Abstract
Invasive candidiasis is an important cause of morbidity and mortality, and its occurrence is increasing due to the growing complexity of patients. In particular, Candida albicans exhibits several virulence factors that facilitate yeast colonization in humans. In this sense, the photodynamic inactivation of yeasts is a promising new alternative to eliminate fungal infections. Herein, the photodynamic activity sensitized by a free-base chlorin (TPCF16) and its complexes with Zn(II) (ZnTPCF16) and Pd(II) (PdTPCF16) was investigated in order to eliminate C. albicans under different forms of cell cultures. A decrease in cell survival of more than 5 log was found in planktonic cells incubated with 5 μM TPCF16 or ZnTPCF16 upon 15 min of white-light irradiation. The mechanism of action mainly involved a type II pathway in the inactivation of C. albicans cells. In addition, the photodynamic action induced by these chlorins was able to suppress the growth of C. albicans in a culture medium. These photosensitizers were also effective to photoinactivate C. albicans pseudohyphae suspended in PBS. Furthermore, the biofilms of C. albicans that incorporated the chlorins during the proliferation stage were completely eradicated using 5 μM TPCF16 or ZnTPCF16 after 60 min of light irradiation. The studies indicated that these chlorins are effective photosensitizing agents to eliminate C. albicans as planktonic cells, pseudohyphae, and biofilms.
1. Introduction
Fungal infections are becoming a major health problem, causing high mortality rates, medical costs for treatments, and an increase in hospitalized patients []. The incidence of fungal skin infections is continuously increasing all over the world, representing a great challenge for health care. In addition, hospital-acquired fungal infections are constantly increasing and are considerably problematic for immunocompromised patients []. In general, cutaneous and subcutaneous fungal diseases are caused by pathogenic or opportunistic organisms. In this sense, Candida albicans is one of the most important human pathogenic fungi []. This microorganism causes millions of cutaneous, mucosal, and systemic diseases that can be life-threatening. The establishment of fungal infections is mediated by virulence factors such as the yeast–hyphal transition and formation of biofilm []. Hyphae are necessary during infections in order to penetrate epithelial cell walls. Furthermore, biofilm production facilitates widespread hyphal growth and acts to cause invasive fungal diseases []. Therefore, biofilm formation represents a major virulence factor during candidiasis. Different biological media are capable of supporting C. albicans biofilm formation. Thus, the incidence of candidiasis increased with the increased use of medical devices in clinical practice []. In addition, these infections are aggravated by the appearance of yeasts resistant to conventional antifungal treatments [,]. This leads to the need to develop new antifungal therapies that can improve clinical outcomes for people with life-threatening fungal diseases worldwide []. In recent years, several antimicrobial strategies were planned to eliminate fungal infections and to decrease the development of virulence factors [].
For this purpose, the photodynamic inactivation (PDI) of yeasts represents an interesting alternative to treat fungal diseases or reduce the formation of virulence factors []. This therapy is based on the administration of a photosensitizing agent (PS) that is rapidly bound to microorganisms. Selective irradiation with an adequate wavelength in the visible region in aerobiosis leads to the generation of reactive oxygen species (ROS), which can then react with biomolecules to cause loss of functionality and, consequently, cell inactivation []. In this procedure, the triplet-excited state of the PS (3PS*) can interact with cell components by electron transfer or hydrogen abstraction to form free-radicals. In addition, these intermediates can react with ground state molecular oxygen (O2(3∑-g)) to produce superoxide anion radicals, hydroxyl radicals, and hydrogen peroxide by a type I pathway []. On the other hand, 3PS* can generate singlet molecular oxygen (O2(1Δg)) by energy transfer to O2(3∑-g) through a type II process [,]. Both mechanisms can act simultaneously, and the prevalence of one of them can depend mainly on the characteristics of the PS, the substrates, and the microenvironmental polarity []. Until now, PDI studies have not shown evidence of the development of microbial resistance related to the application of this therapy []. Therefore, this methodology represents a promising alternative for the elimination of fungal cells.
In particular, porphyrin derivatives and their metal complexes have presented interesting applications as PSs against human microbial pathogens through PDI [,]. In this study, we investigate the photodynamic effect induced by a chlorin derivative (TPCF16) and its complexes with Zn(II) (ZnTPCF16) and Pd(II) (PdTPCF16) (Figure 1) as PSs to eliminate C. albicans. Due to the background with chlorin derivatives, the compounds selected as PSs in this work may present an appropriate role as antifungal agents using photodynamic inactivation treatments. It was previously demonstrated that the free-base chlorin TPCF16 was an efficient PS to produce a high decrease in the cell viability of bacterial cells []. Furthermore, cationic chlorins derived of 5,10,15,20-tetrakis-(pentafluorophenyl)-2,3-[methano(N-methyl)iminomethano]chlorin (TPCF20) exhibited high PDI efficacy towards planktonic and biofilm forms of E. coli [,]. Herein, the spectroscopic properties and photodynamic activity of TPCF16, ZnTPCF16, and PdTPCF16 were established in homogenic solutions. Furthermore, the photoinactivation ability sensitized by these chlorins was evaluated in C. albicans under different cell culture conditions. First, photokilling was tested in planktonic yeast cell suspensions. Insights into the predominant mechanism of photodynamic action were established in cell suspensions using different ROS scavengers. In addition, the photoinactivation activity induced by these chlorins was determined with the growth of C. albicans in culture broth. Considering that reversible cell morphogenesis is an important virulence factor, these compounds were also assayed to eliminate C. albicans pseudohyphae. Lastly, the photoinactivating capacities of the chlorins were investigated in C. albicans biofilms for the prevention and control of these clinical microbial communities.
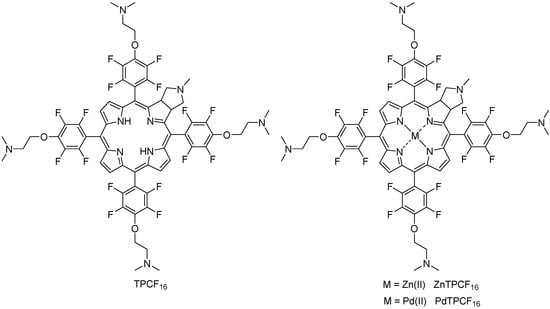
Figure 1.
Molecular structure of TPCF16 and its complexes with Zn(II) and Pd(II).
2. Materials and Methods
Details of materials and instrumentation are available in Supplementary Materials.
2.1. Spectroscopic Measurements
UV-visible absorption and fluorescence spectra were attained in a quartz cell of 1 cm path length at room temperature and by using N,N-dimethylformamide (DMF) as an organic solvent. Emission spectra were measured by exciting the samples at 408 nm. At this wavelength, the absorbances of the samples were approximately 0.05. The emission spectra were recorded and integrated in the range between 600 and 800 nm. The fluorescence quantum yields (ΦF) of the chlorins were determined by comparing the area of the emission spectrum for each PS with that of 5,10,15,20-tetra(4-methoxyphenyl)porphyrin (TMP), which was used as a reference (ΦF = 0.14) []. Excitation spectra were measured by following the fluorescence emissions of the chlorins at 700 nm.
2.2. Photooxidation of DMA
Samples containing 9,10-dimethylanthracene (DMA, 35 μM) and chlorin (A = 0.1 at 610 nm) in 2 mL of DMF were irradiated with light at λirr = 610 nm in a quartz cell of 1 cm path length. The photodecomposition of DMA was evaluated by the decrease in absorbance at λmax = 379 nm (Supplementary Figure S1). The values of the observed rate constants of DMA photo-oxidation (kobsDMA) were determined by a linear least-squares fit of the pseudo-first-order kinetic plots of ln(A0/A) vs. time. The quantum yields of O2(1Δg) production (ΦΔ) were determined by comparing the kobsDMA values for the corresponding chlorins with that of methylene blue (MB, ΦΔ = 0.52), which was used as a reference [].
2.3. Strains and Cultures of C. albicans
The C. albicans (PC31) strain was previously recognized and characterized []. Yeast cells were grown aerobically overnight in 4 mL Sabouraud broth at 37 °C until the stationary phase. The cells were collected via centrifugation of the culture broths (1200× g for 15 min). Then, the yeast cells were resuspended in phosphate-buffered saline (PBS, 4 mL, 10 mM, pH = 7.2) to harvest a cell suspension of ~107 colony forming units (CFU)/mL. After that, cultures were diluted 1/10 in PBS to obtain ~106 CFU/mL [].
2.4. Experiments in C. albicans Planktonic Cells
Assays were attained using 2 mL cell suspensions of ~106 CFU/mL in PBS, which were placed in Pyrex-brand culture tubes (13 × 100 mm). Yeast cells were incubated with 1 and 5 μM PS for 30 min in the dark at 37 °C. Chlorins were added from the stock solution to a volume of 0.5 mM in DMF. The amount of DMF used did not exceed 1% v/v, and this quantity of organic solvent was not toxic to C. albicans cells.
In the studies of the photodynamic mechanism of action, the following tests were performed separately: C. albicans cell suspensions (2 mL, ~106 CFU/mL) in PBS were treated with 50 mM of (1) sodium azide (50 mM), (2) diazabicyclo [2.2.2]octane (DABCO, 50 mM), (3) D-mannitol (50 mM), or (4) L-cysteine (50 mM), of which 1 M each was added from stock solutions into water [,]. Each cell culture was incubated for 30 min at 37 °C in the dark. Subsequently, 1 μM chlorine was added to each culture tube, and they were kept for another 30 min in the dark at 37 °C. Studies in deuterated water (D2O) were performed using cell suspensions (2 mL, ~106 CFU/mL) in PBS, which were centrifuged (1200× g for 15 min) and resuspended in D2O (2 mL). Then, the cell suspensions in D2O were incubated with 1 μM PS for 30 min in the dark at 37 °C.
In all experiments, 200 µL of cell suspensions were placed in wells of a 96-well microtiter plate, and they were irradiated with white light (90 mW/cm2). C. albicans cells were quantified by performing serial dilution in PBS and then counting with the spread plate method. Viable C. albicans cells were determined on Sabouraud agar plates after incubation for 48 h at 37 °C [,].
2.5. Growth Curves of C. albicans
Cultures of C. albicans cells were grown overnight as previously described []. An aliquot (1 mL) of yeast culture was transferred to 20 mL of Sabouraud broth in PBS. The cell suspension was homogenized, and portions of 2 mL each were treated with 5 µM PS in Pyrex brand culture tubes (13 × 100 mm). The tubes were continuously irradiated with white light (90 mW/cm2) at 37 °C. The growth of C. albicans cells was evaluated by analyzing spectroscopic determinations at 660 nm. The values at this wavelength were adjusted by subtracting the absorbance due to the chlorin.
2.6. Studies in C. albicans Pseudohyphae
C. albicans cells (~106 CFU/mL) were incubated in human serum (HS) for 4 h at 37 °C to produce the formation of pseudohyphae []. After this incubation period, the generation of germ tubes was established with optical microscopy []. Pseudohyphae were harvested via centrifugation (1200× g for 15 min) and suspended in PBS. Then, 2 mL of pseudohyphae suspension was placed in Pyrex brand culture tubes (13 × 100 mm). C. albicans pseudohyphae were incubated with 1 and 5 µM chlorin for 30 min in the dark at 37 °C. Culture aliquots (200 µL) were placed in wells of a 96-well microtiter plate and irradiated with white light (90 mW/cm2) for different periods of time (2, 5, 15 and 30 min). Viable C. albicans pseudohyphae were determined as reported [].
2.7. Tests in C. albicans Biofilms
C. albicans cell suspension (~107 CFU/mL) was prepared in PBS supplemented with 7% fetal bovine serum (FBS). Then, 900 µL of this culture was placed in the wells of a 48-well microtiter plate containing a polyvinylchloride (PVC) disc (5mm Ø × 1.5 mm) in each well. For the adhesion step, the plate was incubated for 90 min at 37 °C with shaking (75 r.p.m.). After that, each disc was removed and washed twice with PBS to remove non-adhered cells. In the proliferation step, the PVC discs in the 48-well microtiter plate were treated with 5 μM chlorin in Sabouraud broth supplemented with 7% FBS and incubated for 18 h at 37 °C. After that, the discs were washed with PBS and covered with 900 mL of PBS in the wells. The biofilms were irradiated with white light for 60 min. Then, each disc and the well contents were placed in a test tube, sonicated for 1 min, and vigorously vortexed for 2 min to detach cells from the biofilms on the disc. Viable C. albicans cells were determined as explained above.
2.8. Controls and Statistical Analysis
Controls of yeast cells were performed in the absence and presence of chlorin in the dark. In addition, controls were carried out in irradiated cells with white light (90 mW/cm2) in the absence of chlorin. In all C. albicans photoinactivation experiments, the temperature was maintained at 37 °C. This temperature did not affect the viability of the cells in the absence of PSs. Each experiment was repeated separately three times, and the values were achieved in triplicate. The significance of the differences between the results was established using one-way ANOVA with a confidence level of 95% []. Data in the plots were denoted as the mean ± standard deviation.
3. Results and Discussion
3.1. Molecular Structure of Chlorin Derivatives
The molecular structures of the chlorins are shown in Figure 1. These PSs contain five tertiary amine groups, one in the N-methylpyrrolidine substituent (pKa~10.46) and four in the 3-(N,N-dimethylamine)propanol (pKa~9.51) groups [,]. Considering the pKa values, these basic amine substituents are precursors of cationic groups by protonation in an aqueous medium at physiological pH. Although pKa values are not definitive proof of protonation, similar photoinactivation was previously found between a cationic porphyrin and its analogue without intrinsic charges substituted by basic amine groups []. Furthermore, four of them are covalently attached to the chlorin structure by a two-carbon aliphatic chain. This linker provides mobility to the positively charged precursor groups. Furthermore, this spacer prevents changes in the spectroscopic and photodynamic properties of the chlorin because the amine groups are not conjugated to the macrocycle [,]. Both the formation of cationic groups on the periphery of the chlorin macrocycle and their mobility can allow a better interaction of PS with yeast, thus increasing the photoinactivating capacity of C. albicans cells []. Furthermore, complexation of TPCF16 with Zn(II) and Pd(II) was performed because these metals form stable complexes with tetrapyrrole macrocycles with a 1:1 metal-to-ligand molar ratio [,]. Pd(II) complexes are characterized by a typical four-coordinate metal center, whereas Zn(II) complex centers are mainly five-coordinate []. In TMP, the Zn(II) lies on an inversion center and is coordinated in an almost ideal square planar geometry []. The asymmetric unit also contains one solvent molecule. Complexation with Zn(II) and Pd(II) was used to modify the photodynamic properties of the free-base chlorin because these metals each produce a significant heavy-atom effect []. This phenomenon enhances the intersystem crossing of a PS to 3PS* by spin–orbit coupling []. Therefore, the introduction of heavy atoms can result in an increase of ROS generation [,,].
3.2. Spectroscopic Characterization
UV-visible absorption spectra of TPCF16, ZnTPCF16, and PdTPCF16 were determined in DMF. As shown in Figure 2, the spectra are characterized by a high intensity Soret band around 405–418 nm. Similar absorbance maxima were found for TPCF16 and PdTPCF16, whereas the Zn(II) complex showed a bathochromic shift of 10 nm compared to the free-base chlorin. These absorption peaks presented molar absorption coefficients (ε) in the order of 105 Lmol−1cm−1 (Table 1). Furthermore, these chlorins exhibited Q bands of lower intensity in the visible region between 500 and 700 nm. All spectra showed an intense Q(0-0) band that had ε values of about 104 Lmol−1cm−1 (Table 1), typical of chlorin derivatives [,]. This band is hypsochromically shifted with respect to the free-base chlorin by 30 and 49 nm for ZnTPCF16 and PdTPCF16, respectively. Similar results were previously found for metal complexes of the chlorins’ derivatives [,,,,].
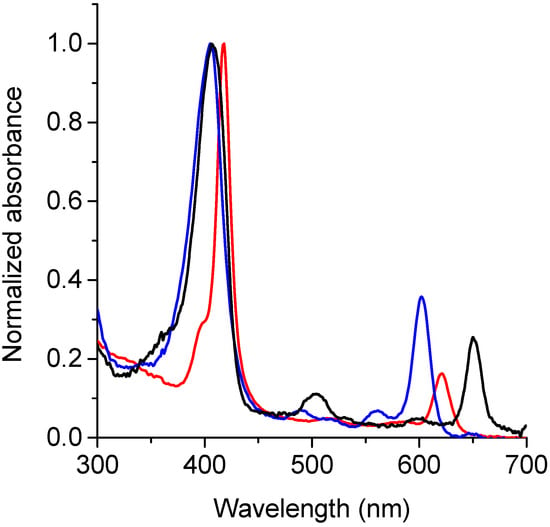
Figure 2.
Absorption spectra of TPCF16 (black line), ZnTPCF16 (red line), and PdTPCF16 (blue line) in DMF.

Table 1.
Spectroscopic characteristics of TPCF16, ZnTPCF16, and PdTPCF16 in DMF.
Fluorescence emission spectra of these chlorins were recorded in DMF (Figure 3A). The spectra of TPCF16 and ZnTPCF16 showed two bands in the red spectral region, with a main emission band of Zn(II) complex hypsochromically shifted by 27 nm compared to free-base chlorin (Table 1). These fluorescence emission bands are typical for similar chlorin derivatives []. These bands can be assigned to the electronic transitions that take place from the first singlet excited state of the chlorin macrocycle to the first two vibrational levels of the ground state, which are denoted as Q(0–0) and Q(0–1), respectively []. In contrast, the emission of PdTPCF16 was negligible under these conditions. This singlet excited state quenching effect was previously observed in Pd(II) complexes with tetrapyrrole macrocycles []. Furthermore, Stokes shifts for TPCF16 and ZnTPCF16 were determined from the wavelengths of the last absorption band and the first emission band, Q(0–0), giving values of 2 and 4 nm, respectively. This behavior indicates that the absorption energy in the first excited singlet state is very similar to its relaxation energy [,]. Therefore, the structural changes between the ground and excited states of these molecules are minimal, mainly as a consequence of the rigidity of the chlorin macrocycle. The ΦF values of TPCF16 and ZnTPCF16 were obtained in DMF (Table 1), using TMP as a reference []. Free-base chlorin presented a ΦF value that agreed with those reported for similar chlorin derivatives []. As expected, this ΦF value decreased in the complex with Zn(II) due to the heavy-atom effect []. These ΦF values are appropriate to determine interactions of these chlorins with microorganisms and to carry out studies using fluorescence microscopy techniques in C. albicans cells [].
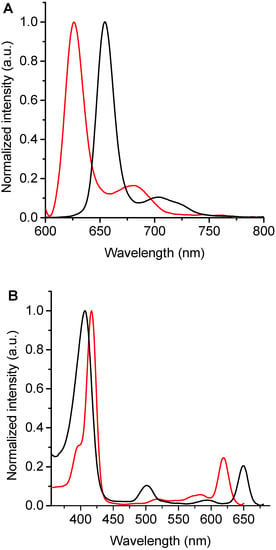
Figure 3.
(A) Fluorescence emission spectra (λexc = 408 nm) and (B) fluorescence excitation spectra (λem = 700 nm) of TPCF16 (black line) and ZnTPCF16 (red line) in DMF.
Moreover, fluorescence excitation spectra of TPCF16 and ZnTPCF16 were recorded in DMF by measuring their emissions at 700 nm (Figure 3B). This methodology allows differentiation of the Q bands of the chlorins in detail, mainly when these compounds are present in very low concentrations. Furthermore, when chlorins are bound to yeast cells, where the absorption spectrum may overlap with that of other chromophores, the excitation spectrum is important for observation of the exact shapes and positions of the PS absorption bands []. Both chlorins showed a close similarity between the fluorescence excitation spectra and the corresponding absorption spectra (Figure 2 and Figure 3B). Therefore, these results confirm that TPCF16 and ZnTPCF16 were mainly dissolved as monomers in this organic solvent.
3.3. Photooxidation of DMA and O2(1Δg) Formation
O2(1Δg) represents one of the main cytotoxic species involved in the photoinactivation of microorganisms [,]. Thus, the generation of O2(1Δg) sensitized by TPCF16, ZnTPCF16, and PdTPCF16 was measured in air-equilibrated solutions of DMF. In these studies, DMA was used as a molecular probe, which quenched O2(1Δg) primarily through a chemical reaction to produce the 9,10-endoperoxide derivative []. Photooxidation of DMA upon reaction with O2(1Δg) was observed following the decrease in absorbance of the quencher at 379 nm (Figure S1) []. The DMA absorption band decreased progressively in the presence of chlorin when the solution was exposed to light at 610 nm, indicating that these PSs generated O2(1Δg). It is important to note that during the experiments, the UV-visible spectra of the chlorins remained without significant changes, indicating that these compounds were stable to photobleaching during these measurements. Figure 4 shows that the reactions followed a pseudo-first-order kinetic for DMA decomposition. The values of the kobsDMA were obtained from the slopes of the linear fits of the data (Table 2). To determine the ΦΔ values, the kinetic results were compared with those attained for MB, which was used as a reference []. As can be seen in Figure 4, the formation of O2(1Δg) sensitized by both chlorin metal complexes was achieved at similar rates. Therefore, comparable ΦΔ values were obtained for ZnTPCF16 and PdTPCF16 (Table 2). Similar improvements of ΦΔ values with respect to free-base chlorin were previously reported for chlorin derivatives forming complexes with Zn(II) and Pd(II) in DMF [,]. While TPCF16 showed a lower ΦΔ value, its Zn(II) and Pd(II) complexes were found to be more effective in forming O2(1Δg) in organic solvent due to the heavy-atom effect. These results reveal that these chlorins are capable of undergoing a type II photomechanism producing O2(1Δg) under irradiation.
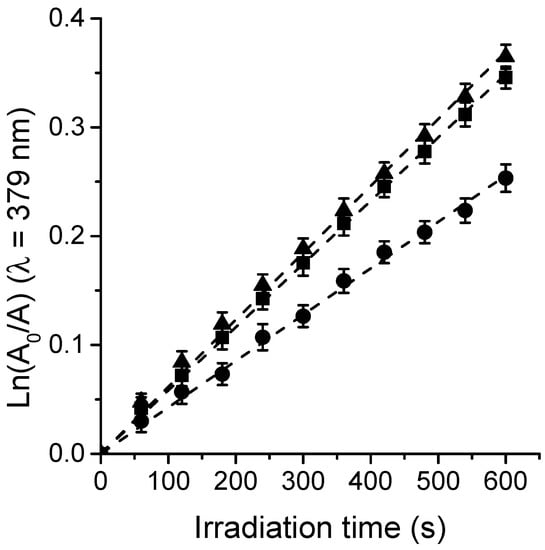
Figure 4.
Kinetic plot for the decomposition of DMA (35 μM) photosensitized by ZnTPCF16 (■), PdTPCF16 (▼), and the reference MB (●) in DMF; λirr = 610 nm.

Table 2.
Photodynamic properties of TPCF16, ZnTPCF16, and PdTPCF16 in DMF.
3.4. Photokilling of C. albicans Planktonic Cells
The cytotoxic effect sensitized by TPCF16, ZnTPCF16, and PdTPCF16 was first evaluated in C. albicans cell suspensions in PBS. The cultures of yeast (~106 UFC/mL) were incubated with 1 and 5 μM PS for 30 min in the dark at 37 °C and irradiated with white light for different periods of time (5, 15, and 30 min, which correspond to 27, 81, and 162 J/cm2, respectively). At these concentrations of chlorins, the viability of C. albicans was not affected by incubation in the dark (Figures S2 and S3). Furthermore, cell survival was not modified by irradiation of the culture without chlorins (Figure 5). Therefore, these control experiments confirm that the photoinactivation of C. albicans was caused by chlorin-induced photodynamic activity. As shown in Figure 5, photokilling of C. albicans was dependent on the chlorin derivative, PS concentration, and the irradiation times. Therefore, by modifying any of these variables, it is possible to change and control the degree of photoinactivation of C. albicans. When cultures were incubated with 1 μM PS and irradiated for 15 min, the photodynamic effect induced by PdTPCF16 and TPCF16 produced reductions of 2.8 and 3.0 log, respectively. In these conditions, ZnTPCF16 sensitized an inactivation of 3.7 log. Moreover, photokilling increased to 3.4 log for PdTPCF16 and 4.1 log for TPCF16, whereas viable C. albicans cells were not detected for ZnTPCF16 after 30 min of irradiation. For the three chlorins, the photoinactivation of fungal cells increased when cells were incubated with 5 μM PS, being more noticeable for TPCF16 and ZnTPCF16. Thus, a decrease of 5 log was found for cultures treated with TPCF16 upon an irradiation of 15 min, whereas viable cells were eliminated using ZnTPCF16. Furthermore, no cell survival was detected in presence of free-base chlorin and its Zn(II) complex after 30 min of irradiation, which represents a cell photoinactivation greater than 99.9996%. It was previously found that 5,10,15,20-tetrakis[4-(3-N,N-dimethylaminopropoxy)phenyl]chlorin (TAPC) was also an effective PS to inactivate C. albicans [].
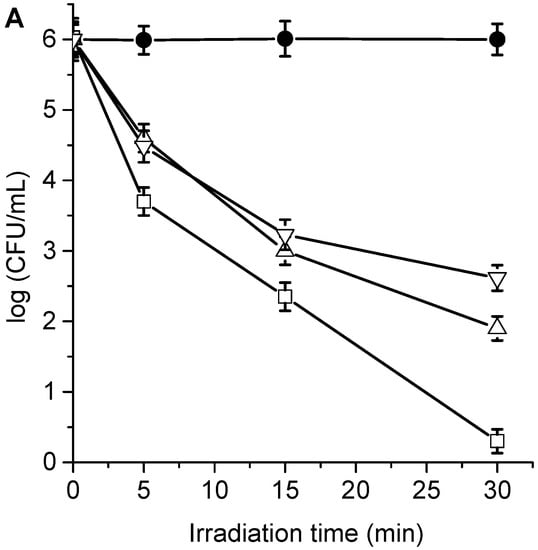
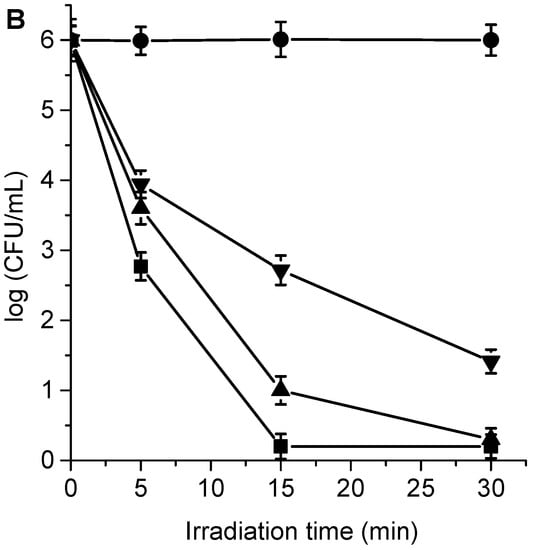
Figure 5.
Survival of C. albicans (~106 UFC/mL) treated with (A) 1 μM TPCF16 (△), 1 μM ZnTPCF16 (☐), 1 μM PdTPCF16 (▽), (B) 5 μM TPCF16 (▲), 5 μM ZnTPCF16 (■), and 5 μM PdTPCF16 (▼) for 30 min at 37 °C in the dark and irradiated with white light for different irradiation times. Control of C. albicans untreated with PS and irradiated (●).
3.5. Photodynamic Mechanism in C. albicans Cells
With the purpose of obtaining information about the photodynamic mechanism of action sensitized by TPCF16, ZnTPCF16, and PdTPCF16 in C. albicans cell suspensions, PDI studies were carried out in the presence of ROS scavengers and D2O (Figure 6, Figure 7 and Figure 8). Cultures were first treated with the additives for 30 min at 37 °C in the dark and then with 1 μM chlorin. Cell viability was not affected in yeast cultures incubated with 50 mM of these compounds in the dark and exposed for 15 min to white light in absence of PSs (Figure 6, Figure 7 and Figure 8, lines 3, 5, 9, and 11). Furthermore, no toxicity was found for the irradiated cell suspensions in D2O (Figure 6, Figure 7 and Figure 8, line 7). In these experiments, a PS concentration of 1 μM and 15 min of irradiation were chosen to produce a photoinactivation level of C. albicans of approximately 3 log and, thus, to be able to visualize the effect produced by the additives or the D2O medium. The PDI results are shown in Figure 6, Figure 7 and Figure 8. Sodium azide and DABCO were used as quenchers of O2(1Δg) [,]. With both additives, a reduction in photoinactivation was found in PDI treatments of C. albicans. The azide ions produced a reduction of about 2.5 log in the inactivation of yeast cells treated with TPCF16 and ZnTPCF16, (Figure 6 and Figure 7, line 4), whereas this effect was slightly lower with PdTPCF16, reaching 2 log of protection (Figure 8, line 4). Similar results were found for cultures incubated with DABCO and chlorins (Figure 6, Figure 7 and Figure 8, line 6), although with a decrease in inactivation somewhat less than that produced by sodium azide. Therefore, azide ions and DABCO produced a significant decrease in chlorin-sensitized photodynamic action by quenching O2(1Δg). To confirm the involvement of a type II mechanism, D2O was used instead of water in order to increase the O2(1Δg) lifetime []. PDI treatments of C. albicans cell suspensions in D2O with chlorin produced a significant increase in yeast photoinactivation relative to cells in PBS (Figure 6, Figure 7 and Figure 8, line 8). The greatest effect was observed for the metalated chlorins, producing an increase of about 2 log in cell inactivation. These results also suggest the participation of O2(1Δg) in the photodynamic pathway that produces cell death. On the other hand, D-mannitol and L-cysteine can act as radical scavengers, and thus these compounds can be used as inhibitors of the type I photoprocess [,]. For all three chlorins, the addition of D-mannitol produced about 0.5 log in C. albicans cell protection (Figure 6, Figure 7 and Figure 8, line 10). Comparable behavior was found in yeast cultures when L-cysteine was used as a free radical scavenger (Figure 6, Figure 7 and Figure 8, line 12). Therefore, the presence of D-mannitol and L-cysteine in C. albicans cell suspensions produce virtually no significant changes in yeast photoinactivation, indicating a negligible contribution from a type I photoprocess.
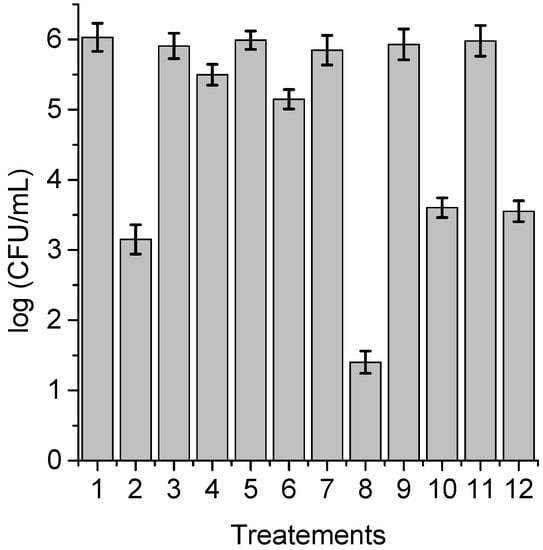
Figure 6.
Survival of C. albicans (~106 UFC/mL) treated with 1 μM TPCF16 for 30 min at 37 °C in the dark and irradiated with white light for 15 min; (1) cells; (2) cells treated with PS; (3) cells treated with 50 mM sodium azide; (4) cells treated with 50 mM sodium azide and PS; (5) cells treated with 50 mM DABCO; (6) cells treated with 50 mM DABCO and PS; (7) cells in D2O; (8) cells in D2O and treated with PS; (9) cells treated with 50 mM D-mannitol; (10) cells treated with 50 mM D-mannitol and PS; (11) cells treated with 50 mM cysteine and PS; (12) cells treated with 50 mM cysteine and PS.
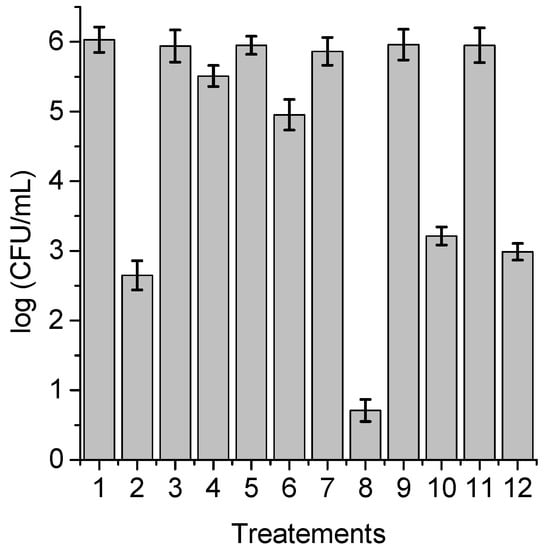
Figure 7.
Survival of C. albicans (~106 UFC/mL) treated with 1 μM ZnTPCF16 for 30 min at 37 °C in the dark and irradiated with white light for 15 min; (1) cells; (2) cells treated with PS; (3) cells treated with 50 mM sodium azide; (4) cells treated with 50 mM sodium azide and PS; (5) cells treated with 50 mM DABCO; (6) cells treated with 50 mM DABCO and PS; (7) cells in D2O; (8) cells in D2O and treated with PS; (9) cells treated with 50 mM D-mannitol; (10) cells treated with 50 mM D-mannitol and PS; (11) cells treated with 50 mM cysteine and PS; (12) cells treated with 50 mM cysteine and PS.
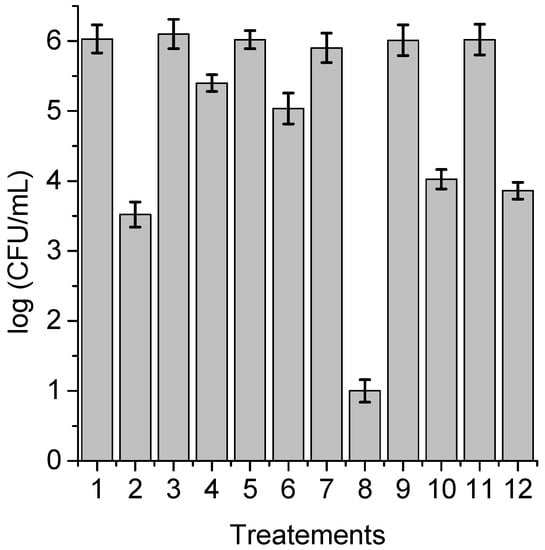
Figure 8.
Survival of C. albicans (~106 UFC/mL) treated with 1 μM PdTPCF16 for 30 min at 37 °C in the dark and irradiated with white light for 15 min; (1) cells; (2) cells treated with PS; (3) cells treated with 50 mM sodium azide; (4) cells treated with 50 mM sodium azide and PS; (5) cells treated with 50 mM DABCO; (6) cells treated with 50 mM DABCO and PS; (7) cells in D2O; (8) cells in D2O and treated with PS; (9) cells treated with 50 mM D-mannitol; (10) cells treated with 50 mM D-mannitol and PS; (11) cells treated with 50 mM cysteine and PS; (12) cells treated with 50 mM cysteine and PS.
In general, this is the behavior mainly found for PSs derived from tetrapyrrole macrocycles []. Analogous results were observed for the photokilling of C. albicans induced by porphyrins bearing cationic substituents or precursor groups of positive charges [,,]. In addition, a type II photoreaction was found to be involved in the photodamage of yeast cells sensitized by TAPC free-base chlorin []. Therefore, the photodynamic action sensitized by chlorins in the present study involves the formation of O2(1Δg) as the main ROS that leads to the death of C. albicans cells.
3.6. Photoinactivation of C. albicans Cells under Growth Conditions
The photocytotoxic effect sensitized by TPCF16, ZnTPCF16, and PdTPCF16 was tested on the growth of C. albicans cultures in Sabouraud broth. These experiments were used to evaluate the ability of these compounds to photoinactivate C. albicans cells when the yeast cultures are in an appropriate medium for their growth []. Therefore, 5 μM chlorin was added to fresh cultures of C. albicans in the growth medium, and the cells were subsequently irradiated with white light at 37 °C. Figure 9 shows the photodynamic activity induced by the chlorin on yeast cell growth. Untreated C. albicans cells irradiated or incubated with PS in the dark behaved similarly to the control in the dark. However, cell growth did not occur when chlorin-containing C. albicans cultures were irradiated with white light. Although the photodynamic effect probably acts in the early part of the growth curve, it was previously demonstrated that these chlorin derivatives were highly photostable in cellular cultures [].
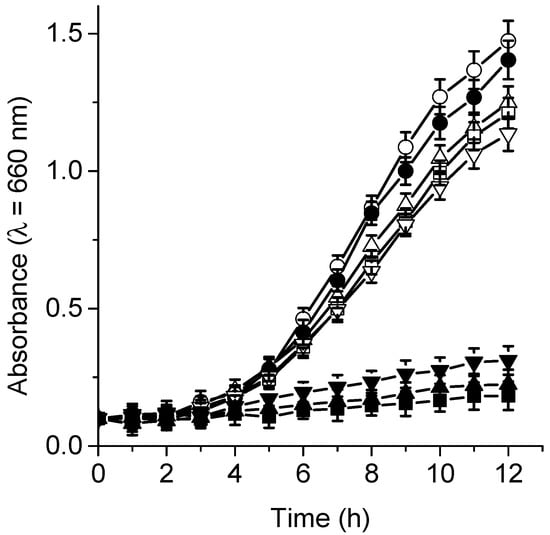
Figure 9.
Growth curves of C. albicans treated with 5 μM TPCF16 (▲), ZnTPCF16 (■), and PdTPCF16 (▼) and irradiated with white light for different irradiation times at 37 °C. Controls: cells untreated with PS and irradiated (●), C. albicans untreated with PS in the dark (◯), cells treated with 5 μM TPCF16 (△), ZnTPCF16 (☐), and PdTPCF16 (▽) in the dark.
In similar tests, a delay was previously found for the growth of C. albicans cells treated with 5 μM tetracationic porphyrin derivatives, although cell growth was not completely stopped [,]. Using the same concentration, 5,10,15,20-tetrakis[4-(3-N,N-dimethylaminopropoxy)phenyl]porphyrin (TAPP) was able to produce a reduction in the growth of C. albicans []. Furthermore, growth of 5 µM TAPC-treated cultures was retarded when the yeast cells were continuously irradiated with white light [].
3.7. Photoinactivation of C. albicans Pseudohyphae
C. albicans has the ability to change its morphological state from a yeast-like state, initially developing a germ tube and going through different elongated forms, called pseudohyphae, until finally forming hyphae [,]. This dimorphism allows certain fungi to switch between the unicellular yeast morphology to the multicellular filamentous form of hyphae in response to environmental changes. At the microbiological level, dimorphism is considered an important virulence factor in this yeast because it allows it to later colonize the mucosal tissues []. It is for this reason that the photodynamic activity induced by TPCF16, ZnTPCF16, and PdTPCF16 was evaluated in C. albicans pseudohyphae suspended in PBS. The formation of the dimorphic state of C. albicans was induced in yeast cultures suspended in HS for 4 h at 37 °C. The development of pseudohyphae was verified with optical microscopy []. C. albicans pseudohyphae cell suspensions (~106 CFU/mL) in PBS were treated with 1 and 5 μM chlorin for 30 min in the dark at 37 °C. Figure 10 shows the survivals of pseudohyphae after different times of irradiations (2, 5, 15, and 30 min) with white light. The viability of the pseudohyphae was not modified by irradiation without incubation with chlorin. Furthermore, no toxicity was found for cells incubated with 5 μM chlorin in the dark for 30 min (Figures S4 and S5). Consequently, the photokilling of pseudohyphae upon irradiation with white light was caused by the photosensitizing action of the chlorins. As can be observed in Figure 10, 1 μM TPCF16 was effective to photoinactivate C. albicans pseudohyphae suspended in PBS, producing a decrease in cell viability of over 5 log after 5 min of irradiation. In addition, 5 μM free-base chlorin was able to eliminate yeast upon an irradiation of 2 min. At the highest concentration used, complexes with Zn(II) and Pd(II) were also active PSs to inactivate pseudohyphae, achieving a decrease in cell survival greater than 5 log after 30 min exposure to white light.
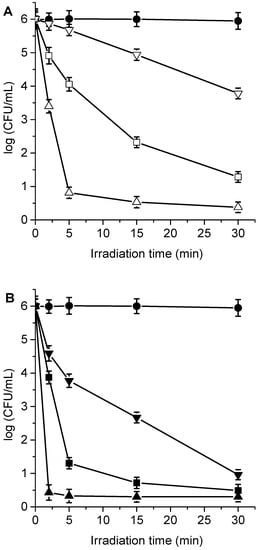
Figure 10.
Survival of C. albicans pseudohyphae (~106 UFC/mL) treated with (A) 1 μM TPCF16 (△), 1 μM ZnTPCF16 (☐), 1 μM PdTPCF16 (▽), (B) 5 μM TPCF16 (▲), 5 μM ZnTPCF16 (■), and 5 μM PdTPCF16 (▼) for 30 min at 37 °C in the dark and irradiated with white light for different irradiation times. Control of C. albicans pseudohyphae untreated with PS and irradiated (●).
In previous investigations, light-activated antimicrobial agent toluidine blue O (TBO) was able to kill the hyphal form of C. albicans, resulting in a 5.2 log reduction in its viability []. In addition, the irradiation of germ tubes of C. albicans incubated with Photofrin produced significant cell damage []. In the present work, photokilling of pseudohyphae sensitized by TPCF16 and ZnTPCF16 was more effective than previously observed results for TAPC-treated cultures, whereas the photoinactivating capacity of PdTPCF16 was similar to the latter free-base chlorin [].
3.8. Photokilling of C. albicans Biofilms
In immunosuppressed or medically compromised individuals, C. albicans infection can lead to the establishment of candidiasis, which can manifest as a superficial or invasive disease []. In this sense, most of the nosocomial septicemias found derive from intravascular and urinary catheters, heart valves, and silicone prostheses, among others. Some of the main reasons that make this type of infection possible are the capacity for filamentous growth, the formation of biofilms, and the production of extracellular material together with the modification of the structure of their cell walls []. Taking this into account, the photoinactivation of C. albicans sensitized by TPCF16, ZnTPCF16, and PdTPCF16 was evaluated in biofilms grown on PVC discs, which is one of the materials commonly used in the manufacture of catheters []. After the adhesion step, the cultures were treated with 5 μM chlorin in Sabouraud broth supplemented with 7% FBS for 18 h at 37 °C in the dark during biofilm proliferation. The survival rates of C. albicans after PDI treatments are indicated in Figure 11. No toxicity was observed for biofilms irradiated for 60 min without PS, nor for those treated with chlorin and kept in the dark. The photodynamic effect mediated by TPCF16 and ZnTPCF16 produced the eradication of C. albicans cells in the biofilms upon 60 min of irradiation. This denotes a greater than 6 log decrease in yeast survival, which represents 99.9997% photoinactivation. In contrast, a lower photokilling capacity was found with PdTPCF16, which induced a 2 log reduction in the viability of C. albicans. Despite the fact that PdTPCF16 presented the highest value of ΦΔ, the photoinactivation induced by the Pd(II) complex was lower than that produced by both the Zn(II) complex and even the free-base chlorin. It was previously found that Pd(II) tetrapyrrole macrocycle complexes can form aggregates in aqueous media, thus decreasing photodynamic activity and the ability to interact with biological media [].
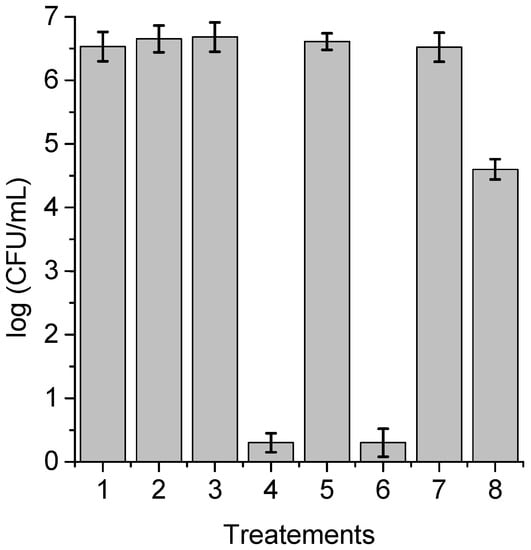
Figure 11.
Survival of C. albicans incubated with 5 μM PS for 18 h at 37 °C in the dark and irradiated with white light for 60 min; (1) cells in the dark; (2) irradiated cells; (3) cells treated with TPCF16 in the dark; (4) irradiated cells treated with TPCF16; (5) cells treated with ZnTPCF16 in the dark; (6) irradiated cells treated with ZnTPCF16; (7) cells treated with PdTPCF16 in the dark; (8) irradiated cells treated with PdTPCF16.
To verify the efficacy of the photodynamic action sensitized by TPCF16 and ZnTPCF16, after the PDI treatments, the PVC discs were deposited on a Sabouraud agar plate and incubated for 48 h at 37 °C in the dark (Figure S6). After that, significant proliferation of viable C. albicans cells was observed in controls of the biofilms kept in the dark, the biofilms irradiated for 60 min, and the biofilms treated with 5 μM chlorin in the dark. In contrast, no cell growth was found on the PVS discs treated with 5 μM TPCF16 or ZnTPCF16 after an irradiation time of 60 min. Furthermore, similar results were obtained by placing the PVC discs in Sabouraud broth after 48 h of incubation at 37 °C in the dark (Figure S7). The characteristic turbidity of yeast cell growth was observed in the test tubes containing the PVC discs with the different biofilm controls (Figure S7A–C and E). However, for the PVC discs subjected to the PDI treatments induced by 5 μM TPCF16 or ZnTPCF16, the tubes did not show growth or development of C. albicans in the liquid medium (Figure S7D,F). These results confirm the efficiency of free-base chlorin and its complex with Zn(II) to eradicate C. albicans biofilms.
The effects of PDI were previously investigated using TBO on the viability of biofilms produced by C. albicans at different stages of development []. The photodynamic activity inhibited biofilm formation, and the PDI treatment was able to decrease the survival of yeast cells and filamentous form in the biofilm in both early and mature biofilms. Furthermore, the photoinactivation produced by 5-aminolevulinic acid (ALA) was investigated on Candida albicans biofilms [,]. The presence of ALA showed a significant increase of protoporphyrin IX (PpIX) in the biofilms. The metabolic activity of C. albicans biofilms treated with ALA confirmed the inhibition efficacy. Cells in C. albicans biofilms were 74.45% inhibited upon radiation at 300 J/cm2 []. Degraded cytoplasmic content, nuclear condensation, and mitochondrial swelling were observed after PDI treatments []. Moreover, a panel of porphyrins was evaluated for PDI applications to control the growth of C. albicans []. In particular, monocationic diaryl-porphyrins were effective PSs to kill the C. albicans cells and inhibit biofilm formation. The tetracationic metalloporphyrin Zn(II) meso-tetrakis(N-n-hexylpyridinium-2-yl)porphyrin (ZnTnHex-2-PyP4+) was also investigated as a PS to inactivate yeasts and biofilms of C. albicans using a blue light-emitting diode []. At 0.8 μM and 4.3 J/cm2 light doses, PDI-treated biofilms showed decreases in cell viability and structural alterations with reduced hyphae. The photodynamic efficiency of a formulation composed of five cationic porphyrins and its combined effect with potassium iodide was tested on a large spectrum of microorganisms []. This combination was efficient in the destruction of C. albicans biofilms. In the present study, TPCF16 and ZnTPCF16 showed interesting properties as potential phototherapeutic agents for the efficient elimination of C. albicans yeast, pseudohyphae, and biofilms.
4. Conclusions
The PDI of yeasts is an interesting new therapy to treat localized fungal infections. In the search for new PSs, in this work we evaluated the photodynamic activity of a free-base chlorin and its complexes with Zn(II) and Pd(II) in C. albicans under different forms of cell cultures. These compounds absorbed intensely in the visible region and produced O2(1Δg) efficiently, mainly in the complexes of free-base chlorin with Zn(II) and Pd(II) due to the heavy-atom effect. Mainly, TPCF16 and ZnTPCF16 were able to kill C. albicans planktonic cells at low PS concentrations and short irradiation periods. The photodynamic mechanism of action involved the formation of O2(1Δg) as the main ROS that causes the inactivation of C. albicans cells. Moreover, the growth of C. albicans was suppressed by the photodynamic effect produced by these chlorins. The morphological plasticity of C. albicans was a determining factor of virulence because the pseudohyphal form has an important role in the infection process. The free-base chlorin and its Zn(II) complex were also effective in eliminating C. albicans pseudohyphae suspended in PBS. On the other hand, C. albicans can form biofilms on solid surfaces in the environment and within mammalian hosts in infections. Biofilms related to medical devices are of great clinical importance due to their high resistance to conventional antifungal agents for hospital use. When chlorins were incorporated during the proliferation stage, the photodynamic effect induced by TPCF16 and ZnTPCF16 was able to eradicate the C. albicans biofilm. Therefore, these results indicated that TPCF16 and ZnTPCF16 are interesting potential phototherapeutic agents to eliminate C. albicans with different morphologies and growth forms.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics12010105/s1. Materials; Instrumentation; Figure S1: Absorption spectral changes during the photo-oxidation of DMA sensitized by ZnTPCF16 and PdTPCF16 in DMF at different irradiation times; Figure S2: Survival of C. albicans treated with 1 μM TPCF16, ZnTPCF16, and PdTPCF16 for 30 min at 37 °C in the dark and kept in the dark for different amounts of time; Figure S3: Survival of C. albicans treated with 5 μM TPCF16, ZnTPCF16, and PdTPCF16 for 30 min at 37 °C in the dark and kept in the dark for different amounts of time; Figure S4: Survival curves of C. albicans pseudohyphae incubated with 1 μM TPCF16, ZnTPCF16, and PdTPCF16 for in PBS for 30 min at 37 °C in dark and kept in the dark for different amounts of time; Figure S5: Survival curves of C. albicans pseudohyphae incubated with 5 μM TPCF16, ZnTPCF16, and PdTPCF16 for in PBS for 30 min at 37 °C in dark and kept in the dark for different amounts of time; Figure S6: Photographs of PVC discs deposited on a Sabouraud agar plate and incubated for 48 h at 37 °C in the dark, control of C. albicans biofilm in the dark, control of C. albicans biofilm irradiated for 60 min with white light, C. albicans biofilm treated with 5 μM TPCF16 in the dark, C. albicans biofilm treated with 5 μM TPCF16 irradiated for 60 min with white light, (E) C. albicans biofilm treated with 5 μM ZnTPCF16 in the dark, C. albicans biofilm treated with 5 μM ZnTPCF16 irradiated for 60 min with white light; Figure S7: Photographs of PVC discs in Sabouraud broth and incubated for 48 h at 37 °C in the dark, control of C. albicans biofilm in the dark, control of C. albicans biofilm irradiated for 60 min with white light, C. albicans biofilm treated with 5 μM TPCF16 in the dark, C. albicans biofilm treated with 5 μM TPCF16 irradiated for 60 min with white light, (E) C. albicans biofilm treated with 5 μM ZnTPCF16 in the dark, C. albicans biofilm treated with 5 μM ZnTPCF16 irradiated for 60 min with white light. References [,,,,,,] are cited in the supplementary materials.
Author Contributions
Conceptualization, P.V.C., M.G.A., E.J.G.L., D.A.H. and E.N.D.; methodology, P.V.C., M.G.A., E.J.G.L., D.A.H. and E.N.D.; Validation, P.V.C., M.G.A., E.J.G.L., D.A.H. and E.N.D.; formal analysis, P.V.C., M.G.A., E.J.G.L., D.A.H. and E.N.D.; investigation, P.V.C., M.G.A., E.J.G.L., D.A.H. and E.N.D.; resources, E.N.D.; data curation, P.V.C., M.G.A., E.J.G.L., D.A.H. and E.N.D.; writing—original draft, E.N.D.; writing—review and editing, D.A.H. and E.N.D.; visualization, P.V.C., M.G.A., E.J.G.L., D.A.H. and E.N.D.; supervision, E.N.D.; project administration, E.N.D.; funding acquisition, E.N.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANPCYT (PICT-2019-02391) and CONICET (PIP 2021-23 PIP 11220200101208CO).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
P.V.C., M.G.A., D.A.H., and E.N.D. are Scientific Members of CONICET. E.J.G.L. thanks CONICET for the research fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ma, Y.; Wang, X.; Li, R. Cutaneous and subcutaneous fungal infections: Recent developments on host-fungus interactions. Curr. Opin. Microbiol. 2021, 62, 93–102. [Google Scholar] [CrossRef]
- Bankaitis, V.A.; Tripathi, A.; Chen, X.-R.; Igumenova, T.I. New strategies for combating fungal infections: Inhibiting inositol lipid signaling by targeting Sec14 phosphatidylinositol transfer proteins. Adv. Biol. Regul. 2022, 84, 100891. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.R.; Gaffen, S.L.; Hube, B. Candidalysin: Discovery and function in Candida albicans infections. Curr. Opin. Microbiol. 2019, 52, 100–109. [Google Scholar] [CrossRef]
- Wijnants, S.; Vreys, J.; Van Dijck, P. Interesting antifungal drug targets in the central metabolism of Candida albicans. Trends Pharmacol. Sci. 2022, 43, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Arita, G.S.; Faria, D.R.; Capoci, I.R.G.; Kioshima, E.S.; Bonfim-Mendonça, P.S.; Svidzinski, T.I.E. Cell wall associated proteins involved in filamentation with impact on the virulence of Candida albicans. Microbiol. Res. 2022, 258, 126996. [Google Scholar] [CrossRef]
- Wall, G.; Montelongo-Jauregui, D.; Bonifacio, B.V.; Lopez-Ribot, J.L.; Uppuluri, P. Candida albicans biofilm growth and dispersal: Contributions to pathogenesis. Curr. Opin. Microbiol. 2019, 52, 1–6. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Morace, G.; Perdoni, F.; Borghi, E. Antifungal drug resistance in Candida species. J. Glob. Antimicrob. Resist. 2014, 2, 254–259. [Google Scholar] [CrossRef]
- Robbins, N.; Cowen, L.E. Antifungal discovery. Curr. Opin. Microbiol. 2022, 69, 102198. [Google Scholar] [CrossRef]
- Capoor, M.R.; Subudhi, C.P.; Collier, A.; Bal, A.M. Antifungal stewardship with an emphasis on candidaemia. J. Glob. Antimicrob. Resist. 2019, 19, 262–268. [Google Scholar] [CrossRef]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial photodynamic therapy: Latest developments with a focus on combinatory strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef] [PubMed]
- Durantini, A.M.; Heredia, D.A.; Durantini, J.E.; Durantini, E.N. BODIPYs to the rescue: Potential applications in photodynamic inactivation. Eur. J. Med. Chem. 2018, 144, 651–661. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Liu, Y.-C.; Sun, H.; Guo, D.-S. Type I photodynamic therapy by organic-inorganic hybrid materials: From strategies to applications. Coord. Chem. Rev. 2019, 395, 46–62. [Google Scholar] [CrossRef]
- Heredia, D.A.; Durantini, A.M.; Durantini, J.E.; Durantini, E.N. Fullerene C60 derivatives as antimicrobial photodynamic agents. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100471. [Google Scholar] [CrossRef]
- Kashef, N.; Hamblin, M.R. Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist. Updates 2017, 31, 31–42. [Google Scholar] [CrossRef]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Porphyrinoid photosensitizers mediated photodynamic inactivation against bacteria. Eur. J. Med. Chem. 2019, 175, 72–106. [Google Scholar] [CrossRef]
- Souza, T.H.S.; Sarmento-Neto, J.F.; Souza, S.O.; Raposo, B.L.; Silva, B.P.; Borges, C.P.F.; Santos, B.S.; Cabral Filho, P.E.; Rebouças, J.S.; Fontes, A. Advances on antimicrobial photodynamic inactivation mediated by Zn(II) porphyrins. J. Photochem. Photobiol. C Photochem. Rev. 2021, 49, 100454. [Google Scholar] [CrossRef]
- Heredia, D.A.; Durantini, J.E.; Ferreyra, D.D.; Reynoso, E.; Gonzalez Lopez, E.J.; Durantini, A.M.; Milanesio, M.E.; Durantini, E.N. Charge density distribution effect in pyrrolidine-fused chlorins on microbial uptake and antimicrobial photoinactivation of microbial pathogens. J. Photochem. Photobiol. B Biol. 2021, 225, 112321. [Google Scholar] [CrossRef]
- Costa, D.C.S.; Gomes, M.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Cavaleiro, J.A.S.; Almeida, A.; Tomé, J.P.C. Comparative photodynamic inactivation of antibiotic resistant bacteria by first and second generation cationic photosensitizers. Photochem. Photobiol. Sci. 2012, 11, 1905–1913. [Google Scholar] [CrossRef]
- Santos, I.; Gamelas, S.R.D.; Vieira, C.; Faustino, M.A.F.; Tomé, J.P.C.; Almeida, A.; Gomes, A.T.P.C.; Lourenço, L.M.O. Pyrazole-pyridinium porphyrins and chlorins as powerful photosensitizers for photoinactivation of planktonic and biofilm forms of E. coli. Dyes Pigment. 2021, 193, 109557. [Google Scholar] [CrossRef]
- Ballatore, M.B.; Spesia, M.B.; Milanesio, M.E.; Durantini, E.N. Synthesis, spectroscopic properties and photodynamic activity of porphyrinefullerene C60 dyads with application in the photodynamic inactivation of Staphylococcus aureus. Eur. J. Med. Chem. 2014, 83, 685–694. [Google Scholar] [CrossRef]
- Ballatore, M.B.; Milanesio, M.E.; Fujita, H.; Lindsey, J.S.; Durantini, E.N. Bacteriochlorin-bis(spermine) conjugate affords an effective photodynamic action to eradicate microorganisms. J. Biophotonics 2020, 13, e201960061. [Google Scholar] [CrossRef] [PubMed]
- Cormick, M.P.; Alvarez, M.G.; Rovera, M.; Durantini, E.N. Photodynamic inactivation of Candida albicans sensitized by tri- and tetra-cationic porphyrin derivatives. Eur. J. Med. Chem. 2009, 44, 1592–1599. [Google Scholar] [CrossRef]
- Quiroga, E.D.; Cordero, P.; Mora, S.J.; Alvarez, M.G.; Durantini, E.N. Mechanistic aspects in the photodynamic inactivation of Candida albicans sensitized by a dimethylaminopropoxy porphyrin and its equivalent with cationic intrinsic charges. Photodiagn. Photodyn. Ther. 2020, 31, 101877. [Google Scholar] [CrossRef] [PubMed]
- Agazzi, M.L.; Durantini, J.E.; Quiroga, E.D.; Alvarez, M.G.; Durantini, E.N. A novel tricationic fullerene C60 as broad-spectrum antimicrobial photosensitizer: Mechanisms of action and potentiation with potassium iodide. Photochem. Photobiol. Sci. 2021, 20, 327–341. [Google Scholar] [CrossRef]
- Quiroga, E.D.; Mora, S.J.; Alvarez, M.G.; Durantini, E.N. Photodynamic inactivation of Candida albicans by a tetracationictentacle porphyrin and its analogue without intrinsic charges inpresence of fluconazole. Photodiagn. Photodyn. Ther. 2016, 13, 334–340. [Google Scholar] [CrossRef]
- Samaranayake, Y.H.; Cheung, B.P.K.; Yau, J.Y.Y.; Yeung, S.K.W.; Samaranayake, L.P. Human serum promotes Candida albicans biofilm growth and virulence gene expression on silicone biomaterial. PLoS ONE 2013, 8, e62902. [Google Scholar] [CrossRef]
- Cordero, P.V.; Ferreyra, D.D.; Pérez, M.E.; Alvarez, M.G.; Durantini, E.N. Photodynamic Effect of 5,10,15,20-Tetrakis[4-(3-N,N-dimethylaminopropoxy) phenyl]chlorin towards the human pathogen Candida albicans under different culture conditions. Photochem 2021, 1, 505–522. [Google Scholar] [CrossRef]
- Heredia, D.A.; Durantini, A.M.; Sarotti, A.M.; Gsponer, N.S.; Ferreyra, D.D.; Bertolotti, S.G.; Milanesio, M.E.; Durantini, E.N. Proton-dependent switching of a novel amino chlorin derivative as a fluorescent probe and photosensitizer for acidic media. Chem. Eur. J. 2018, 24, 5950–5961. [Google Scholar] [CrossRef]
- Milanesio, M.E.; Alvarez, M.G.; Bertolotti, S.G.; Durantini, E.N. Photophysical characterization and photodynamic activity of metallo 5-(4-(trimethylammonium)phenyl)-10,15,20-tris(2,4,6-trimethoxyphenyl)porphyrin in homogeneous and biomimetic media. Photochem. Photobiol. Sci. 2008, 7, 963–972. [Google Scholar] [CrossRef]
- Hirohara, S.; Kawasaki, Y.; Funasako, R.; Yasui, N.; Totani, M.; Alitomo, H.; Yuasa, J.; Kawai, T.; Oka, C.; Kawaichi, M.; et al. Sugar and heavy atom effects of glycoconjugated chlorin palladium complex on photocytotoxicity. Bioconjug. Chem. 2012, 23, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Senge, M.O.; Zawadzka, M. Structural investigation of 5,10-A2B2-type porphyrins: Palladium(II) and zinc(II) complexes of 5,10-dibromo-15,20-bis(4-methylphenyl)porphyrin. Acta Crystallogr. C Struct. Chem. 2014, 70, 1143–1146. [Google Scholar] [CrossRef]
- McGill, S.; Nesterov, V.N.; Gould, S.L. [5,10,15,20-Tetrakis(4-methoxyphenyl)-porphyrinato]zinc dichloromethane disolvate. Acta Crystallogr. E Crystallogr. Commun. 2013, 70, m470. [Google Scholar] [CrossRef]
- Obata, M.; Hirohara, S.; Tanaka, R.; Kinoshita, I.; Ohkubo, K.; Fukuzumi, S.; Tanihara, M.; Yano, S. In vitro heavy-atom effect of palladium(II) and platinum(II) complexes of pyrrolidine-fused chlorin in photodynamic therapy. J. Med. Chem. 2009, 52, 2747–2753. [Google Scholar] [CrossRef]
- Almeida, J.; Silva, A.M.N.; Rebelo, S.L.H.; Cunha-Silva, L.; Rangel, M.; de Castro, B.; Leite, A.; Silva, A.M.G. Synthesis and coordination studies of 5-(40-carboxyphenyl)-10,15,20-tris(pentafluorophenyl)porphyrin and its pyrrolidine-fused chlorin derivative. New J. Chem. 2018, 42, 8169–8179. [Google Scholar] [CrossRef]
- Ferreyra, D.D.; Reynoso, E.; Cordero, P.; Spesia, M.B.; Alvarez, M.G.; Milanesio, M.E.; Durantini, E.N. Synthesis and properties of 5,10,15,20-tetrakis[4-(3-N,N-dimethylaminopropoxy)phenyl]chlorin as potential broad-spectrum antimicrobial photosensitizers. J. Photochem. Photobiol. B Biol. 2016, 158, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Novaira, M.; Cormick, M.P.; Durantini, E.N. Spectroscopic and time-resolved fluorescence emission properties of a cationic and an anionic porphyrin in biomimetic media and Candida albicans cells. J. Photochem. Photobiol. A Chem. 2012, 246, 67–74. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef]
- Heredia, D.A.; Martínez, S.R.; Durantini, A.M.; Pérez, M.E.; Mangione, M.I.; Durantini, J.E.; Gervaldo, M.A.; Otero, L.A.; Durantini, E.N. Antimicrobial photodynamic polymeric films bearing biscarbazol triphenylamine end-capped dendrimeric Zn(II) porphyrin. ACS Appl. Mater. Interfaces 2019, 11, 27574–27587. [Google Scholar] [CrossRef]
- da Silva, E.F.F.; Pedersen, B.W.; Breitenbach, T.; Toftegaard, R.; Kuimova, M.K.; Arnaut, L.G.; Ogilby, P.R. Irradiation- and sensitizer-dependent changes in the lifetime of intracellular singlet oxygen produced in a photosensitized process. J. Phys. Chem. B 2012, 116, 445–461. [Google Scholar] [CrossRef]
- Cormick, M.P.; Quiroga, E.D.; Bertolotti, S.G.; Alvarez, M.G.; Durantini, E.N. Mechanistic insight of the photodynamic effect induced by tri- and tetra-cationic porphyrins on Candida albicans cells. Photochem. Photobiol. Sci. 2011, 10, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Di Palma, M.A.; Alvarez, M.G.; Durantini, E.N. Photodynamic action mechanism mediated by zinc(II) 2,9,16,23-tetrakis [4-(N-methylpyridyloxy)]phthalocyanine in Candida albicans cells. Photochem. Photobiol. 2015, 91, 1203–1209. [Google Scholar] [CrossRef]
- Gsponer, N.S.; Agazzi, M.L.; Spesia, M.B.; Durantini, E.N. Approaches to unravel pathways of reactive oxygen species in the photoinactivation of bacteria induced by a dicationic fulleropyrrolidinium derivative. Methods 2016, 109, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Almeida, A. Photodynamic inactivation of mammalian viruses and bacteriophages. Viruses 2012, 4, 1034–1074. [Google Scholar] [CrossRef]
- Costa, L.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A. Involvement of type I and type II mechanisms on the photoinactivation of non-enveloped DNA and RNA bacteriophages. J. Photochem. Photobiol. B Biol. 2013, 120, 10–16. [Google Scholar] [CrossRef]
- Scanone, A.C.; Gsponer, N.S.; Alvarez, M.G.; Durantini, E.N. Porphyrins containing basic aliphatic amino groups as potential broad spectrum antimicrobial agents. Photodiagn. Photodyn. Ther. 2018, 24, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, E.D.; Alvarez, M.G.; Durantini, E.N. Susceptibility of Candida albicans to photodynamic action of 5,10,15,20-tetra(4-N-methylpyridyl)porphyrin in different media. FEMS Immunol. Med. Microbiol. 2010, 60, 123–131. [Google Scholar] [CrossRef]
- Sudbery, P.E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011, 9, 737–748. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, M.; Wang, Y.; Chen, Y.; Gao, J.; Ying, C. ERG11 couples oxidative stress adaptation, hyphal elongation and virulence in Candida albicans. FEMS Yeast Res. 2018, 18, 1–7. [Google Scholar] [CrossRef]
- Noble, S.M.; Gianetti, B.A.; Witchley, J.N. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 2017, 15, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Jackson, Z.; Meghji, S.; MacRobert, A.; Henderson, B.; Wilson, M. Killing of the yeast and hyphal forms of Candida albicans using a light-activated antimicrobial agent. Lasers Med. Sci. 1999, 14, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Bliss, J.M.; Bigelow, C.E.; Foster, T.H.; Haidaris, C.G. Susceptibility of Candida species to photodynamic effects of Photofrin. Antimicrob. Agents Chemother. 2004, 48, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Swidergall, M. Candida albicans at host barrier sites: Pattern recognition receptors and beyond. Pathogens 2019, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, C.N.; Kosovski, I.B.; Mare, A.D.; Toma, F.; Pintea-Simon, I.A.; Man, A. Candida and candidiasis-opportunism versus pathogenicity: A review of the virulence traits. Microorganisms 2020, 8, 857. [Google Scholar] [CrossRef]
- Duarte-Peña, L.; López-Saucedo, F.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Modification of indwelling PVC catheters by ionizing radiation with temperature- and pH-responsive polymers for antibiotic delivery. Radiat. Phys. Chem. 2022, 193, 110005. [Google Scholar] [CrossRef]
- Pinto, A.P.; Bueno Rosseti, I.; Lopes Carvalho, M.; Graziele Marques da Silva, B.; Alberto-Silva, C.; Silva Costa, M. Photodynamic Antimicrobial Chemotherapy (PACT), using Toluidine blue O inhibits the viability of biofilm produced by Candida albicans at different stages of development. Photodiagn. Photodyn. Ther. 2018, 21, 182–189. [Google Scholar] [CrossRef]
- Shi, H.; Li, J.; Zhang, H.; Zhang, J.; Sun, H. Effect of 5-aminolevulinic acid photodynamic therapy on Candida albicans biofilms: An in vitro study. Photodiagn. Photodyn. Ther. 2016, 15, 40–45. [Google Scholar] [CrossRef]
- Shi, H.; Li, J.; Peng, C.; Xu, B.; Sun, H. The inhibitory activity of 5-aminolevulinic acid photodynamic therapy (ALA-PDT) on Candida albicans biofilms. Photodiagn. Photodyn. Ther. 2021, 34, 102271. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Martegani, E.; Bolognese, F.; Trivellin, N.; Mat’átková, O.; Paldrychová, M.; Baj, A.; Caruso, E. Photodynamic therapy by diaryl-porphyrins to control the growth of Candida albicans. Cosmetics 2020, 7, 31. [Google Scholar] [CrossRef]
- Souza, S.O.; Raposo, B.L.; Sarmento-Neto, J.F.; Rebouças, J.S.; Macêdo, D.P.C.; Figueiredo, R.C.B.Q.; Santos, B.S.; Freitas, A.Z.; Cabral Filho, P.E.; Ribeiro, M.S.; et al. Photoinactivation of yeast and biofilm communities of Candida albicans mediated by ZnTnHex-2-PyP4+ porphyrin. J. Fungi 2022, 8, 556. [Google Scholar] [CrossRef]
- Vieira, C.; Bartolomeu, M.; Santos, A.R.; Mesquita, M.Q.; Gomes, A.T.P.C.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Photoinactivation of bacterial and fungal planktonic/biofilm forms using the combination of a porphyrinic formulation with potassium iodide. Med. Sci. Forum 2022, 12, 13. [Google Scholar]
- Milanesio, M.E.; Alvarez, M.G.; Yslas, E.I.; Borsarelli, C.D.; Silber, J.J.; Rivarola, V.; Durantini, E.N. Photodynamic studies of metallo 5,10,15,20-tetrakis(4-methoxyphenyl) porphyrin: Photochemical characterization and biological consequences in a human carcinoma cell line. Photochem. Photobiol. 2001, 74, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.E.; Durantini, J.E.; Reynoso, E.; Alvarez, M.G.; Milanesio, M.E.; Durantini, E.N. Porphyrin-schiff base conjugates bearing basic amino groups as antimicrobial phototherapeutic agents. Molecules 2021, 26, 5877. [Google Scholar] [CrossRef] [PubMed]
- Santamarina, S.C.; Heredia, D.A.; Durantini, A.M.; Durantini, E.N. Antimicrobial photosensitizing material based on conjugated Zn(II) porphyrins. Antibiotics 2022, 11, 91. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).