Microbial Resistance to Antibiotics and Biofilm Formation of Bacterial Isolates from Different Carp Species and Risk Assessment for Public Health
Abstract
1. Introduction
2. Results and Discussion
2.1. Microbiological Quality of the Tested Fresh Fish Samples
2.2. Antibiotic Resistance of the Isolates from Different Carp Biofilms
3. Materials and Methods
3.1. Design of Study and Fish Sampling
3.2. Isolation and Identification of Bacteria
3.3. Antibiotic Susceptibility
3.4. In Vitro Biofilm Formation and Recovery
3.5. Antibiotic Susceptibility Pattern of the Isolates after Biofilm Formation
3.6. Analysis of Antibiotics
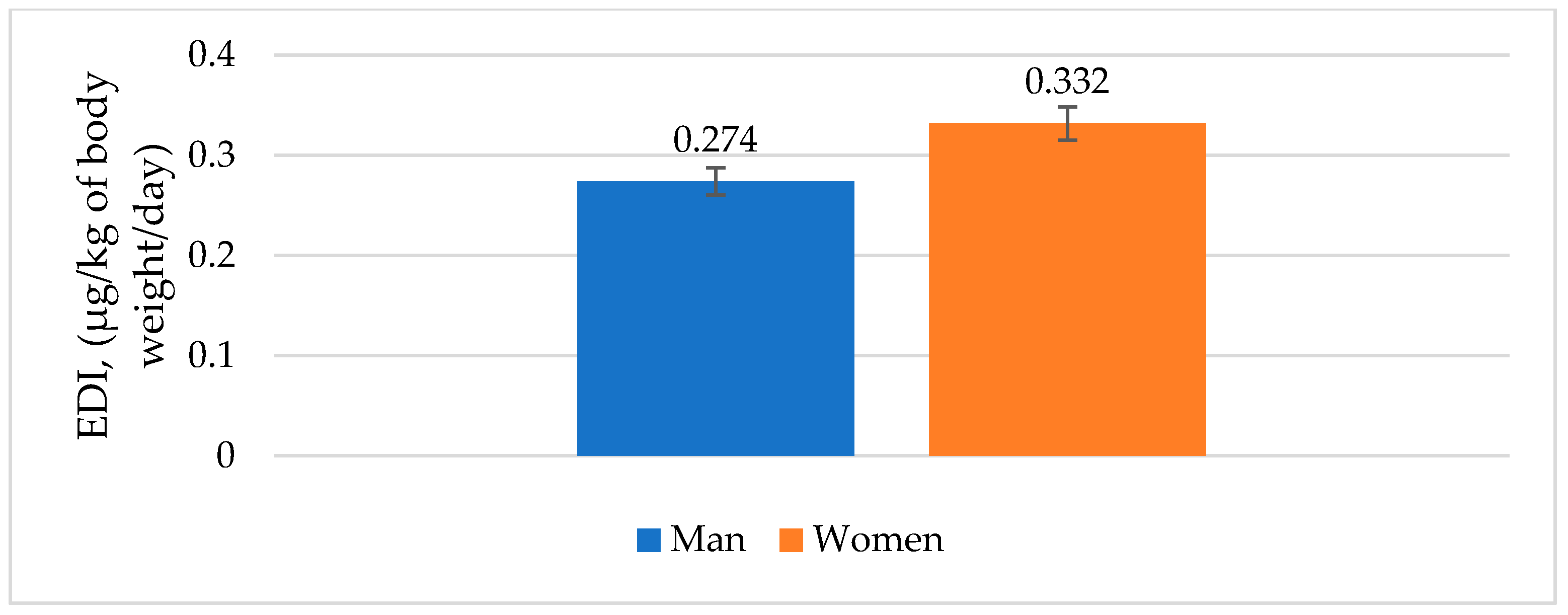
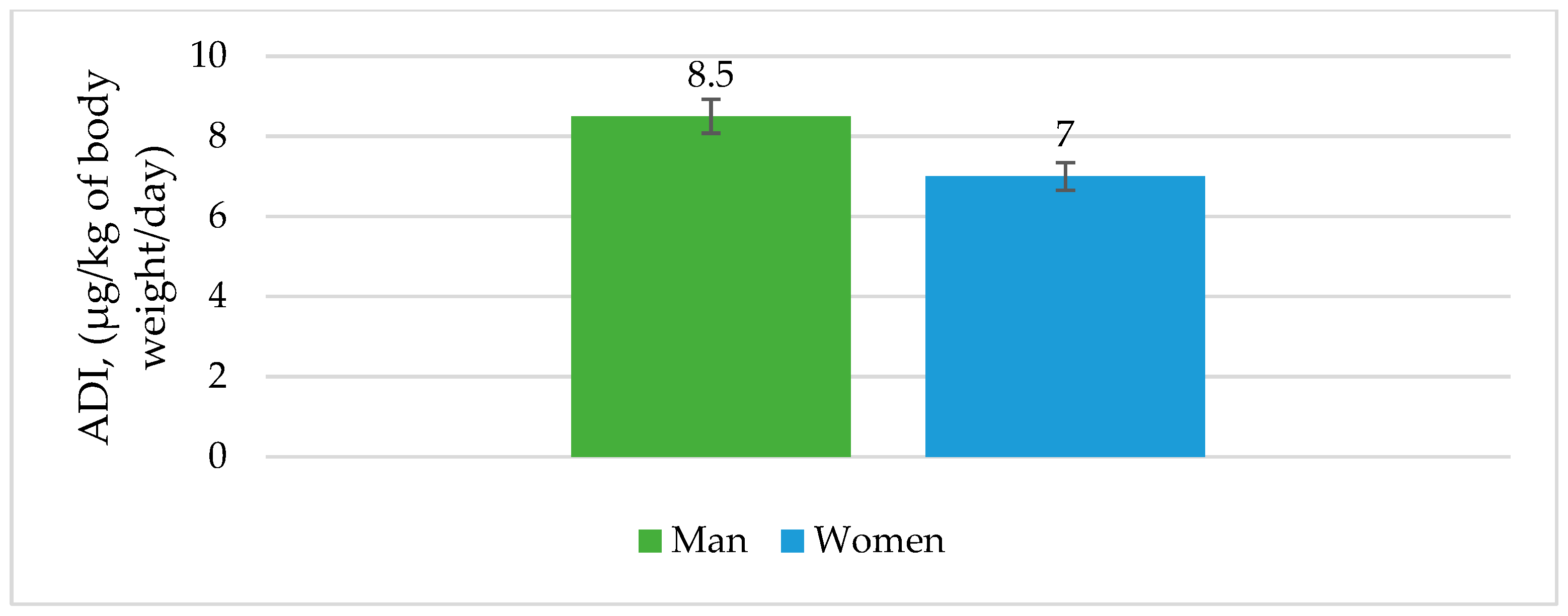
3.7. Risk Assessment for Fish Consumption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tavelli, R.; Callens, M.; Grootaert, C.; Abdallah, M.F.; Rajkovic, A. Foodborne Pathogens in the Plastisphere: Can Microplastics in the Food Chain Threaten Microbial Food Safety? Trends Food Sci. Technol. 2022, 129, 1–10. [Google Scholar] [CrossRef]
- Gargiulo, A.H.; Duarte, S.G.; Campos, G.Z.; Landgraf, M.; Franco, B.D.G.M.; Pinto, U.M. Food Safety Issues Related to Eating In and Eating Out. Microorganisms 2022, 10, 2118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-L.; Yang, L.; Huang, K.-X.; Chen, D.-Z.; Gao, F. Mechanisms and Application of Microalgae on Removing Emerging Contaminants from Wastewater: A Review. Bioresour. Technol. 2022, 364, 128049. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Hansen, M.F.; Røder, H.L.; Wang, N.; Burmølle, M.; He, G. Mixed-Species Biofilms in the Food Industry: Current Knowledge and Novel Control Strategies. Crit. Rev. Food Sci. Nutr. 2020, 60, 2277–2293. [Google Scholar] [CrossRef]
- Puvača, N.; de Llanos Frutos, R. Antimicrobial Resistance in Escherichia coli Strains Isolated from Humans and Pet Animals. Antibiotics 2021, 10, 69. [Google Scholar] [CrossRef]
- Štefánek, M.; Wenner, S.; Borges, V.; Pinto, M.; Gomes, J.P.; Rodrigues, J.; Faria, I.; Pessanha, M.A.; Martins, F.; Sabino, R.; et al. Antimicrobial Resistance and Biofilms Underlying Catheter-Related Bloodstream Coinfection by Enterobacter Cloacae Complex and Candida Parapsilosis. Antibiotics 2022, 11, 1245. [Google Scholar] [CrossRef]
- González-Rivas, F.; Ripolles-Avila, C.; Fontecha-Umaña, F.; Ríos-Castillo, A.G.; Rodríguez-Jerez, J.J. Biofilms in the Spotlight: Detection, Quantification and Removal Methods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1261–1276. [Google Scholar] [CrossRef]
- Yawata, Y.; Nguyen, J.; Stocker, R.; Rusconi, R. Microfluidic Studies of Biofilm Formation in Dynamic Environments. J. Bacteriol. 2016, 198, 2589–2595. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting Microbial Biofilms: Current and Prospective Therapeutic Strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Cai, W.; Arias, C.R. Biofilm Formation on Aquaculture Substrates by Selected Bacterial Fish Pathogens. J. Aquat. Anim. Health 2017, 29, 95–104. [Google Scholar] [CrossRef]
- Wang, K.; Pang, S.; Mu, X.; Qi, S.; Li, D.; Cui, F.; Wang, C. Biological Response of Earthworm, Eisenia Fetida, to Five Neonicotinoid Insecticides. Chemosphere 2015, 132, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Bassler, B.L. Surviving as a Community: Antibiotic Tolerance and Persistence in Bacterial Biofilms. Cell Host Microbe. 2019, 26, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef]
- Machat, R.; Pojezdal, L.; Piackova, V.; Faldyna, M. Carp Edema Virus and Immune Response in Carp (Cyprinus carpio): Current Knowledge. J. Fish Dis. 2021, 44, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Ghimpețeanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Drăgotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic Use in Livestock and Residues in Food—A Public Health Threat: A Review. Foods 2022, 11, 1430. [Google Scholar] [CrossRef]
- Radosavljevic, V.; Glišić, D.; Maksimović Zorić, J.; Veljović, L.; Nešić, K.; Milićević, V. Carp EDEMA virus disease in Serbia—Disease out of control. Arch. Vet. Med. 2021, 14, 37–52. [Google Scholar] [CrossRef]
- Furlan, J.P.R.; Gallo, I.F.L.; de Campos, A.C.L.P.; Passaglia, J.; Falcão, J.P.; Navarro, A.; Nakazato, G.; Stehling, E.G. Molecular Characterization of Multidrug-Resistant Shiga Toxin-Producing Escherichia Coli Harboring Antimicrobial Resistance Genes Obtained from a Farmhouse. Pathog. Glob. Health 2019, 113, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Puvača, N.; Lika, E.; Tufarelli, V.; Bursić, V.; Ljubojević Pelić, D.; Nikolova, N.; Petrović, A.; Prodanović, R.; Vuković, G.; Lević, J.; et al. Influence of Different Tetracycline Antimicrobial Therapy of Mycoplasma (Mycoplasma Synoviae) in Laying Hens Compared to Tea Tree Essential Oil on Table Egg Quality and Antibiotics Residues. Foods 2020, 9, 612. [Google Scholar] [CrossRef] [PubMed]
- Puvača, N.; Vapa Tankosić, J.; Ignjatijević, S.; Carić, M.; Prodanović, R. Antimicrobial Resistance in the Environment: Review of the Selected Resistance Drivers and Public Health Concerns. J. Agron. Technol. Eng. Manag. 2022, 5, 793–802. [Google Scholar] [CrossRef]
- Falgenhauer, L.; Schwengers, O.; Schmiedel, J.; Baars, C.; Lambrecht, O.; Heß, S.; Berendonk, T.U.; Falgenhauer, J.; Chakraborty, T.; Imirzalioglu, C. Multidrug-Resistant and Clinically Relevant Gram-Negative Bacteria Are Present in German Surface Waters. Front Microbiol. 2019, 10, 2779. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Yusaimi, Y.A.; Zulkeflle, S.N.M.; Sugiura, N.; Iwamoto, K.; Goto, M.; Utsumi, M.; bin Othman, N.; Zakaria, Z. Molecular Characterization of Multi-Drug Resistant Escherichia coli Isolates from Tropical Environments in Southeast Asia. J. Gen. Appl. Microbiol. 2018, 64, 284–292. [Google Scholar] [CrossRef]
- Pelić, M.; Puvača, N.; Kartalović, B.; Živkov Baloš, M.; Novakov, N.; Ljubojević Pelić, D. Antibiotics and Sulfonamides in Water, Sediment and Fish in an Integrated Production System. J. Agron. Technol. Eng. Manag. 2023, 6, 851–856. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic Resistance—The Need for Global Solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, D.; Haagensen, J.A.J.; Rich, C.; Forestier, C. Characterization of Type 2 Quorum Sensing in Klebsiella Pneumoniae and Relationship with Biofilm Formation. J. Bacteriol. 2005, 187, 2870–2880. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-Cell Communication in Bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef]
- Sztajer, H.; Szafranski, S.P.; Tomasch, J.; Reck, M.; Nimtz, M.; Rohde, M.; Wagner-Döbler, I. Cross-Feeding and Interkingdom Communication in Dual-Species Biofilms of Streptococcus Mutans and Candida Albicans. ISME J. 2014, 8, 2256–2271. [Google Scholar] [CrossRef]
- Antonova, E.S.; Hammer, B.K. Quorum-Sensing Autoinducer Molecules Produced by Members of a Multispecies Biofilm Promote Horizontal Gene Transfer to Vibrio Cholerae: Vibrio Autoinducers Promote Horizontal Gene Transfer. FEMS Microbi. Lett. 2011, 322, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Burmølle, M.; Ren, D.; Bjarnsholt, T.; Sørensen, S.J. Interactions in Multispecies Biofilms: Do They Actually Matter? Trends Microbiol. 2014, 22, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Dufour, D.; Leung, V.; Lévesque, C.M. Bacterial Biofilm: Structure, Function and Antimicrobial Resistance: Bacterial Biofilm. Endod. Top. 2010, 22, 2–16. [Google Scholar] [CrossRef]
- Seneviratne, G.; Zavahir, J.S.; Bandara, W.M.M.S.; Weerasekara, M.L.M.A.W. Fungal-Bacterial Biofilms: Their Development for Novel Biotechnological Applications. World J. Microbiol. Biotechnol. 2008, 24, 739–743. [Google Scholar] [CrossRef]
- Paniagua-Michel, J. Wastewater Treatment Using Phototrophic–Heterotrophic Biofilms and Microbial Mats. In Prospects and Challenges in Algal Biotechnology; Tripathi, B.N., Kumar, D., Eds.; Springer Singapore: Singapore, 2017; pp. 257–275. ISBN 978-981-10-1949-4. [Google Scholar]
- Raghupathi, P.K.; Liu, W.; Sabbe, K.; Houf, K.; Burmølle, M.; Sørensen, S.J. Synergistic Interactions within a Multispecies Biofilm Enhance Individual Species Protection against Grazing by a Pelagic Protozoan. Front Microbiol. 2018, 8, 2649. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Chen, L.; Yuk, H.-G. Stress Response and Survival of Salmonella Enteritidis in Single and Dual Species Biofilms with Pseudomonas Fluorescens Following Repeated Exposure to Quaternary Ammonium Compounds. Int. J. Food Microbiol. 2020, 325, 108643. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Røder, H.L.; Madsen, J.S.; Bjarnsholt, T.; Sørensen, S.J.; Burmølle, M. Interspecific Bacterial Interactions Are Reflected in Multispecies Biofilm Spatial Organization. Front Microbiol. 2016, 7, 1336. [Google Scholar] [CrossRef]
- Poursat, B.A.J.; van Spanning, R.J.M.; de Voogt, P.; Parsons, J.R. Implications of Microbial Adaptation for the Assessment of Environmental Persistence of Chemicals. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2220–2255. [Google Scholar] [CrossRef]
- Bacanlı, M.; Başaran, N. Importance of Antibiotic Residues in Animal Food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef]
- Menkem, Z.E.; Ngangom, B.L.; Tamunjoh, S.S.A.; Boyom, F.F. Antibiotic Residues in Food Animals: Public Health Concern. Acta Ecol. Sin. 2019, 39, 411–415. [Google Scholar] [CrossRef]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ştefan, M.G.; Loghin, F. Antibiotics in the Environment: Causes and Consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef]
- Iwu, C.D.; Korsten, L.; Okoh, A.I. The Incidence of Antibiotic Resistance within and beyond the Agricultural Ecosystem: A Concern for Public Health. MicrobiologyOpen 2020, 9, e1035. [Google Scholar] [CrossRef]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L.B.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a New Generation of Antimicrobials: Toxicity Aspects and Regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef]
- Bilal, M.; Mehmood, S.; Rasheed, T.; Iqbal, H.M.N. Antibiotics Traces in the Aquatic Environment: Persistence and Adverse Environmental Impact. Curr. Opin. Environ. Sci. Health 2020, 13, 68–74. [Google Scholar] [CrossRef]
- Forster, S.C.; Liu, J.; Kumar, N.; Gulliver, E.L.; Gould, J.A.; Escobar-Zepeda, A.; Mkandawire, T.; Pike, L.J.; Shao, Y.; Stares, M.D.; et al. Strain-Level Characterization of Broad Host Range Mobile Genetic Elements Transferring Antibiotic Resistance from the Human Microbiome. Nat. Commun. 2022, 13, 1445. [Google Scholar] [CrossRef]
- Boto, L.; Pineda, M.; Pineda, R. Potential Impacts of Horizontal Gene Transfer on Human Health and Physiology and How Anthropogenic Activity Can Affect It. FEBS J. 2019, 286, 3959–3967. [Google Scholar] [CrossRef] [PubMed]
- Sadrekarimi, H.; Gardanova, Z.R.; Bakhshesh, M.; Ebrahimzadeh, F.; Yaseri, A.F.; Thangavelu, L.; Hasanpoor, Z.; Zadeh, F.A.; Kahrizi, M.S. Emerging Role of Human Microbiome in Cancer Development and Response to Therapy: Special Focus on Intestinal Microflora. J. Transl. Med. 2022, 20, 301. [Google Scholar] [CrossRef] [PubMed]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human Health Risk Assessment of Antibiotic Resistance Associated with Antibiotic Residues in the Environment: A Review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Vapa Tankosić, J.; Puvača, N.; Giannenas, I.; Tufarelli, V.; Ignjatijević, S. Food Safety Policy in the European Union. J. Agron. Technol. Eng. Manag. 2022, 5, 712–717. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, K.; Zhang, J.; Liu, X.; Yang, L.; Wei, R.; Wang, S.; Zhang, D.; Xie, S.; Tao, F. Antibiotic Body Burden of Elderly Chinese Population and Health Risk Assessment: A Human Biomonitoring-Based Study. Environ. Pollut. 2020, 256, 113311. [Google Scholar] [CrossRef]
- Lin, L.; Wang, S.-F.; Yang, T.-Y.; Hung, W.-C.; Chan, M.-Y.; Tseng, S.-P. Antimicrobial Resistance and Genetic Diversity in Ceftazidime Non-Susceptible Bacterial Pathogens from Ready-to-Eat Street Foods in Three Taiwanese Cities. Sci. Rep. 2017, 7, 15515. [Google Scholar] [CrossRef]
- Harnisz, M.; Korzeniewska, E. The Prevalence of Multidrug-Resistant Aeromonas Spp. in the Municipal Wastewater System and Their Dissemination in the Environment. Sci. Total Environ. 2018, 626, 377–383. [Google Scholar] [CrossRef]
- Bollache, L.; Bardet, E.; Depret, G.; Motreuil, S.; Neuwirth, C.; Moreau, J.; Hartmann, A. Dissemination of CTX-M-Producing Escherichia Coli in Freshwater Fishes From a French Watershed (Burgundy). Front Microbiol. 2019, 9, 3239. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Zhu, L.; Wang, J. Field-Based Evidence for Enrichment of Antibiotic Resistance Genes and Mobile Genetic Elements in Manure-Amended Vegetable Soils. Sci. Total Environ. 2019, 654, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Lika, E.; Rosić, M.; Cocoli, S.; Puvača, N.; Vuković, G.; Shtylla Kika, T.; Bursić, V. Antimicrobial Resistance of Staphylococcus Aureus Strains Isolated from Cow Raw Milk Samples from Albania and Serbia. Mljekarstvo 2021, 71, 248–256. [Google Scholar] [CrossRef]
- Cornejo, J.; Pokrant, E.; Figueroa, F.; Riquelme, R.; Galdames, P.; Di Pillo, F.; Jimenez-Bluhm, P.; Hamilton-West, C. Assessing Antibiotic Residues in Poultry Eggs from Backyard Production Systems in Chile, First Approach to a Non-Addressed Issue in Farm Animals. Animals 2020, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Projahn, M.; von Tippelskirch, P.; Semmler, T.; Guenther, S.; Alter, T.; Roesler, U. Contamination of Chicken Meat with Extended-Spectrum Beta-Lactamase Producing- Klebsiella Pneumoniae and Escherichia coli during Scalding and Defeathering of Broiler Carcasses. Food Microbiol. 2019, 77, 185–191. [Google Scholar] [CrossRef]
- European Commission Commission Regulation (EU). No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. Off J. Eur. Union. 2010, 15, 1–72. [Google Scholar]
- Ramalho Ribeiro, A.; Altintzoglou, T.; Mendes, J.; Nunes, M.L.; Dinis, M.T.; Dias, J. Farmed Fish as a Functional Food: Perception of Fish Fortification and the Influence of Origin—Insights from Portugal. Aquaculture 2019, 501, 22–31. [Google Scholar] [CrossRef]
- Woodhead, A.J.; Abernethy, K.E.; Szaboova, L.; Turner, R.A. Health in Fishing Communities: A Global Perspective. Fish Fish. 2018, 19, 839–852. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- Aglago, E.K.; Huybrechts, I.; Murphy, N.; Casagrande, C.; Nicolas, G.; Pischon, T.; Fedirko, V.; Severi, G.; Boutron-Ruault, M.-C.; Fournier, A.; et al. Consumption of Fish and Long-Chain n-3 Polyunsaturated Fatty Acids Is Associated with Reduced Risk of Colorectal Cancer in a Large European Cohort. Clin. Gastroenterol. Hepatol. 2020, 18, 654–666.e6. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Wu, J.; Li, X.; Yu, L.; Xie, K.; Zhang, M.; Ren, L.; Ji, Y.; Li, Y. Exposure to Veterinary Antibiotics via Food Chain Disrupts Gut Microbiota and Drives Increased Escherichia coli Virulence and Drug Resistance in Young Adults. Pathogens 2022, 11, 1062. [Google Scholar] [CrossRef]
- Wang, H.; Ren, L.; Yu, X.; Hu, J.; Chen, Y.; He, G.; Jiang, Q. Antibiotic Residues in Meat, Milk and Aquatic Products in Shanghai and Human Exposure Assessment. Food Control. 2017, 80, 217–225. [Google Scholar] [CrossRef]
- Wang, H.; Wang, N.; Wang, B.; Fang, H.; Fu, C.; Tang, C.; Jiang, F.; Zhou, Y.; He, G.; Zhao, Q.; et al. Antibiotics Detected in Urines and Adipogenesis in School Children. Environ. Int. 2016, 89–90, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Cockerill, F.R.; Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Tests: Approved Standard, 11th ed.; Clinical and Laboratory Standards Institute, CLSI: Wayne, PA, USA, 2012; ISBN 978-1-56238-782-2. [Google Scholar]
- Chowdhury, F.F.K.; Acharjee, M.; Noor, R. Maintenance of Environmental Sustainability Through Microbiological Study of Pharmaceutical Solid Wastes: General. Clean Soil. Air Water 2016, 44, 309–316. [Google Scholar] [CrossRef]
- Oceans and Fisheries. Available online: https://oceans-and-fisheries.ec.europa.eu/index_en (accessed on 12 December 2022).
- Average Height of Men and Women Worldwide. Available online: https://www.worlddata.info/average-bodyheight.php (accessed on 12 December 2022).
| Species | Microbial Load (CFU/g) | |||||
|---|---|---|---|---|---|---|
| E. coli | A. hydrophila | Salmonella spp. | Shewanella putrefaciens | Vibrio spp. | Staphylococcus spp. | |
| Common carp | 3.3 × 103 | 8.8 × 103 | nd | nd | 1.3 × 103 | 1.0 × 102 |
| Silver carp | 6.9 × 104 | 7.8 × 104 | nd | 1.6 × 102 | 3.6 × 102 | 2.2 × 103 |
| Grass carp | 4.4 × 104 | 6.6 × 104 | nd | nd | 1.8 × 102 | 1.2 × 102 |
| Isolates | Antibiotics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMC | AM | CTX | CIP | CHL | CN | STR | TE | TMP | ||
| Bulk sample | E. coli | I | R | S | S | S | S | S | R | S |
| A. hydrophila | I | R | S | S | S | S | S | R | S | |
| Vibrio spp. | I | R | S | S | S | S | S | R | S | |
| Staphylococcus spp. | S | R | S | S | S | S | S | R | S | |
| Biofilm | E. coli | R | R | S | S | S | S | R | R | R |
| Staphylococcus spp. | R | R | S | S | S | S | I | R | I | |
| Isolates | Antibiotics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMC | AM | CTX | CIP | CHL | CN | STR | TE | TMP | ||
| Bulk sample | E. coli | R | R | S | S | S | S | I | R | S |
| A. hydrophila | S | S | S | S | S | S | S | R | S | |
| Shewanella putrefaciens | S | S | S | S | S | S | S | S | S | |
| Vibrio spp. | R | I | S | S | S | S | R | R | S | |
| Staphylococcus spp. | R | R | S | S | S | S | R | R | S | |
| Biofilm | E. coli | R | R | S | S | I | S | R | R | I |
| A. hydrophila | R | R | S | S | S | S | R | R | I | |
| Staphylococcus spp. | R | R | S | S | S | S | R | R | I | |
| Isolates | Antibiotics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMC | AM | CTX | CIP | CHL | CN | STR | TE | TMP | ||
| Bulk sample | E. coli | R | R | S | S | S | S | S | R | S |
| A. hydrophila | S | S | S | S | S | S | S | S | S | |
| Shewanella putrefaciens | S | S | S | S | S | S | I | S | S | |
| Vibrio spp. | S | S | S | S | S | S | I | S | S | |
| Staphylococcus spp. | S | R | S | S | S | S | I | R | S | |
| Biofilm | E. coli | R | R | S | S | I | S | R | R | I |
| A. hydrophila | R | R | S | S | S | S | R | R | I | |
| Antibiotics | Sum of Antibiotics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMC | AM | CTX | CIP | CHL | CN | STR | TE | TMP | ||
| Concentration, µg/kg | 11.4 | 4.9 | - | 52.3 | - | 31.1 | 199.4 | 92.4 | 25.6 | 417.1 |
| SD | 0.02 | 0.00 | - | 0.28 | - | 0.43 | 0.95 | 0.18 | 0.02 | |
| Recovery, % | 90.12 | 89.31 | - | 90.22 | - | 78.61 | 81.33 | 95.12 | 98.38 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puvača, N.; Ljubojević Pelić, D.; Pelić, M.; Bursić, V.; Tufarelli, V.; Piemontese, L.; Vuković, G. Microbial Resistance to Antibiotics and Biofilm Formation of Bacterial Isolates from Different Carp Species and Risk Assessment for Public Health. Antibiotics 2023, 12, 143. https://doi.org/10.3390/antibiotics12010143
Puvača N, Ljubojević Pelić D, Pelić M, Bursić V, Tufarelli V, Piemontese L, Vuković G. Microbial Resistance to Antibiotics and Biofilm Formation of Bacterial Isolates from Different Carp Species and Risk Assessment for Public Health. Antibiotics. 2023; 12(1):143. https://doi.org/10.3390/antibiotics12010143
Chicago/Turabian StylePuvača, Nikola, Dragana Ljubojević Pelić, Miloš Pelić, Vojislava Bursić, Vincenzo Tufarelli, Luca Piemontese, and Gorica Vuković. 2023. "Microbial Resistance to Antibiotics and Biofilm Formation of Bacterial Isolates from Different Carp Species and Risk Assessment for Public Health" Antibiotics 12, no. 1: 143. https://doi.org/10.3390/antibiotics12010143
APA StylePuvača, N., Ljubojević Pelić, D., Pelić, M., Bursić, V., Tufarelli, V., Piemontese, L., & Vuković, G. (2023). Microbial Resistance to Antibiotics and Biofilm Formation of Bacterial Isolates from Different Carp Species and Risk Assessment for Public Health. Antibiotics, 12(1), 143. https://doi.org/10.3390/antibiotics12010143










