Antibacterial and Antifungal Alkaloids from Asian Angiosperms: Distribution, Mechanisms of Action, Structure-Activity, and Clinical Potentials
Abstract
1. Introduction
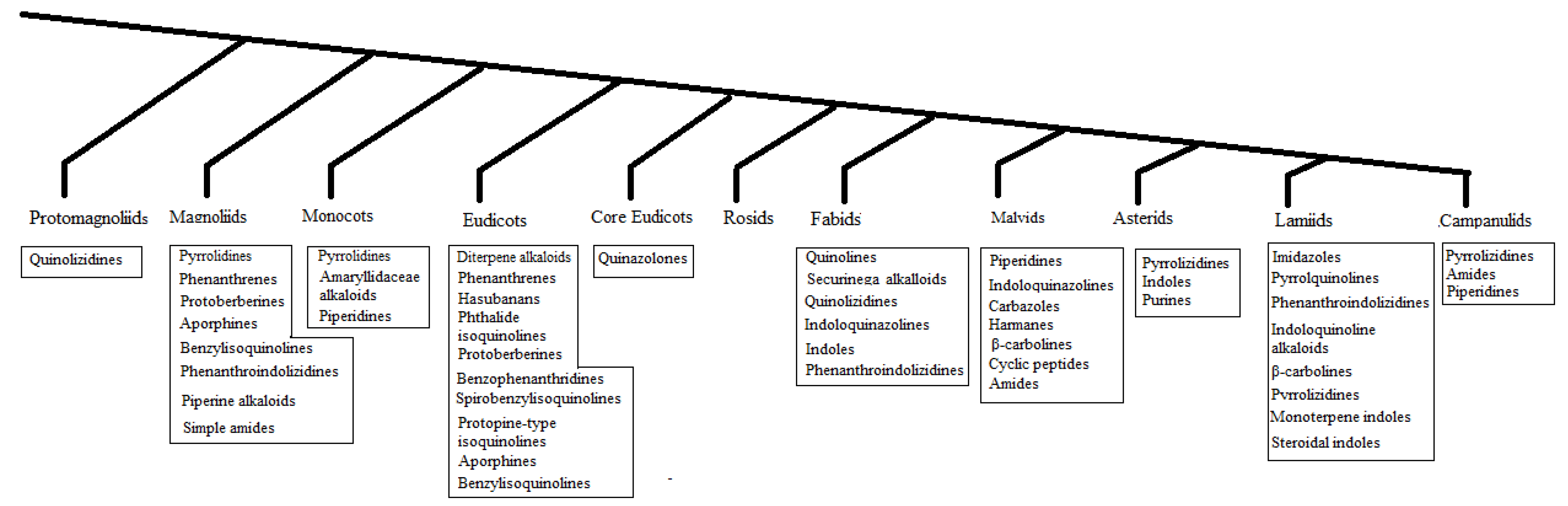
2. Distribution
- All clades yielded antibacterial and/or antifungal alkaloids, except for the Rosids.
- Most antibacterial and/or antifungal alkaloids can be found in basal angiosperms, which are isoquinolines.
- Some clades yielded a specific class of antibacterial and/or antifungal alkaloids such as the Amaryllidaceae alkaloids in the Monocots, phenanthrene alkaloid in the Magnoliids, Securinega alkaloids in Fabids, carbazoles in the Malvids, and monoterpene indole alkaloids in the Lamiids.
- Core angiosperms and upper angiosperms use various classes of alkaloids and phytoalexins.
- Tripathi et al. (1994) observed changes in the antibacterial alkaloid concentrations in plants over time [11].
3. The Strongest Antibacterial and/or Antifungal Alkaloids Identified and Spectrum of Activity
4. Influence of Molecular Mass
5. Influence of Solubility and Polar Surface
6. Mechanisms of Action and Structure-Activity Relationships
6.1. Alkaloids Targeting DNA and/or Topoisomerase
6.2. Alkaloids Targeting the Cytoplasmic Membrane
6.3. Miscellaneous Targets
7. Efflux Pumps Inhibitors
8. Amide Alkaloids
8.1. Simple Amide Alkaloids
8.2. Cyclopeptides
9. Indole Alkaloids
9.1. Simple Indoles
9.1.1. Brassicaceous Indoles
9.1.2. β-Carbolines
9.1.3. Carbazoles
9.1.4. Monoterpene Indole Alkaloids
9.1.5. Miscellaneous
10. Piperidine Alkaloids
10.1. Piperine Alkaloids
10.2. Quinolizidine Alkaloids
10.3. Phenanthroindolizidine Alkaloids
10.4. Securinega Alkaloids
10.5. Miscellaneous
11. Quinoline Alkaloids
11.1. Simple Quinolines
11.2. Benzylisoquinolines
11.3. Bisbenzylisoquinolines
11.4. Aporphines
11.5. Protopines
11.6. Protoberberines
11.7. Spirobenzylisoquinolines
11.8. Benzophenanthridines
11.9. Protoberberines
11.10. Phthalides
11.11. Hasubanans
11.12. Amaryllidaceae Alkaloids
11.13. Miscellaneous
11.13.1. Quinolinones
11.13.2. Acridanones
11.13.3. Phenanthrene Alkaloids
12. Pyrrolidines and Imidazole Alkaloids
13. Diterpene Alkaloids
14. Steroidal Alkaloids
15. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Angiosperm Phylogeny Group. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 105–121. [Google Scholar]
- Tiku, A.R. Antimicrobial compounds (phytoanticipins and phytoalexins) and their role in plant defense. In Co-Evolution of Secondary Metabolites; Springer: Cham, Switzerland, 2020; pp. 845–868. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yamada, Y. Alkaloid biogenesis: Molecular aspects. Annu. Rev. Plant Biol. 1994, 45, 257–285. [Google Scholar] [CrossRef]
- Denyer, S.P.; Maillard, J.Y. Cellular impermeability and uptake of biocides and antibiotics in Gram-negative bacteria. J. Appl. Microbiol. 2002, 92, 35S–45S. [Google Scholar] [CrossRef] [PubMed]
- Ahirrao, P.; Tambat, R.; Chandal, N.; Mahey, N.; Kamboj, A.; Jain, U.K.; Singh, I.P.; Jachak, S.M.; Nandanwar, H.S. MsrA efflux pump inhibitory activity of Piper cubeba lf and its phytoconstituents against S. aureus RN4220. Chem. Biodivers. 2020, 17, e2000144. [Google Scholar]
- Rios, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Ablordeppey, S.Y.; Fan, P.; Li, S.; Clark, A.M.; Hufford, C.D. Substituted indoloquinolines as new antifungal agents. Bioorg. Med. Chem. 2002, 10, 1337–1346. [Google Scholar] [CrossRef]
- Adeleye, A.; Ikotun, T. Antifungal activity of dihydrodioscorine extracted from a wild variety of Dioscorea bulbifera L. J. Basic Microbiol. 1989, 29, 265–267. [Google Scholar] [CrossRef]
- Adesanya, S.A.; Nia, R.; Martin, M.T.; Boukamcha, N.; Montagnac, A.; Païs, M. Stilbene derivatives from Cissus quadrangularis. J. Nat. Prod. 1999, 62, 1694–1695. [Google Scholar] [CrossRef]
- Aeschlimann, J.R.; Dresser, L.D.; Kaatz, G.W.; Rybak, M.J. Effects of NorA inhibitors on in vitro antibacterial activities and postantibiotic effects of levofloxacin, ciprofloxacin, and norfloxacin in genetically related strains of S. aureus. Antimicrob. Agents Chemother. 1999, 43, 335–340. [Google Scholar] [CrossRef]
- Tripathi, Y.C.; Rathore, M.; Kumar, H. On the variation of alkaloidal contents of Fumaria indica at different stages of life span. Anc. Sci. Life 1994, 13, 271. [Google Scholar]
- Kuete, V.; Poumale, H.P.; Guedem, A.N.; Shiono, Y.; Randrianasolo, R.; Ngadjui, B.T. Antimycobacterial, antibacterial and antifungal activities of the methanol extract and compounds from Thecacoris annobonae (Euphorbiaceae). S. Afr. J. Bot. 2010, 76, 536–542. [Google Scholar] [CrossRef]
- Joosten, L.; van Veen, J.A. Defensive properties of pyrrolizidine alkaloids against microorganisms. Phytochem. Rev. 2011, 10, 127–136. [Google Scholar] [CrossRef]
- Maiti, M.; Nandi, R.; Chaudhuri, K. Sanguinarine: A monofunctional intercalating alkaloid. FEBS Lett. 1982, 142, 280–284. [Google Scholar] [CrossRef]
- Cao, R.; Peng, W.; Wang, Z.; Xu, A. β-Carboline alkaloids: Biochemical and pharmacological functions. Curr. Med. Chem. 2007, 14, 479–500. [Google Scholar] [CrossRef]
- Tanious, F.A.; Ding, D.; Patrick, D.A.; Tidwell, R.R.; Wilson, W.D. A new type of DNA minor-groove complex: Carbazole dication−DNA interactions. Biochemistry 1997, 36, 15315–15325. [Google Scholar] [CrossRef]
- Bandekar, P.P.; Roopnarine, K.A.; Parekh, V.J.; Mitchell, T.R.; Novak, M.J.; Sinden, R.R. Antimicrobial activity of tryptanthrins in E. coli. J. Med. Chem. 2010, 53, 3558–3565. [Google Scholar] [CrossRef]
- Bailly, C.; Laine, W.; Baldeyrou, B.; Pauw-Gillet, D.; Colson, P.; Houssier, C.; Cimanga, K.; Van Miert, S.; Vlietinck, A.J.; Pieters, L. DNA intercalation, topoisomerase II inhibition and cytotoxic activity of the plant alkaloid neocryptolepine. Anti-Cancer Drug Des. 2000, 15, 191–201. [Google Scholar]
- Sawer, I.K.; Berry, M.I.; Ford, J.L. The killing effect of cryptolepine on Staphylococcus aureus. Lett. Appl. Microbiol. 2005, 40, 24–29. [Google Scholar] [CrossRef]
- Chan, A.L.F.; Chang, W.S.; Chen, L.M.; Lee, C.M.; Chen, C.E.; Lin, C.M.; Hwang, J.L. Evodiamine stabilizes topoisomerase I-DNA cleavable complex to inhibit topoisomerase I activity. Molecules 2009, 14, 1342–1352. [Google Scholar] [CrossRef]
- Towers, G.H.N.; Whitehead, F.W.; Abramowski, Z.A.; Mitchell, J.C. Dictamnine, an alkaloid which crosslinks DNA in the presence of ultraviolet light. Biochem. Biophy. Res. Comm. 1980, 95, 603–607. [Google Scholar] [CrossRef]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef]
- Woo, S.H.; Reynolds, M.C.; Sun, N.J.; Cassady, J.M.; Snapka, R.M. Inhibition of topoisomerase II by liriodenine. Biochem. Pharmacol. 1997, 54, 467–473. [Google Scholar] [CrossRef]
- Kang, S.; Li, Z.; Yin, Z.; Jia, R.; Song, X.; Li, L.; Chen, Z.; Peng, L.; Qu, J.; Hu, Z.; et al. The antibacterial mechanism of berberine against Actinobacillus pleuropneumoniae. Nat. Prod. Res. 2015, 29, 2203–2206. [Google Scholar] [CrossRef]
- Peng, L.; Kang, S.; Yin, Z.; Jia, R.; Song, X.; Li, L.; Li, Z.; Zou, Y.; Liang, X.; Li, L.; et al. Antibacterial activity and mechanism of berberine against Streptococcus agalactiae. Int. J. Clin. Exp. Pathol. 2015, 8, 5217. [Google Scholar]
- Chen, B.H.; Chang, H.W.; Huang, H.M.; Chong, I.W.; Chen, J.S.; Chen, C.Y.; Wang, H.M. (−)-Anonaine induces DNA damage and inhibits growth and migration of human lung carcinoma h1299 cells. J. Agric. Food Chem. 2011, 59, 2284–2290. [Google Scholar] [CrossRef]
- Okon, E.; Kukula-Koch, W.; Jarzab, A.; Halasa, M.; Stepulak, A.; Wawruszak, A. Advances in chemistry and bioactivity of magnoflorine and magnoflorine-containing extracts. Int. J. Mol. Sci. 2020, 21, 1330. [Google Scholar] [CrossRef]
- Woo, S.H.; Sun, N.J.; Cassady, J.M.; Snapka, R.M. Topoisomerase II inhibition by aporphine alkaloids. Biochem. Pharmacol. 1999, 57, 1141–1145. [Google Scholar] [CrossRef]
- Chen, G.L.; Tian, Y.Q.; Wu, J.L.; Li, N.; Guo, M.Q. Antiproliferative activities of Amaryllidaceae alkaloids from Lycoris radiata targeting DNA topoisomerase I. Sci. Rep. 2016, 6, 38284. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, D.; Jiang, L.; Yang, D. DNA topoisomerase I inhibitory alkaloids from Corydalis saxicola. Chem. Biodivers. 2008, 5, 1335–1344. [Google Scholar] [CrossRef]
- Okamura, S.; Nishiyama, E.; Yamazaki, T.; Otsuka, N.; Taniguchi, S.; Ogawa, W.; Hatano, T.; Tsuchiya, T.; Kuroda, T. Action mechanism of 6, 6′-dihydroxythiobinupharidine from Nuphar japonicum, which showed anti-MRSA and anti-VRE activities. Biochim. Biophys. Acta Gene. Subj. 2015, 1850, 1245–1252. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, H.; Li, H.; Zhang, Y.; Zhang, H.; Su, F.; Xu, S.; Li, Y.; Si, Y.; Yu, S.; et al. Interaction studies of an anticancer alkaloid, (+)-(13aS)-deoxytylophorinine, with calf thymus DNA and four repeated double-helical DNAs. Chemotherapy 2011, 57, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Mitton-Fry, M.J.; Brickner, S.J.; Hamel, J.C.; Barham, R.; Brennan, L.; Casavant, J.M.; Ding, X.; Finegan, S.; Hardink, J.; Hoang, T.; et al. Novel 3-fluoro-6-methoxyquinoline derivatives as inhibitors of bacterial DNA gyrase and topoisomerase IV. Bioorganic Med. Chem. Lett. 2017, 27, 3353–3358. [Google Scholar] [CrossRef]
- Chen, C.; Qi, W.; Peng, X.; Chen, J.; Wan, C. Inhibitory effect of 7-demethoxytylophorine on Penicillium italicum and its possible mechanism. Microorganisms 2019, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Thakre, A.; Jadhav, V.; Kazi, R.; Shelar, A.; Patil, R.; Kharat, K.; Zore, G.; Karuppayil, S.M. Oxidative stress induced by piperine leads to apoptosis in Candida albicans. Med. Myc. J. 2021, 59, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.V.; Oliveira, A.D.; Raggi, L.; Young, M.; Kato, M.J. Antifungal activity of natural and synthetic amides from Piper species. J. Braz. Chem. Soc. 2010, 21, 1807–1813. [Google Scholar] [CrossRef]
- Vinche, A.D.L.; de-La-Cruz-Chacón, I.; González-Esquinca, A.R.; Silva, J.D.F.D.; Ferreira, G.; Santos, D.C.D.; Garces, H.G.; Oliveira, D.V.M.D.; Marçon, C.; Cavalcante, R.D.S.; et al. Antifungal activity of liriodenine on agents of systemic mycoses, with emphasis on the genus Paracoccidioides. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20200023. [Google Scholar] [CrossRef]
- Ashraf, S.M.; Mahanty, S.; Rathinasamy, K. Securinine induces mitotic block in cancer cells by binding to tubulin and inhibiting microtubule assembly: A possible mechanistic basis for its anticancer activity. Life Sci. 2021, 287, 120105. [Google Scholar] [CrossRef]
- Nishino, K.; Yamaguchi, A. Role of xenobiotic transporters in bacterial drug resistance and virulence. IUBMB Life 2008, 60, 569–574. [Google Scholar] [CrossRef]
- Hirasawa, Y.; Dai, X.; Deguchi, J.; Hatano, S.; Sasaki, T.; Ohtsuka, R.; Nugroho, A.E.; Kaneda, T.; Morita, H. New vasorelaxant indole alkaloids, taberniacins A and B, from Tabernaemontana divaricata. J. Nat. Med. 2019, 73, 627–632. [Google Scholar] [CrossRef]
- Gupta, S.; Tyagi, S.; Almeida, D.V.; Maiga, M.C.; Ammerman, N.C.; Bishai, W.R. Acceleration of tuberculosis treatment by adjunctive therapy with verapamil as an efflux inhibitor. Am. J. Respir. Crit. Care Med. 2013, 188, 600–607. [Google Scholar] [CrossRef]
- Dwivedi, G.R.; Singh, D.P.; Sharma, S.A.; Darokar, M.P. Efflux Pumps: Warheads of Gram-Negative Bacteria and Efflux Pump Inhibitors. In New Approaches in Biological Research; Sinha, R.P., Richa, Eds.; Nova Science Publishers: New York, NY, USA, 2017; Chapter 2; pp. 35–72. [Google Scholar]
- Jones, H.E.; Holland, I.B.; Jacq, A.; Wall, T.; Campbell, A.K. Escherichiacoli lacking the AcrAB multidrug efflux pump also lacks nonproteinaceous, PHB–polyphosphate Ca2+ channels in the membrane. Biochim. Biophys. Acta (BBA)-Biomembr. 2003, 1612, 90–97. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, V.K.; Pathania, R. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J. Med. Res. 2019, 149, 129. [Google Scholar] [PubMed]
- Michalet, S.; Cartier, G.; David, B.; Mariotte, A.M.; Dijoux-Franca, M.G.; Kaatz, G.W.; Stavri, M.; Gibbons, S. N-caffeoylphenalkylamide derivatives as bacterial efflux pump inhibitors. Bioorganic Med. Chem. Lett. 2007, 17, 1755–1758. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Casadevall, A. Growth of Cryptococcus neoformans in presence of L-dopa decreases its susceptibility to amphotericin B. Antimicrob. Agents Chemother. 1994, 38, 2648–2650. [Google Scholar] [CrossRef]
- Srinivasa Reddy, P.; Jamil, K.; Madhusudhan, P.; Anjani, G.; Das, B. Antibacterial activity of isolates from Piper longum and Taxus baccata. Pharm. Biol. 2001, 39, 236–238. [Google Scholar] [CrossRef]
- Oh, J.; Hwang, I.H.; Kim, D.C.; Kang, S.C.; Jang, T.S.; Lee, S.H.; Na, M. Anti-listerial compounds from Asari radix. Arch. Pharmacal Res. 2010, 33, 1339–1345. [Google Scholar] [CrossRef]
- Nna, P.J.; Tor-Anyiin, T.A.; Igoli, J.O. Fagaramide and pellitorine from the stem bark of Zanthoxylum zanthoxyloides and their antimicrobial activities. S. Asian Res. J. Nat. Prod. 2019, 2, 1–8. [Google Scholar]
- Saranya, A.M.A.D.; Yuenyongsawad, S.; Wattanapiromsakul, C. Investigation of antitubercular and cytotoxic activities of fruit extract and isolated compounds from Piper retrofractum Vahl. Walailak J. Sci. Technl. 2017, 14, 731–739. [Google Scholar]
- Reddy, S.V.; Srinivas, P.V.; Praveen, B.; Kishore, K.H.; Raju, B.C.; Murthy, U.S.; Rao, J.M. Antibacterial constituents from the berries of Piper nigrum. Phytomedicine 2004, 11, 697–700. [Google Scholar] [CrossRef]
- Rukachaisirikul, T.; Siriwattanakit, P.; Sukcharoenphol, K.; Wongvein, C.; Ruttanaweang, P.; Wongwattanavuch, P.; Suksamrarn, A. Chemical constituents and bioactivity of Piper sarmentosum. J. Ethnopharmacol. 2004, 93, 173–176. [Google Scholar] [CrossRef]
- Walters, D.; Meurer-Grimes, B.; Rovira, I. Antifungal activity of three spermidine conjugates. FEMS Microbiol. Lett. 2001, 201, 255–258. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, A.K.; Sadik, G.; Islam, M.R.; Khondkar, P.; Hossain, M.A.; Rashid, M.A. Antimicrobial and cytotoxic activities of Achyranthes ferruginea. Fitoterapia 2007, 78, 260–262. [Google Scholar] [CrossRef]
- Dahmer, J.; do Carmo, G.; Mostardeiro, M.A.; Neto, A.T.; da Silva, U.F.; Dalcol, I.I.; Morel, A.F. Antibacterial activity of Discaria americana Gillies ex Hook (Rhamnaceae). J. Ethnopharmacol. 2019, 239, 111635. [Google Scholar] [CrossRef]
- Emile, A.; Waikedre, J.; Herrenknecht, C.; Fourneau, C.; Gantier, J.C.; Hnawia, E.; Cabalion, P.; Hocquemiller, R.; Fournet, A. Bioassay-guided isolation of antifungal alkaloids from Melochia odorata. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 398–400. [Google Scholar]
- Panseeta, P.; Lomchoey, K.; Prabpai, S.; Kongsaeree, P.; Suksamrarn, A.; Ruchirawat, S.; Suksamrarn, S. Antiplasmodial and antimycobacterial cyclopeptide alkaloids from the root of Ziziphus mauritiana. Phytochemistry 2011, 72, 909–915. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Sarwar, M.G.; Suchy, M.; Adio, A.M. The phytoalexins from cauliflower, caulilexins A, B and C: Isolation, structure determination, syntheses and antifungal activity. Phytochemistry 2006, 67, 1503–1509. [Google Scholar] [CrossRef]
- Jimenez, L.D.; Ayer, W.A.; Tewari, J.P. Phytoalexins produced in the leaves of Capsella bursa-pastoris (shepherd’s purse). Phytoprotection 1997, 78, 99–103. [Google Scholar] [CrossRef]
- O’Donnell, G.; Gibbons, S. Antibacterial activity of two canthin-6-one alkaloids from Allium neapolitanum. Phytother. Res. 2007, 21, 653–657. [Google Scholar] [CrossRef]
- Su, X.L.; Xu, S.; Shan, Y.; Yin, M.; Chen, Y.; Feng, X.; Wang, Q.Z. Three new quinazolines from Evodia rutaecarpa and their biological activity. Fitoterapia 2018, 127, 186–192. [Google Scholar] [CrossRef]
- Maynart, G.; Pousset, J.L.; Mboup, S.; Denis, F. Antibacterial effect of borreverine, an alkaloid isolated from Borreria verticillata (Rubiaceae). Comptes Rendus Des Seances La Soc. Biol. Ses Fil. 1980, 174, 925–928. [Google Scholar]
- Bhattacharyya, P.; Chakrabartty, P.K.; Chowdhury, B.K. Glycozolidol, an antibacterial carbazole alkaloid from Glycosmis pentaphylla. Phytochemistry 1985, 24, 882–883. [Google Scholar] [CrossRef]
- Sunthitikawinsakul, A.; Kongkathip, N.; Kongkathip, B.; Phonnakhu, S.; Daly, J.W.; Spande, T.F.; Nimit, Y.; Rochanaruangrai, S. Coumarins and carbazoles from Clausena excavata exhibited antimycobacterial and antifungal activities. Planta Med. 2003, 69, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Maneerat, W.; Phakhodee, W.; Ritthiwigrom, T.; Cheenpracha, S.; Promgool, T.; Yossathera, K.; Deachathai, S.; Laphookhieo, S. Antibacterial carbazole alkaloids from Clausena harmandiana twigs. Fitoterapia 2012, 83, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Case, R.J.; Wang, Y.; Zhang, H.J.; Tan, G.T.; Van Hung, N.; Cuong, N.M.; Franzblau, S.G.; Soejarto, D.D.; Fong, H.H.; et al. Anti-tuberculosis constituents from the stem bark of Micromelum hirsutum. Planta Med. 2005, 71, 261–267. [Google Scholar] [CrossRef]
- Nalli, Y.; Khajuria, V.; Gupta, S.; Arora, P.; Riyaz-Ul-Hassan, S.; Ahmed, Z.; Ali, A. Four new carbazole alkaloids from Murraya koenigii that display anti-inflammatory and anti-microbial activities. Org. Biomol. Chem. 2016, 14, 3322–3332. [Google Scholar] [CrossRef]
- Joshi, T.; Jain, T.; Mahar, R.; Singh, S.K.; Srivastava, P.; Shukla, S.K.; Mishra, D.K.; Bhatta, R.S.; Banerjee, D.; Kanojiya, S. Pyranocarbazoles from Murraya koenigii (L.) Spreng. as antimicrobial agents. Nat. Prod. Res. 2018, 32, 430–434. [Google Scholar] [CrossRef]
- Rahman, M.M.; Gray, A.I. A benzoisofuranone derivative and carbazole alkaloids from Murraya koenigii and their antimicrobial activity. Phytochemistry 2005, 66, 1601–1606. [Google Scholar] [CrossRef]
- Aassila, H.; Bourguet-Kondracki, M.L.; Rifai, S.; Fassouane, A.; Guyot, M. Identification of harman as the antibiotic compound produced by a tunicate-associated bacterium. Mar. Biotechnol. 2003, 5, 163–166. [Google Scholar] [CrossRef]
- Cruz, K.S.; Lima, E.S.; Silva, M.D.J.A.D.; Souza, E.S.D.; Montoia, A.; Pohlit, A.M.; Souza, J.V.B.D. Screening and antifungal activity of a β-carboline derivative against Cryptococcus neoformans and C. gattii. Int. J. Microb. 2019, 7157845, 2019. [Google Scholar] [CrossRef]
- de Carvalho Junior, A.R.; Oliveira Ferreira, R.; de Souza Passos, M.; da Silva Boeno, S.I.; Glória das Virgens, L.D.L.; Ventura, T.L.B.; Calixto, S.D.; Lassounskaia, E.; de Carvalho, M.G.; Braz-Filho, R.; et al. Antimycobacterial and nitric oxide production inhibitory activities of triterpenes and alkaloids from Psychotria nuda (Cham. & Schltdl.) Wawra. Molecules 2019, 24, 1026. [Google Scholar]
- Rastogi, N.; Abaul, J.; Goh, K.S.; Devallois, A.; Philogène, E.; Bourgeois, P. Antimycobacterial activity of chemically defined natural substances from the Caribbean flora in Guadeloupe. FEMS Immun. Med. Microbiol. 1998, 20, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; A Sharma, R.; K Vyas, G. Antimicrobial, antineoplastic and cytotoxic activities of indole alkaloids from Tabernaemontana divaricata (L.) R. Br. Curr. Pharm. Anal. 2011, 7, 125–132. [Google Scholar] [CrossRef]
- Karaket, N.; Supaibulwatana, K.; Ounsuk, S.; Bultel-Poncé, V.; Pham, V.C.; Bodo, B. Chemical and bioactivity evaluation of the bark of Neonauclea purpurea. Nat. Prod. Commun. 2012, 7, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.J.; Zhao, Y.L.; Lunga, P.K.; Yang, X.W.; Song, C.W.; Cheng, G.G.; Liu, L.; Chen, Y.Y.; Liu, Y.P.; Luo, X.D. Indole alkaloids with antibacterial activity from aqueous fraction of Alstonia scholaris. Tetrahedron 2015, 71, 4372–4378. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.Y.; Qin, X.J.; Wang, B.; Jin, Q.; Liu, Y.P.; Luo, X.D. Antibacterial monoterpenoid indole alkaloids from Alstonia scholaris cultivated in temperate zone. Fitoterapia 2015, 105, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Macabeo, A.P.G.; Krohn, K.; Gehle, D.; Read, R.W.; Brophy, J.J.; Franzblau, S.G.; Aguinaldo, M.A.M. Activity of the extracts and indole alkaloids from Alstonia scholaris against Mycobacterium tuberculosis H37Rv. Philip. Agric. Sci. 2008, 91, 348–351. [Google Scholar]
- Kawakami, J.; Matsushima, N.; Ogawa, Y.; Kakinami, H.; Nakane, A.; Kitahara, H.; Nagaki, M.; Ito, S. Antibacterial and antifungal activities of tryptanthrin derivatives. Trans. Mater. Res. Soc. Jpn. 2011, 36, 603–606. [Google Scholar] [CrossRef]
- Honda, G.; Tabata, M. Isolation of antifungal principle tryptanthrin, from Strobilanthes cusia O. Kuntze. Planta Med. 1979, 36, 85–86. [Google Scholar] [CrossRef]
- Hao, Y.; Guo, J.; Wang, Z.; Liu, Y.; Li, Y.; Ma, D.; Wang, Q. Discovery of tryptanthrins as novel antiviral and anti-phytopathogenic-fungus agents. J. Agri. Food Chem. 2020, 68, 5586–5595. [Google Scholar] [CrossRef]
- Lin, C.J.; Chang, Y.L.; Yang, Y.L.; Chen, Y.L. Natural alkaloid tryptanthrin exhibits novel anticryptococcal activity. Med. Myc. J. 2021, 59, 545–556. [Google Scholar] [CrossRef]
- Wu, J.Y.; Chang, M.C.; Chen, C.S.; Lin, H.C.; Tsai, H.P.; Yang, C.C.; Yang, C.H.; Lin, C.M. Topoisomerase I inhibitor evodiamine acts as an antibacterial agent against drug-resistant Klebsiella pneumoniae. Planta Med. 2013, 79, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Paulo, M.D.Q.; Barbosa-Filho, J.; Lima, E.O.; Maia, R.F.; de Cassia, R.; Barbosa, B.B.C.; Kaplan, M.A.C. Antimicrobial activity of benzylisoquinoline alkaloids from Annona salzmanii DC. J. Ethnopharmacol. 1992, 36, 39–41. [Google Scholar] [CrossRef]
- Chen, Y.J.; Liu, H.; Zhang, S.Y.; Li, H.; Ma, K.Y.; Liu, Y.Q.; Yin, X.D.; Zhou, R.; Yan, Y.F.; Wang, R.X.; et al. Design, synthesis, and antifungal evaluation of cryptolepine derivatives against phytopathogenic fungi. J. Agri. Food Chem. 2021, 69, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Mgbeahuruike, E.E.; Stålnacke, M.; Vuorela, H.; Holm, Y. Antimicrobial and synergistic effects of commercial piperine and piperlongumine in combination with conventional antimicrobials. Antibiotics 2019, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Xiong, H.; Song, D.; Cao, X. Natural phenolic derivatives based on piperine scaffold as potential antifungal agents. BMC Chem. 2020, 14, 24. [Google Scholar] [CrossRef]
- Ramírez-Betancourt, A.; Hernández-Sánchez, A.M.; Salcedo-Morales, G.; Ventura-Zapata, E.; Robledo, N.; Wink, M.; Bermúdez-Torres, K. Unraveling the biosynthesis of quinolizidine alkaloids using the genetic and chemical diversity of Mexican lupins. Diversity 2021, 13, 375. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Li, W.; Nie, H.; Liao, M.; Qiu, B.; Yang, Y.L.; Chen, Y.F. Five new alkaloids from the roots of Sophora flavescens. Chem. Biodivers. 2018, 15, e1700577. [Google Scholar] [CrossRef]
- Teles, M.M.R.S.; Pinheiro, A.A.V.; Dias, C.D.S.; Tavares, J.F.; Barbosa Filho, J.M.; Da Cunha, E.V.L. Alkaloids of the Lauraceae. Alkal. Chem. Biol. 2019, 82, 147–304. [Google Scholar]
- Dhiman, M.; Parab, R.R.; Manju, S.L.; Desai, D.C.; Mahajan, G.B. Antifungal activity of hydrochloride salts of tylophorinidine and tylophorinine. Nat. Prod. Commun. 2012, 7, 1171–1172. [Google Scholar] [CrossRef]
- Xin, Z.; OuYang, Q.; Wan, C.; Che, J.; Li, L.; Chen, J.; Tao, N. Isolation of antofine from Cynanchum atratum BUNGE (Asclepiadaceae) and its antifungal activity against Penicillium digitatum. Postharvest Biol. Technol. 2019, 157, 110961. [Google Scholar] [CrossRef]
- Mensah, J.L.; Lagarde, I.; Ceschin, C.; Michelb, G.; Gleye, J.; Fouraste, I. Antibacterial activity of the leaves of Phyllanthus discoideus. J. Ethnopharmacol. 1990, 28, 129–133. [Google Scholar] [CrossRef]
- Singh, A.K.; Pandey, M.B.; Singh, U.P. Antifungal activity of an alkaloid allosecurinine against some fungi. Mycobiology 2007, 35, 62–64. [Google Scholar] [CrossRef][Green Version]
- Goel, M.; Maurya, S.; Pandey, V.B.; Singh, V.P.; Singh, A.K.; Singh, U.P. Effect of Ent-norsecurinine, an alkaloid, on spore germination of some fungi. Mycobiology 2002, 30, 225–227. [Google Scholar] [CrossRef]
- Laluces, H.M.C.; Nakayama, A.; Nonato, M.G.; dela Cruz, T.E.; Tan, M.A. Antimicrobial alkaloids from the leaves of Pandanus amaryllifolius. J. Appl. Pharm. Sci. 2015, 5, 151–153. [Google Scholar] [CrossRef]
- Bibi, N.; Tanoli, S.A.K.; Farheen, S.; Afza, N.; Siddiqi, S.; Zhang, Y.; Kazmi, S.U.; Malik, A. In vitro antituberculosis activities of the constituents isolated from Haloxylon salicornicum. Bioorg. Med. Chem. Lett. 2010, 20, 4173–4176. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Lee, S.E.; Yang, J.Y.; Lee, H.S. Antimicrobial potentials of active component isolated from Citrullus colocynthis fruits and structure–activity relationships of its analogues against foodborne bacteria. J. Sci. Food Agric. 2014, 94, 2529–2533. [Google Scholar] [CrossRef] [PubMed]
- Aguinaldo, A.M.; Dalangin-Mallari, V.M.; Macabeo, A.P.G.; Byrne, L.T.; Abe, F.; Yamauchi, T.; Franzblau, S.G. Quinoline alkaloids from Lunasia amara inhibit Mycobacterium tuberculosis H37Rv in vitro. Int. J. Antimicrob. Agents 2007, 29, 744–746. [Google Scholar] [CrossRef]
- Tantapakul, C.; Sripisut, T.; Maneerat, W.; Ritthiwigrom, T.; Laphookhieo, S. Antibacterial compounds from Glycosmis puberula Twigs. Nat. Prod. Commun. 2014, 9, 1705–1707. [Google Scholar] [CrossRef]
- Huang, H.Y.; Ishikawa, T.; Peng, C.F.; Tsai, I.L.; Chen, I.S. Constituents of the root wood of Zanthoxylum wutaiense with antitubercular activity. J. Nat. Prod. 2008, 71, 1146–1151. [Google Scholar] [CrossRef]
- Ramesha, B.T.; Suma, H.K.; Senthilkumar, U.; Priti, V.; Ravikanth, G.; Vasudeva, R.; Kumar, T.S.; Ganeshaiah, K.N.; Shaanker, R.U. New plant sources of the anti-cancer alkaloid, camptothecine from the Icacinaceae taxa, India. Phytomedicine 2013, 20, 521–527. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Z.; Cain, A.; Wang, B.; Long, M.; Taylor, J. Antifungal activity of camptothecin, trifolin, and hyperoside isolated from Camptotheca acuminata. J. Agric. Food Chem. 2005, 53, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Özçelik, B.; Karaoğlu, T.; Şener, B. Antiviral and antimicrobial profiles of selected isoquinoline alkaloids from Fumaria and Corydalis species. Z. Naturforsch. 2007, 62, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ari, S.N.; Yuvita, D.D.; Phurpa, W.; Paul, A.K. Anti-infective and anti-cancer properties of the Annona species: Their ethnomedicinal uses, alkaloid diversity, and pharmacological activities. Molecules 2019, 24, 4419. [Google Scholar] [CrossRef]
- Pandey, M.B.; Singh, A.K.; Singh, A.K.; Singh, U.P. Inhibitive effect of fuyuziphine isolated from plant (Pittapapra) (Fumaria indica) on spore germination of some fungi. Mycobiology 2007, 35, 157–158. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Wang, K.; Zhang, G.; Ho, H.I.; Gao, A. Synergistic anti-candidal activity of tetrandrine on ketoconazole: An experimental study. Planta Med. 2010, 76, 53–61. [Google Scholar] [CrossRef]
- Sureram, S.; Senadeera, S.P.; Hongmanee, P.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Antimycobacterial activity of bisbenzylisoquinoline alkaloids from Tiliacora triandra against multidrug-resistant isolates of Mycobacterium tuberculosis. Bioorganic Med. Chem. Lett. 2012, 22, 2902–2905. [Google Scholar] [CrossRef]
- Singh, K.P.; Pandey, V.B.; Tripathi, Y.C.; Singh, U.P. Tiliacorinine, a new systemic fungicide effective against Alternaria blight of pigeon pea (Cajanus cajan)/Tiliacorinine, ein neues systemisches Fungizid mit Wirkung gegen die Alternaria-Blattfleckenkrankheit an Taubenerbsen (Cajanus cajan). J. Plant Dis. Protec. 1991, 98, 213–219. [Google Scholar]
- Rahman, M.M.; Lopa, S.S.; Sadik, G.; Islam, R.; Khondkar, P.; Alam, A.K.; Rashid, M.A. Antibacterial and cytotoxic compounds from the bark of Cananga odorata. Fitoterapia 2005, 76, 758–761. [Google Scholar] [CrossRef]
- De la Cruz-Chacón, I.; González-Esquinca, A.R.; Fefer, P.G.; Garcia, L.F.J. Liriodenine, early antimicrobial defence in Annona diversifolia. Z. Naturforsch. C 2011, 66, 377–384. [Google Scholar] [CrossRef]
- Costa, E.V.; Pinheiro, M.L.B.; Barison, A.; Campos, F.R.; Salvador, M.J.; Maia, B.H.L.; Cabral, E.C.; Eberlin, M.N. Alkaloids from the bark of Guatteria hispida and their evaluation as antioxidant and antimicrobial agents. J. Nat. Prod. 2010, 73, 1180–1183. [Google Scholar] [CrossRef]
- Tan, K.K.; Khoo, T.J.; Rajagopal, M.; Wiart, C. Antibacterial alkaloids from Artabotrys crassifolius Hook. f. & Thomson. Nat. Prod. Res. 2015, 29, 2346–2349. [Google Scholar] [PubMed]
- Omar, H.; Hashim, N.M.; Zajmi, A.; Nordin, N.; Abdelwahab, S.I.; Azizan, A.H.S.; Hadi, A.H.A.; Ali, H.M. Aporphine alkaloids from the leaves of Phoebe grandis (Nees) Mer. (Lauraceae) and their cytotoxic and antibacterial activities. Molecules 2013, 18, 8994–9009. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.L.; Liou, Y.F.; Lu, S.T. Screening of isoquinoline alkaloids and their derivatives for antibacterial and antifungal activities. Gaoxiong Yi Xue Ke Xue Za Zhi Kaohsiung J. Med. Sci. 1989, 5, 132–145. [Google Scholar]
- Hossain, M.S.; Ferdous, A.J.; Hasan, C.M. In vitro antimicrobial activities of alkaloids from the stem bark of Desmos longiflorus (Roxb.). Bangladesh J. Bot. 1993, 22, 37–40. [Google Scholar]
- Agarwal, A.K.; Xu, T.; Jacob, M.R.; Feng, Q.; Lorenz, M.C.; Walker, L.A.; Clark, A.M. Role of heme in the antifungal activity of the azaoxoaporphine alkaloid sampangine. Eukaryot. Cell 2008, 7, 387–400. [Google Scholar] [CrossRef]
- Khan, M.R.; Kihara, M.; Omoloso, A.D. Antimicrobial activity of the alkaloidal constituents of the root bark of Eupomatia laurina. Pharm. Biol. 2003, 41, 277–280. [Google Scholar] [CrossRef]
- Lekphrom, R.; Kanokmedhakul, S.; Kanokmedhakul, K. Bioactive styryllactones and alkaloid from flowers of Goniothalamus laoticus. J. Ethnopharmacol. 2009, 125, 47–50. [Google Scholar] [CrossRef]
- Puvanendran, S.; Wickramasinghe, A.; Karunaratne, D.N.; Carr, G.; Wijesundara, D.S.A.; Andersen, R.; Karunaratne, V. Antioxidant constituents from Xylopia championii. Pharm. Biol. 2008, 46, 352–355. [Google Scholar] [CrossRef][Green Version]
- Camacho-Corona, M.D.R.; Favela-Hernández, J.M.D.J.; González-Santiago, O.; Garza-González, E.; Molina-Salinas, G.M.; Said-Fernández, S.; Delgado, G.; Luna-Herrera, J. Evaluation of some plant-derived secondary metabolites against sensitive and multidrug-resistant Mycobacterium tuberculosis. J. Mex. Chem. Soc. 2009, 53, 71–75. [Google Scholar] [CrossRef]
- Makarasen, A.; Sirithana, W.; Mogkhuntod, S.; Khunnawutmanotham, N.; Chimnoi, N.; Techasakul, S. Cytotoxic and antimicrobial activities of aporphine alkaloids isolated from Stephania venosa (Blume) Spreng. Planta Med. 2011, 77, 1519–1524. [Google Scholar] [CrossRef]
- Mollataghi, A.; Coudiere, E.; Hadi, A.H.A.; Mukhtar, M.R.; Awang, K.; Litaudon, M.; Ata, A. Anti-acetylcholinesterase, anti-α-glucosidase, anti-leishmanial and anti-fungal activities of chemical constituents of Beilschmiedia species. Fitoterapia 2012, 83, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Du, F.; Yan, L.; He, G.; He, J.; Wang, C.; Rao, G.; Jiang, Y.; Xu, G. Potent activities of roemerine against Candida albicans and the underlying mechanisms. Molecules 2015, 20, 17913–17928. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, V.K.; ElSohly, H.N.; Khan, S.I.; Jacob, M.R.; Joshi, V.C.; Smillie, T.; Khan, I.A.; Walker, L.A. Constituents of Nelumbo nucifera leaves and their antimalarial and antifungal activity. Phytochem. Lett. 2008, 1, 89–93. [Google Scholar] [CrossRef]
- Kim, J.; Ha Quang Bao, T.; Shin, Y.K.; Kim, K.Y. Antifungal activity of magnoflorine against Candida strains. World J. Microbiol. Biotechnol. 2018, 34, 167. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Jin, L.; Yang, C.; Zhu, Y.; Ye, X.; Li, X.; Zhang, B. Antifungal activity and potential mechanism of magnoflorine against Trichophyton rubrum. J. Antibiot. 2021, 74, 206–214. [Google Scholar] [CrossRef]
- Kosina, P.; Gregorova, J.; Gruz, J.; Vacek, J.; Kolar, M.; Vogel, M.; Roos, W.; Naumann, K.; Simanek, V.; Ulrichova, J. Phytochemical and antimicrobial characterization of Macleaya cordata herb. Fitoterapia 2010, 81, 1006–1012. [Google Scholar] [CrossRef]
- Faizi, S.; Khan, R.A.; Azher, S.; Khan, S.A.; Tauseef, S.; Ahmad, A. New antimicrobial alkaloids from the roots of Polyalthia longifolia var. pendula. Planta Med. 2003, 69, 350–355. [Google Scholar] [CrossRef]
- Park, K.M.; You, J.S.; Lee, H.Y.; Baek, N.I.; Hwang, J.K. Kuwanon G: An antibacterial agent from the root bark of Morus alba against oral pathogens. J. Ethnopharmacol. 2003, 84, 181–185. [Google Scholar] [CrossRef]
- Choi, J.G.; Kang, O.H.; Chae, H.S.; Obiang-Obounou, B.; Lee, Y.S.; Oh, Y.C.; Kim, M.S.; Shin, D.W.; Kim, J.A.; Kim, Y.H.; et al. Antibacterial activity of Hylomecon hylomeconoides against methicillin-resistant S. aureus. Appl. Biochem. Biotech. 2010, 160, 2467–2474. [Google Scholar] [CrossRef]
- Meng, F.; Zuo, G.; Hao, X.; Wang, G.; Xiao, H.; Zhang, J.; Xu, G. Antifungal activity of the benzo [c] phenanthridine alkaloids from Chelidonium majus Linn against resistant clinical yeast isolates. J. Ethnopharmacol. 2009, 125, 494–496. [Google Scholar] [CrossRef]
- Xue, X.; Zhang, H.; Zhang, X.; Liu, X.; Xi, K.; Han, Y.; Guo, Z. TLC bioautography-guided isolation and antimicrobial, antifungal effects of 12 alkaloids from Hylomecon japonica roots. Nat. Prod. Commun. 2017, 12, 1439–1442. [Google Scholar] [CrossRef]
- Tavares, L.D.C.; Zanon, G.; Weber, A.D.; Neto, A.T.; Mostardeiro, C.P.; Da Cruz, I.B.; Oliveira, R.M.; Ilha, V.; Dalcol, I.I.; Morel, A.F. Structure-activity relationship of benzophenanthridine alkaloids from Zanthoxylum rhoifolium having antimicrobial activity. PLoS ONE 2014, 9, e97000. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.Y.; Li, Y.; Wang, T.; Han, J.; Wang, G.C.; Zhang, Y.L.; Pan, W.D. Synergistic antibacterial and antibiotic effects of bisbenzylisoquinoline alkaloids on clinical isolates of methicillin-resistant S. aureus (MRSA). Molecules 2018, 16, 9819–9826. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Yang, X.J.; Zhou, L.; Hu, H.J.; Zheng, F.; Ding, X.D.; Sun, D.M.; Zhou, C.D.; Sun, W. Structural modification of sanguinarine and chelerythrine and their antibacterial activity. Nat. Prod. Res. 2011, 25, 863–875. [Google Scholar] [CrossRef]
- Guang Ma, W.; Fukushi, Y.; Tahara, S. Fungitoxic alkaloids from Hokkaido corydalis species. Fitoterapia 1999, 70, 258–265. [Google Scholar]
- Phatchana, R.; Yenjai, C. Cytotoxic coumarins from Toddalia asiatica. Planta Med. 2014, 80, 719–722. [Google Scholar] [CrossRef]
- Hu, J.; Shi, X.; Chen, J.; Mao, X.; Zhu, L.; Yu, L.; Shi, J. Alkaloids from Toddalia asiatica and their cytotoxic, antimicrobial and antifungal activities. Food Chem. 2014, 148, 437–444. [Google Scholar] [CrossRef]
- Gu, J.Q.; Graf, T.N.; Lee, D.; Chai, H.B.; Mi, Q.; Kardono, L.B.; Setyowati, F.M.; Ismail, R.; Riswan, S.; Farnsworth, N.R.; et al. Cytotoxic and antimicrobial constituents of the bark of Diospyros maritima collected in two geographical locations in Indonesia. J. Nat. Prod. 2004, 67, 1156–1161. [Google Scholar] [CrossRef]
- Čerňáková, M.; Košťálová, D. Antimicrobial activity of berberine—A constituent of Mahonia aquifolium. Folia Microbiol. 2002, 47, 375–378. [Google Scholar] [CrossRef]
- Villinski, J.; Dumas, E.; Chai, H.B.; Pezzuto, J.; Angerhofer, C.; Gafner, S. Antibacterial activity and alkaloid content of Berberis thunbergii, Berberis vulgaris and Hydrastis canadensis. Pharm. Biol. 2003, 41, 551–557. [Google Scholar] [CrossRef]
- Slobodníková, L.; KoSt’álová, D.; Labudová, D.; Kotulová, D.; Kettmann, V. Antimicrobial activity of Mahonia aquifolium crude extract and its major isolated alkaloids. Phytother. Res. 2004, 18, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.H.; Kim, K.J.; Cha, J.D.; Kim, H.K.; Lee, Y.E.; Choi, N.Y.; You, Y.O. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant S. aureus. J. Med. Food 2005, 8, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Chomnawang, M.T.; Trinapakul, C.; Gritsanapan, W. In vitro antigonococcal activity of Coscinium fenestratum stem extract. J. Ethnopharmacol. 2009, 122, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Wojtyczka, R.D.; Dziedzic, A.; Kępa, M.; Kubina, R.; Kabała-Dzik, A.; Mularz, T.; Idzik, D. Berberine enhances the antibacterial activity of selected antibiotics against coagulase-negative Staphylococcus strains in vitro. Molecules 2014, 19, 6583–6596. [Google Scholar] [CrossRef]
- Blanco, P.; Hernando-Amado, S.; Reales-Calderon, J.A.; Corona, F.; Lira, F.; Alcalde-Rico, M.; Bernardini, A.; Sanchez, M.B.; Martinez, J.L. Bacterial multidrug efflux pumps: Much more than antibiotic resistance determinants. Microorganisms 2016, 4, 14. [Google Scholar] [CrossRef]
- da Silva, A.R.; de Andrade Neto, J.B.; da Silva, C.R.; de Sousa Campos, R.; Silva, R.A.C.; Freitas, D.D.; do Nascimento, F.B.S.A.; de Andrade, L.N.D.; Sampaio, L.S.; Grangeiro, T.B.; et al. Berberine antifungal activity in fluconazole-resistant pathogenic yeasts: Action mechanism evaluated by flow cytometry and biofilm growth inhibition in Candida spp. Antimicrob. Agents Chemother. 2016, 60, 3551–3557. [Google Scholar] [CrossRef]
- Iwazaki, R.S.; Endo, E.H.; Ueda-Nakamura, T.; Nakamura, C.V.; Garcia, L.B.; Dias Filho, B.P. In vitro antifungal activity of the berberine and its synergism with fluconazole. Antonie Van Leeuwenhoek 2010, 97, 201. [Google Scholar] [CrossRef]
- Gentry, E.J.; Hanuman, B.; Keshavarz-Shokri, J.A.; Morton, M.D.; David, V.V.; Hanumaiah, T.; Lester, A.; Mitscher, R.S.; Debbi, H.; William, B. Antitubercular natural products: Berberine from the roots of commercial Hydrastis c anadensis powder. Isolation of inactive 8-oxotetrahydrothalifendine, canadine, β-hydrastine, and two new quinic acid esters, hycandinic acid esters-1 and-2. J. Nat. Prod. 1998, 61, 1187–1193. [Google Scholar] [CrossRef]
- Wang, L.J.; Ye, X.L.; Li, X.G.; Sun, Q.L.; Yu, G.; Cao, X.G.; Liang, Y.T.; Zhou, J.Z. Synthesis and antimicrobial activity of 3-alkoxyjatrorrhizine derivatives. Planta Med. 2008, 74, 290–292. [Google Scholar] [CrossRef]
- Volleková, A.; Košt’álová, D.; Kettmann, V.; Tóth, J. Antifungal activity of Mahonia aquifolium extract and its major protoberberine alkaloids. Phytother. Res. 2003, 17, 834–837. [Google Scholar] [CrossRef]
- Basha, S.A.; Mishra, R.K.; Jha, R.N.; Pandey, V.B.; Singh, U.P. Effect of berberine and (±)-bicuculline isolated from Corydalis chaerophylla on spore germination of some fungi. Folia Microbiol. 2002, 47, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Semwal, D.K.; Badoni, R.; Semwal, R.; Kothiyal, S.K.; Singh, G.J.P.; Rawat, U. The genus Stephania (Menispermaceae): Chemical and pharmacological perspectives. J. Ethnopharmacol. 2010, 132, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.W.; Ruan, Y.; Ren, W.; Ma, B.J.; Wang, X.L.; Zheng, C.F. Lycorine: A potential broad-spectrum agent against crop pathogenic fungi. J. Microbiol. Biotechnol. 2014, 24, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.J.; van Staden, J. Antifungal constituents of the plant family Amaryllidaceae. Phytother. Res. 2018, 32, 976–984. [Google Scholar] [CrossRef]
- Pettit, G.R.; Melody, N.; Herald, D.L. Antineoplastic agents. synthesis of (+)-pancratistatin from (+)-narciclasine as relay1a. J. Org. Chem. 2001, 66, 2583–2587. [Google Scholar] [CrossRef]
- Liang, C.; Yang, L.; Shao, Y.; Zhu, X.; Zhao, H.; Chen, B.; Song, W.; Song, X.; Ding, X.; Sun, R. Broad-spectrum antifungal activity of dichloromethane extract of Waltheria indica stems and isolated compounds. Ind. Crops Prod. 2019, 142, 111855. [Google Scholar] [CrossRef]
- Zhao, Z.; He, X.; Han, W.; Chen, X.; Liu, P.; Zhao, X.; Wang, X.; Zhang, L.; Wu, S.; Zheng, X. Genus Tetradium L.: A comprehensive review on traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol. 2019, 231, 337–354. [Google Scholar] [CrossRef]
- Pan, X.; Bligh, S.A.; Smith, E. Quinolone alkaloids from Fructus euodiae show activity against methicillin-resistant Staphylococcus aureus. Phytother. Res. 2014, 28, 305–307. [Google Scholar] [CrossRef]
- Adams, M.; Mahringer, A.; Kunert, O.; Fricker, G.; Efferth, T.; Bauer, R. Cytotoxicity and p-glycoprotein modulating effects of quinolones and indoloquinazolines from the Chinese herb Evodia rutaecarpa. Planta Med. 2007, 73, 1554–1557. [Google Scholar] [CrossRef]
- Sripisut, T.; Ritthiwigrom, T.; Promgool, T.; Yossathera, K.; Deachathai, S.; Phakhodee, W.; Cheenpracha, S.; Laphookhieo, S. Glycopentaphyllone: The first isolation of hydroperoxyquinolone from the fruits of Glycosmis pentaphylla. Phytochem. Lett. 2012, 5, 379–381. [Google Scholar] [CrossRef]
- Suliman Mohamed, M.; Timan Idriss, M.; Khedr, A.I.; Abd AlGadir, H.; Takeshita, S.; Shah, M.M.; Ichinose, Y.; Maki, T. Activity of Aristolochia bracteolata against Moraxella catarrhalis. Int. J. Bacteriol. 2014, 2014, 481686. [Google Scholar] [CrossRef] [PubMed]
- Angalaparameswari, S.; Saleem, T.M.; Alagusundaram, M.; Ramkanth, S.; Thiruvengadarajan, V.; Gnanaprakash, K.; Chetty, C.M.; Pratheesh, G. Anti-microbial activity of aristolochic acid from root of Aristolochia bracteata Retz. Int. J. Biomed. Life Sci. 2012, 8, 2–4. [Google Scholar]
- Hinou, J.; Demetzos, C.; Harvala, C.; Roussakis, C. Cytotoxic and antimicrobial principles from the roots of Aristolochia longa. Int. J. Crude Drug Res. 1990, 28, 149–151. [Google Scholar] [CrossRef]
- Lee, Y.S.; Han, S.H.; Lee, S.H.; Kim, Y.G.; Park, C.B.; Kang, O.H.; Keum, J.H.; Kim, S.B.; Mun, S.H.; Seo, Y.S.; et al. The mechanism of antibacterial activity of tetrandrine against Staphylococcus aureus. Foodborne Pathog. Dis. 2012, 9, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Attaluri, S.; Bonala, R.R.; Yang, I.Y.; Lukin, M.A.; Wen, Y.; Grollman, A.P.; Moriya, M.; Iden, C.R.; Johnson, F. DNA adducts of aristolochic acid II: Total synthesis and site-specific mutagenesis studies in mammalian cells. Nucleic Acids Res. 2010, 38, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Pfau, W.; Schmeiser, H.H.; Wiessler, M. Aristolochic acid binds covalently to the exocyclic amino group of purine nucleotides in DNA. Carcinogenesis 1990, 11, 313–319. [Google Scholar] [CrossRef]
- Zeng, Y.B.; Wei, D.J.; Dong, W.H.; Cai, C.H.; Yang, D.L.; Zhong, H.M.; Mei, W.L.; Dai, H.F. Antimicrobial glycoalkaloids from the tubers of Stephania succifera. Arch. Pharmacal Res. 2017, 40, 429–434. [Google Scholar] [CrossRef]
- Tuntiwachwuttikul, P.; Phansa, P.; Pootaeng-on, Y.; Taylor, W.C. Chemical constituents of the roots of Piper sarmentosum. Chem. Pharm. Bull. 2006, 54, 149–151. [Google Scholar] [CrossRef]
- Shi, Y.N.; Liu, F.F.; Jacob, M.R.; Li, X.C.; Zhu, H.T.; Wang, D.; Cheng, R.R.; Yang, C.R.; Xu, M.; Zhang, Y.J. Antifungal amide alkaloids from the aerial parts of Piper flaviflorum and Piper sarmentosum. Planta Med. 2017, 83, 143–150. [Google Scholar] [CrossRef]
- Lakshmanan, G.; Sivaraj, C.; Ammar, A.; Gopinath, S.; Saravanan, K.; Gunasekaran, K.; Murugesan, K. Isolation and structural elucidation of allantoin a bioactive compound from Cleome viscosa L.: A combined experimental and computational investigation. Pharmacogn. J. 2019, 11, 1391–1400. [Google Scholar] [CrossRef]
- Atta-ur-Rahman Nasreen, A.; Akhtar, F.; Shekhani, M.S.; Clardy, J.; Parvez, M.; Choudhary, M.I. Antifungal diterpenoid alkaloids from Delphinium denudatum. J. Nat. Prod. 1997, 60, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Li-Na, Z.H.O.U.; Xiao-Lei, G.E.; Ting-Ting, D.O.N.G.; Hui-Yuan, G.A.O.; Bo-Hang, S.U.N. Antibacterial steroidal alkaloids from Holarrhena antidysenteriaca. Chin. J. Nat. Med. 2017, 15, 540–545. [Google Scholar]
- Weinstein, L.I.; Albersheim, P. Host-pathogen interactions: XXIII. The mechanism of the antibacterial action of glycinol, a pterocarpan phytoalexin synthesized by soybeans. Plant Physiol. 1983, 72, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.; Ausubel, F.M. Prospects for plant-derived antibacterials. Nat. Biotechnol. 2006, 24, 1504–1507. [Google Scholar] [CrossRef] [PubMed]
| CLASS Compound | Chemical Structure | Genus, Species | Family |
|---|---|---|---|
| ACRIDANONES | |||
| 1-Hydroxy-3,4-dimethoxy-10-methylacridan-9-one | 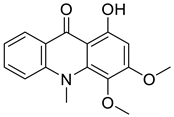 | Limonia acidissima L. | Rutaceae |
| 1-Hydroxy-3-methoxy-10-methylacridan-9-one |  | Limonia acidissima L. | Rutaceae |
| AMARYLLIDACEAE ALKALOIDS | |||
| Crinamine |  | Crinum asiaticum L. | Amaryllidaceae |
| Lycoricidine |  | Lycoris radiata (L’Hér.) Herb. | Amaryllidaceae |
| Lycorine | 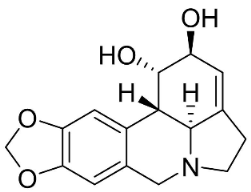 | Lycoris radiata (L’Hér.) Herb. | Amaryllidaceae |
| Narciclasine | 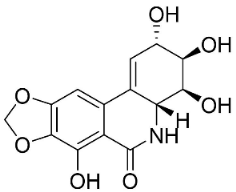 | Lycoris radiata (L’Hér.) Herb. | Amaryllidaceae |
| Tazettine | 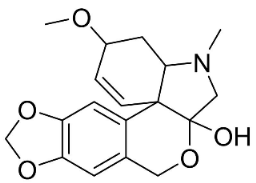 | Narcissus tazetta L. | Amaryllidaceae |
| APORPHINES | |||
| Anonaine | 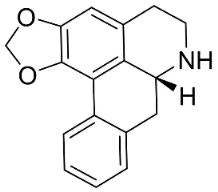 | Michelia alba DC. | Magnolicaceae |
| Artabotrine | 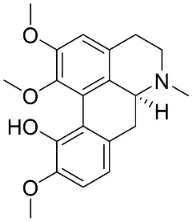 | Artabotrys suaveolens (Bl.) Bl. | Annonaceae |
| Bulbocapnine | 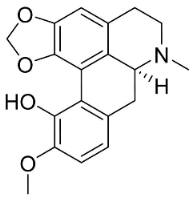 | Corydalis bulbosa DC. | Fumariaceae |
| Dicentrinone |  | Phoebe lanceolata (Nees) Nees | Lauraceae |
| Isoboldine |  | Corydalis bulbosa DC. | Fumariaceae |
| Lanuginosine | 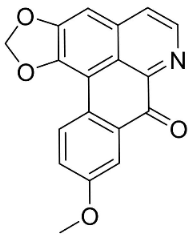 | Eupomatia laurina R. Br. | Eupomatiaceae |
| Liriodenine | 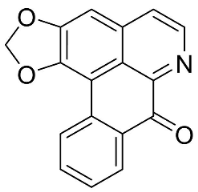 | Cananga odorata Hook. F. & Thomson | Annonaceae |
| Lysicamine | 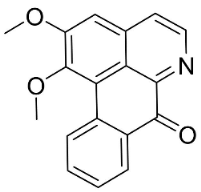 | Phoebe grandis (Nees) Merr. | Lauraceae |
| Magnoflorine | 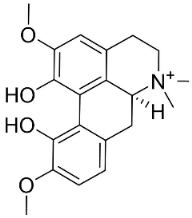 | Mahonia bealei (Fortune) Carrière | Papaveraceae |
| Nordicentrine | 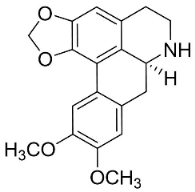 | Phoebe lanceolata (Nees) Nees | Lauraceae |
| O-Methylmoschatoline | 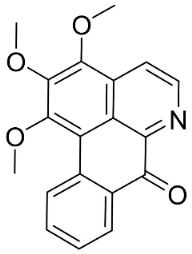 | Cananga odorata (Lam.) Hook. F. & Thomson | Annonaceae |
| Roemerine |  | Papaver rhoeas L. | Papaveraceae |
| Sampangine | 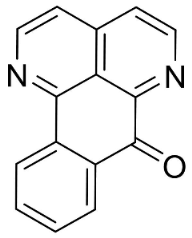 | Eupomatia laurina R. Br. | Eupomatiaceae |
| Thailandine | 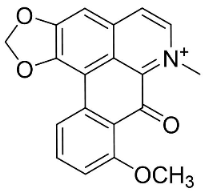 | Stephania venosa (Bl.) Spreng | Menispermaceae |
| Xylopine |  | Artabotrys suaveolens (Bl.) Bl. | Annonaceae |
| BENZOPHENANTHRIDINES | |||
| 8-Acetylnorchelerythrine | 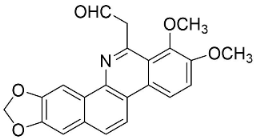 | Toddalia asiatica (L.) Lam. | Rutaceae |
| Avicine | 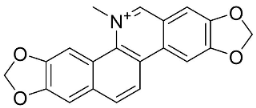 | Toddalia asiatica (L.) Lam. | Rutaceae |
| Chelerythrine |  | Macleaya cordata (Willd.) R. Br. | Papaveraceae |
| Corynoline | 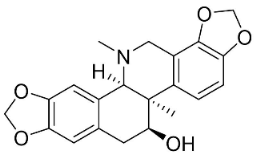 | Corydalis incisa (Thunb.) Pers. | Fumariaceae |
| Dihydrochelerythrine | 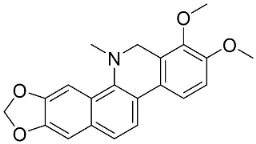 | Macleaya cordata (Willd.) R. Br. | Papaveraceae |
| Dihydrosanguinarine | 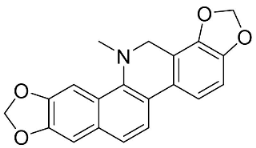 | Macleaya cordata (Willd.) R. Br | Papaveraceae |
| 8-Hydroxydihydrochelerythrine | 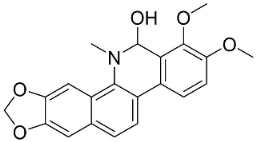 | Chelidonium majus L. | Fumariaceae |
| 8-Hydroxydihydrosanguinarine |  | Chelidonium majus L. | Fumariaceae |
| 6-Methoxydihydrosanguinarine |  | Chelidonium japonicum Thunb | Fumariaceae |
| Nitidine | 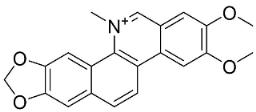 | Zanthoxylum L. | Rutaceae |
| Norchelerythrine | 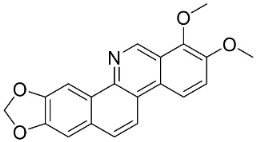 | Toddalia asiatica (L.) Lam. | Rutaceae |
| Norsanguinarine | 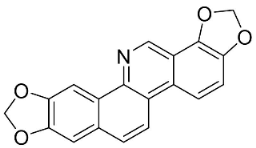 | Fumaria indica Pugsley | Fumariaceae |
| Rhoifoline B | 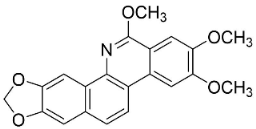 | Toddalia asiatica (L.) Lam. | Rutaceae |
| Sanguinarine | 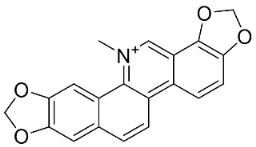 | Fumaria officinalis L. | Fumariaceae |
| Stylopine |  | Fumaria officinalis L. | Fumariaceae |
| CARBAZOLES | |||
| 3,3′-[Oxybis(methylene)]bis(9-methoxy-9H-carbazole) | 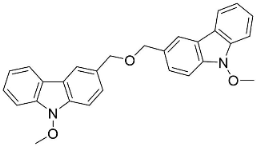 | Murraya koenigii (L.) Spreng. | Rutaceae |
| Clausamine A | 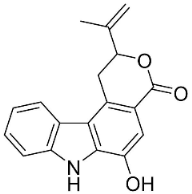 | Clausena harmandiana (Pierre) Guillaumin | Rutaceae |
| Clausamine B | 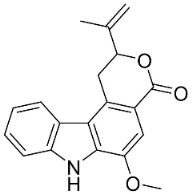 | Clausena harmandiana (Pierre) Guillaumin | Rutaceae |
| Clausine F | 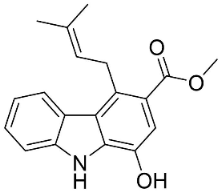 | Clausena harmandiana (Pierre) Guillaumin | Rutaceae |
| Clauszoline N | 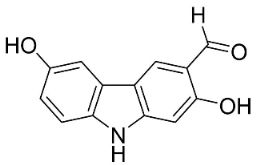 | Clausena harmandiana (Pierre) Guillaumin | Rutaceae |
| 3-Formylcarbazole |  | Clausena mexicana Burm.f. | Rutaceae |
| 3-Formyl-1-methoxycarbazole | 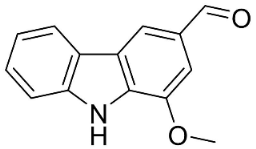 | Murraya koenigii (L.) Spreng. | Rutaceae |
| Girinimbine |  | Murraya koenigii (L.) Spreng. | Rutaceae |
| Glycozolidol | 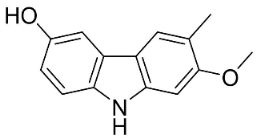 | Glycosmis pentaphylla (Retz.) DC. | Rutaceae |
| Harmane | 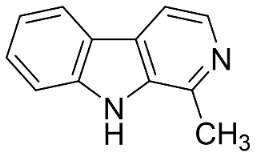 | Murraya mexicana (L.) Jack | Rutaceae |
| Koenimbine | 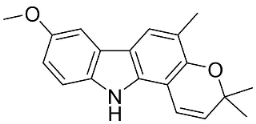 | Murraya koenigii (L.) Spreng. | Rutaceae |
| Koenigine | 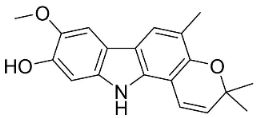 | Murraya koenigii (L.) Spreng. | Rutaceae |
| Lansine | 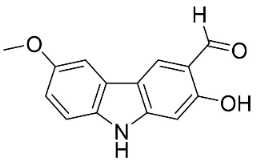 | Micromelum pubescens Bl. | Rutaceae |
| Murrayamine J | 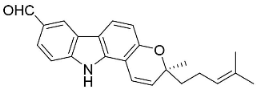 | Murraya mexicana (L.) Jack | Rutaceae |
| BENZYLISOQUINOLINES | |||
| Reticuline | 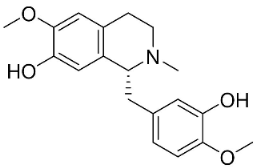 | Annona squamosa L. | Annonaceae |
| Fuyuziphine | 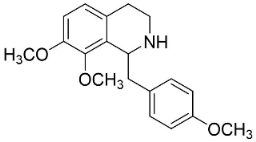 | Fumaria indica Pugsley | Fumariaceae |
| BISBENZYLISOQUINOLINES | |||
| 2′-Nortiliacorinine | 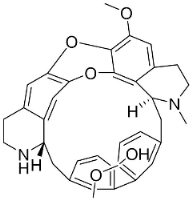 | Tiliacora triandra Diels | Menispermaceae |
| Tetrandrine | 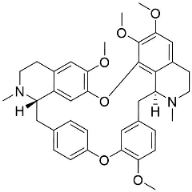 | Cyclea barbata Miers | Menispermaceae |
| Tiliacorine | 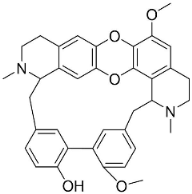 | Tiliacora triandra Diels | Menispermaceae |
| Tiliacorinine | 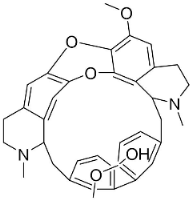 | Tiliacora triandra Diels | Menispermaceae |
| DITERPENE ALKALOIDS | |||
| 8-Acetylheterophyllisine | 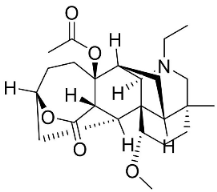 | Delphinium denudatum Wall. Ex Hook. F. & Thomson yields | Ranunculaceae |
| Panicutine | 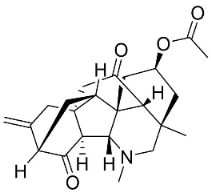 | Delphinium denudatum Wall. Ex Hook. F. & Thomson yields | Ranunculaceae |
| Vilmorrianone | 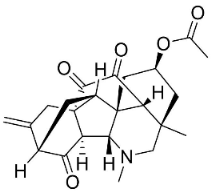 | Delphinium denudatum Wall. Ex Hook. F. & Thomson yields | Ranunculaceae |
| HASUBANANS | |||
| Glabradine |  | Stephania glabra (Roxb.) Miers | Menispermaceae |
| IMIDAZOLES | |||
| Allantoin |  | Tournefortia sarmentosa Lam | Borraginaceae |
| INDOLOQUINAZOLINES | |||
| Dehydroevodiamine |  | Euodia rutaecarpa Benth | Rutaceae |
| Evodiamine |  | Euodia rutaecarpa Benth | Rutaceae |
| Tryptanthrin |  | Indigofera tinctoria L. | Fabaceae |
| INDOLOQUINOLINES | |||
| Cryptolepine |  | Cryptolepis sanguinolenta (Lindl.) Schltr. | Apocynaceae |
| MONOTERPENE INDOLE ALKALOIDS | |||
| Alstoniascholarine A | 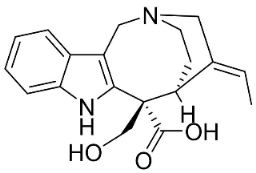 | Alstonia scholaris (L.) R.Br. | Apocynaceae |
| Alstoniascholarine E |  | Alstonia scholaris (L.) R.Br. | Apocynaceae |
| Alstoniascholarine J | 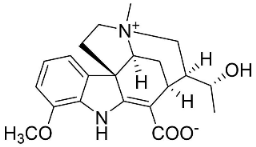 | Alstonia scholaris (L.) R.Br. | Apocynaceae |
| Cadambine | 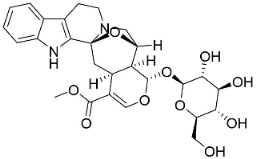 | Neolamarckia cadamba (Roxb.) Bosser | Rubiaceae |
| Ibogaine | 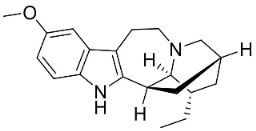 | Ervatamia mexicana (L.) Burkill | Apocynaceae |
| 3-Oxocoronaridine | 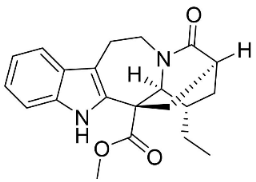 | Ervatamia mexicana (L.) Burkill | Apocynaceae |
| 5-Oxocoronaridine | 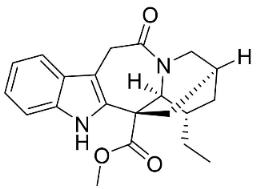 | Ervatamia mexicana (L.) Burkill | Apocynaceae |
| Strictosidine | 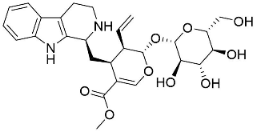 | Guettarda speciosa L. | Rubiaceae |
| Vallesamine | 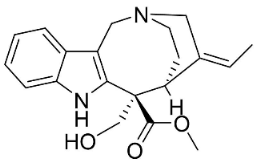 | Ervatamia mexicana (L.) Burkill | Apocynaceae |
| Voacamine | 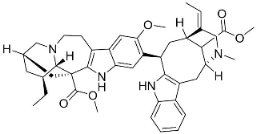 | Ervatamia mexicana (L.) Burkill | Apocynaceae |
| Voacangine |  | Ervatamia exicana (L.) Burkill | Apocynaceae |
| Vobasine | 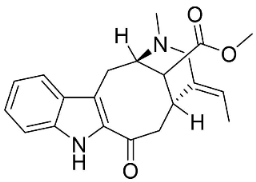 | Ervatamia mexicana (L.) Burkill | Apocynaceae |
| Tubotaiwine |  | Alstonia scholaris (L.) R.Br. | Apocynaceae |
| PHENANTHRENE ALKALOIDS | |||
| Aristolochic acid |  | Aristolochia L. | Aristolochiaceae |
| Aristolactam N-(6′-trans-p-coumaroyl)-β-d-glucopyranoside |  | Aristolochia L. | Aristolochiaceae |
| 1-N-monomethylcarbamate-argentinine-3-O-β-d-glucoside | 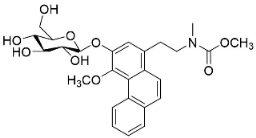 | Stephania succifera H.S. Lo & Y. Tsoong | Meninspermaceae |
| PHENANTHROINDOLIZIDINE ALKALOIDS | |||
| 7-Demethoxytylophorine |  | Cynanchum atratum Bunge | Asclepiadaceae |
| Tylophorinine | 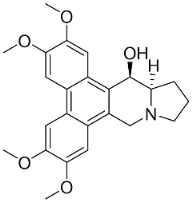 | Tylophora indica (Burm.f.) Merr. | Asclepiadaceae |
| Tylophorinidine |  | Tylophora indica (Burm.f.) Merr | Asclepiadaceae |
| PIPERINE ALKALOIDS | |||
| Piperine | 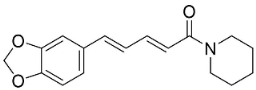 | Piper nigrum L. | Piperaceae |
| Piperlongumine | 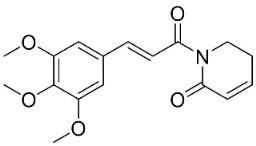 | Piper longum L. | Piperaceae |
| PHTHALIDES | |||
| Adlumidine | 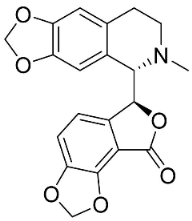 | Fumaria officinalis L. t | Fumariaceae |
| Bicuculline | 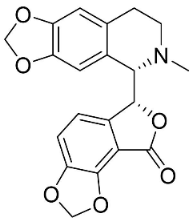 | Corydalis bulbosa DC. | Fumariaceae |
| PROTOBERBERINES | |||
| Berberine |  | Coptis chinensis Franch. | Ranunculaceae |
| Jatrorrhizine |  | Coptis chinensis Franch. | |
| Palmatine | 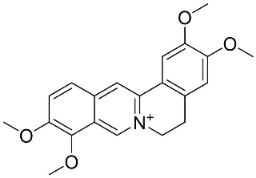 | Corydalis exicana (Thunb.) Pers. | Fumariaceae |
| Sinactine | 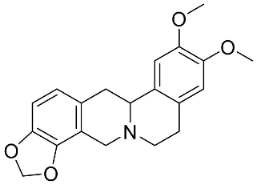 | Fumaria officinalis L. | Fumariaceae |
| PYRROLIDINES | |||
| Brachyamide B |  | Piper nigrum L. | Piperaceae |
| Isopiperolein B | 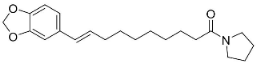 | Piper nigrum L. | Piperaceae |
| N-[9-(3,4-Methylenedioxyphenyl)-2E,4E,8E-nonatrienoyl]pyrrolidine | 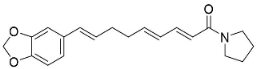 | Piper nigrum L. | Piperaceae |
| Pandamarilactonine A | 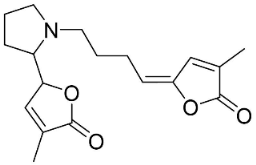 | Pandanus odorus Ridl. | Pandanaceae |
| Trachyone |  | Piper nigrum L. | Piperaceae |
| QUINOLINONES | |||
| Antidesmone | 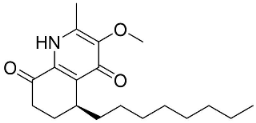 | Waltheria indica L. | Malvaceae |
| Evocarpine | 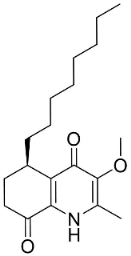 | Euodia rutaecarpa Benth | Rutaceae |
| 1-Methyl-2-nonyl-4(1H)-quinolone |  | Euodia rutaecarpa Benth | Rutaceae |
| 1-Methyl-2-[(Z)-5′-pentadecenyl]-4(1H)-quinolone | 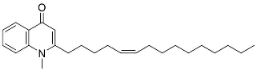 | Euodia rutaecarpa Benth | Rutaceae |
| Waltherione C | 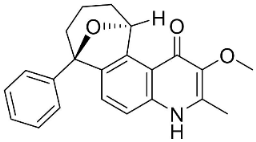 | Waltheria indica L. | Malvaceae |
| QUINOLIZIDINE ALKALOIDS | |||
| 6,6′-Dihydroxythiobinupharidine | 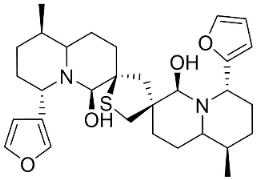 | Nuphar japonica DC. | Nymphaeaceae |
| 7-Hydroxylupanine |  | Sophora flavescens Aiton | Fabaceae |
| N-Butylcytisine |  | Sophora flavescens Aiton | Fabaceae |
| SECURINEGA ALKALOIDS | |||
| Securinine | 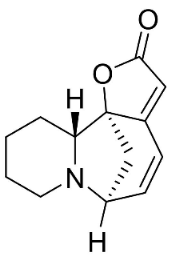 | Flueggea virosa (Roxb. Ex Willd.) Royle | Phyllanthaceae |
| Viroallosecurinine | 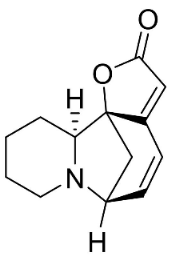 | Flueggea virosa (Roxb. Ex Willd.) Royle | Phyllanthaceae |
| MISCELLANEOUS PIPERINE ALKALOIDS | |||
| Dihydrodioscorine | 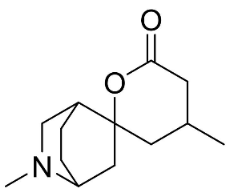 | Dioscorea bulbifera L. | Dioscoreaceae |
| Haloxyline B |  | Haloxylon salicornicum (Moq.) Bunge ex Boiss. | Chenopodiaceae |
| Pandamarilactone-1 | 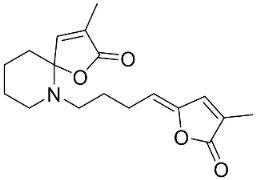 | Pandanus odorus Ridl. | Pandanaceae |
| SIMPLE QUINOLINE ALKALOIDS | |||
| Camptothecin | 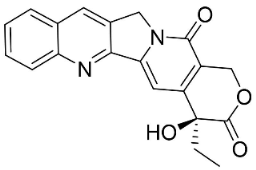 | Gomphandra Wall. Ex Lindl. | Icacinaceae |
| Dictamine | 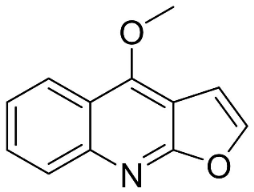 | Dictamnus albus L. | Rutaceae |
| γ-Fagarine | 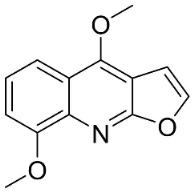 | Dictamnus albus L. | Rutaceae |
| 4-Methoxy-2-phenylquinoline | 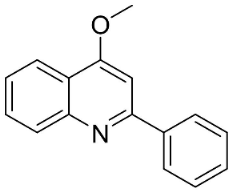 | Lunasia amara Blanco | Rutaceae |
| 4-Methylquinoline |  | Citrullus colocynthis (L.) Schrad. | Cucurbitaceae |
| Robustine |  | Dictamnus albus L. | Rutaceae |
| PROTOBERBERINES | |||
| Pendulamine A | 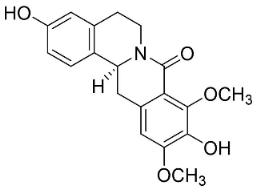 | Polyalthia longifolia (Sonn.) | Annonaceae |
| Pendulamine B | 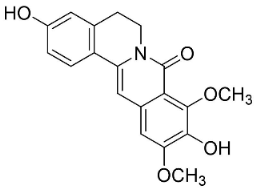 | Polyalthia longifolia (Sonn.) | Annonaceae |
| PROTOPINES | |||
| Allocryptopine |  | Macleaya cordata (Willd.) R. Br | Papaveraceae |
| Protopine | 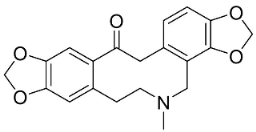 | Argemone mexicana L. | |
| SPIROBENZYLISOQUINOLINES | |||
| (+)-Fumariline |  | Fumaria officinalis L. | Fumariaceae |
| Fumarophycine | 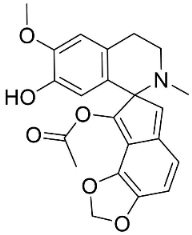 | Fumaria officinalis L. yields | Fumariaceae |
| (+)-Parfumine |  | Fumaria indica Pugsley | Fumariaceae |
| STEROIDAL ALKALOIDS | |||
| Conimine |  | Holarrhena pubescens Wall. Ex G. Don | Apocynaceae |
| N-Formylconessimine | 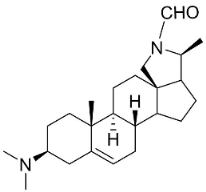 | Holarrhena pubescens Wall. Ex G. Don | Apocynaceae |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulaiman, M.; Jannat, K.; Nissapatorn, V.; Rahmatullah, M.; Paul, A.K.; de Lourdes Pereira, M.; Rajagopal, M.; Suleiman, M.; Butler, M.S.; Break, M.K.B.; et al. Antibacterial and Antifungal Alkaloids from Asian Angiosperms: Distribution, Mechanisms of Action, Structure-Activity, and Clinical Potentials. Antibiotics 2022, 11, 1146. https://doi.org/10.3390/antibiotics11091146
Sulaiman M, Jannat K, Nissapatorn V, Rahmatullah M, Paul AK, de Lourdes Pereira M, Rajagopal M, Suleiman M, Butler MS, Break MKB, et al. Antibacterial and Antifungal Alkaloids from Asian Angiosperms: Distribution, Mechanisms of Action, Structure-Activity, and Clinical Potentials. Antibiotics. 2022; 11(9):1146. https://doi.org/10.3390/antibiotics11091146
Chicago/Turabian StyleSulaiman, Mazdida, Khoshnur Jannat, Veeranoot Nissapatorn, Mohammed Rahmatullah, Alok K. Paul, Maria de Lourdes Pereira, Mogana Rajagopal, Monica Suleiman, Mark S. Butler, Mohammed Khaled Bin Break, and et al. 2022. "Antibacterial and Antifungal Alkaloids from Asian Angiosperms: Distribution, Mechanisms of Action, Structure-Activity, and Clinical Potentials" Antibiotics 11, no. 9: 1146. https://doi.org/10.3390/antibiotics11091146
APA StyleSulaiman, M., Jannat, K., Nissapatorn, V., Rahmatullah, M., Paul, A. K., de Lourdes Pereira, M., Rajagopal, M., Suleiman, M., Butler, M. S., Break, M. K. B., Weber, J.-F., Wilairatana, P., & Wiart, C. (2022). Antibacterial and Antifungal Alkaloids from Asian Angiosperms: Distribution, Mechanisms of Action, Structure-Activity, and Clinical Potentials. Antibiotics, 11(9), 1146. https://doi.org/10.3390/antibiotics11091146










