Abstract
Carbapenem-resistant Enterobacterales (CRE) species are top priority pathogens according to the World Health Organization. Rapid detection is necessary and useful for their surveillance and control globally. This study developed a multiplex polymerase chain reaction (mPCR) detection of the common carbapenemase genes NDM, KPC, and OXA-48-like, together with identification of Escherichia coli, and distinguished a Klebsiella pneumoniae complex to be K. pneumoniae, K. quasipneumoniae, and K. variicola. Of 840 target Enterobacterales species, 190 E. coli, 598 K. pneumoniae, 28 K. quasipneumoniae, and 23 K. variicola. with and without NDM, KPC, or OXA-48-like were correctly detected for their species and carbapenemase genes. In contrast, for the Enterobacterales species other than E. coli or K. pneumoniae complex with carbapenemase genes, the mPCR assay could detect only NDM, KPC, or OXA-48-like. This PCR method should be useful in clinical microbiology laboratories requiring rapid detection of CRE for epidemiological investigation and for tracking the trends of carbapenemase gene dynamics.
Keywords:
PCR; carbapenemase; NDM; KPC; OXA-48-like; Escherichia coli; Klebsiella pneumoniae complex 1. Introduction
The current emergence of carbapenem-resistant Enterobacterales (CRE) is an especially concerning antimicrobial resistant (AMR) threat that can result in an important clinical problem associated with resistance to many last-resort antibiotics, making it difficult to treat and leading to high mortality rates and expensive hospital stays [1]. The World Health Organization (WHO) considers the growing AMR issue one of the three major public health challenges of the 21st century, responsible for healthcare costs, long hospitalizations, treatment failures, and death [2]. In addition, the WHO has listed CRE as critical priority pathogens, necessitating the development of new antibiotics against such organisms [3]. The Enterobacterales, especially Escherichia coli, Klebsiella pneumoniae, and Enterobacter cloacae, are major causes of hospital-acquired infections that have spread around the globe [1,4].
CRE develops antibiotic resistance through several mechanisms, including through the main mechanism of production of carbapenemases which are enzymes that degrade carbapenem antibiotics [4,5]. They can be divided into CRE with and CRE without carbapenemase production [1,4,5]. Carbapenemase-producing CRE (CP-CRE) are more associated with higher levels of antimicrobial resistance, worse outcomes, and rapid spread via plasmid transmission among bacterial strains, while non-carbapenemase-producing CRE (non-CP-CRE) have been associated with asymptomatic carriage and perhaps less person-to-person transmission [1,6]. Among the carbapenemase types, NDM, OXA-48-like, IMP, VIM, and KPC have been commonly found in different parts of the world [2,4,5]. KPC is the most common carbapenemase in the United States, China, Italy, Greece, and the UK, whereas NDM has now spread worldwide [4,5,7]. VIM and IMP are most prevalent in Southern Europe and Asia, while OXA-48-like is most prevalent in the Mediterranean region, Europe, and Asia, including India, China, Vietnam, and Thailand [4,5,6,7]. Of these carbapenemase types, NDM, KPC, and OXA-48-like have been very frequently detected worldwide [4,5,7]. Furthermore, the major Enterobacterales carriers of these carbapenemases are E. coli and K. pneumoniae [4,5,6].
Surveillance is a crucial element of national prevention and control strategies to control dissemination of CP-CRE. Several countries have systems to monitor acquired carbapenem resistance [8]. For example, diagnostic laboratories in England have a duty to report carbapenemase-producing Gram-negative bacteria isolated from human samples to Public Health England [8]. Therefore, rapid detection and reporting of CP-CRE is one of the top priorities of clinical microbiology laboratories. Recently, there have been increasing numbers of tests available for carbapenemase detection, including colorimetric tests, immunochromatographic assays, matrix-assisted laser desorption ionization-time of flight MS-based tests, phenotypic and molecular methods [9,10]. However, the Clinical and Laboratory Standards Institute (CLSI) recommends the modified carbapenem inactivation method (mCIM), Carba NP, and molecular assay, such as PCR, to determine CP-CRE [11].
PCR is a technique with high accuracy and a rapid turnaround time that is currently inexpensive. Several studies have developed a multiplex PCR (mPCR) to detect the relevant carbapenemase genes [12,13,14,15,16]. However, there is no current PCR approach that detects both carbapenemase genes and Enterobacterales species in the same reaction. Herein, we developed an mPCR assay to detect the most prevalent carbapenemase genes, including KPC, NDM, and OXA-48-like, and identify E. coli, K. pneumoniae, K. quasipneumoniae, and K. variicola in the same reaction. This mPCR technique could be applied usefully in clinical diagnostic laboratories to determine both common CRE species and the relevant carbapenemase genes at the same time, as well as reducing the turnaround time and saving on costs.
2. Results and Discussion
In the current study, an mPCR assay was designed to detect common carbapenemase genes and, frequently, the Enterobacterales species carrying those genes. E. coli and K. pneumoniae complex (K. pneumoniae, K. quasipneumoniae, K. variicola), NDM, OXA-48-like, and KPC, which are common carbapenemase genes disseminated globally, were the target for this mPCR assay. According to several studies, K. pneumoniae- and E. coli-carrying NDM, KPC, and OXA-48-like, were prevalent in the Netherlands, Spain, the USA, Thailand, China, Korea, Tunisia, Iran, Nepal, and India [6,17,18,19,20,21,22,23,24,25,26,27]. This assay could be applied in these countries, especially in Asia, where there are high prevalence levels of these bacteria carrying the aforementioned carbapenemase genes.
We demonstrated the potential utility of the mPCR assay to detect either E. coli or K. pneumoniae complex with and without carbapenemase genes. The mPCR assay identified the E. coli and K. pneumoniae complex together with detected NDM, OXA-48-like, and KPC in the same reaction (Figure 1 and Table 1). Our mPCR assay could distinguish the K. pneumoniae complex into K. pneumoniae, K. quasipneumoniae, and K. variicola (Figure 1). In the case of E. coli, K. pneumoniae, K. quasipneumoniae, and K. variicola without carbapenemase genes, the mPCR assay could identify the species correctly with no carbapenemase genes NDM, OXA-48-like, or KPC (Table 1). In addition, the mPCR assay detected NDM, KPC, or OXA-48-like in C. fruendii, C. werkmanii, E. cloacae, and E. asburiae, without bands of E. coli or the K. pneumoniae complex (Table 1). A limitation of this mPCR assay is that it could not identify C. freundii, E. cloacae, E. asburaie, and Enterobacterales species other than the 4 mPCR target organisms. The expansion of this mPCR to detect other Enterobacterales species should be the subject of future investigations. In addition, no cross reactivity was observed in other bacteria except the target bacteria and carbapenemase genes.
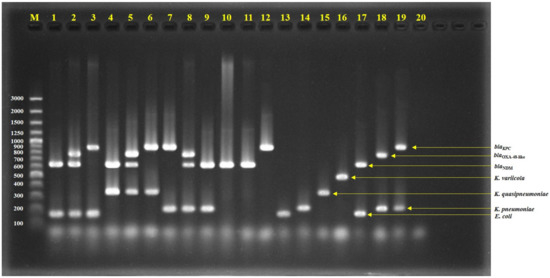
Figure 1.
Agarose gel electrophoresis of multiplex PCR-amplified products; lane 1 = Isolate No. C76 (E. coli with NDM), lane 2 = Isolate No. C163 (E. coli with NDM and OXA-48-like), lane 3 = Isolate No. C1992 (E. coli with KPC), lane 4 = Isolate No. C34 (K. quasipneumoniae with NDM), lane 5 = Isolate No. C110 (K. quasipneumoniae with NDM and OXA-48-like), lane 6 = Isolate No. AMR353 (K. quasipneumoniae with KPC), lane 7 = Isolate No. C1985 (K. pneumoniae with KPC), lane 8 = Isolate No. C73 (K. pneumoniae with NDM and OXA-48-like), lane 9 = Isolate No. C75 (K. pneumoniae with NDM), lane 10 = Isolate No. C19 (E. cloacae with NDM), lane 11 = Isolate No. C487 (C. freundii with NDM), lane 12 = Isolate No. C2135 (E. asburiae with KPC), lane 13 = E. coli ATCC25922, lane 14 = K. pneumoniae ATCC27335, lane 15 = K. quasipneumoniae ATCC700603, lane 16 = K. variicola ATCC-BAA830, lane 17 = E. coli ATCC-BAA2469 (contain NDM), lane 18 = K. pneumoniae ATCC-BAA2524 (contain OXA-48), lane 19 = K. pneumoniae ATCC-BAA1705 (contain KPC), lane 20 = negative control, and lane M = 100 bp DNA ladder.

Table 1.
Enterobacterales species used in this study and mPCR results.
In addition, we also tried to use the mPCR to directly detect the E. coli and K. pneumoniae complex together with detected NDM, OXA-48-like, and KPC from pure colonies. As shown in Figure 2, the colony PCR has successfully detected representative strains. This is an alternative method for laboratories because it is not necessary to purify DNA from bacteria growths, and shortens the time and renders unnecessary the equipment required for DNA purification.
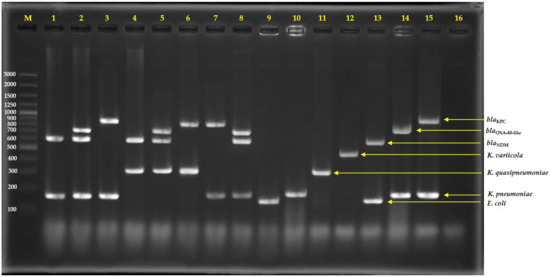
Figure 2.
Agarose gel electrophoresis of multiplex PCR-amplified products from direct colonies; lane 1 = Isolate No. C76 (E. coli with NDM), lane 2 = Isolate No. C163 (E. coli with NDM and OXA-48-like), lane 3 = Isolate No. C1992 (E. coli with KPC), lane 4 = Isolate No. C34 (K. quasipneumoniae with NDM), lane 5 = Isolate No. C110 (K. quasipneumoniae with NDM and OXA-48-like), lane 6 = Isolate No. AMR353 (K. quasipneumoniae with KPC), lane 7 = Isolate No. C1985 (K. pneumoniae with KPC), lane 8 = Isolate No. C73 (K. pneumoniae with NDM and OXA-48-like), lane 9 = E. coli ATCC25922, lane 10 = K. pneumoniae ATCC27335, lane 11 = K. quasipneumoniae ATCC700603, lane 12 = K. variicola ATCC-BAA830, lane 13 = E. coli ATCC-BAA2469 (contain NDM), lane 14 = K. pneumoniae ATCC-BAA2524 (contain OXA-48), lane 15 = K. pneumoniae ATCC-BAA1705 (contain KPC), lane 16 = negative control, and lane M = 100 bp DNA ladder.
The spread of CP-CRE is a global threat to public health. Among CP-CREs, the carbapenemase genes, NDM, OXA-48-like, and KPC, are widely spread globally, as mentioned above. Several phenotypic and genotypic techniques have been applied to detect CRE and CP-CRE [28]. It is vital to diagnose CP-CRE early to undertake the appropriate measures to prevent transmission and to help in implementing effective countermeasures at an appropriate time to initiate early treatment interventions. In addition, the plasmids harboring carbapenemase genes can be transmitted easily among patients. Therefore, a method which can search for genetically diverse CP-CRE isolates would be useful in terms of epidemiological investigation and in tracking the trends of carbapenemase gene dynamics.
PCR is an ideal tool that saves on costs, is rapid, and can identify the type of carbapenemase genes to trace epidemiological dynamics, be proactive in the control and prevention of spread, and provide prompt treatment [12,13,14,15,16]. However, the currently available PCR-detectable carbapenemase genes have not identified the bacterial species [12,13,14,15,16]. Our mPCR assay allows for the rapid testing of common carbapenemase genes (NDM, KPC, OXA-48-like) together with identification of clinical E. coli and K. pneumoniae complex isolates that frequently harbor these genes; furthermore, the assay does not require prior phenotypical characterization, thus constituting a rapid and valuable tool in the management of infections in hospitals. However, this mPCR has limitations due to the fact that it does not detect IMP genes. The IMP gene is more prevalent in Asia and the South Pacific region than other continents [29]. Therefore, future research directions should include developing this mPCR to detect IMP genes.
3. Materials and Methods
3.1. Bacterial Strains
As shown in Table 1, this study included 780 Enterobacterales strains with known species and carbapenemase genes based on whole-genome sequencing from another study [26,30]: 578 K. pneumoniae, 16 K. quasipneumoniae, 171 E. coli, 6 Enterobacter cloacae, 1 Enterobacter asburiae, 5 Citrobacter werkmanii, and 3 Citrobacter freundii.
An additional 159 Enterobacterales species without carbapenemase genes including 20 E. coli, 20 K. pneumoniae, 10 K. oxytoca, 23 K. variicola, 12 K. quasipneumoniae, 20 K. aerogenes, 14 E. cloacae, 10 Serratia marcescens, 20 Salmonella enterica, and 10 C. freundii, were identified at the species level using conventional biochemical tests; PCR-detected carbapenemase genes described elsewhere were included in the current study [31,32,33]. These bacterial isolates were resistant to either carbapenems or cephalosporins.
We also included reference strains of other bacterial species to evaluate possible non-specific reactions. These strains consisted of: Achromobacter xylosoxidans ATCC27061, Pseudomonas aeruginosa ATCC9027, Acinetobacter baumannii ATCC19606, Burkholderia cepacia LMG0122, Haemophilus influenzae ATCC10211, Elizabethkingia meningoseptica ATCC13253, Micrococcus luteus ATCC10240, Bacillus subtilis ATCC6633, Staphylococcus aureus ATCC700698, Enterococcus faecalis ATCC29212, Streptococcus pneumoniae ATCC33400, Leuconostoc lactis ATCC19256, and Listeria monocytogenes ATCC7644. In addition, E. coli ATCC-BAA2469 (NDM-1), K. pneumoniae ATCC-BAA2524 (OXA-48), K. pneumoniae ATCC-BAA1705 (KPC), K. quasipneumoniae ATCC700603, and K. variicola ATCC-BAA830 were used for PCR reaction control.
The bacterial stains from stock at −80 °C were cultured overnight on sheep blood agar and incubated at 37 °C before performing the DNA extraction.
3.2. Primer Design
As shown in Table 2, only primers for NDM and E. coli were designed in the current study. The NDM and E. coli uidA were retrieved from GenBank under accession numbers NC_023908 and S69414, respectively. These sequences were used as the templates for design of primers by using Primer-BLAST program (http://www.ncbi.nlm.nih.gov/tools/primer-blast/; accessed on 9 December 2022).

Table 2.
Primers used for detection of antibiotic resistance genes.
3.3. Multiplex PCR
Bacterial genomic DNA samples were extracted using ZymoBIOMICS DNA Kits (Zymo Research, CA, USA), following the manufacturer’s instruction. The PCR reaction mixture (25 µL) contained a 1× JumpStart REDTaq ReadyMix (Sigma) and each primer (Table 2). The list of primers used in the mPCR are also presented in Table 2 [34,35,36,37]. The PCR reaction was performed as follows: initial activation of DNA polymerase at 95 °C for 3 min, plus 35 cycles of denaturation at 95 °C for 30 sec, primer annealing and extension at 62.5 °C for 1.30 min, and a final extension at 72 °C for 5 min. A negative control was included in each run, consisting of the same reaction mixture but with water instead of template DNA.
The PCR products (5 µL) were analyzed using gel electrophoresis on 1.5% (w/v) agarose gel in 0.5 × TBE buffer at a constant voltage of 100 V for 30 min (Mupid exU system; Takara; Tokyo, Japan). The gels were stained with ethidium bromide and visualized under ultraviolet light (GeneGenius Bioimaging System; SynGene; Cambridge, UK). The sizes of the PCR products were determined by comparison with molecular-sized standards (GeneRuler™ 100 bp Plus DNA ladder; Thermo Fisher Scientific; Vilnius, Lithuania).
3.4. Colony PCR
Bacteria were cultured on Tryptic Soy Agar for 18 h at 37 °C. A small amount of pure colony was picked up using a toothpick and then suspended in the PCR reaction mixture of each tube. The PCR reaction and gel electrophoresis were done as described above.
4. Conclusions
The mPCR assay developed in the current study could detect NDM, KPC, and OXA-48-like together with distinguishing E. coli, K. pneumoniae, K. quasipneumoniae, and K. variicola in a single reaction. It should be useful for clinical microbiology laboratories requiring the rapid detection of CRE as one of the critical priority pathogens according to the WHO. In addition, the developed assay should be useful for epidemiological investigation and tracking the trends of carbapenemase gene dynamics.
Author Contributions
Conceptualization, R.H. and A.K.; methodology, R.H.; validation, A.K.; formal analysis, R.H. and P.C.; resources, R.H. and A.K.; writing—original draft preparation, R.H., P.C. and P.B.; writing—review and editing, R.H. and A.K.; supervision, A.K.; funding acquisition, R.H. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Kasetsart University, Bangkok, Thailand under the research program of Establishment of Knowledge and Innovation Products for Detection of Antimicrobial Resistant Bacteria.
Institutional Review Board Statement
Not applicable because this study did not involve humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The Kasetsart University Research and Development Institute (KURDI) provided English editing and the article processing charge (APC).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Suay-García, B.; Pérez-Gracia, M.T. Present and Future of Carbapenem-resistant Enterobacteriaceae (CRE) Infections. Antibiotics 2019, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Neidhöfer, C.; Buechler, C.; Neidhöfer, G.; Bierbaum, G.; Hannet, I.; Hoerauf, A.; Parčina, M. Global Distribution Patterns of Carbapenemase-Encoding Bacteria in a New Light: Clues on a Role for Ethnicity. Front. Cell Infect. Microbiol. 2021, 11, 659753. [Google Scholar] [CrossRef]
- World Health Organization WHO Priority Pathogens List for R&D of New Antibiotics. 2017. Available online: http://www.who.int/bulletin/volumes/94/9/16-020916.pdf (accessed on 20 November 2022).
- Logan, L.K.; Weinstein, R.A. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.T. Continuous Evolution: Perspective on the Epidemiology of Carbapenemase Resistance among Enterobacterales and Other Gram-Negative Bacteria. Infect. Dis. Ther. 2021, 10, 75–92. [Google Scholar] [CrossRef]
- Paveenkittiporn, W.; Lyman, M.; Biedron, C.; Chea, N.; Bunthi, C.; Kolwaite, A.; Janejai, N. Molecular epidemiology of carbapenem-resistant Enterobacterales in Thailand, 2016–2018. Antimicrob. Resist. Infect. Control 2021, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Doi, Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017, 8, 460–469. [Google Scholar] [CrossRef]
- Patel, B.; Hopkins, K.L.; Freeman, R.; People, D.; Brown, C.S.; Robotham, J.V. Carbapenemase-producing Enterobacterales: A challenge for healthcare now and for the next decade. Infect. Prev. Pract. 2020, 2, 100089. [Google Scholar] [CrossRef]
- Baeza, L.L.; Pfennigwerth, N.; Greissl, C.; Göttig, S.; Saleh, A.; Stelzer, Y.; Gatermann, S.G.; Hamprecht, A. Comparison of five methods for detection of carbapenemases in Enterobacterales with proposal of a new algorithm. Clin. Microbiol. Infect. 2019, 25, 1286-e9. [Google Scholar] [CrossRef]
- Tamma, P.D.; Opene, B.N.; Gluck, A.; Chambers, K.K.; Carroll, K.C.; Simner, P.J. Comparison of 11 Phenotypic Assays for Accurate Detection of Carbapenemase-Producing Enterobacteriaceae. J. Clin. Microbiol. 2017, 55, 1046–1055. [Google Scholar] [CrossRef]
- Clinical Laboratory Standard Institute. Performance standards for antimicrobial susceptibility testing. In CLSI Supplement M100, 32nd ed.; Clinical Laboratory Standard Institute: Wayne, PA, USA, 2022; ISBN 978-1-68440-066-9. [Google Scholar]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef]
- Smiljanic, M.; Kaase, M.; Ahmad-Nejad, P.; Ghebremedhin, B. Comparison of in-house and commercial real time-PCR based carbapenemase gene detection methods in Enterobacteriaceae and non-fermenting gram-negative bacterial isolates. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 48. [Google Scholar] [CrossRef]
- Watahiki, M.; Kawahara, R.; Suzuki, M.; Aoki, M.; Uchida, K.; Matsumoto, Y.; Kumagai, Y.; Noda, M.; Masuda, K.; Fukuda, C.; et al. Single-Tube Multiplex Polymerase Chain Reaction for the Detection of Genes Encoding Enterobacteriaceae Carbapenemase. Jpn. J. Infect. Dis. 2020, 73, 166–172. [Google Scholar] [CrossRef]
- Cerezales, M.; Biniossek, L.; Gerson, S.; Xanthopoulou, K.; Wille, J.; Wohlfarth, E.; Kaase, M.; Seifert, H.; Higgins, P.G. Novel multiplex PCRs for detection of the most prevalent carbapenemase genes in Gram-negative bacteria within Germany. J. Med. Microbiol. 2021, 70, 001310. [Google Scholar] [CrossRef]
- Yoshioka, N.; Hagiya, H.; Deguchi, M.; Hamaguchi, S.; Kagita, M.; Nishi, I.; Akeda, Y.; Tomono, K. Multiplex Real-Time PCR Assay for Six Major Carbapenemase Genes. Pathogens 2021, 10, 276. [Google Scholar] [CrossRef]
- Vlek, A.L.; Frentz, D.; Haenen, A.; Bootsma, H.J.; Notermans, D.W.; Frakking, F.N.; de Greeff, S.C.; Leenstra, T. ISIS-AR study group. Detection and epidemiology of carbapenemase producing Enterobacteriaceae in the Netherlands in 2013–2014. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1089–1096. [Google Scholar] [CrossRef]
- Hernández-García, M.; Pérez-Viso, B.; Carmen Turrientes, M.; Díaz-Agero, C.; López-Fresneña, N.; Bonten, M.; Malhotra-Kumar, S.; Ruiz-Garbajosa, P.; Cantón, R. Characterization of carbapenemase-producing Enterobacteriaceae from colonized patients in a university hospital in Madrid, Spain, during the R-GNOSIS project depicts increased clonal diversity over time with maintenance of high-risk clones. J. Antimicrob. Chemother. 2018, 73, 3039–3043. [Google Scholar] [CrossRef]
- Sekar, R.; Srivani, S.; Kalyanaraman, N.; Thenmozhi, P.; Amudhan, M.; Lallitha, S.; Mythreyee, M. New Delhi Metallo-β-lactamase and other mechanisms of carbapenemases among Enterobacteriaceae in rural South India. J. Glob. Antimicrob. Resist. 2019, 18, 207–214. [Google Scholar] [CrossRef]
- Dziri, O.; Dziri, R.; Ali, E.L.; Salabi, A.; Chouchani, C. Carbapenemase Producing Gram-Negative Bacteria in Tunisia: History of Thirteen Years of Challenge. Infect. Drug Resist. 2020, 13, 4177–4191. [Google Scholar] [CrossRef]
- Gurung, S.; Kafle, S.; Dhungel, B.; Adhikari, N.; Thapa Shrestha, U.; Adhikari, B.; Banjara, M.R.; Rijal, K.R.; Ghimire, P. Detection of OXA-48 Gene in Carbapenem-Resistant Escherichia coli and Klebsiella pneumoniae from Urine Samples. Infect. Drug Resist. 2020, 13, 2311–2321. [Google Scholar] [CrossRef]
- Han, R.; Shi, Q.; Wu, S.; Yin, D.; Peng, M.; Dong, D.; Zheng, Y.; Guo, Y.; Zhang, R.; Hu, F. China Antimicrobial Surveillance Network (CHINET) Study Group. Dissemination of Carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) among Carbapenem-Resistant Enterobacteriaceae Isolated From Adult and Children Patients in China. Front. Cell Infect. Microbiol. 2020, 10, 314. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, G.R.; Jeong, J.; Kim, S.; Shin, J.H. Prevalence and Characteristics of Carbapenemase-Producing Enterobacteriaceae in Three Tertiary-Care Korean University Hospitals between 2017 and 2018. Jpn. J. Infect. Dis. 2020, 73, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Solgi, H.; Nematzadeh, S.; Giske, C.G.; Badmasti, F.; Westerlund, F.; Lin, Y.L.; Goyal, G.; Nikbin, V.S.; Nemati, A.H.; Shahcheraghi, F. Molecular Epidemiology of OXA-48 and NDM-1 Producing Enterobacterales Species at a University Hospital in Tehran, Iran, between 2015 and 2016. Front. Microbiol. 2020, 11, 936. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Deshpande, L.M.; Mendes, R.E.; Doyle, T.B.; Sader, H.S. Prevalence of carbapenemase genes among carbapenem-nonsusceptible Enterobacterales collected in US hospitals in a five-year period and activity of ceftazidime/avibactam and comparator agents. JAC. Antimicrob. Resist. 2022, 4, dlac098. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, D.; Kerdsin, A.; Akeda, Y.; Sugawara, Y.; Sakamoto, N.; Matsumoto, Y.; Motooka, D.; Ishihara, T.; Nishi, I.; Laolerd, W.; et al. Nationwide surveillance in Thailand revealed genotype-dependent dissemination of carbapenem-resistant Enterobacterales. Microb. Genom. 2022, 8, 000797. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Upreti, M.K.; Bimali, N.K.; Shrestha, B.; Sah, A.K.; Nepal, K.; Dhungel, B.; Adhikari, S.; Adhikari, N.; Lekhak, B.; et al. Detection of NDM Variants (blaNDM-1, blaNDM-2, blaNDM-3) from Carbapenem-Resistant Escherichia coli and Klebsiella pneumoniae: First Report from Nepal. Infect. Drug Resist. 2022, 15, 4419–4434. [Google Scholar] [CrossRef] [PubMed]
- Osei Sekyere, J.; Govinden, U.; Essack, S.Y. Review of established and innovative detection methods for carbapenemase-producing Gram-negative bacteria. J. Appl. Microbiol. 2015, 119, 1219–1233. [Google Scholar] [CrossRef]
- Matsumura, Y.; Peirano, G.; Motyl, M.R.; Adams, M.D.; Chen, L.; Kreiswirth, B.; DeVinney, R.; Pitout, J.D. Global Molecular Epidemiology of IMP-Producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2017, 61, e02729-16. [Google Scholar] [CrossRef]
- Kerdsin, A.; Deekae, S.; Chayangsu, S.; Hatrongjit, R.; Chopjitt, P.; Takeuchi, D.; Akeda, Y.; Tomono, K.; Hamada, S. Genomic characterization of an emerging blaKPC-2 carrying Enterobacteriaceae clinical isolates in Thailand. Sci. Rep. 2019, 9, 18521. [Google Scholar] [CrossRef]
- Abbott, S. Klebsiella, Enterobacter, Citrobacter, Serratia, Plesiomonas, and other Enterobacteriaceae. In Manual of Clinical Microbiology, 10th ed.; Versalovic, J., Carroll, K., Funke, G., Jorgensen, J., Landry, M.L., Warnock, D., Eds.; ASM Press: Washington, DC, USA, 2011; Volume 2, pp. 639–657. [Google Scholar]
- Hatrongjit, R.; Kerdsin, A.; Akeda, Y.; Hamada, S. Detection of plasmid-mediated colistin-resistant and carbapenem-resistant genes by multiplex PCR. MethodsX 2018, 5, 532–536. [Google Scholar] [CrossRef]
- Phetburom, N.; Boueroy, P.; Chopjitt, P.; Hatrongjit, R.; Akeda, Y.; Hamada, S.; Nuanualsuwan, S.; Kerdsin, A. Klebsiella pneumoniae Complex Harboring mcr-1, mcr-7, and mcr-8 Isolates from Slaughtered Pigs in Thailand. Microorganisms 2021, 9, 2436. [Google Scholar] [CrossRef]
- Pechorsky, A.; Nitzan, Y.; Lazarovitch, T. Identification of pathogenic bacteria in blood cultures: Comparison between conventional and PCR methods. J. Microbiol. Methods 2009, 78, 325–330. [Google Scholar] [CrossRef]
- Fonseca, E.L.; Ramos, N.D.; Andrade, B.G.; Morais, L.L.; Marin, M.F.; Vicente, A.C. A one-step multiplex PCR to identify Klebsiella pneumoniae, Klebsiella variicola, and Klebsiella quasipneumoniae in the clinical routine. Diagn. Microbiol. Infect. Dis. 2017, 87, 315–317. [Google Scholar] [CrossRef]
- Poirel, L.; Heritier, C.; Tol’un, V.; Nordmann, P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents. Chemother. 2004, 48, 15–22. [Google Scholar] [CrossRef]
- Bradford, P.A.; Bratu, S.; Urban, C.; Visalli, M.; Mariano, N.; Landman, D.; Rahal, J.J.; Brooks, S.; Cebular, S.; Quale, J. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 beta-lactamases in New York City. Clin. Infect. Dis. 2004, 39, 55–60. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).