Is There a Relationship between Antimicrobial Use and Antibiotic Resistance of the Most Common Mastitis Pathogens in Dairy Cows?
Abstract
1. Introduction
2. Results
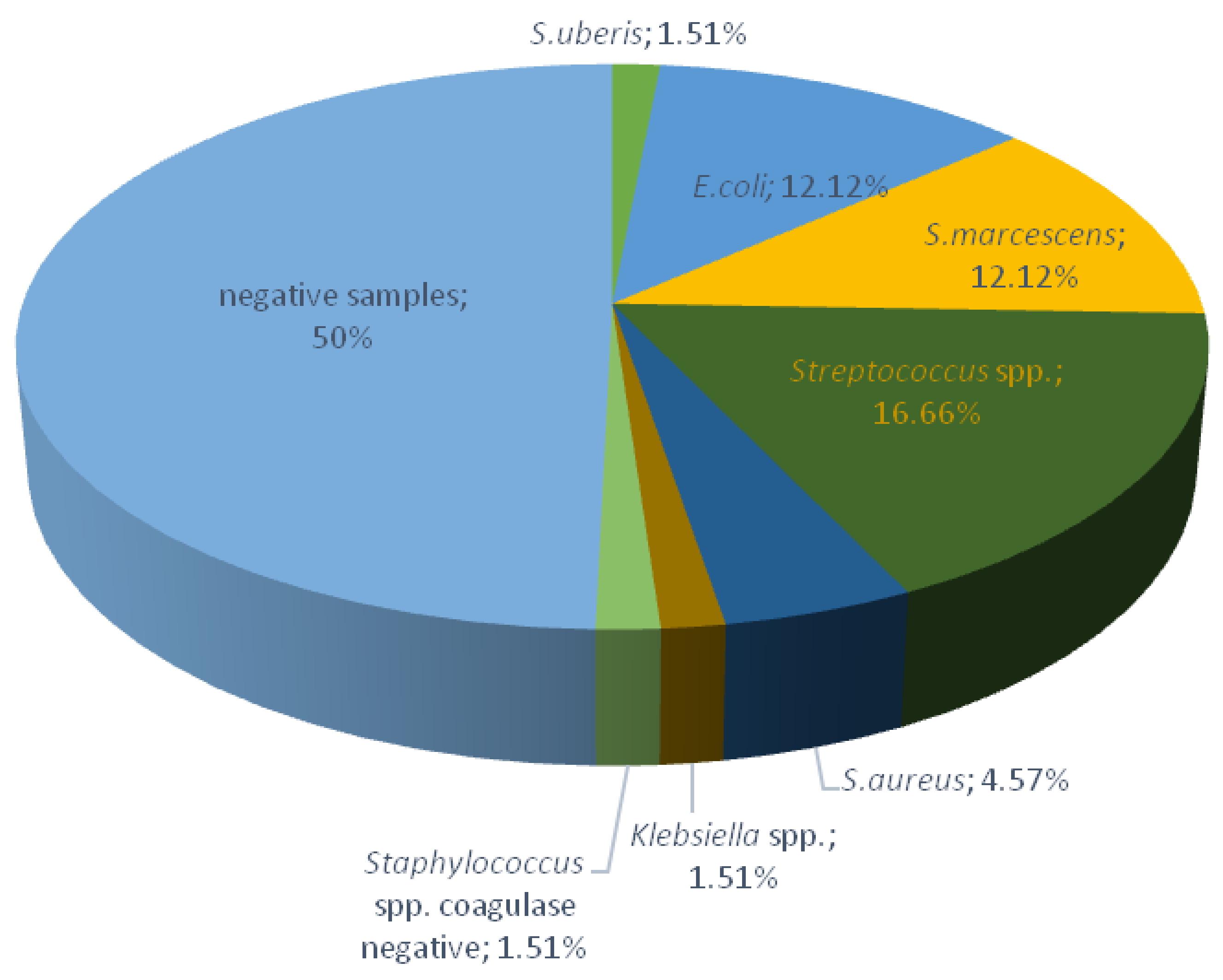
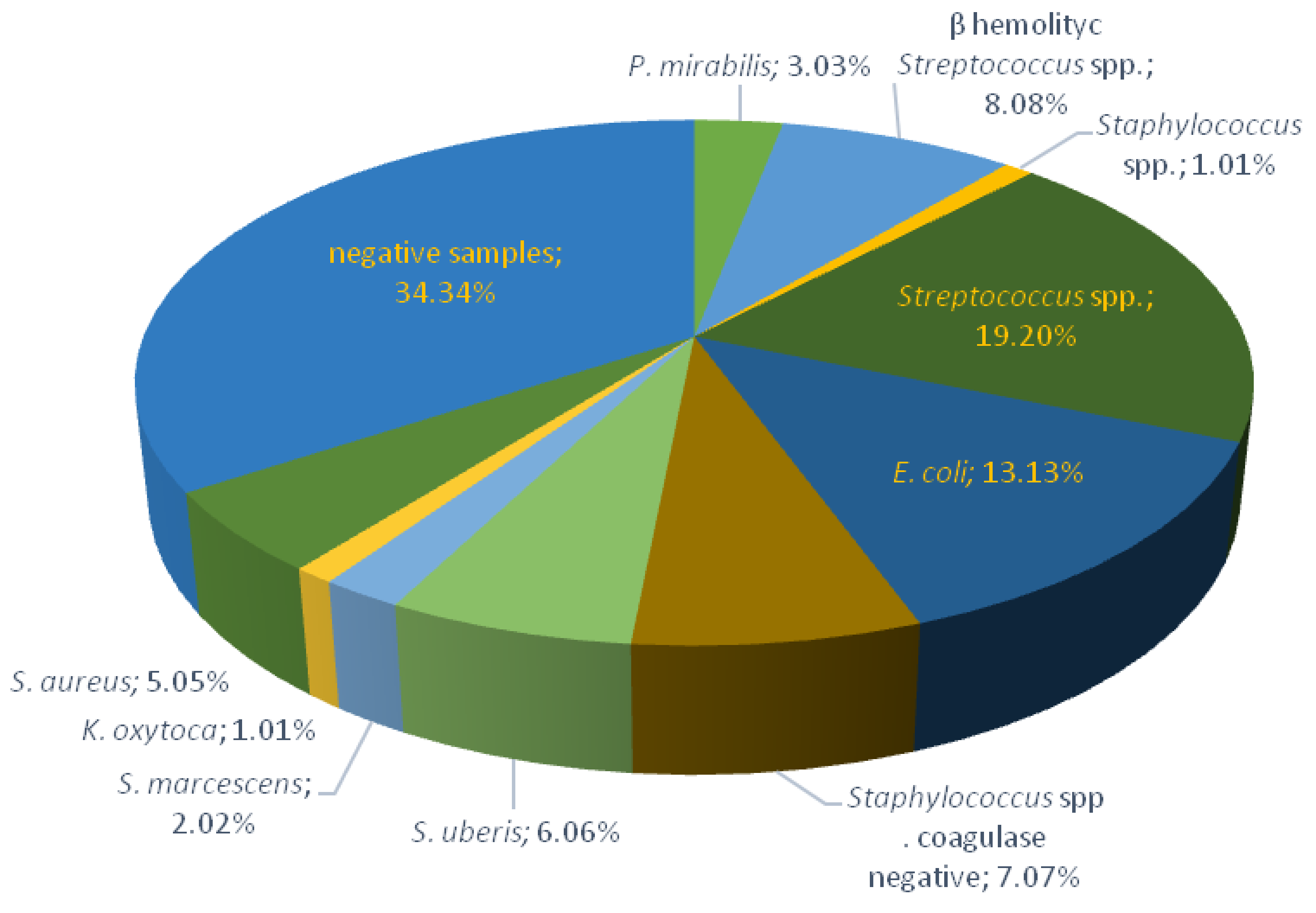
2.1. Bacteriological Testing of Milk Samples
2.2. Antibiotics Use at the Farm Level
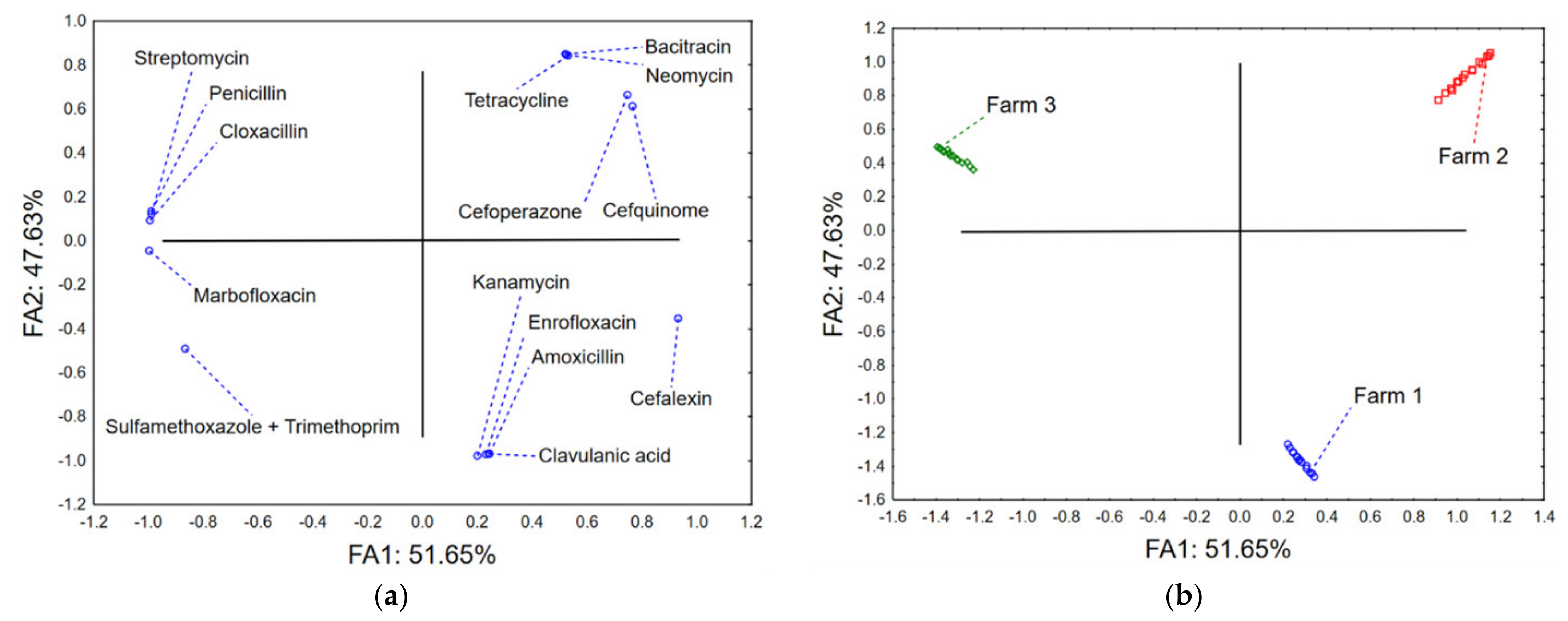
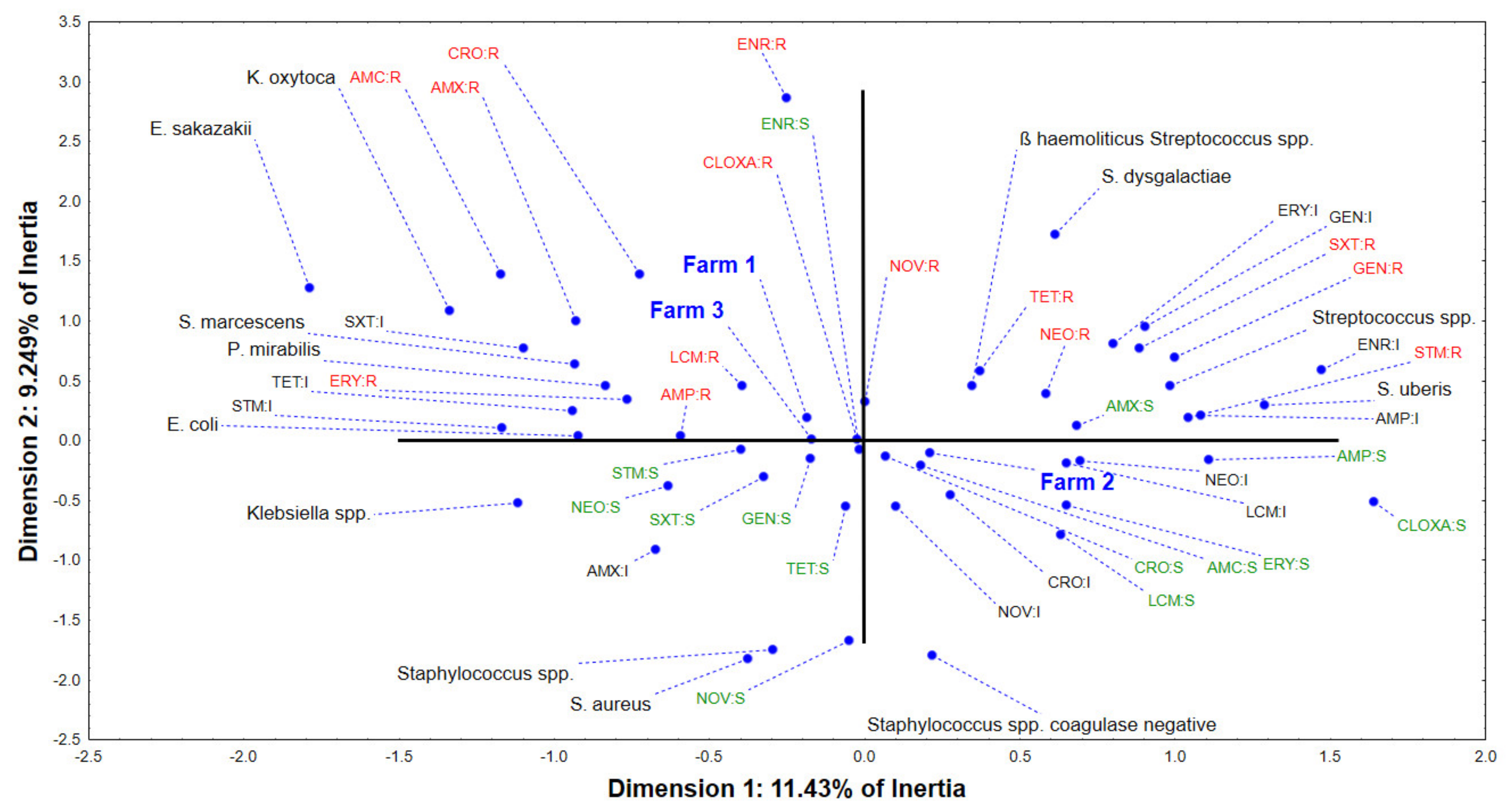
2.3. Multivariate Approach
3. Discussion
4. Materials and Methods
4.1. Sampling Procedures
4.2. Isolation and Identification of Mastitis Pathogens and Antibiotic Susceptibility Pattern
4.3. Data on Antibiotics Use at the Farm Level and Calculation of the Defined Daily Dose
4.4. Data Analysis
4.5. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EMA Committee for Medicinal Products for Veterinary Use (CVMP); EFSA Panel on Biological Hazards (BIOHAZ); Murphy, D.; Ricci, A.; Auce, Z.; Beechinor, J.G.; Bergendahl, H.; Breathnach, R.; Bureš, J.; Duarte Da Silva, J.P.; et al. EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA). EFSA J. 2017, 15, e04666. [Google Scholar] [PubMed]
- Schwarz, S.; Loeffler, A.; Kadlec, K. Bacterial resistance to antimicrobial agents and its impact on veterinary and human medicine. Adv. Vet. Dermatol. 2017, 8, 95–110. [Google Scholar]
- Pomba, C.; Rantala, M.; Greko, C.; Baptiste, K.E.; Catry, B.; Van Duijkeren, E.; Mateus, A.; Moreno, M.A.; Pyörälä, S.; Ružauskas, M. Public health risk of antimicrobial resistance transfer from companion animals. J. Antimicrob. Chemother. 2017, 72, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Kramer, T.; Jansen, L.E.; Lipman, L.J.; Smit, L.A.; Heederik, D.J.; Dorado-García, A. Farmers’ knowledge and expectations of antimicrobial use and resistance are strongly related to usage in Dutch livestock sectors. Prev. Vet. Med. 2017, 147, 142–148. [Google Scholar] [CrossRef] [PubMed]
- ECDC. The Bacterial Challenge: Time to React; European Center for Disease Prevention and Control: Stockholm, Sweden, 2009. [Google Scholar]
- Robinson, T.P.; Bu, D.; Carrique-Mas, J.; Fèvre, E.M.; Gilbert, M.; Grace, D.; Hay, S.I.; Jiwakanon, J.; Kakkar, M.; Kariuki, S. Antibiotic resistance is the quintessential One Health issue. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 377–380. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention; National Center for Emerging and Zoonotic Infectious Diseases (NCEZID). One Health Basics. Available online: https://www.cdc.gov/onehealth/basics/index.html (accessed on 1 November 2022).
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2018-trends-2010-2018-tenth-esvac-report_en.pdf (accessed on 1 November 2022).
- Cusini, A.; Rampini, S.K.; Bansal, V.; Ledergerber, B.; Kuster, S.P.; Ruef, C.; Weber, R. Different patterns of inappropriate antimicrobial use in surgical and medical units at a tertiary care hospital in Switzerland: A prevalence survey. PLoS ONE 2010, 5, e14011. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Forestry and Water Management. National Program for the Control of Bacterial Resistance to Antibiotics for the Period 2019–2021. Available online: https://www.vet.minpolj.gov.rs/AMR/amr.pdf (accessed on 1 November 2022).
- Timbrook, T.T.; Caffrey, A.R.; Ovalle, A.; Beganovic, M.; Curioso, W.; Gaitanis, M.; LaPlante, K.L. Assessments of opportunities to improve antibiotic prescribing in an emergency department: A period prevalence survey. Infect. Dis. Ther. 2017, 6, 497–505. [Google Scholar] [CrossRef]
- Sl. Glasnik RS 30/2010. Law on Medicines and Medical Devices of Serbia. Available online: https://www.paragraf.rs/propisi/zakon_o_lekovima_i_medicinskim_sredstvima.html (accessed on 1 November 2022).
- Van Boeckel, T.P.; Glennon, E.E.; Chen, D.; Gilbert, M.; Robinson, T.P.; Grenfell, B.T.; Levin, S.A.; Bonhoeffer, S.; Laxminarayan, R. Reducing antimicrobial use in food animals. Science 2017, 357, 1350–1352. [Google Scholar] [CrossRef]
- Gomes, F.; Henriques, M. Control of bovine mastitis: Old and recent therapeutic approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef]
- Cervinkova, D.; Vlkova, H.; Borodacova, I.; Makovcova, J.; Babak, V.; Lorencova, A.; Vrtkova, I.; Marosevic, D.; Jaglic, Z. Prevalence of mastitis pathogens in milk from clinically healthy cows. Vet. Med. 2013, 58, 567–575. [Google Scholar] [CrossRef]
- Benić, M.; Maćešić, N.; Cvetnić, L.; Habrun, B.; Cvetnić, Ž.; Turk, R.; Đuričić, D.; Lojkić, M.; Dobranić, V.; Valpotić, H. Bovine mastitis: A persistent and evolving problem requiring novel approaches for its control—A review. Vet. Arh. 2018, 88, 535–557. [Google Scholar] [CrossRef]
- Maletić, M.; Magaš, V.; Maletić, J. The role of veterinarian in the monitoring programs of mastitis control. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017; p. 012035. [Google Scholar]
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments—A review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Hillerton, J.; Berry, E. A review. Treating mastitis in the cow-a tradition or an archaism. J. Appl. Microbiol. 2005, 98, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Abdi, R.D.; Gillespie, B.E.; Ivey, S.; Pighetti, G.M.; Almeida, R.A.; Kerro Dego, O. Antimicrobial resistance of major bacterial pathogens from dairy cows with high somatic cell count and clinical mastitis. Animals 2021, 11, 131. [Google Scholar] [CrossRef]
- Iwu, C.D.; Korsten, L.; Okoh, A.I. The incidence of antibiotic resistance within and beyond the agricultural ecosystem: A concern for public health. Microbiologyopen 2020, 9, e1035. [Google Scholar] [CrossRef]
- Graham, D.W.; Bergeron, G.; Bourassa, M.W.; Dickson, J.; Gomes, F.; Howe, A.; Kahn, L.H.; Morley, P.S.; Scott, H.M.; Simjee, S. Complexities in understanding antimicrobial resistance across domesticated animal, human, and environmental systems. Ann. N. Y. Acad. Sci. 2019, 1441, 17–30. [Google Scholar] [CrossRef]
- Schar, D.; Sommanustweechai, A.; Laxminarayan, R.; Tangcharoensathien, V. Surveillance of antimicrobial consumption in animal production sectors of low-and middle-income countries: Optimizing use and addressing antimicrobial resistance. PLoS Med. 2018, 15, e1002521. [Google Scholar] [CrossRef]
- Medicines and Medical Devices Agency of Serbia. Summary of Product Characteristics-DRYCLOXA-KEL. Available online: https://www.alims.gov.rs/doc_file/lekovi_veterina/smpc/323-01-00034-20-001.pdf (accessed on 1 November 2022).
- Medicines and Medical Devices Agency of Serbia. Summary of Product Characteristics-RILEXINE 500. Available online: https://www.alims.gov.rs/doc_file/lekovi_veterina/smpc/323-01-00018-17-001.pdf (accessed on 1 November 2022).
- Zoopharm. Summary of Product Characteristics-CEFIMAM®. Available online: http://zoopharm.rs/cefimam-lc.html (accessed on 1 November 2022).
- Medicines and Medical Devices Agency of Serbia. Summary of Product Characteristics-DAIRYMED. Available online: https://www.alims.gov.rs/doc_file/lekovi_veterina/smpc/323-01-00005-18-003.pdf (accessed on 1 November 2022).
- Medicines and Medical Devices Agency of Serbia. Summary of Product Characteristics-MASTIJET FORTE. Available online: https://www.alims.gov.rs/doc_file/lekovi_veterina/smpc/323-01-00433-16-001.pdf (accessed on 1 August 2022).
- Medicines and Medical Devices Agency of Serbia. Summary of Product Characteristics-RILEXINE 200 LC. Available online: https://www.alims.gov.rs/doc_file/lekovi_veterina/smpc/323-01-00317-19-001.pdf (accessed on 1 August 2022).
- Medicines and Medical Devices Agency of Serbia. Summary of Product Characteristics-UBROLEXIN. Available online: https://www.alims.gov.rs/doc_file/lekovi_veterina/smpc/323-01-00321-18-001.pdf (accessed on 1 November 2022).
- Medicines and Medical Devices Agency of Serbia. Summary of Product Characteristics-COBACTAN. Available online: https://www.alims.gov.rs/doc_file/lekovi_veterina/smpc/323-01-00109-19-002.pdf (accessed on 1 November 2022).
- Medicines and Medical Devices Agency of Serbia. Summary of Product Characteristics-ENROCIN S 10%. Available online: https://www.alims.gov.rs/doc_file/lekovi_veterina/smpc/323-01-00384-21-001.pdf (accessed on 1 August 2022).
- Medicines and Medical Devices Agency of Serbia. Summary of Product Characteristics-KELBOMAR. Available online: https://www.alims.gov.rs/doc_file/lekovi_veterina/smpc/323-01-00143-21-001.pdf (accessed on 1 August 2022).
- Zoetis. Summary of Product Characteristics-SYNULOX RTU. Available online: https://www2.zoetis.rs/ (accessed on 1 November 2022).
- Medicines and Medical Devices Agency of Serbia. Summary of Product Characteristics-PATHOZONE. Available online: http://www.korvetteam.rs/img/pathozone%20SmPC.pdf (accessed on 1 November 2022).
- Medicines and Medical Devices Agency of Serbia. Summary of Product Characteristics-RILEXINE. Available online: https://www.alims.gov.rs/doc_file/lekovi_veterina/smpc/323-01-00319-19-001.pdf (accessed on 1 November 2022).
- Medicines and Medical Devices Agency of Serbia. Summary of Product Characteristics-Baytril MAX®. Available online: https://www.alims.gov.rs/doc_file/lekovi_veterina/smpc/323-01-00248-21-002.pdf (accessed on 1 November 2022).
- Medicines and Medical Devices Agency of Serbia. Summary of Product Characteristics-PENSTREP. Available online: https://www.alims.gov.rs/doc_file/lekovi_veterina/smpc/323-01-00324-18-001.pdf (accessed on 1 August 2022).
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e316–e327. [Google Scholar] [CrossRef]
- Scott, A.M.; Beller, E.; Glasziou, P.; Clark, J.; Ranakusuma, R.W.; Byambasuren, O.; Bakhit, M.; Page, S.W.; Trott, D.; Del Mar, C. Is antimicrobial administration to food animals a direct threat to human health? A rapid systematic review. Int. J. Antimicrob. Agents 2018, 52, 316–323. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dölz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef]
- Bennani, H.; Mateus, A.; Mays, N.; Eastmure, E.; Stärk, K.D.; Häsler, B. Overview of evidence of antimicrobial use and antimicrobial resistance in the food chain. Antibiotics 2020, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, U.; Moodley, A.; Osbjer, K. Antimicrobial resistance at the livestock-human interface: Implications for Veterinary Services. Rev. Sci. Tech. 2021, 40, 511–521. [Google Scholar] [CrossRef]
- Ungemach, F.R.; Müller-Bahrdt, D.; Abraham, G. Guidelines for prudent use of antimicrobials and their implications on antibiotic usage in veterinary medicine. Int. J. Med. Microbiol. 2006, 296, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J. Mastitis therapy and antimicrobial susceptibility: A multispecies review with a focus on antibiotic treatment of mastitis in dairy cattle. J. Mammary Gland Biol. Neoplasia 2011, 16, 383–407. [Google Scholar] [CrossRef]
- Beuron, D.C.; Cortinhas, C.S.; Botaro, B.G.; Macedo, S.N.; Gonçalves, J.L.; Brito, M.A.; Santos, M.V. Risk factors associated with the antimicrobial resistance of Staphylococcus aureus isolated from bovine mastitis. Pesqui. Veterinária Bras. 2014, 34, 947–952. [Google Scholar] [CrossRef][Green Version]
- Kovačević, Z.; Radinović, M.; Čabarkapa, I.; Kladar, N.; Božin, B. Natural agents against bovine mastitis pathogens. Antibiotics 2021, 10, 205. [Google Scholar] [CrossRef]
- Tomanić, D.; Božin, B.; Kladar, N.; Stanojević, J.; Čabarkapa, I.; Stilinović, N.; Apić, J.; Božić, D.D.; Kovačević, Z. Environmental Bovine Mastitis Pathogens: Prevalence, Antimicrobial Susceptibility, and Sensitivity to Thymus vulgaris L., Thymus serpyllum L., and Origanum vulgare L. Essential Oils. Antibiotics 2022, 11, 1077. [Google Scholar] [CrossRef]
- Pol, M.; Ruegg, P. Treatment practices and quantification of antimicrobial drug usage in conventional and organic dairy farms in Wisconsin. J. Dairy Sci. 2007, 90, 249–261. [Google Scholar] [CrossRef]
- Radinović, M.; Davidov, I.; Kovačević, Z.; Stojanović, D.; Galfi, A.; Erdeljan, M. Osnovni principi terapije mastitisa krava. Ветеринарски Журнал Републике Српске 2019, 19, 105–109. [Google Scholar] [CrossRef]
- Martins, L.; Gonçalves, J.L.; Leite, R.F.; Tomazi, T.; Rall, V.L.; Santos, M.V. Association between antimicrobial use and antimicrobial resistance of Streptococcus uberis causing clinical mastitis. J. Dairy Sci. 2021, 104, 12030–12041. [Google Scholar] [CrossRef]
- Mišić, M.; Arsović, A.; Čukić, J.; Rosić, M.I.; Tošić-Pajić, J.; Manojlović, N.; Čekerevac, I.; Vidanović, D.; Šekler, M.; Baskić, D. The prevalence of resistance to macrolides and lincosamides among community-and hospital-acquired staphylococci and streptococci isolates in southeast Serbia. Srp. Arh. Za Celok. Lek. 2018, 146, 384–390. [Google Scholar] [CrossRef]
- Versporten, A.; Bolokhovets, G.; Ghazaryan, L.; Abilova, V.; Pyshnik, G.; Spasojevic, T.; Korinteli, I.; Raka, L.; Kambaralieva, B.; Cizmovic, L. Antibiotic use in eastern Europe: A cross-national database study in coordination with the WHO Regional Office for Europe. Lancet Infect. Dis. 2014, 14, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Vakanjac, S.; Pavlović, V.; Magaš, V.; Pavlović, M.; Đurić, M.; Maletić, M.; Nedić, S.; Sočo, I. Investigations of efficacy of intramammary applied antimicrobials and glucocorticosteroides in the treatment of subclinical and clinical mastitis in cows. Vet. Glas. 2013, 67, 15–27. [Google Scholar] [CrossRef]
- Anđelković, J.; Radonjić, V. Usage of intramammary antimicrobial veterinary medicinal products in the republic of Serbia from 2011 to 2014. Serb. J. Exp. Clin. Res. 2017, 18, 27–31. [Google Scholar] [CrossRef]
- Saini, V.; McClure, J.T.; Scholl, D.; DeVries, T.; Barkema, H. Herd-level association between antimicrobial use and antimicrobial resistance in bovine mastitis Staphylococcus aureus isolates on Canadian dairy farms. J. Dairy Sci. 2012, 95, 1921–1929. [Google Scholar] [CrossRef]
- Harbarth, S.; Balkhy, H.H.; Goossens, H.; Jarlier, V.; Kluytmans, J.; Laxminarayan, R.; Saam, M.; Van Belkum, A.; Pittet, D. Antimicrobial Resistance: One world, One Fight! Antimicrob. Resist. Infect. Control 2015, 4, 49. [Google Scholar] [CrossRef]
- WHO. Worldwide Country Situation Analysis: Response to Antimicrobial Resistance: Summary; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Hameed, K.G.A.; Sender, G.; Korwin-Kossakowska, A. Public health hazard due to mastitis in dairy cows. Anim. Sci. Pap. Rep. 2007, 25, 73–85. [Google Scholar]
- McEwen, S.A.; Fedorka-Cray, P.J. Antimicrobial use and resistance in animals. Clin. Infect. Dis. 2002, 34, S93–S106. [Google Scholar] [CrossRef]
- Scott, H.M.; Acuff, G.; Bergeron, G.; Bourassa, M.W.; Gill, J.; Graham, D.W.; Kahn, L.H.; Morley, P.S.; Salois, M.J.; Simjee, S. Critically important antibiotics: Criteria and approaches for measuring and reducing their use in food animal agriculture. Ann. N. Y. Acad. Sci. 2019, 1441, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Schalm, O. Experiments and observations leading to development of the California Mastitis Test. J. Am. Vet. Med. Assoc. 1957, 130, 199–204. [Google Scholar]
- National Mastitis Council. Microbiological Procedures for the Diagnosis of Udder Infection, 3rd ed.; National Mastitis Council Inc.: New Prague, MN, USA, 2004. [Google Scholar]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Protocol; American Society for Microbiology (ASM): Washington, DC, USA, 2009. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 3rd ed.; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard M02-A12; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Allerton, F.; Prior, C.; Bagcigil, A.F.; Broens, E.; Callens, B.; Damborg, P.; Dewulf, J.; Filippitzi, M.-E.; Carmo, L.P.; Gómez-Raja, J. Overview and evaluation of existing guidelines for rational antimicrobial use in small-animal veterinary practice in Europe. Antibiotics 2021, 10, 409. [Google Scholar] [CrossRef] [PubMed]

| Brand Name | Intramammary Antibiotic | Amount per Syringe or mL | Number of Syringe or mL | Dose * | Frequency, Times/Day | DDD | Quantity |
|---|---|---|---|---|---|---|---|
| Intramammary dry cow therapy | |||||||
| Drycloxa-kel® [24] | cloxacillin | 1000 mg | 4 | - | 1 | 4000 mg | 36 im |
| Rilexine 500® [25] | cefalexin | 375 mg | 1 | - | 1 | 375 mg | 11 im |
| Intramammary lactating mastitis | |||||||
| Cefimam® [26] | cefquinome | 75 mg | 3 | - | 1 | 225 mg | 192 im |
| Dairymed® [27] | amoxicillin | 200 mg | 3 | - | 1 | 600 mg | 5 im |
| clavulanic acid | 50 mg | 3 | - | 1 | 150 mg | ||
| Mastijet® forte [28] | tetracycline | 200 mg | 2 | - | 2 | 800 mg | 20 im |
| neomycin | 250 mg | 2 | - | 2 | 1000 mg | ||
| bacitracin | 2000 IU | 2 | - | 2 | 8000 IU | ||
| Rilexine® 200 [29] | cefalexin | 200 mg | 2 | - | 2 | 800 mg | 195 im |
| Ubrolexin® [30] | cefalexin | 200 mg | 2 | - | 1 | 400 mg | 40 im |
| kanamycin | 100,000 IU | 2 | - | 1 | 200,000 IU | ||
| Parenteral therapy | |||||||
| Cobactan® [31] | cefquinome | 25 mg | - | 1 mg/kg | 1 | 680 mg | 37 inj |
| Enrocin S® [32] | enrofloxacin | 100 mg | - | 5 mg/kg | 1 | 3400 mg | 24 inj |
| Kelbomar® [33] | marbofloxacin | 100 mg | - | 2 mg/kg | 1 | 1360 mg | 36 inj |
| Synulox® RTU [34] | amoxicillin | 140 mg | - | 7 mg | 1 | 4760 mg | 39 inj |
| clavulanic acid | 35 mg | - | 1.75 mg | 1 | 1190 mg |
| Brand Name | Intramammary Antibiotic | Amount per Syringe or mL | Number of Syringe or mL | Dose * (mg/kg) | Frequency, Times/Day | DDD | Quantity |
|---|---|---|---|---|---|---|---|
| Intramammary dry cow therapy | |||||||
| Rilexine 500® [25] | cefalexin | 375 mg | 1 | - | 1 | 375 mg | 20 im |
| Intramammary lactating mastitis | |||||||
| Mastijet forte® [28] | tetracycline | 200 mg | 2 | - | 2 | 800 mg | 1588 inj |
| neomycin | 250 mg | 2 | - | 2 | 1000 mg | ||
| bacitracin | 2000 IU | 2 | - | 2 | 8000 IU | ||
| Dairymed® [27] | amoxicillin | 200 mg | 3 | - | 1 | 600 mg | 54 inj |
| clavulanic acid | 50 mg | 3 | - | 1 | 150 mg | ||
| Pathozone® [35] | cefoperazone | 250 mg | 1 | - | 1 | 250 mg | 6 inj |
| Parenteral therapy | |||||||
| Cobactan® [31] | cefquinome | 25 mg | - | 1 | 1 | 680 mg | 7920 mL |
| Rilexine® [36] | cefalexin | 150 mg | - | 15 | 1 | 10,200 mg | 1200 mL |
| Baytril® MAX [37] | enrofloxacin | 100 mg | - | 5 | 1 | 3400 mg | 90 mL |
| Brand Name | Intramammary Antibiotic | Amount per Syringe or mL | Number of Syringe or mL | Dose * (mg/kg) | Frequency, Times/Day | DDD | Quantity |
|---|---|---|---|---|---|---|---|
| Intramammary dry cow therapy | |||||||
| Drycloxa-kel® [24] | cloxacillin | 1000 mg | 4 | - | 1 | 4000 mg | 156 im |
| Intramammary lactating mastitis | |||||||
| Cefimam® [26] | cefquinome | 75 mg | 3 | - | 1 | 225 mg | 1296 im |
| Mastijet forte® [28] | tetracycline | 200 mg | 2 | - | 2 | 800 mg | 37 im |
| neomycin | 250 mg | 2 | - | 2 | 1000 mg | ||
| bacitracin | 2000 IU | 2 | - | 2 | 8000 IU | ||
| Parenteral therapy | |||||||
| Kelbomar® [33] | marbofloxacin | 100 mg | - | 2 | 1 | 1360 mg | 93 inj |
| Penstrep® [38] | penicillin | 200 mg | - | 8 | 1 | 5400 mg | 298 |
| streptomycin | 250 mg | - | 10 | 1 | 6800 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačević, Z.; Samardžija, M.; Horvat, O.; Tomanić, D.; Radinović, M.; Bijelić, K.; Vukomanović, A.G.; Kladar, N. Is There a Relationship between Antimicrobial Use and Antibiotic Resistance of the Most Common Mastitis Pathogens in Dairy Cows? Antibiotics 2023, 12, 3. https://doi.org/10.3390/antibiotics12010003
Kovačević Z, Samardžija M, Horvat O, Tomanić D, Radinović M, Bijelić K, Vukomanović AG, Kladar N. Is There a Relationship between Antimicrobial Use and Antibiotic Resistance of the Most Common Mastitis Pathogens in Dairy Cows? Antibiotics. 2023; 12(1):3. https://doi.org/10.3390/antibiotics12010003
Chicago/Turabian StyleKovačević, Zorana, Marko Samardžija, Olga Horvat, Dragana Tomanić, Miodrag Radinović, Katarina Bijelić, Annamaria Galfi Vukomanović, and Nebojša Kladar. 2023. "Is There a Relationship between Antimicrobial Use and Antibiotic Resistance of the Most Common Mastitis Pathogens in Dairy Cows?" Antibiotics 12, no. 1: 3. https://doi.org/10.3390/antibiotics12010003
APA StyleKovačević, Z., Samardžija, M., Horvat, O., Tomanić, D., Radinović, M., Bijelić, K., Vukomanović, A. G., & Kladar, N. (2023). Is There a Relationship between Antimicrobial Use and Antibiotic Resistance of the Most Common Mastitis Pathogens in Dairy Cows? Antibiotics, 12(1), 3. https://doi.org/10.3390/antibiotics12010003







