, and E. coli [30].
The genetic determinants for enterocin NKR-5-3-A/B/C/D/Z were detected in two of our E. faecium strains. These enterocins have been purified and studied previously [31]. NKR-5-3-A (identical to brochocin A) and NKR-5-3-Z are class IIb bacteriocins and exhibit synergistic antimicrobial activity. NKR-3-5-B is a novel circular bacteriocin belonging to class IIc bacteriocins with a broad spectrum of antimicrobial activities against Bacillus spp., Enterococcus spp., and gram-negative bacteria (E. coli and Salmonella). NKR-5-3-C is a class IIa bacteriocin with strong antimicrobial activity against L. monocytogenes. NKR-5-3-D, a class IId bacteriocin, has a weak antimicrobial activity but can be produced even under unfavorable conditions [32,33]. NKR-5-3-A, D, and Z variant genes were detected in the two E. faecium strains. The genetic determinants of enterocins NKR-5-3-A/C/D/Z are closely located in a gene cluster (13 kb long) and include specific bacteriocin biosynthetic genes, such as an ABC transporter gene (enkT), two immunity-related genes (enkIaz and enkIc), a response regulator (enkR), and a histidine protein kinase (enkK). This gene cluster is essential for the biosynthesis and regulation of NKR-5-3 enterocins [34].
Genes encoding enterocin SE-K4 and staphylococcin C55a/b were identified in the two E. faecalis strains in this study. Enterocin SE-K4 was first identified in E. faecalis K-4 isolated from grass silage [35]; it grows at 43–45 °C and exhibits antimicrobial activities against E. faecium, E. faecalis, B. subtilis, C. beijerinckii, and L. monocytogenes. This enterocin has a high degree of homology to bacteriocin 31 and T8/43 [10]. Staphylococcin C55a/b was originally found to be produced by S. aureus C55 [36], consisting of three distinct peptide components termed staphylococcins C55a, C55b, and C55g. Staphylococcins C55a and C55b (lantibiotic components) acted synergistically against S. aureus and M. luteus [36]. It is a plasmid-encoded bacteriocin [37]; thus, the plasmid transfer between the producer, Staphylococcus, and the E. faecalis strains could account for the presence of the genetic determinants of this bacteriocin.
3.2.2. BP+ Enterococcus Resistance Phenotype and Resistome
Five of the twelve BP+ enterococci, all from E. durans isolates, were susceptible to the nine antibiotics tested. The remaining strains showed resistance to at least one of the antibiotics. Generally, the enterococci of poultry origin have more resistance genes than those of other origins (camel and camel milk). The only gene discovered in the E. durans strains of milk origin was aac(6′)-Iih, which is intrinsically present in E durans [38,39]. Antibiotics are commonly used in poultry farming, leading to the development of acquired resistance mechanisms in poultry-derived strains.
The genus Enterococcus is characterized by its intrinsic resistance to several antibiotics and ability to acquire new resistance mechanisms [40]. Enterococci are naturally resistant to semisynthetic penicillins (a reduced susceptibility), aminoglycosides (in low levels), vancomycin (at a low level and only in the species E. gallinarum and E. casseliflavus/E. flavescens, which are carriers of vanC genes), to lincosamides, polymyxins, and streptogramins (the species E. faecalis) [41]. In addition, E. faecium carries some intrinsic genes, such as msrC and aac(6′)-Ii, whereas E. durans harbors the gene aac(6′)-Iih [38,39]. Antibiotic resistance can occur either through the acquisition of genetic elements containing the resistance genes or via DNA mutations (mostly in genes encoding antibiotic targets), which are favored when there is a selective antibiotic pressure [40].
Among the acquired resistance genes detected in the E. faecium and E. faecalis strains, the genes associated with erythromycin [erm(B)], chloramphenicol [fexB and cat], tetracycline [tet(M) and tet(L)], streptomycin [str], gentamicin and tobramycin [aac(6′)-aph(2″)], and linezolid resistance (poxtA) have been reported. Vancomycin resistance genes have not been reported [42].
E. faecium strains X2893 and X2906 carry only chromosomal and intrinsic resistance genes (msr(C) and aac(6′)-Ii), which are non-transferable; therefore, these strains are excellent candidates for use as potential protective cultures.
Specific mutations in the pbp5 and gyrA/parC genes are associated with resistance to beta-lactams and fluoroquinolones, respectively [43,44,45]. Different mutations in the pbp5, gyrA, and parC genes have been detected in our strains, although, in most cases, with an unknown resistance phenotype associated.
3.2.3. Virulence of BP+ Enterococci
Different virulence factors are involved in the attachment to host cells and extracellular matrix proteins (AS, Esp, Hyl, and EfaA), macrophage resistance (AS), and cell and tissue damage (Cyl and GelE) [46,47]. Thus, although enterococci are commensal bacteria found in the intestine, they can still cause infections. Therefore, the Food and Drug Administration (FDA) has not yet assigned them to the GRAS category. Genes encoding these virulence factors are located in conjugative plasmids (agg, cyl, or hyl), in the chromosome (gelE or fsr), or in regions of the chromosome called pathogenic islands (esp and cyl) [48,49].
In the 12 enterococcal strains, virulence genes were detected in E. faecium and E. faecalis but not in E. durans. E. faecalis has already been described as more virulent than other species [50]. Fifteen virulence genes were detected in both E. faecalis strains. However, the presence of these genes is not always related to the virulence potential, as they are sometimes silenced and not associated with the phenotype [49]. Both strains carried the gelE gene, which is associated with gelatinase activity, but only strain X3198 was positive for gelatinase activity.
All the E. faecium strains carried the functional collagen adhesin gene, acm, which plays an essential role in colonization by binding to collagen type I, with less affinity to collagen type IV [51]. As these E. faecium strains did not carry other virulence factors, the presence of acm might be positive, as it could facilitate the colonization of this beneficial strain. Nevertheless, as mentioned before, the presence of a virulence gene does not always indicate that it is being expressed [49]. Therefore, further studies must uncover whether acm is, in fact, expressed as a virulence factor.
3.2.4. Plasmidome of the BP+ Enterococci
Ten of the BP+ enterococci harbored at least one plasmid. Interestingly, strains X2893 and X2906 did not present any mobile genetic elements, which, along with the other characteristics, makes them good candidates for potential protective cultures [52].
4. Materials and Methods
4.1. Enterococcus Sampling and Identification
In total, 251 enterococcal isolates of poultry origin were used in this study: (a) 60 isolates were collected during this study from poultry carcass samples obtained from different supermarkets and butchers in the La Rioja Region (Spain), the isolates recovered in the Slanetz–Bartley agar (OXOID); (b) 166 isolates were previously obtained from poultry carcasses at the slaughterhouses’ level in Tunisia; (c) 25 poultry isolates were obtained from the University of La Rioja’s collection (Spain). Additionally, 5 BP+ enterococci of other origins (2 isolates from cow milk and 3 from camel milk) were obtained from the University of LAVAL’s strain collection (Canada).
4.2. Screening for Anti-C. perfringens Activity Using the “Spot on the Lawn” Method
The antimicrobial activity of the 256 Enterococcus isolates against the indicator strain, C. perfringens X2967 (a clinical strain obtained from the Hospital San Pedro, Logroño, Spain), was analyzed using the “spot-on-the-lawn” method [53]. The active isolates were identified as BP+. Briefly, a fresh culture of C. perfringens strain X2967 was suspended in brain–heart infusion broth (BHI) (turbidity 0.5 MacFarland). Subsequently, 10 µL of this indicator microorganism solution was added to tubes containing 5 mL of semi-solid melted tryptic soy broth (TSB) and supplemented with 0.7% agar and 0.3% yeast extract. Finally, the semi-solid TSB medium with the indicator microorganism was poured onto tryptic soy agar plates (TSA). Once the plates were dried, the enterococcal microorganisms were sting-seeded, and the plates were incubated at 37 °C for 24 h under strict, anaerobic conditions.
Strains that showed inhibitory activity against C. perfringens strain X2967 were tested against other relevant pathogens and multidrug-resistant (MDR) bacteria using the same test. This panel included E. casseliflavus C1232, E. gallinarum C2310, E. faecium C2321, E. faecalis C410, E. durans C1433, E. hirae C1436, MSSA C411, MRSA C1570, M. luteus C157, L. monocytogenes C137, S. suis C2058, E. coli C408, S. enterica C660, Y. enterocolitica X3080, and P. aeruginosa X3282. A blood agar plate was used for S. suis testing. All strains used as indicator bacteria came from the University of La Rioja’s collection.
4.3. Screening for Anti-C. perfringens Activity Using the Agar Diffusion Method
NHS and HS extracts were prepared from Enterococcus isolates showing inhibitory activity in the spot-on-the-lawn assay. These supernatants were tested against a collection of 20 C. perfringens isolates using the previously described agar diffusion method [54], with nisin as a positive control. The C. perfringens isolates were collected from the NE of poultry origin (University of Laval, Quebec, QC, Canada).
To prepare the NHS, enterococci were inoculated in 10 mL of TSB in sterile tubes and were incubated overnight at 37 °C. Then, the culture medium was centrifuged at 5000× g rpm for 5 min and filtrated using 0.20 µm filters. Next, a fraction of this supernatant was heated at 100 °C for 15 min and used as the HS. For the concentrated supernatants, the culture cell media were concentrated 10 times using a Speed Vac (Thermo Scientific Savant, Asheville, NC, United States) after centrifugation.
For the agar well-diffusion method, C. perfringens was cultured in a reinforced clostridial medium (RCM) (Himedia, Kennett Square, PA, USA) supplemented with 10% agar. The plates were incubated overnight at 37 ºC under strict, anaerobic conditions.
4.4. Anti-C. perfringens Activity Determination Using Microtitration Assay
A microtitration assay was performed to determine the total activity (AU/mL) of the active supernatant of BP+ enterococci against the C. perfringens ATCC 13124 strain, as described previously [55,56]. The BHI was used as the growth medium for C. perfringens and was added to the wells, with a final bacterial concentration of ~105 CFU/well. The microplate was incubated for 24 h at 37 °C under strict, anaerobic conditions. After incubation, the optical density was measured at 595 nm using a microplate reader (Infinite M200, Tecan, Männedorf, Switzerland) to determine the number of wells in which inhibition occurred.
The following formula was used to calculate the total arbitrary activity:
where 2 is the dilution factor, n is the number of inhibition wells, 1000 is the factor for reporting the result per mL, and 125 is the volume of the solution tested in microliters.
4.5. Characterization of BP+ Enterococci
Twelve BP+ enterococci were chosen for further characterization based on their antimicrobial activity against C. perfringens strains.
4.5.1. Susceptibility to Antibiotics
The susceptibility of BP+ enterococci to nine antibiotics was tested using the disk diffusion method according to the Clinical and Laboratory Standard Institute (CLSI) guidelines (2020) [57]. The antibiotics tested were as follows (disk charge): penicillin (10 units), tetracycline (30 µg), erythromycin (15 µg), chloramphenicol (30 µg), linezolid (30 µg), high-level gentamicin (120 µg), high-level streptomycin (300 µg), vancomycin (30 µg), and ciprofloxacin (5 µg). Strains were then identified as susceptible (S), resistant (R), or intermediate (I) using the protocol interpretation guidelines [57].
4.5.2. Gelatinase Activity and Hemolysis
The gelatinase activity and hemolytic capacity of BP+ enterococci strains were determined as reported previously [58], using TSA supplemented with 3% skim milk and blood agar, respectively.
4.5.3. Whole Genome Sequencing (WGS) Analysis
DNA from BP+ enterococci was extracted using a DNeasy Blood & Tissue Kit (QIAGEN, Hilden, Germany), following the manufacturer’s instructions for gram-positive bacteria. The DNA was subjected to WGS using an Illumina sequencing system at the Hospital Center of University Laval (CHUL). Data were analyzed using the following programs; fastp for trimming and quality check of the trimming [59], SPAdes for the assembly [60], QUAS for checking the assembled quality [61], and prokka for annotation [62]. Anti-SMASH 6.0 [63] and BAGEL4 [64] were used to detect genes encoding bacteriocins. ResFinder 4.1 [65,66,67] was used to detect genes associated with antibiotic resistance and mutations in the pbps, parC, and gyrA genes. VirulenceFinder 2.0 was used to detect virulence factors [67,68,69] and PlasmidFinder 2.1 for plasmid detection [67,70]. Multi-locus sequence typing (MLST) was performed using MLST 2.0 [71,72,73,74,75,76]. Representation in the phylogenetic tree was performed using R version 4.2.1 [77], and the phylogenetic distances were calculated using the average nucleotide identity (ANI) method.
5. Conclusions
Among the 12 enterococci that showed inhibitory activity against C. perfringens, the strains E. faecium X2893 and X2906 seem to be the most promising candidates for use as protective cultures in poultry farming. Both strains belong to the sequence type ST722 and harbor enterocin A and Enterocin B genetic determinants. These strains also do not have acquired resistance genes, do not carry plasmids, and only carry the acm gene, which is implicated in host colonization and might be a desirable feature for protective strains. Both are gelatinase-negative and gamma-hemolytic.
The strains derived from other origins (milk and camel milk) and belonging to the species E. durans might be also good candidates as protective cultures, as they do not harbor any virulence factors or resistance genes, and they produce bacteriocins. However, these strains carry more than one plasmid and have not been isolated from poultry.
Concluding, E. faecium X2893 and X2906 showed potential to be considered in further studies as protective cultures in poultry farming, a promising alternative to antibiotic use in this sector.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12020231/s1, File S1: Supplemented material on mutations in pbp5, gyrA and parC found in the 12 BP+ enterococci analyzed by WGS.
Author Contributions
Conceptualization, C.T. and I.F.; Formal analysis, C.T. and I.F.; Investigation, S.G.-V., L.B.S., R.F.-F. and H.B.Y.; Methodology, S.G.-V. and K.B.S.; Software, S.G.-V., S.S. and R.G.; Supervision, C.T. and I.F.; Validation, C.T. and I.F.; Visualization, C.T.; Writing—original draft, S.G.-V., C.T. and I.F.; Writing—review & editing, S.G.-V., L.B.S., S. S., R.G., R.F.-F., H.B.Y., K.B.S., C.T. and I.F. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the financial support received for this work from the International Development Research Center (IDRC). Sara García-Vela has a predoctoral fellowship financed by IDRC. Rosa Fernández-Fernández has a predoctoral fellowship, FPU, from the Ministerio de Ciencia, Innovación y Universidades of Spain (FPU18/05438).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, F.; Wang, Y.; Wan, Z.; Shao, H.; Qian, K.; Ye, J.; Qin, A. Generation of a recombinant chickenized monoclonal antibody against the neuraminidase of H9N2 avian influenza virus. AMB Express 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Gochez, D.; Moulin, G.; Erlacher-Vindel, E. OIE Annual Report on Antimicrobial Agents Intended for Use in Animals: Methos Used. Front. Vet. Sci. 2021, 6, 317. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J. Antimicrobial resistance: A one health perspective. Microbiol. Spectr. 2018, 6, 10. [Google Scholar] [CrossRef]
- Mora, Z.V.-d.l.; Macías-Rodríguez, M.E.; Arratia-Quijada, J.; Gonzalez-Torres, Y.S.; Nuño, K.; Villarruel-López, A. Clostridium perfringens as foodborne pathogen in broiler production: Pathophysiology and potential strategies for controlling necrotic enteritis. Animals 2020, 10, 1718. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Shojadoost, B.; Boodhoo, N.; Astill, J.; Taha-Abdelaziz, K.; Hodgins, D.C.; Kulkarni, R.R.; Sharif, S. Necrotic enteritis in chickens: A review of pathogenesis, immune responses and prevention, focusing on probiotics and vaccination. Anim. health Res. Rev. 2021, 22, 147–162. [Google Scholar] [CrossRef]
- Immerseel, F.V.; Buck, J.D.; Pasmans, F.; Huyghebaert, G.; Haesebrouck, F.; Ducatelle, R. Clostridium perfringens in poultry: An emerging threat for animal and public health. Avian. Pathol. 2004, 33, 537–549. [Google Scholar] [CrossRef]
- Agunos, A.; Pierson, F.W.; Lungu, B.; Dunn, P.A.; Tablante, N. Review of nonfoodborne zoonotic and potentially zoonotic poultry diseases. Avian. Dis. 2016, 60, 553–575. [Google Scholar] [CrossRef]
- Young, N.; O’sullivan, G. The influence of ingredients on product stability and shelf life. In Food and Beverage Stability and Shelf Life; Elsevier: Amsterdam, The Netherlands, 2011; pp. 132–183. [Google Scholar]
- Braiek, O.B.; Smaoui, S. Enterococci: Between emerging pathogens and potential probiotics. Biomed. Res. Int. 2019, 2019, 5938210. [Google Scholar]
- Franz, C.M.; Van Belkum, M.J.; Holzapfel, W.H.; Abriouel, H.; Gálvez, A. Diversity of enterococcal bacteriocins and their grouping in a new classification scheme. FEMS Microbiol. Rev. 2007, 31, 293–310. [Google Scholar] [CrossRef]
- Silva, C.C.; Silva, S.P.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef]
- Hammami, R.; Fliss, I.; Corsetti, A. Application of protective cultures and bacteriocins for food biopreservation. Front. Microbiol. 2019, 10, 1561. [Google Scholar] [CrossRef] [PubMed]
- Hanchi, H.; Mottawea, W.; Sebei, K.; Hammami, R. The genus Enterococcus: Between probiotic potential and safety concerns—An update. Front. Microbiol. 2018, 9, 1791. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, M.S.; Clewell, D.B.; Ike, Y.; Shankar, N. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Multiple bacteriocin production in lactic acid bacteria. J. Biosci. Bioeng. 2022, 134, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, antimicrobial peptides from bacterial origin: Overview of their biology and their impact against multidrug-resistant bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef] [PubMed]
- Cintas, L.M.; Casaus, P.; Håvarstein, L.S.; Hernandez, P.E.; Nes, I.F. Biochemical and genetic characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl. Environ. Microbiol. 1997, 63, 4321–4330. [Google Scholar] [CrossRef]
- Cintas, L.M.; Casaus, P.; Herranz, C.; Håvarstein, L.S.; Holo, H.; Hernández, P.E.; Nes, I.F. Biochemical and genetic evidence that Enterococcus faecium L50 produces enterocins L50A and L50B, the sec-dependent enterocin P, and a novel bacteriocin secreted without an N-terminal extension termed enterocin Q. J. Bacteriol. 2000, 182, 6806–6814. [Google Scholar] [CrossRef] [PubMed]
- Criado, R.; Gutiérrez, J.; Budin-Verneuil, A.; Hernandez, P.; Hartke, A.; Cintas, L.; Auffray, Y.; Benachour, A. Molecular analysis of the replication region of the pCIZ2 plasmid from the multiple bacteriocin producer strain Enterococcus faecium L50. Plasmid 2008, 60, 181–189. [Google Scholar] [CrossRef]
- Abriouel, H.; Ben Omar, N.; Lucas, R.; Martínez-Cañamero, M.; Galvez, A. Bacteriocin production, plasmid content and plasmid location of enterocin P structural gene in enterococci isolated from food sources. Lett. Appl. Microbiol. 2006, 42, 331–337. [Google Scholar] [CrossRef]
- Ness, I.F.; Diep, D.B.; Ike, Y. Enterococcal Bacteriocins and Antimicrobial Proteins that Contribute to Niche Control; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Cintas, L.M.; Casaus, P.; Holo, H.; Hernandez, P.E.; Nes, I.F.; Håvarstein, L.S. Enterocins L50A and L50B, two novel bacteriocins from Enterococcus faecium L50, are related to staphylococcal hemolysins. J. Bacteriol. 1998, 180, 1988–1994. [Google Scholar] [CrossRef]
- Criado, R.; Gutiérrez, J.; Martín, M.; Herranz, C.; Hernández, P.E.; Cintas, L.M. Immunochemical characterization of temperature-regulated production of enterocin L50 (EntL50A and EntL50B), enterocin P, and enterocin Q by Enterococcus faecium L50. Appl. Environ. Microbiol. 2006, 72, 7634–7643. [Google Scholar] [CrossRef]
- Inoue, T.; Tomita, H.; Ike, Y. Bac 32, a novel bacteriocin widely disseminated among clinical isolates of Enterococcus faecium. Antimicrob. Agents Chemoter. 2006, 50, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, E.; Cai, Y.; Marchioni, E.; Ennahar, S. Genetic identification of the bacteriocins produced by Enterococcus faecium IT62 and evidence that bacteriocin 32 is identical to enterocin IT. Antimicrob. Agents Chemoter. 2009, 53, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Hanchi, H.; Hammami, R.; Fernandez, B.; Kourda, R.; Ben Hamida, J.; Fliss, I. Simultaneous production of formylated and nonformylated enterocins L50A and L50B as well as 61A, a new glycosylated durancin, by Enterococcus durans 61A, a strain isolated from artisanal fermented milk in Tunisia. J. Agric. Food Chem. 2016, 64, 3584–3590. [Google Scholar] [CrossRef]
- Hanchi, H.; Hammami, R.; Gingras, H.; Kourda, R.; Bergeron, M.G.; Ben Hamida, J.; Ouellette, M.; Fliss, I. Inhibition of MRSA and of Clostridium difficile by durancin 61A: Synergy with bacteriocins and antibiotics. Future Microbiol. 2017, 12, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Aymerich, T.; Holo, H.; Håvarstein, L.S.; Hugas, M.; Garriga, M.; Nes, I.F. Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl. Environ. Microbiol. 1996, 62, 1676–1682. [Google Scholar] [CrossRef]
- Casaus, P.; Nilsen, T.; Cintas, L.M.; Nes, I.F.; Hernández, P.E.; Holo, H. Enterocin B, a new bacteriocin from Enterococcus faecium T136 which can act synergistically with enterocin A. Microbiology 1997, 143, 2287–2294. [Google Scholar] [CrossRef]
- Ankaiah, D.; Palanichamy, E.; Antonyraj, C.B.; Ayyanna, R.; Perumal, V.; Ahamed, S.I.B.; Arul, V. Cloning, overexpression, purification of bacteriocin enterocin-B and structural analysis, interaction determination of enterocin-A, B against pathogenic bacteria and human cancer cells. Int. J. Biol. Macromol. 2018, 116, 502–512. [Google Scholar] [CrossRef]
- Ishibashi, N.; Himeno, K.; Fujita, K.; Masuda, Y.; Perez, R.H.; Zendo, T.; Wilaipun, P.; Leelawatcharamas, V.; Nakayama, J.; Sonomoto, K. Purification and characterization of multiple bacteriocins and an inducing peptide produced by Enterococcus faecium NKR-5-3 from Thai fermented fish. Biosci. Biotechnol. Biochem. 2012, 76, 947–953. [Google Scholar] [CrossRef]
- Himeno, K.; Rosengren, K.J.; Inoue, T.; Perez, R.H.; Colgrave, M.L.; Lee, H.S.; Chan, L.Y.; Henriques, S.n.T.; Fujita, K.; Ishibashi, N. Identification, characterization, and three-dimensional structure of the novel circular bacteriocin, enterocin NKR-5-3B, from Enterococcus faecium. Biochemistry 2015, 54, 4863–4876. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, N.; Matsumoto, N.; Perez, R.H.; Iwatani, S.; Sugino, H.; Zendo, T.; Wilaipun, P.; Leelawatcharamas, V.; Nakayama, J.; Sonomoto, K. Molecular characterization of the possible regulation of multiple bacteriocin production through a three-component regulatory system in Enterococcus faecium NKR-5-3. J. Biosci. Bioeng. 2021, 131, 131–138. [Google Scholar] [CrossRef]
- Ishibashi, N.; Himeno, K.; Masuda, Y.; Perez, R.H.; Iwatani, S.; Zendo, T.; Wilaipun, P.; Leelawatcharamas, V.; Nakayama, J.; Sonomoto, K. Gene cluster responsible for secretion of and immunity to multiple bacteriocins, the NKR-5-3 enterocins. Appl. Environ. Microbiol. 2014, 80, 6647–6655. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Kaminaka, K.; Shima, J.; KAwAMoTo, S.; MoRI, K.; Choi, S.-H.; Doi, K.; OHMoMo, S.; Ogata, S. Isolation and characterization of enterocin SE-K4 produced by thermophilic enterococci, Enterococcus faecalis K-4. Biosci. Biotechnol. Biochem. 2001, 65, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Navaratna, M.A.; Sahl, H.-G.; Tagg, J.R. Two-component anti-Staphylococcus aureus lantibiotic activity produced by Staphylococcus aureus C55. Appl. Environ. Microbiol. 1998, 64, 4803–4808. [Google Scholar] [CrossRef]
- Kawada-Matsuo, M.; Shammi, F.; Oogai, Y.; Nakamura, N.; Sugai, M.; Komatsuzawa, H. C55 bacteriocin produced by ETB—plasmid positive Staphylococcus aureus strains is a key factor for competition with S. aureus strains. Microbiol. Immunol. 2016, 60, 139–147. [Google Scholar] [CrossRef]
- Portillo, A.; Ruiz-Larrea, F.; Zarazaga, M.; Alonso, A.; Martinez, J.L.; Torres, C. Macrolide resistance genes in Enterococcus spp. Antimicrob. Agents Chemoter. 2000, 44, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, R.; Galán, J.C.; Tenorio, C.; Ruiz-Garbajosa, P.; Zarazaga, M.; Torres, C.; Baquero, F. New aac(6′)-I genes in Enterococcus hirae and Enterococcus durans: Effect on β-lactam/aminoglycoside synergy. J. Antimicrob. Chemother. 2005, 55, 1053–1055. [Google Scholar] [CrossRef]
- Miller, W.R.; Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance in enterococci. Expert. Rev. Anti Infect. Ther. 2014, 12, 1221–1236. [Google Scholar] [CrossRef]
- Fontana, R.; Ligozzi, M.; Pittaluga, F.; Satta, G. Intrinsic penicillin resistance in enterococci. Microb. Drug Resist. 1996, 2, 209–213. [Google Scholar] [CrossRef]
- Hollenbeck, B.L.; Rice, L.B. Intrinsic and acquired resistance mechanisms in Enterococcus. Virulence 2012, 3, 421–569. [Google Scholar] [CrossRef]
- Poeta, P.; Costa, D.; Igrejas, G.; Saenz, Y.; Zarazaga, M.; Rodrigues, J.; Torres, C. Polymorphisms of the pbp5 gene and correlation with ampicillin resistance in Enterococcus faecium isolates of animal origin. J. Med. Microbiol. 2007, 56, 236–240. [Google Scholar] [CrossRef]
- López, M.; Tenorio, C.; Del Campo, R.; Zarazaga, M.; Torres, C. Characterization of the mechanisms of fluoroquinolone resistance in vancomycin-resistant enterococci of different origins. J. Chemother. 2011, 23, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Cercenado, E. Enterococcus: Resistencias fenotípicas y genotípicas y epidemiología en España. Enferm. Infecc. Microbiol. Clin. 2011, 29, 59–65. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. The ecology, epidemiology and virulence of Enterococcus. Microbiology 2009, 155, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, M.S.; Clewell, D.B.; Courvalin, P.; Dunny, G.M.; Murray, B.E.; Rice, L.B. The enterococci: Pathogenesis, Molecular Biology, and Antibiotic Resistance; ASM Press: Washington, DC, USA, 2002; Volume 10. [Google Scholar]
- Nakayama, J.; Kariyama, R.; Kumon, H. Description of a 23.9-kilobase chromosomal deletion containing a region encoding fsr genes which mainly determines the gelatinase-negative phenotype of clinical isolates of Enterococcus faecalis in urine. Appl. Environ. Microbiol. 2002, 68, 3152–3155. [Google Scholar] [CrossRef] [PubMed]
- Shankar, N.; Baghdayan, A.S.; Gilmore, M.S. Modulation of virulence within a pathogenicity island in vancomycin-resistant Enterococcus faecalis. Nature 2002, 417, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Eaton, T.J.; Gasson, M.J. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl. Environ. Microbiol. 2001, 67, 1628–1635. [Google Scholar] [CrossRef]
- Nallapareddy, S.R.; Singh, K.V.; Okhuysen, P.C.; Murray, B.E. A functional collagen adhesin gene, acm, in clinical isolates of Enterococcus faecium correlates with the recent success of this emerging nosocomial pathogen. Infect. Immun. 2008, 76, 4110–4119. [Google Scholar] [CrossRef]
- Krawczyk, B.; Wityk, P.; Gałęcka, M.; Michalik, M. The many faces of Enterococcus spp.—Commensal, probiotic and opportunistic pathogen. Microorganisms 2021, 9, 1900. [Google Scholar] [CrossRef]
- Poeta, P.; Costa, D.; Rojo-Bezares, B.; Zarazaga, M.; Klibi, N.; Rodrigues, J.; Torres, C. Detection of antimicrobial activities and bacteriocin structural genes in faecal enterococci of wild animals. Microbiol. Res. 2007, 162, 257–263. [Google Scholar] [CrossRef]
- Bennett, S.; Ben Said, L.; Lacasse, P.; Malouin, F.; Fliss, I. Susceptibility to nisin, bactofencin, pediocin and reuterin of multidrug resistant Staphylococcus aureus, Streptococcus dysgalactiae and Streptococcus uberis causing bovine mastitis. Antibiotics 2021, 10, 1418. [Google Scholar] [CrossRef]
- Lo Verso, L.; Lessard, M.; Talbot, G.; Fernandez, B.; Fliss, I. Isolation and selection of potential probiotic bacteria from the pig gastrointestinal tract. Probiotics Antimicrob. Proteins 2018, 10, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Biron, E.; Ben Said, L.; Subirade, M.; Fliss, I. Bacteriocin-Based Synergetic Consortia: A Promising Strategy to Enhance Antimicrobial Activity and Broaden the Spectrum of Inhibition. Microbiol. Spectr. 2022, 10, e00406–e00421. [Google Scholar] [CrossRef]
- Humphries, R.; Bobenchik, A.M.; Hindler, J.A.; Schuetz, A.N. Overview of changes to the clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing, M100. Clin. Microbiol. Newsl. 2021, 59, e00213–e00221. [Google Scholar] [CrossRef]
- Poeta, P.; Costa, D.; Klibi, N.; Rodrigues, J.; Torres, C. Phenotypic and Genotypic Study of Gelatinase and β--Haemolysis Activities in Faecal Enterococci of Poultry in Portugal. J. Vet. Med. 2006, 53, 203–208. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; Van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. Spec. Publ. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- van Heel, A.J.; de Jong, A.; Song, C.; Viel, J.H.; Kok, J.; Kuipers, O.P. BAGEL4: A user-friendly web server to thoroughly mine RiPPs and bacteriocins. Nucleic Acids Res. Spec. Publ. 2018, 46, W278–W281. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Zankari, E.; Allesøe, R.; Joensen, K.G.; Cavaco, L.M.; Lund, O.; Aarestrup, F.M. PointFinder: A novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J. Antimicrob. Chemother. 2017, 72, 2764–2768. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. Clin. Microbiol. Newsl. 2014, 52, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Malberg Tetzschner, A.M.; Johnson, J.R.; Johnston, B.D.; Lund, O.; Scheutz, F. In silico genotyping of Escherichia coli isolates for extraintestinal virulence genes by use of whole-genome sequencing data. Clin. Microbiol. Newsl. 2020, 58, e01220–e01269. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemoter. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Pontén, T.; Ussery, D.W.; Aarestrup, F.M. Multilocus sequence typing of total-genome-sequenced bacteria. Clin. Microbiol. Newsl. 2012, 50, 1355–1361. [Google Scholar] [CrossRef]
- Bartual, S.G.; Seifert, H.; Hippler, C.; Luzon, M.A.D.n.; Wisplinghoff, H.; Rodríguez-Valera, F. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. Clin. Microbiol. Newsl. 2005, 43, 4382–4390. [Google Scholar] [CrossRef]
- Griffiths, D.; Fawley, W.; Kachrimanidou, M.; Bowden, R.; Crook, D.W.; Fung, R.; Golubchik, T.; Harding, R.M.; Jeffery, K.J.; Jolley, K.A. Multilocus sequence typing of Clostridium difficile. Clin. Microbiol. Newsl. 2010, 48, 770–778. [Google Scholar] [CrossRef]
- Lemee, L.; Dhalluin, A.; Pestel-Caron, M.; Lemeland, J.-F.; Pons, J.-L. Multilocus sequence typing analysis of human and animal Clostridium difficile isolates of various toxigenic types. Clin. Microbiol. Newsl. 2004, 42, 2609–2617. [Google Scholar] [CrossRef]
- Wirth, T.; Falush, D.; Lan, R.; Colles, F.; Mensa, P.; Wieler, L.H.; Karch, H.; Reeves, P.R.; Maiden, M.C.; Ochman, H. Sex and virulence in Escherichia coli: An evolutionary perspective. Mol. Microbiol. 2006, 60, 1136–1151. [Google Scholar] [CrossRef] [PubMed]
- Jaureguy, F.; Landraud, L.; Passet, V.; Diancourt, L.; Frapy, E.; Guigon, G.; Carbonnelle, E.; Lortholary, O.; Clermont, O.; Denamur, E. Phylogenetic and genomic diversity of human bacteremic Escherichia coli strains. BMC Genom. 2008, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).