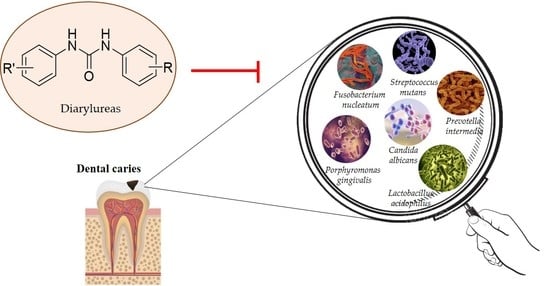
Diarylureas: New Promising Small Molecules against Streptococcus mutans for the Treatment of Dental Caries
Abstract
1. Introduction
2. Dental Caries
2.1. Diagnostics and Risk Factors
2.2. Streptococcus mutans
2.3. Prevention of Dental Caries
2.4. Treatment Strategies
2.4.1. Antimicrobials
2.4.2. Diarylureas
| Structure | Name or Number | Ref | Mechanism of Action |
|---|---|---|---|
 | Schreiner’s Catalyst | Sigma Aldrich (Italy) | |
| 19 | Nelson et al., 2015 [92] |
| |
| BPU | Liao et al., 2022 [24] |
| |
 | ComAI | Kaur et al., 2016 [102] |
|
| DMTU | Kaur et al., 2017 [103]; Kalimuthu et al., 2020 [25] |
| |
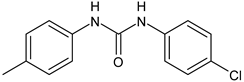 | ComAI1 | Kaur et al., 2016 [102] |
|
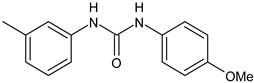 | ComAI2 | Kaur et al., 2016 [102] | |
 | ComAI3 | Kaur et al., 2016 [102] | |
 | ComAI4 | Kaur et al., 2016 [102] | |
 | 5 | Pujol et al., 2018 [105] |
|
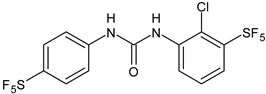 | 6 | Pujol et al., 2018 [105] | |
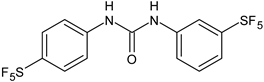 | 9 | Pujol et al., 2018 [105] | |
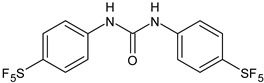 | 10 | Pujol et al., 2018 [105] | |
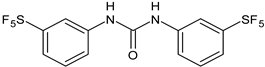 | 11 | Pujol et al., 2018 [105] | |
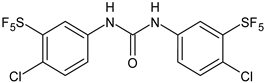 | 14 | Pujol et al., 2018 [105] |
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, L.J.; Lamster, I.B.; Greenspan, J.S.; Pitts, N.B.; Scully, C.; Warnakulasuriya, S. Global burden of oral diseases: Emerging concepts, management and interplay with systemic health. Oral Dis. 2016, 22, 609–619. [Google Scholar] [CrossRef]
- Wen, P.Y.F.; Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M. Global Burden and Inequality of Dental Caries, 1990 to 2019. J. Dent. Res. 2022, 101, 392–399. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreno, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Hajishengallis, E.; Parsaei, Y.; Klein, M.I.; Koo, H. Advances in the microbial etiology and pathogenesis of early childhood caries. Mol. Oral Microbiol. 2017, 32, 24–34. [Google Scholar] [CrossRef]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Van Houte, J. Role of micro-organisms in caries etiology. J. Dent. Res. 1994, 73, 672–681. [Google Scholar] [CrossRef]
- Soleimani, B.; Goli, H.; Naranjian, M.; Mousavi, S.J.; Nahvi, A. Comparison of antimicrobial activity of fluoride varnishes against Streptococcus mutans and Lactobacillus acidophilus: An in vitro study. Iran. J. Pediatr. 2021, 31, e111422. [Google Scholar] [CrossRef]
- Metwalli, K.H.; Khan, S.A.; Krom, B.P.; Jabra-Rizk, M.A. Streptococcus mutans, Candida albicans, and the human mouth: A sticky situation. PLoS Pathog. 2013, 9, e1003616. [Google Scholar] [CrossRef]
- Xiao, J.; Moon, Y.; Li, L.; Rustchenko, E.; Wakabayashi, H.; Zhao, X.; Feng, C.; Gill, S.R.; McLaren, S.; Malmstrom, H.; et al. Candida albicans carriage in children with severe early childhood caries (S-ECC) and maternal relatedness. PLoS ONE 2016, 11, e0164242. [Google Scholar] [CrossRef]
- Deng, L.; Li, W.; He, Y.; Wu, J.; Ren, B.; Zou, L. Cross-kingdom interaction of Candida albicans and Actinomyces viscosus elevated cariogenic virulence. Arch. Oral Biol. 2019, 100, 106–112. [Google Scholar] [CrossRef]
- Hwang, G. In it together: Candida-bacterial oral biofilms and therapeutic strategies. Environ. Microbiol. Rep. 2022, 14, 183–196. [Google Scholar] [CrossRef]
- Hernandez, P.; Sanchez, M.C.; Llama-Palacios, A.; Ciudad, M.J.; Collado, L. Strategies to combat caries by maintaining the integrity of biofilm and homeostasis during the rapid phase of supragingival plaque formation. Antibiotics 2022, 11, 880. [Google Scholar] [CrossRef]
- Whelton, H.P.; Spencer, A.J.; Do, L.G.; Rugg-Gunn, A.J. Fluoride revolution and dental caries: Evolution of policies for global use. J. Dent. Res. 2019, 98, 837–846. [Google Scholar] [CrossRef]
- Shahmoradi, M.; Hunter, N.; Swain, M. Efficacy of Fluoride Varnishes with Added Calcium Phosphate in the Protection of the Structural and Mechanical Properties of Enamel. BioMed Res. Int. 2017, 2017, 7834905. [Google Scholar] [CrossRef]
- Johnston, N.R.; Strobel, S.A. Principles of fluoride toxicity and the cellular response: A review. Arch. Toxicol. 2020, 94, 1051–1069. [Google Scholar] [CrossRef]
- Qiu, W.; Zhou, Y.; Li, Z.; Huang, T.; Xiao, Y.; Cheng, L.; Peng, X.; Zhang, L.; Ren, B. Application of antibiotics/antimicrobial agents on dental caries. BioMed Res. Int. 2020, 2020, 5658212. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug resistance (MDR): A widespread phenomenon in pharmacological therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef]
- Gostemeyer, G.; Schulze, F.; Paris, S.; Schwendicke, F. Arrest of root carious lesions via sodium fluoride, chlorhexidine and silver diamine fluoride in vitro. Materials 2017, 11, 9. [Google Scholar] [CrossRef]
- Pukallus, M.L.; Plonka, K.A.; Barnett, A.G.; Walsh, L.J.; Holcombe, T.F.; Seow, W.K. A randomised, controlled clinical trial comparing chlorhexidine gel and low-dose fluoride toothpaste to prevent early childhood caries. Int. J. Paediatr. Dent. 2013, 23, 216–224. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.; Yang, R.; Xu, X. Small molecule compounds, a novel strategy against Streptococcus mutans. Pathogens 2021, 10, 1540. [Google Scholar] [CrossRef] [PubMed]
- Atta, L.; Khalil, R.; Khan, K.M.; Zehra, M.; Saleem, F.; Nur, E.A.M.; Ul-Haq, Z. Virtual screening, synthesis and biological evaluation of Streptococcus mutans mediated biofilm inhibitors. Molecules 2022, 27, 1455. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lyu, X.; Zhang, J.; Shui, Y.; Yang, R.; Xu, X. The Application of Small Molecules to the Control of Typical Species Associated with Oral Infectious Diseases. Front. Cell. Infect. Microbiol. 2022, 12, 816386. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zhang, M.; Lin, X.; Yan, F. Diaryl urea derivative molecule inhibits cariogenic Streptococcus mutans by affecting exopolysaccharide synthesis, stress response, and nitrogen metabolism. Front. Cell. Infect. Microbiol. 2022, 12, 904488. [Google Scholar] [CrossRef]
- Kalimuthu, S.; Cheung, B.P.K.; Yau, J.Y.Y.; Shanmugam, K.; Solomon, A.P.; Neelakantan, P. A novel small molecule, 1,3-di-m-tolyl-urea, inhibits and disrupts multispecies oral biofilms. Microorganisms 2020, 8, 1261. [Google Scholar] [CrossRef]
- Nagata, J.Y.; Hioka, N.; Kimura, E.; Batistela, V.R.; Terada, R.S.; Graciano, A.X.; Baesso, M.L.; Hayacibara, M.F. Antibacterial photodynamic therapy for dental caries: Evaluation of the photosensitizers used and light source properties. Photodiagn. Photodyn. Ther. 2012, 9, 122–131. [Google Scholar] [CrossRef]
- Terra-Garcia, M.; de Souza, C.M.; Ferreira Goncalves, N.M.; Pereira, A.H.C.; de Barros, P.P.; Borges, A.B.; Miyakawa, W.; Strixino, J.F.; Junqueira, J.C. Antimicrobial effects of photodynamic therapy with Fotoenticine on Streptococcus mutans isolated from dental caries. Photodiagn. Photodyn. Ther. 2021, 34, 102303. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Pellegrino, M.; Mariconda, A.; Longo, P.; Sinicropi, M.S.; Aquaro, S. COVID-19 at a glance: An up-to-date overview on variants, drug design and therapies. Viruses 2022, 14, 573. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Maio, A.C.; Basile, G.; Giuzio, F.; Bonomo, M.G.; Aquaro, S.; Walsh, T.J.; Sinicropi, M.S.; et al. Are nutraceuticals effective in COVID-19 and post-COVID prevention and treatment? Foods 2022, 11, 2884. [Google Scholar] [CrossRef]
- Dickson-Swift, V.; Kangutkar, T.; Knevel, R.; Down, S. The impact of COVID-19 on individual oral health: A scoping review. BMC Oral Health 2022, 22, 422. [Google Scholar] [CrossRef]
- Wdowiak-Szymanik, A.; Wdowiak, A.; Szymanik, P.; Grocholewicz, K. Pandemic COVID-19 influence on adult’s oral hygiene, dietary habits and caries disease-literature review. Int. J. Environ. Res. Public Health 2022, 19, 12744. [Google Scholar] [CrossRef] [PubMed]
- Rathee, M.; Sapra, A. Dental Caries. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2020. [Google Scholar]
- Jenkinson, H.F.; Lamont, R.J. Oral microbial communities in sickness and in health. Trends Microbiol. 2005, 13, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef]
- Bernabe, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; Arora, A.; et al. Global, Regional, and National levels and trends in burden of oral conditions from 1990 to 2017: A systematic analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar]
- WHO. Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 18 November 2022).
- Uribe, S.E.; Innes, N.; Maldupa, I. The global prevalence of early childhood caries: A systematic review with meta-analysis using the WHO diagnostic criteria. Int. J. Paediatr. Dent. 2021, 31, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Cui, T.; Zeng, J.; Chen, L.; Zhang, W.; Xu, X.; Cheng, L.; Li, M.; Li, J.; Zhou, X.; et al. Molecule targeting glucosyltransferase inhibits Streptococcus mutans biofilm formation and virulence. Antimicrob. Agents Chemother. 2016, 60, 126–135. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, J.; Zhou, X.; Li, Y. Inhibition of Streptococcus mutans biofilm formation by strategies targeting the metabolism of exopolysaccharides. Crit. Rev. Microbiol. 2021, 47, 667–677. [Google Scholar] [CrossRef]
- Momeni-Moghaddam, M.; Hashemi, C.; Fathi, A.; Khamesipour, F. Diagnostic accuracy, available treatment, and diagnostic methods of dental caries in practice: A meta-analysis. Beni Suef Univ. J. Basic Appl. Sci. 2022, 11, 62. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, L.; Yue, L.; Ling, J.; Fan, M.; Yang, D.; Huang, Z.; Niu, Y.; Liu, J.; Zhao, J.; et al. Expert consensus on dental caries management. Int. J. Oral Sci. 2022, 14, 17. [Google Scholar] [CrossRef]
- Ahmad, P.; Hussain, A.; Carrasco-Labra, A.; Siqueira, W.L. Salivary proteins as dental caries biomarkers: A Systematic review. Caries Res. 2022, 56, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Chismirina, S.; Sungkar, S.; Andayani, R.; Rezeki, S.; Darmawi. Existence of Streptococcus mutans and Streptococcus sobrinus in oral cavity as main cariogenc bacteria of dental caries. In Proceedings of the 1st Aceh International Dental Meeting (AIDEM 2019), Oral Health International Conference on Art, Nature and Material Science Development 2019. Adv. Health Sci. Res. 2021, 32, 90–92. [Google Scholar]
- Zhou, Q.; Qin, X.; Qin, M.; Ge, L. Genotypic diversity of Streptococcus mutans and Streptococcus sobrinus in 3–4-year-old children with severe caries or without caries. Int. J. Paediatr. Dent 2011, 21, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Szkaradkiewicz, A.K. Microbiology of dental caries. J. Biol. Earth Sci. 2013, 3, M21–M24. [Google Scholar]
- Zhu, B.; Macleod, L.C.; Kitten, T.; Xu, P. Streptococcus sanguinis biofilm formation & interaction with oral pathogens. Future Microbiol. 2018, 13, 915–932. [Google Scholar]
- Diaz-Garrido, N.; Lozano, C.P.; Kreth, J.; Giacaman, R.A. Extended biofilm formation time by Streptococcus sanguinis modifies its non-cariogenic behavior, in vitro. Braz. Oral Res. 2022, 36, e107. [Google Scholar] [CrossRef]
- Paster, B.J.; Boches, S.K.; Galvin, J.L.; Ericson, R.E.; Lau, C.N.; Levanos, V.A.; Sahasrabudhe, A.; Dewhirst, F.E. Bacterial diversity in human subgingival plaque. J. Bacteriol. 2001, 183, 3770–3783. [Google Scholar] [CrossRef]
- Hoshino, E. Predominant obligate anaerobes in human carious dentin. J. Dent. Res. 1985, 64, 1195–1198. [Google Scholar] [CrossRef]
- Bachtiar, E.W.; Bachtiar, B.M. Relationship between Candida albicans and Streptococcus mutans in early childhood caries, evaluated by quantitative PCR. F1000Research 2018, 7, 1645. [Google Scholar] [CrossRef]
- Chokshi, A.; Mahesh, P.; Sharada, P.; Chokshi, K.; Anupriya, S.; Ashwini, B.K. A correlative study of the levels of salivary Streptococcus mutans, lactobacilli and Actinomyces with dental caries experience in subjects with mixed and permanent dentition. J. Oral Maxillofac. Pathol. 2016, 20, 25–28. [Google Scholar] [CrossRef]
- Garcia, B.A.; Acosta, N.C.; Tomar, S.L.; Roesch, L.F.W.; Lemos, J.A.; Mugayar, L.R.F.; Abranches, J. Association of Candida albicans and Cbp(+) Streptococcus mutans with early childhood caries recurrence. Sci. Rep. 2021, 11, 10802. [Google Scholar] [CrossRef]
- Clarke, J.K. On the Bacterial Factor in the Ætiology of Dental Caries. Br. J. Exp. Pathol. 1924, 5, 141–147. [Google Scholar]
- Forssten, S.D.; Björklund, M.; Ouwehand, A.C. Streptococcus mutans, caries and simulation models. Nutrients 2010, 2, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Prince, A.; Roy, S.; McDonald, D. Exploration of the antimicrobial synergy between selected natural substances on Streptococcus mutans to identify candidates for the control of dental caries. Microbiol. Spectr. 2022, 10, e0235721. [Google Scholar] [CrossRef] [PubMed]
- Elyassi, M.; Babaeekhou, L.; Ghane, M. Streptococcus mutans and Streptococcus sobrinus contributions in dental caries in Iranian and Afghan children: A report from serotype distribution and novel STs. Arch. Oral Biol. 2022, 139, 105431. [Google Scholar] [CrossRef] [PubMed]
- Babaeekhou, L.; Ghane, M.; Ezatzade, F.; Eftekhari Toroghi, S. Streptococcus mutans and Streptococcus sobrinus distribution in the saliva and plaque of Iranian population: Higher prevalence of S. mutans serotypes f and k. Int. J. Dent. Hyg 2021, 19, 193–200. [Google Scholar] [CrossRef]
- Kusuma Eriwati, Y.; Putriani, D.; Geraldine, K.; Hermansyah, H. Fluoride and calcium release from peppermint-flavored fluoride varnish containing dicalcium-phosphate-dihydrate coated with xylitol. Saudi Dent. J. 2022, 34, 68–73. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, M.; Ma, Y.; Cao, L.; Xu, H.H.K.; Zhang, K.; Bai, Y. Effects of Fluoride and Calcium Phosphate Materials on Remineralization of Mild and Severe White Spot Lesions. BioMed Res. Int. 2019, 2019, 1271523. [Google Scholar] [CrossRef]
- Hamilton, I.R. Biochemical effects of fluoride on oral bacteria. J. Dent. Res. 1990, 69, 660–667. [Google Scholar] [CrossRef]
- Shah, S.G.; Bhaskar, V.; Chawla, S.; Venkataraghavan, K.; Choudhary, P.; Ganesh, M.; Trivedi, K. Efficacy of silver diamine fluoride as a topical fluoride agent compared to fluoride varnish and acidulated phosphate fluoride gel: An in vivo study. J. Dent. Child. 2014, 2, 5–12. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Herford, A.S.; Laino, L.; Cicciu, M. Stannous fluoride effects on enamel: A systematic review. Biomimetics 2020, 5, 41. [Google Scholar] [CrossRef]
- Zuo, H.; Chen, L.; Kong, M.; Qiu, L.; Lu, P.; Wu, P.; Yang, Y.; Chen, K. Toxic effects of fluoride on organisms. Life Sci. 2018, 198, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Toumba, K.J.; Twetman, S.; Splieth, C.; Parnell, C.; van Loveren, C.; Lygidakis, N. Guidelines on the use of fluoride for caries prevention in children: An updated EAPD policy document. Eur. Arch. Paediatr. Dent. 2019, 20, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Achmad, H. The impact of using fluoride in pediatric dentistry: A Systematic review. Ann. Roman. Soc. Cell. Biol. 2021, 25, 2816–2839. [Google Scholar]
- Seifo, N.; Robertson, M.; MacLean, J.; Blain, K.; Grosse, S.; Milne, R.; Seeballuck, C.; Innes, N. The use of silver diamine fluoride (SDF) in dental practice. Br. Dent. J. 2020, 228, 75–81. [Google Scholar] [CrossRef]
- Roshni, R.; Shetty, P.J. Nano Silver Fluoride for Arresting Dental Caries. J. Pharm. Sci. Res. 2020, 12, 1448–1451. [Google Scholar]
- Burns, J.; Hollands, K. Nano Silver Fluoride for preventing caries. Evid. Based Dent. 2015, 16, 8–9. [Google Scholar] [CrossRef]
- Zameer, M.; Birajdar, S.B.; Basheer, S.N.; Peeran, S.W.; Peeran, S.A.; Reddy, A. Nanosilver fluoride as a caries arresting agent: A narrative. Contemp. Pediatr. 2021, 2, 1–13. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, X.; Li, Y. Strategies for Streptococcus mutans biofilm dispersal through extracellular polymeric substances disruption. Mol. Oral Microbiol. 2022, 37, 1–8. [Google Scholar] [CrossRef]
- Piewngam, P.; Chiou, J.; Chatterjee, P.; Otto, M. Alternative approaches to treat bacterial infections: Targeting quorum-sensing. Expert Rev. Anti Infect. Ther. 2020, 18, 499–510. [Google Scholar] [CrossRef]
- Prazdnova, E.V.; Gorovtsov, A.V.; Vasilchenko, N.G.; Kulikov, M.P.; Statsenko, V.N.; Bogdanova, A.A.; Refeld, A.G.; Brislavskiy, Y.A.; Chistyakov, V.A.; Chikindas, M.L. Quorum-sensing inhibition by Gram-positive bacteria. Microorganisms 2022, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, G.M.; Horowitz, R.A.; Johnson, R.; Prestiano, R.A.; Klein, B.I. Oral biofilms and their connection to systemic health. Med. Res. Arch. 2022, 10, 1–18. [Google Scholar] [CrossRef]
- Balagopal, S.; Arjunkumar, R. Chlorhexidine: The gold standard antiplaque agent. J. Pharm. Sci. Res. 2013, 5, 270. [Google Scholar]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance Toward chlorhexidine in oral bacteria—Is there cause for concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Rema, T.; Medihala, P.; Lawrence, J.R.; Vidovic, S.; Leppard, G.G.; Reid, M.; Korber, D.R. Proteomic analyses of chlorhexidine tolerance mechanisms in Delftia acidovorans biofilms. mSphere 2016, 1, e00017-15. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Linares, M.; Ferrer-Luque, C.M.; Arias-Moliz, T.; de Castro, P.; Aguado, B.; Baca, P. Antimicrobial activity of alexidine, chlorhexidine and cetrimide against Streptococcus mutans biofilm. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 41. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Szkaradkiewicz, A.K. Chlorhexidine--pharmaco-biological activity and application. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1321–1326. [Google Scholar]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-bacterial properties of cannabigerol toward Streptococcus mutans. Front. Microbiol. 2021, 12, 656471. [Google Scholar] [CrossRef]
- Luong, A.D.; Buzid, A.; Luong, J.H.T. Important roles and potential uses of natural and synthetic antimicrobial peptides (AMPs) in oral diseases: Cavity, periodontal disease, and thrush. J. Funct. Biomater. 2022, 13, 175. [Google Scholar] [CrossRef]
- Niu, J.Y.; Yin, I.X.; Wu, W.K.K.; Li, Q.L.; Mei, M.L.; Chu, C.H. Antimicrobial peptides for the prevention and treatment of dental caries: A concise review. Arch. Oral Biol. 2021, 122, 105022. [Google Scholar] [CrossRef]
- Catalano, A. Diarylurea: A privileged scaffold in drug discovery and therapeutic development. Curr. Med. Chem. 2022, 29, 4302–4306. [Google Scholar] [CrossRef] [PubMed]
- Garuti, L.; Roberti, M.; Bottegoni, G.; Ferraro, M. Diaryl urea: A privileged structure in anticancer agents. Curr. Med. Chem. 2016, 23, 1528–1548. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Sinicropi, M.S.; Franchini, C. Diarylureas as antitumor agents. Appl. Sci. 2021, 11, 374. [Google Scholar] [CrossRef]
- York, E.; McNaughton, D.A.; Roseblade, A.; Cranfield, C.G.; Gale, P.A.; Rawling, T. Structure-Activity Relationship and Mechanistic studies of bisaryl urea anticancer agents indicate mitochondrial uncoupling by a fatty acid-activated mechanism. ACS Chem. Biol. 2022, 17, 2065–2073. [Google Scholar] [CrossRef]
- Gömeç, M.; Yulak, F.; Gezegen, H.; Özkaraca, M.; Sayin, K.; Ataseven, H. Synthesis of diaryl urea derivatives and evaluation of their antiproliferative activities in colon adenocarcinoma. J. Mol. Struct. 2022, 1254, 132318. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Pellegrino, M.; Aquaro, S.; Franchini, C.; Sinicropi, M.S. Diarylureas: Repositioning from antitumor to antimicrobials or multi-target agents against new pandemics. Antibiotics 2021, 10, 92. [Google Scholar] [CrossRef]
- Du, X.; Wang, M.; Hu, X.; Nie, T.; Zhu, M.; Zhang, G.; You, X.; Wang, Y. Synthesis and biological evaluation of novel N, N’-diarylurea derivatives as potent antibacterial agents against MRSA. Bioorg. Med. Chem. Lett. 2022, 75, 128975. [Google Scholar] [CrossRef]
- Catalano, A.; Ceramella, J.; Iacopetta, D.; Mariconda, A.; Scali, E.; Bonomo, M.G.; Saturnino, C.; Longo, P.; Aquaro, S.; Sinicropi, M.S. Thidiazuron: New trends and future perspectives to fight Xylella fastidiosa in olive trees. Antibiotics 2022, 11, 947. [Google Scholar] [CrossRef]
- Wang, C.; Hu, T.; Lu, J.; Lv, Y.; Ge, S.; Hou, Y.; He, H. Convenient diaryl ureas as promising anti-pseudo-allergic agents. J. Med. Chem. 2022, 65, 10626–10637. [Google Scholar] [CrossRef]
- Nelson, J.W.; Plummer, M.S.; Blount, K.F.; Ames, T.D.; Breaker, R.R. Small molecule fluoride toxicity agonists. Chem. Biol. 2015, 22, 527–534. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, G.; Yang, X.; Jiang, S.; Xue, W.; Chi, Y.R.; Jin, Z. Carbene-Catalyzed enantioselective aromatic N-nucleophilic addition of heteroarenes to ketones. Angew. Chem. Int. Ed. Engl. 2020, 59, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wu, Y.; Wu, X.; Zhou, M.; Zhou, R.; Wang, J.; Xiao, X.; Yuan, Y.; Liu, R. Facile synthesis of polypeptoids bearing bulky sidechains via urea accelerated ring-opening polymerization of α-amino acid N-substituted N-carboxyanhydrides. Polym. Chem. 2022, 13, 420–426. [Google Scholar] [CrossRef]
- Kavanagh, S.A.; Piccinini, A.; Fleming, E.M.; Connon, S.J. Urea derivatives are highly active catalysts for the base-mediated generation of terminal epoxides from aldehydes and trimethylsulfonium iodide. Org. Biomol. Chem. 2008, 6, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Horwitz, M.A.; Durr, A.B.; Ibba, F.; Pupo, G.; Gao, Y.; Ricci, P.; Christensen, K.E.; Pathak, T.P.; Claridge, T.D.W.; et al. Asymmetric azidation under hydrogen bonding phase-transfer catalysis: A combined experimental and computational study. J. Am. Chem. Soc. 2022, 144, 4572–4584. [Google Scholar] [CrossRef] [PubMed]
- Chandi, A.K.; Kaur, A. Hormone analogues and chitin synthesis inhibitors. In Molecular Approaches for Sustainable Insect Pest Management; Springer: Berlin/Heidelberg, Germany, 2021; pp. 253–282. [Google Scholar]
- Umar, W.; Majid, A. Effects of worker-soldier termite ratio on the mortality rate exposed to chlorfluazuron baits. In Proceedings of the 1st International Electronic Conference on Entomology, online, 1–15 July 2021; pp. 1–15. [Google Scholar]
- Busschaert, N.; Kirby, I.L.; Young, S.; Coles, S.J.; Horton, P.N.; Light, M.E.; Gale, P.A. Squaramides as potent transmembrane anion transporters. Angew. Chem. 2012, 124, 4502–4506. [Google Scholar] [CrossRef]
- Zhang, M.; Liao, Y.; Tong, X.; Yan, F. Novel urea derivative-loaded PLGA nanoparticles to inhibit caries-associated Streptococcus mutans. RSC Adv. 2022, 12, 4072–4080. [Google Scholar] [CrossRef] [PubMed]
- Denoyelle, S.; Chen, T.; Chen, L.; Wang, Y.; Klosi, E.; Halperin, J.A.; Aktas, B.H.; Chorev, M. In vitro inhibition of translation initiation by N,N′-diarylureas—Potential anti-cancer agents. Bioorg. Med. Chem. Lett. 2012, 22, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Balamurugan, P.; Uma Maheswari, C.; Anitha, A.; Princy, S.A. Combinatorial effects of aromatic 1,3-disubstituted ureas and fluoride on in vitro inhibition of Streptococcus mutans biofilm formation. Front. Microbiol. 2016, 7, 861. [Google Scholar] [CrossRef]
- Kaur, G.; Balamurugan, P.; Princy, S.A. Inhibition of the quorum sensing system (ComDE Pathway) by aromatic 1,3-di-m-tolylurea (DMTU): Cariostatic effect with fluoride in Wistar rats. Front. Cell. Infect. Microbiol. 2017, 7, 313. [Google Scholar] [CrossRef]
- Iacopetta, D.; Catalano, A.; Ceramella, J.; Saturnino, C.; Salvagno, L.; Ielo, I.; Drommi, D.; Scali, E.; Plutino, M.R.; Rosace, G.; et al. The different facets of triclocarban: A review. Molecules 2021, 26, 2811. [Google Scholar] [CrossRef]
- Pujol, E.; Blanco-Cabra, N.; Julián, E.; Leiva, R.; Torrents, E.; Vázquez, S. Pentafluorosulfanyl-containing triclocarban analogs with potent antimicrobial activity. Molecules 2018, 23, 2853. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Rosato, A.; Salvagno, L.; Ceramella, J.; Longo, F.; Sinicropi, M.S.; Franchini, C. Searching for small molecules as antibacterials: Non-cytotoxic diarylureas analogues of triclocarban. Antibiotics 2021, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Rosato, A.; Salvagno, L.; Iacopetta, D.; Ceramella, J.; Fracchiolla, G.; Sinicropi, M.S.; Franchini, C. Benzothiazole-Containing analogues of triclocarban with potent antibacterial activity. Antibiotics 2021, 10, 803. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacopetta, D.; Ceramella, J.; Catalano, A.; D’Amato, A.; Lauria, G.; Saturnino, C.; Andreu, I.; Longo, P.; Sinicropi, M.S. Diarylureas: New Promising Small Molecules against Streptococcus mutans for the Treatment of Dental Caries. Antibiotics 2023, 12, 112. https://doi.org/10.3390/antibiotics12010112
Iacopetta D, Ceramella J, Catalano A, D’Amato A, Lauria G, Saturnino C, Andreu I, Longo P, Sinicropi MS. Diarylureas: New Promising Small Molecules against Streptococcus mutans for the Treatment of Dental Caries. Antibiotics. 2023; 12(1):112. https://doi.org/10.3390/antibiotics12010112
Chicago/Turabian StyleIacopetta, Domenico, Jessica Ceramella, Alessia Catalano, Assunta D’Amato, Graziantonio Lauria, Carmela Saturnino, Inmaculada Andreu, Pasquale Longo, and Maria Stefania Sinicropi. 2023. "Diarylureas: New Promising Small Molecules against Streptococcus mutans for the Treatment of Dental Caries" Antibiotics 12, no. 1: 112. https://doi.org/10.3390/antibiotics12010112
APA StyleIacopetta, D., Ceramella, J., Catalano, A., D’Amato, A., Lauria, G., Saturnino, C., Andreu, I., Longo, P., & Sinicropi, M. S. (2023). Diarylureas: New Promising Small Molecules against Streptococcus mutans for the Treatment of Dental Caries. Antibiotics, 12(1), 112. https://doi.org/10.3390/antibiotics12010112