Clinical Impact of a Pharmacist-Driven Prospective Audit with Intervention and Feedback on the Treatment of Patients with Bloodstream Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preintervention
2.2. Pharmacist-Driven Prospective Audit with Intervention and Feedback for BSI Patients
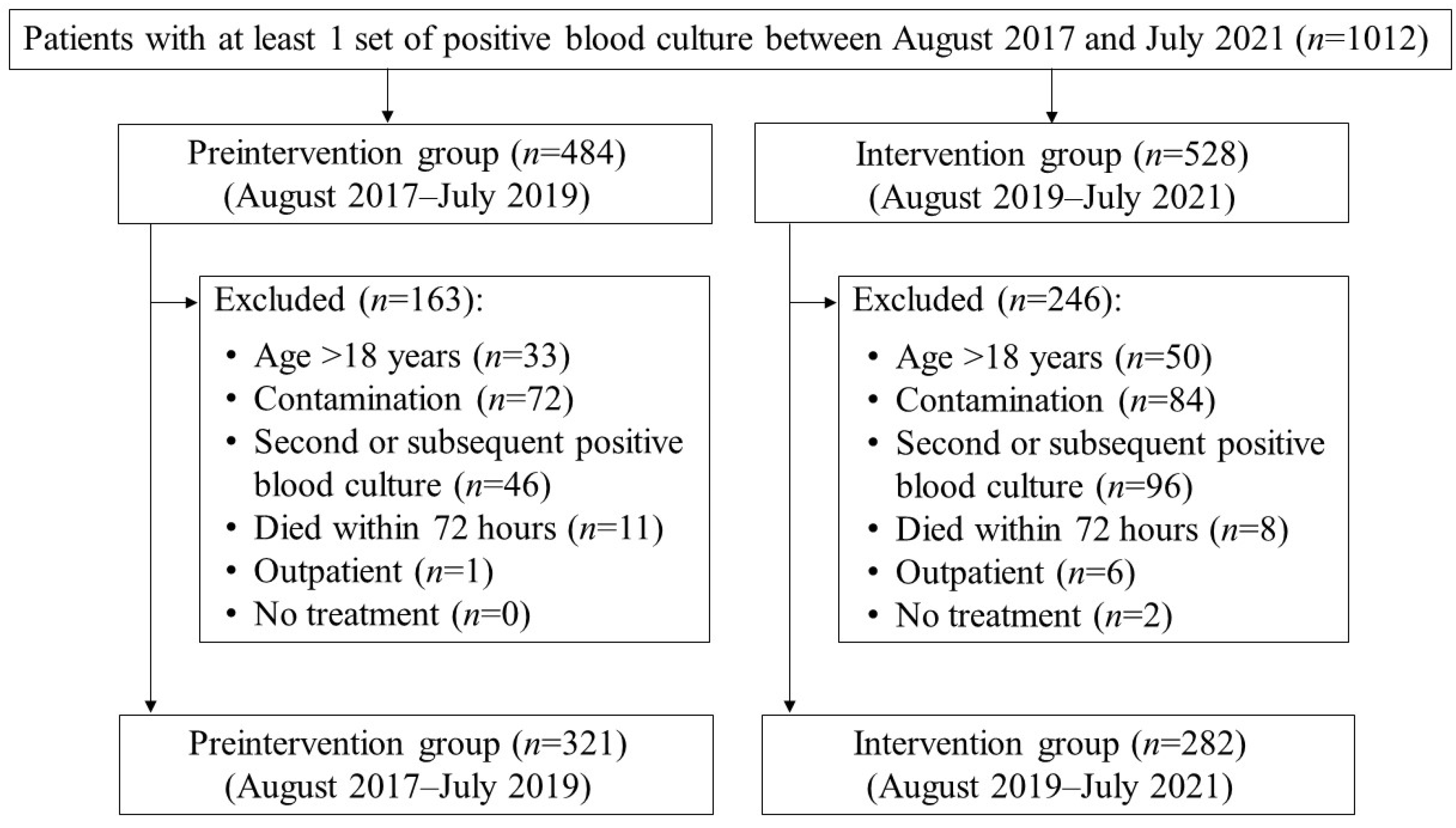
2.3. Study Population
2.4. Outcome Measures
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Primary Outcome Measures
3.3. Secondary Outcome Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, R.; Coast, J. The True Cost of Antimicrobial Resistance. BMJ 2013, 346, f1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, L.Y.; Kwa, A.L.; Lye, D.; Chlebicki, M.P.; Tan, T.Y.; Ling, M.L.; Wong, S.Y.; Goh, L.G. Reducing Antimicrobial Resistance Through Appropriate Antibiotic Usage in Singapore. Singap. Med. J. 2008, 49, 749–755. [Google Scholar]
- Ohji, G.; Doi, A.; Yamamoto, S.; Iwata, K. Is De-Escalation of Antimicrobials Effective? A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2016, 49, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef]
- Garau, J.; Bassetti, M. Role of Pharmacists in Antimicrobial Stewardship Programmes. Int. J. Clin. Pharm. 2018, 40, 948–952. [Google Scholar] [CrossRef]
- Hashimoto, M.; Asai, S.; Umezawa, K.; Kohara, K.; Miyazawa, M.; Suzuki, Y.; Miyachi, H. Impact of Ward Pharmacist-Led Antimicrobial Stewardship in Intensive Care Units. J. Chemother. 2022, 1–10. [Google Scholar] [CrossRef]
- Paul, M.; Dickstein, Y.; Raz-Pasteur, A. Antibiotic De-Escalation for Bloodstream Infections and Pneumonia: Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2016, 22, 960–967. [Google Scholar] [CrossRef]
- Kufel, W.D.; Mastro, K.A.; Steele, J.M.; Wang, D.; Riddell, S.W.; Paolino, K.M.; Thomas, S.J. Impact of a Pharmacist-Facilitated, Evidence-Based Bundle Initiative on Staphylococcus Aureus Bacteremia Management. Diagn. Microbiol. Infect. Dis. 2021, 101, 115535. [Google Scholar] [CrossRef]
- Shinoda, Y.; Ohashi, K.; Matsuoka, T.; Arai, K.; Hotta, N.; Asano, I.; Yoshimura, T. Impact of Continuous Pharmacist Intervention for Injectable Antimicrobials on the Treatment of Patients with Escherichia coli Bacteremia. Am. J. Infect. Control 2022. [Google Scholar] [CrossRef]
- Pien, B.C.; Sundaram, P.; Raoof, N.; Costa, S.F.; Mirrett, S.; Woods, C.W.; Reller, L.B.; Weinstein, M.P. The Clinical and Prognostic Importance of Positive Blood Cultures in Adults. Am. J. Med. 2010, 123, 819–828. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Chow, J.; Fine, M.J.; Shlaes, D.M.; Quinn, J.P.; Hooper, D.C.; Johnson, M.P.; Ramphal, R.; Wagener, M.M.; Miyashiro, D.K.; Yu, V.L. Enterobacter Bacteremia: Clinical Features and Emergence of Antibiotic Resistance during Therapy. Ann. Intern. Med. 1991, 115, 585–590. [Google Scholar] [CrossRef]
- Suzuki, A.; Maeda, M.; Yokoe, T.; Hashiguchi, M.; Togashi, M.; Ishino, K. Impact of the Multidisciplinary Antimicrobial Stewardship Team Intervention Focusing on Carbapenem De-Escalation: A Single-Centre and Interrupted Time Series Analysis. Int. J. Clin. Pract. 2020, 75, e13693. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Thorlacius-Ussing, L.; Sandholdt, H.; Nissen, J.; Rasmussen, J.; Skov, R.; Frimodt-Møller, N.; Knudsen, J.D.; Østergaard, C.; Benfield, T. Comparable Outcomes of Short-Course and Prolonged-Course Therapy in Selected Cases of Methicillin-Susceptible Staphylococcus aureus Bacteremia: A Pooled Cohort Study. Clin. Infect. Dis. 2021, 73, 866–872. [Google Scholar] [CrossRef]
- Von Dach, E.; Albrich, W.C.; Brunel, A.-S.; Prendki, V.; Cuvelier, C.; Flury, D.; Gayet-Ageron, A.; Huttner, B.; Kohler, P.; Lemmenmeier, E.; et al. Effect of C-Reactive Protein–Guided Antibiotic Treatment Duration, 7-Day Treatment, or 14-Day Treatment on 30-Day Clinical Failure Rate in Patients with Uncomplicated Gram-Negative Bacteremia: A Randomized Clinical Trial. JAMA 2020, 323, 2160–2169. [Google Scholar] [CrossRef]
- Cardozo, C.; Cuervo, G.; Salavert, M.; Merino, P.; Gioia, F.; Fernández-Ruiz, M.; López-Cortés, L.E.; Escolà-Vergé, L.; Montejo, M.; Muñoz, P.; et al. An Evidence-Based Bundle Improves the Quality of Care and Outcomes of Patients with Candidaemia. J. Antimicrob. Chemother. 2019, 75, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, R.K.; Gillani, S.W.; Alzaabi, M.J.; Gulam, S.M. Evaluation of Inappropriate Antibiotic Prescribing and Management Through Pharmacist-Led Antimicrobial Stewardship Programmes: A Meta-Analysis of Evidence. Eur. J. Hosp. Pharm. 2021, 29, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Kooda, K.; Canterbury, E.; Bellolio, F. Impact of Pharmacist-Led Antimicrobial Stewardship on Appropriate Antibiotic Prescribing in the Emergency Department: A Systematic Review and Meta-Analysis. Ann. Emerg. Med. 2022, 79, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Monmaturapoj, T.; Scott, J.; Smith, P.; Abutheraa, N.; Watson, M. Pharmacist-Led Education-Based Antimicrobial Stewardship Interventions and Their Effect on Antimicrobial Use in Hospital Inpatients: A Systematic Review and Narrative Synthesis. J. Hosp. Infect. 2021, 115, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Smit, C.C.H.; De Vos, S.; Benko, R.; Llor, C.; Paget, W.J.; Briant, K.; Pont, L.; Van Dijk, L.; Taxis, K. A Systematic Literature Review and Meta-Analysis of Community Pharmacist-Led Interventions to Optimise the Use of Antibiotics. Br. J. Clin. Pharmacol. 2022, 88, 2617–2641. [Google Scholar] [CrossRef]
- Cantudo-Cuenca, M.R.; Jiménez-Morales, A.; la Plata, J.E.M.-D. Pharmacist-Led Antimicrobial Stewardship Programme in a Small Hospital Without Infectious Diseases Physicians. Sci. Rep. 2022, 12, 9501. [Google Scholar] [CrossRef]
- Maeda, M.; Muraki, Y.; Kosaka, T.; Yamada, T.; Aoki, Y.; Kaku, M.; Seki, M.; Tanabe, Y.; Fujita, N.; Niki, Y.; et al. Essential Human Resources for Antimicrobial Stewardship Teams in Japan: Estimates from A Nationwide Survey Conducted by the Japanese Society of Chemotherapy. J. Infect. Chemother. 2019, 25, 653–656. [Google Scholar] [CrossRef] [Green Version]
- Garwan, Y.M.; Alsalloum, M.A.; Thabit, A.K.; Jose, J.; Eljaaly, K. Effectiveness of Antimicrobial Stewardship Interventions on Early Switch from Intravenous-To-Oral Antimicrobials in Hospitalized Adults: A Systematic Review. Am. J. Infect. Control 2022. [Google Scholar] [CrossRef]
- Spellberg, B.; Rice, L.B. Duration of Antibiotic Therapy: Shorter Is Better. Ann. Intern. Med. 2019, 171, 210–211. [Google Scholar] [CrossRef]
- Burke, J.P. Antibiotic Resistance—Squeezing the Balloon? JAMA 1998, 280, 1270–1271. [Google Scholar] [CrossRef]
- Yamada, K.; Imoto, W.; Yamairi, K.; Shibata, W.; Namikawa, H.; Yoshii, N.; Fujimoto, H.; Nakaie, K.; Okada, Y.; Fujita, A.; et al. The Intervention by an Antimicrobial Stewardship Team Can Improve Clinical and Microbiological Outcomes of Resistant Gram-Negative Bacteria. J. Infect. Chemother. 2019, 25, 1001–1006. [Google Scholar] [CrossRef]
- Remtulla, S.; Zurek, K.; Cervera, C.; Hernandez, C.; Lee, M.-C.; Hoang, H.L. Impact of an Unsolicited, Standardized Form–Based Antimicrobial Stewardship Intervention to Improve Guideline Adherence in the Management of Staphylococcus aureus Bacteremia. Open Forum Infect. Dis. 2019, 6, ofz098. [Google Scholar] [CrossRef]
- Jindai, K.; Kusama, Y.; Gu, Y.; Honda, H.; Ohmagari, N. Narrative Review: The Process of Expanding the Manual of Antimicrobial Stewardship by the Government of Japan. Intern. Med. 2021, 60, 181–190. [Google Scholar] [CrossRef]
- Bernal, J.L.; Cummins, S.; Gasparrini, A. Interrupted time series regression for the evaluation of public health interventions: A tutorial. Int. J. Epidemiol. 2017, 46, 348–355. [Google Scholar] [CrossRef]

| Variables | Preintervention Group (n = 321) | Intervention Group (n = 282) | p-Value |
|---|---|---|---|
| Age (years), mean (SD) | 67.05 (14.22) | 66.90 (14.29) | 0.85 a |
| Male sex (%) | 199 (62.0) | 174 (61.7) | 1.00 b |
| eGFR (ml/min/1.73 m2), mean (SD) | 65.71 (35.04) | 67.65 (41.58) | 0.55 a |
| Chronic kidney disease stage 4, n (%) | 44 (13.7) | 49 (17.4) | 0.22 b |
| Diabetes mellitus, n (%) | 100 (31.2) | 100 (35.5) | 0.30 b |
| Department, n (%) | |||
| Gastroenterology | 52 (16.2) | 51 (18.1) | 0.54 b |
| Gastrointestinal surgery | 40 (12.5) | 35 (12.4) | 0.99 b |
| Hematology | 44 (13.7) | 35 (12.4) | 0.64 b |
| Respiratory | 31 (9.6) | 28 (10.0) | 0.91 b |
| Urology | 29 (9.0) | 26 (9.2) | 0.94 b |
| Neurosurgery | 20 (6.2) | 10 (3.5) | 0.13 b |
| Obstetrics and gynecology | 11 (3.4) | 18 (6.4) | 0.091 b |
| Orthopedic surgery | 2 (0.6) | 8 (2.8) | 0.051 c |
| Others | 92 (28.7) | 71 (25.2) | 0.34 b |
| Pathogen, n (%) | |||
| Methicillin-sensitive Staphylococcus aureus | 26 (8.1) | 18 (6.4) | 0.42 b |
| Methicillin-resistant Staphylococcus aureus | 9 (2.8) | 7 (2.5) | 0.81 b |
| Coagulase-negative staphylococci | 52 (16.2) | 39 (13.8) | 0.42 b |
| Streptococcus spp. | 29 (9.0) | 16 (5.7) | 0.12 b |
| Enterococcus spp. | 17 (5.3) | 21 (7.4) | 0.28 b |
| E. coli | 35 (10.9) | 41 (14.5) | 0.18 b |
| ESBL-producing E. coli | 14 (4.4) | 21 (7.4) | 0.11 b |
| NDM-1-producing E. coli | 1 (0.3) | 0 (0) | 1.00 c |
| Klebsiella spp. | 38 (11.8) | 21 (7.4) | 0.075 b |
| Enterobacter spp. | 15 (4.7) | 15 (5.3) | 0.72 b |
| Acinetobacter spp. | 9 (2.8) | 4 (1.4) | 0.27 c |
| Serratia spp. | 8 (2.5) | 5 (1.8) | 0.54 c |
| Pseudomonas aeruginosa | 10 (3.1) | 10 (3.5) | 0.77 b |
| Candida spp. | 8 (2.5) | 7 (2.5) | 0.99 b |
| Mixed infection | 26 (8.1) | 23 (8.2) | 1.00 b |
| Others | 24 (7.5) | 34 (12.1) | 0.057 b |
| Pitt bacteremia score (IQR) | 1 (0–2) | 1 (0–2) | 0.351 a |
| Pitt bacteremia score ≥ 4 (%) | 35 (10.9) | 29 (10.3) | 1.00 b |
| Treatment | Preintervention Group (n = 321) | Intervention Group (n = 282) | p-Value |
|---|---|---|---|
| Empiric therapy, n (%) | |||
| Carbapenem | 67 (20.9) | 64 (22.7) | 0.59 a |
| Tazobactam/piperacillin | 87 (27.1) | 104 (36.9) | 0.01 a |
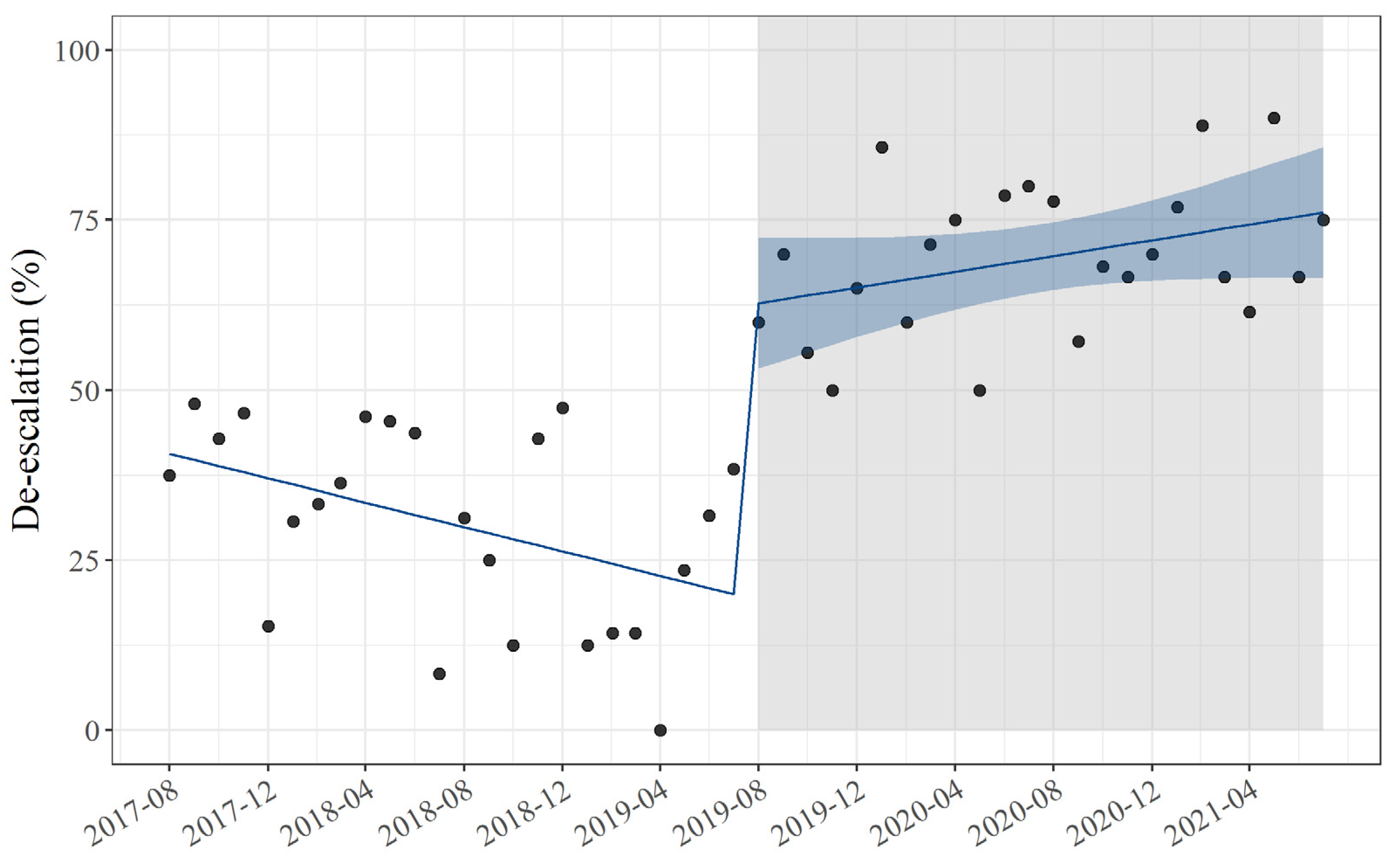
| De-escalation, n (%) | 104 (32.4) | 193 (68.4) | <0.01 a |
| Switch to narrow-spectrum agents | 84 (80.8) | 149 (77.2) | 0.48 a |
| Switch to oral agents | 12 (11.5) | 21 (14.1) | 0.86 a |
| Stop | 8 (7.7) | 23 (11.9) | 0.26 a |
| DOT per 100 patient days, mean (SD) | |||
| Carbapenem | 26.9 (13.1) | 13.5 (6.9) | <0.01 b |
| Tazobactam/piperacillin | 22.3 (12.5) | 11.7 (5.3) | <0.01 c |
| Nonrecommended empiric therapy (%) | 30 (9.3) | 0 (0) | <0.01 a |
| Nonrecommended definitive therapy (%) | 82 (25.5) | 17 (6.0) | <0.01 a |
| Parameter | Estimate | 95% CI | p-Value |
|---|---|---|---|
| De-escalation | |||
| Level change | 44 | 30, 58 | <0.01 |
| Slope change | 1.5 | 0.46, 2.5 | <0.01 |
| DOT of carbapenem | |||
| Level change | −16 | −28, −3.5 | 0.012 |
| Slope change | −0.78 | −1.6, 0.08 | 0.076 |
| DOT of tazobactam/piperacillin | |||
| Level change | −15 | −26, −4.9 | <0.01 |
| Slope change | −0.86 | −1.6, −0.11 | 0.026 |
| Outcome | Preintervention Group | Intervention Group | p-Value |
|---|---|---|---|
| 30-day mortality | 21 (6.5) | 20 (7.1) | 0.79 c |
| Staphylococcus aureus a | 1 (2.8) | 3 (11.1) | 0.31 d |
| Candida spp.b | 2 (25.0) | 0 (0) | 0.15 d |
| Bundle compliance | |||
| Staphylococcus aureus a | |||
| Re-test of blood culture | 18 (50.0) | 25 (92.6) | <0.01 c |
| Echocardiography | 16 (44.4) | 15 (55.6) | 0.38 c |
| Source control | 19 (52.8) | 24 (88.9) | <0.01 c |
| Optimal antimicrobial agents | 26 (72.2) | 27 (100.0) | <0.01 c |
| Candida spp.b | |||
| Re-test of blood culture | 5 (62.5) | 11 (91.7) | 0.26 c |
| Consult to ophthalmology | 4 (50.0) | 7 (58.3) | 1.00 d |
| Source control | 6 (75.0) | 6 (50.0) | 0.37 c |
| Optimal antifungal agents | 7 (87.5) | 12 (100.0) | 0.40 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okada, N.; Azuma, M.; Tsujinaka, K.; Abe, A.; Takahashi, M.; Yano, Y.; Sato, M.; Shibata, T.; Goda, M.; Ishizawa, K. Clinical Impact of a Pharmacist-Driven Prospective Audit with Intervention and Feedback on the Treatment of Patients with Bloodstream Infection. Antibiotics 2022, 11, 1144. https://doi.org/10.3390/antibiotics11091144
Okada N, Azuma M, Tsujinaka K, Abe A, Takahashi M, Yano Y, Sato M, Shibata T, Goda M, Ishizawa K. Clinical Impact of a Pharmacist-Driven Prospective Audit with Intervention and Feedback on the Treatment of Patients with Bloodstream Infection. Antibiotics. 2022; 11(9):1144. https://doi.org/10.3390/antibiotics11091144
Chicago/Turabian StyleOkada, Naoto, Momoyo Azuma, Kaito Tsujinaka, Akane Abe, Mari Takahashi, Yumiko Yano, Masami Sato, Takahiro Shibata, Mitsuhiro Goda, and Keisuke Ishizawa. 2022. "Clinical Impact of a Pharmacist-Driven Prospective Audit with Intervention and Feedback on the Treatment of Patients with Bloodstream Infection" Antibiotics 11, no. 9: 1144. https://doi.org/10.3390/antibiotics11091144
APA StyleOkada, N., Azuma, M., Tsujinaka, K., Abe, A., Takahashi, M., Yano, Y., Sato, M., Shibata, T., Goda, M., & Ishizawa, K. (2022). Clinical Impact of a Pharmacist-Driven Prospective Audit with Intervention and Feedback on the Treatment of Patients with Bloodstream Infection. Antibiotics, 11(9), 1144. https://doi.org/10.3390/antibiotics11091144






