Abstract
We have previously identified substantial antibiotic treatment heterogeneity, even among organism-specific and site-specific infections with treatment guidelines. Therefore, we sought to quantify the extent of treatment heterogeneity among patients hospitalized with P. aeruginosa pneumonia in the national Veterans Affairs Healthcare System from Jan-2015 to Apr-2018. Daily antibiotic exposures were mapped from three days prior to culture collection until discharge. Heterogeneity was defined as unique patterns of antibiotic treatment (drug and duration) not shared by any other patient. Our study included 5300 patients, of whom 87.5% had unique patterns of antibiotic drug and duration. Among patients receiving any initial antibiotic/s with a change to at least one anti-pseudomonal antibiotic (n = 3530, 66.6%) heterogeneity was 97.2%, while heterogeneity was 91.5% in those changing from any initial antibiotic/s to only anti-pseudomonal antibiotics (n = 576, 10.9%). When assessing heterogeneity of anti-pseudomonal antibiotic classes, irrespective of other antibiotic/s received (n = 4542, 85.7%), 50.5% had unique patterns of antibiotic class and duration, with median time to first change of three days, and a median of two changes. Real-world evidence is needed to inform the development of treatment pathways and antibiotic stewardship initiatives based on clinical outcome data, which is currently lacking in the presence of such treatment heterogeneity.
1. Introduction
Pseudomonas aeruginosa is an important nosocomial pathogen, often causing pneumonia in hospitalized patients [1,2]. Second only to Staphylococcus aureus, P. aeruginosa is the most common Gram-negative pathogen isolated from hospitalized patients with bacterial pneumonia [3]. P. aeruginosa is the fourth most common cause of nosocomial infections, and the leading Gram-negative cause of both hospital-acquired (HABP) and ventilator-acquired bacterial pneumonia (VABP) in the United States (US) and an important nosocomial pathogen worldwide [3,4,5,6,7]. Further, P. aeruginosa is significant respiratory tract pathogen in community-acquired pneumonia (CAP) among patients with certain comorbidities and/or history of P. aeruginosa colonization or infection [3,8,9].
The greatest challenges with P. aeruginosa infections include its virulence and its intrinsic, acquired, and adaptive mechanisms of antibiotic resistance, often expressed simultaneously; which, have made infections caused by this pathogen particularly difficult to treat [4,10]. The frequency of multi-drug resistant (MDR) strains is increasing, and MDR P. aeruginosa has been recognized by the Centers for Disease Control and Prevention (CDC) as a serious public health threat [11,12,13]. Between 17% and 39% of P. aeruginosa VABP isolates are MDR in the US [4]. MDR P. aeruginosa nosocomial pneumonia is associated with increased morbidity, mortality, length of hospital and intensive care unit stay, and increased health care costs, likely related to inappropriate empiric therapy [14,15,16,17,18,19,20].
Existing treatment guidelines for P. aeruginosa include the 2019 Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) CAP guidelines and newer treatment guidelines from IDSA for resistant Gram-negative infections [3,8,21,22]. However, treatment of P. aeruginosa pneumonia can vary based on patient-specific factors, such as antibiotic allergies, drug toxicities and interactions, comorbidities, and the substantial heterogeneity of possible resistance mechanisms at play with P. aeruginosa. While there is frequent discussion surrounding heterogeneity of patients and heterogeneity of treatment effects, there is very little work evaluating differences in how patients are treated (treatment heterogeneity). We do not have a good understanding of how patients are treated beyond a single hospital or clinic. Furthermore, definitions of treatment in research tend to be overly simplistic and broad, leading to substantial misclassification of treatment definitions. Therefore, real-world evidence must be interpreted with caution. Over the past several years, our group has been studying treatment heterogeneity in serious bacterial infections, defined as drug-resistant infections requiring hospitalization and associated with high mortality such as pneumonia and/or bloodstream infections [23]. We have found that even in the presence of clinical guidelines, society guidelines, and/or hospital protocols to guide treatment for the optimal management of serious bacterial infections, substantial heterogeneity exists. We therefore wanted to assess whether heterogeneity persists in infections where there is limited evidence to support treatment guidelines, specifically P. aeruginosa pneumonia.
2. Results
2.1. Study Population
Our study included 5300 hospitalized patients from 112 hospitals in the national Veterans Affairs (VA) Healthcare system, with a P. aeruginosa isolated from respiratory tract cultures collected between January 2015 and April 2018. Our study population was mostly male (n = 5167, 97.5%), older (mean age 70.4 years, standard deviation [SD] 10.3; Table 1), and White (n = 3956, 74.6%). About one-third (n = 1800, 34.0%) of patients received care in intensive care units (ICU), and the comorbidity burden was high (median Charlson 3, interquartile range [IQR] 2–5; median Elixhauser 5, IQR 3–7]). Multidrug-resistant P. aeruginosa respiratory isolates were present in 12.4% (n = 636/5117) patients, with 22.0% (n = 1136/5157) demonstrating fluoroquinolone resistance and 19.0% (n = 874/4601) demonstrating carbapenem resistance. The median time from admission to P. aeruginosa culture was 2 days (IQR 0–7).

Table 1.
Demographics and comorbidities among patients with Pseudomonas aeruginosa pneumonia.
2.2. Treatment Heterogeneity
When evaluating all antibiotics, 87.5% (n = 4635) of patients with P. aeruginosa pneumonia had unique antibiotic treatments, in terms of the specific antibiotic, timing of receipt of each antibiotic (which day of admission), and duration of therapy (Table 2). Most (n = 4473, 84.4%) patients had a change in therapy, with a median of three changes over the course of the hospitalization. Most changes (43.5%) were made the day after culture collection, with a median time to first change of 1 day (IQR 0–2), median time to second change of 2 days (IQR 1–4), and median time to third change of 4 days (2–6). Figure 1 demonstrates the 50 most common unique antibiotic treatment patterns (patterns alone without duration of therapy), including changes in therapy, over the course of the admission [24].

Table 2.
Treatment heterogeneity in patients with Pseudomonas aeruginosa pneumonia.
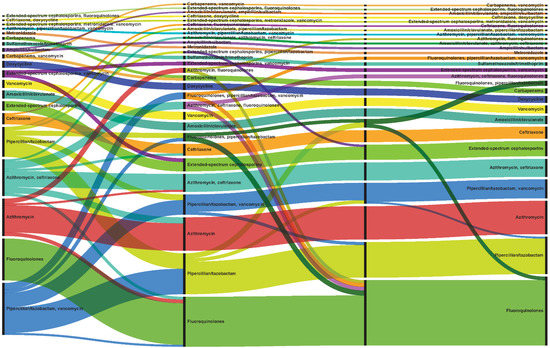
Figure 1.
Common treatment patterns. Alluvial chart demonstrates the 50 most common unique antibiotic treatment patterns (patterns alone without duration of therapy) observed among all patients (n = 5300), including changes in treatment, from three days prior to the culture collection date until discharge, or 30 days from culture for longer hospital stays. The width of each line represents the number of patients receiving that specific treatment. Patients without changes in treatment are depicted in each segment as having the same antibiotic as the previous segment, while those with changes, move to another antibiotic in the next segment. Different colors represent different antibiotics or concomitant antibiotics.
Among patients with any initial antibiotic therapy (monotherapy or combination therapy), and changing to at least one anti-pseudomonal antibiotic (n = 3530, 66.6%), heterogeneity was higher at 97.2% (n = 3430/3530), with a median of three changes. Among patients initially treated with any antibiotic and then changing to only anti-pseudomonal antibiotics (n = 576, 10.9%), heterogeneity was 91.5% (n = 527/576). When assessing anti-pseudomonal antibiotic classes only, irrespective of other antibiotics received (n = 4542, 85.7%), heterogeneity was 50.5% (n = 2294/4542) and among those with changes in therapy, there were a median of 2 changes, with the first change most commonly occurring 3 days after culture.
The most commonly utilized antibiotics (Figure 2) [24] were vancomycin (n = 3106, 58.6%), piperacillin/tazobactam (n = 2563, 48.4%), levofloxacin (n = 1766, 33.3%), cefepime (n = 1475, 27.8%), azithromycin (n = 1447, 27.3%), ceftriaxone (n = 1259, 23.8%), ciprofloxacin (n = 931, 17.6%), meropenem (n = 876, 16.5%), and metronidazole (n = 809, 15.3%). Most patients (88.9%) were treated more than one antibiotic over the course of the admission (median antibiotics received 3, IQR 2–5). The utilization distribution of anti-pseudomonal antibiotic classes was 47.4% (n = 2510) for fluoroquinolones, 31.9% (n = 1692) for extended-spectrum cephalosporins, 19.7% (n = 1045) for carbapenems, 8.3% (n = 440) for aminoglycosides.
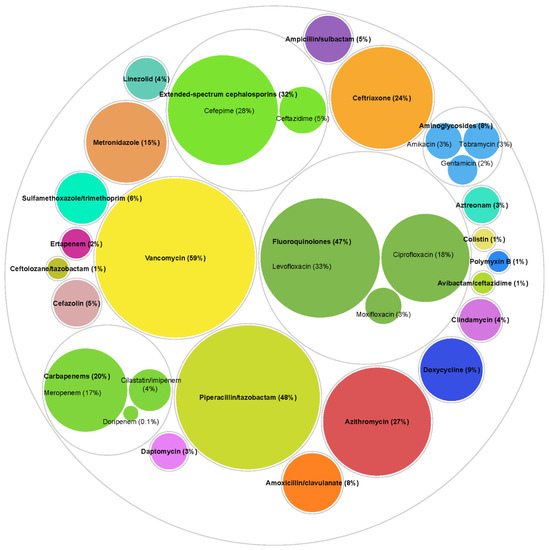
Figure 2.
Utilization of specific antibiotics. Antibiotic exposures were assessed from three days prior to Pseudomonas aeruginosa culture collection date until discharge, or 30 days from culture for longer hospital stays among all patients (n = 5300). Counts and percentages are not mutually exclusive as 88.9% patients received more than one antibiotic over the course of the admission (median number of antibiotics received 3, interquartile range 2–5). Different colors represent different antibiotics. Other antibiotics not included in the figure were used in <2% of patients.
When assessing anti-pseudomonal antibiotic classes only (n = 4542, 85.7%), irrespective of other antibiotics received and without considering duration of each antibiotic, only eight patterns were observed in more than 2% of patients: (1) piperacillin/tazobactam (15.8%), (2) fluoroquinolone (13.3%), (3) extended-spectrum cephalosporin (8.0%), (4) initial treatment with piperacillin/tazobactam alone, then a change to combination therapy of a fluoroquinolone and piperacillin/tazobactam, and then a change to a fluoroquinolone alone (discontinuation of piperacillin/tazobactam) (4.8%), (5) initial treatment with piperacillin/tazobactam alone, then a change to combination therapy of a fluoroquinolone and piperacillin/tazobactam (2.9%), (6) initial treatment with an extended-spectrum cephalosporin, then a change to combination therapy of a fluoroquinolone and an extended-spectrum cephalosporin, and then a change to a fluoroquinolone alone (discontinuation of extended-spectrum cephalosporin) (2.4%), (7) carbapenem (2.3%), and (8) initial treatment with piperacillin/tazobactam, then a change to combination therapy of an extended-spectrum cephalosporin and piperacillin/tazobactam, and then a change to an extended-spectrum cephalosporin alone (discontinuation of piperacillin/tazobactam) (2.1%).
2.3. Clinical Outcomes
Most patients had changes in therapy (n = 4473, 84.4%). Length of hospital stay, mortality, and persistent positive cultures were significantly higher among those who had changes in therapy (Table 3, median length of stay admission to discharge 12 versus 4 days, inpatient mortality 17.1% versus 10.3%, persistent positive culture 38.1% versus 9.2%) as compared to those without changes in therapy. Among those without changes in therapy (n = 827), length of stay and mortality were significantly lower among those treated with monotherapy (n = 591, 71.5%) (median length of stay admission to discharge 5 versus 3 days, inpatient mortality 21.2% versus 5.9%) as compared to those treated with combination therapy.

Table 3.
Clinical outcomes in patients with Pseudomonas aeruginosa pneumonia.
3. Discussion
Among hospitalized patients with positive P. aeruginosa respiratory cultures, 87.5% had different antibiotic treatment patterns, in terms of all antibiotics received each day and the duration of each antibiotic therapy. This heterogeneity was largely driven by differences in antibiotic treatment patterns as opposed to duration of therapy (75.5% heterogeneity when not considering length of therapy versus 87.5% heterogeneity when including length of therapy). Treatment heterogeneity remained high, at approximately 50%, even when restricting the analysis to anti-pseudomonal antibiotic classes only (not accounting for other antibiotics received) and duration of therapy, with duration accounting for approximately half of the heterogeneity observed (heterogeneity of 23.1% when not considering length of therapy). Most changes were made the day after culture collection, which was likely when preliminary culture results were communicated to the treating clinicians. Current methods to assess treatment, including intent-to-treat, as-treated, and even time-dependent exposures, do not adequately account for the extensive heterogeneity observed in the treatment of infectious diseases. This misclassification has important implications as clinical outcomes may vary between heterogeneous treatment approaches, which makes it difficult to attribute outcomes to one specific treatment approach. Future work should investigate the impact of specific treatment approaches on clinical outcomes in patients with P. aeruginosa pneumonia.
Inpatient mortality was highest among those initially receiving combination therapy and continuing with that same combination therapy (no changes in therapy), and lowest among those receiving only monotherapy (no changes in therapy). While this could indicate that initial targeted anti-pseudomonal monotherapy (monotherapy not requiring changes in therapy) may be associated with better clinical outcomes, these patients may have also been less complex, with less severe infections. It is also possible that since patients exposed to combination therapy were exposed to more antibiotics, they had a higher risk for toxicity and adverse drug events than those exposed to monotherapy. As such, these differences in outcomes require further study.
We observed higher rates of mortality in our study compared to the 8% mortality rate reported by the CDC in 2019 for MDR P. aeruginosa infections, which included both respiratory and non-respiratory infections [13]. As worse outcomes, including mortality, have been associated with MDR P. aeruginosa infections, and only 12.4% of our population had MDR P. aeruginosa, the clinical significance of pneumonias caused by all P. aeruginosa, including both MDR and susceptible strains, cannot be understated [25,26,27,28,29].
Empiric treatment recommendations for P. aeruginosa pneumonia endorsed by the IDSA include piperacillin-tazobactam (4.5 g every 6 h), cefepime (2 g every 8 h), ceftazidime (2 g every 8 h), imipenem (500 mg every 6 h), meropenem (1 g every 8 h), or aztreonam (2 g every 8 h) [3,8]. Newer treatment guidelines from IDSA for resistant Gram-negative infections recommend monotherapy with ceftolozane-tazobactam, ceftazidime-avibactam, or imipenem-cilastatin-relebactam for infections outside the urinary tract caused by difficult to treat (DTR) P. aeruginosa (defined as non-susceptibility to all of the following: piperacillin-tazobactam, ceftazidime, cefepime, aztreonam, meropenem, imipenem-cilastatin, ciprofloxacin, and levofloxacin) [21,22]. If none of these newer beta-lactam/beta-lactamase inhibitors are options due to intolerance or resistance, alternative treatment options include cefiderocol or an aminoglycoside, such as plazomicin [21]. However, it should be noted there is limited clinical data supporting treatment recommendations of DTR P. aeruginosa and older treatment options, such as aminoglycosides and colistin, are recognized to have significant toxicity issues [21].
While existing guidelines address empiric treatment options for P. aeruginosa pneumonias, given the complexity and variability of antibiotic resistance patterns among P. aeruginosa strains causing infection, guidelines may benefit from specific and tailored recommendations. For example, the use of guideline-recommended empiric therapy for highly resistant P. aeruginosa strains could lead to initial treatment failure, while empiric therapy may be excessively broad for more sensitive P. aeruginosa strains, which could lead to longer hospital stays, and increased risk of developing additional resistance or Clostridioides difficile infections [30,31,32,33].
The fundamental principle in treatment of serious P. aeruginosa infections is early administration of appropriate antibiotics [34]. Previous work has demonstrated that the odds of mortality in patients with P. aeruginosa pneumonia is up to 3 times higher in those treated with inappropriate versus appropriate empiric treatment [35,36]. As such, best practice guidelines for community-acquired and HABP/VABP both recommend empiric treatment with conventional anti-pseudomonal β-lactams (meropenem, imipenem, doripenem, piperacillin-tazobactam, cefepime, and ceftazidime), potentially in combination with a second agent (aminoglycoside, fluoroquinolone, or polymyxin), when P. aeruginosa is suspected [8,34,37]. Two agents are generally recommended until bacterial susceptibly results are available, if the patient has specific risk factors for MDR P. aeruginosa, such as high risk for mortality, previous intravenous anti-pseudomonal antibiotic therapy, and/or based on local susceptibility data [8,34,37,38,39]. These recommendations are centered around the goals of achieving early appropriate therapy while also limiting superfluous coverage, which increases the risk of adverse drug effects, Clostridioides difficile infections, and antimicrobial resistance [37].
Use of newer β-lactam antibiotics, such as ceftolozane/tazobactam, ceftazidime/avibactam, cefiderocol, and imipenem-cilastatin-relebactam, may also be considered for treatment of P. aeruginosa pneumonias. Empiric or targeted use of newer agents may increase earlier receipt of appropriate therapy, particularly for resistant P. aeruginosa infections, while decreasing the risk of unintended effects from combination therapy with older, more toxic antibiotics [40,41]. However, their role in therapy has yet to be fully elucidated and these agents remain largely absent from guidelines [34]. For directed therapy, while it has been postulated that continued combination therapy may minimize the emergence of resistance and promote antimicrobial synergy, there is a lack of data to support these hypotheses [42,43]. As such, once culture results are available, it is generally recommended to streamline treatment to a single, highly active agent for the directed treatment of P. aeruginosa pneumonia [34]. Despite these general recommendations, there is a lack of high-level evidence to firmly guide treatment decisions, especially for patients with infections due to resistant phenotypes, in part, because it is not a requirement for the approval of new antibiotics [44].
Treatment patterns observed in our study were consistent with best practice guidelines for the treatment of CAP and HABP/VABP guidelines [3,8,9,21,37]. It is generally recommended that a β-lactam plus a macrolide or a respiratory fluroquinolone are used for empiric treatment of CAP in hospitalized patients without suspicion for P. aeruginosa, which is consistent with our findings regarding the frequency of use of ceftriaxone (23.8%), azithromycin (27.3%), and levofloxacin (33.3%). For HABP/VABP, piperacillin/tazobactam or cefepime are generally recommended for empiric treatment, with the addition of vancomycin if there are risk factors for methicillin-resistant Staphylococcus aureus (MRSA) or the local prevalence of MRSA is high (>20%) [8]. While P. aeruginosa is intrinsically resistant to ceftriaxone, azithromycin, and vancomycin, these represent recommended empiric treatment options for CAP (ceftriaxone and azithromycin) [8] and MRSA (vancomycin) [37], and do not preclude their use for the treatment of concomitant infections or syndromes, such as chronic obstructive pulmonary disease or another infection type. For directed anti-pseudomonal therapy, single-agent conventional therapy (piperacillin-tazobactam, cefepime, and ceftazidime) based on susceptibilities is generally recommended, with carbapenems (meropenem, imipenem, doripenem) potentially being reserved for more resistant phenotypes or polymicrobial infections [8,37].
Real-world data evaluating P. aeruginosa pneumonia treatments are limited. A recent, observational cohort study compared ceftolozane/tazobactam to polymyxin or aminoglycoside-based regimens for the treatment of resistant P. aeruginosa infections (52% VABP) [45]. This study similarly demonstrated the frequent use of conventional anti-pseudomonal therapy in the polymyxin or aminoglycoside-based combination treatment group with carbapenems, piperacillin/tazobactam, and extended-spectrum cephalosporins most commonly being used. However, detailed descriptions of treatment patterns were not described, nor were additional agents used for any polymicrobial infections or concomitant infections.
Resistance rates in our cohort were lower than previous reports, with 12.4% demonstrating MDR, 13.1% with aminoglycoside resistance, 19.0% with carbapenem resistance, 17.2% with extended-spectrum cephalosporin resistance, 22.0% with fluoroquinolone resistance, and 15.3% with piperacillin resistance. Alternatively, of 1412 P. aeruginosa isolates from adult patients with VAP submitted to the National Healthcare Safety Network (NHSN) between 2015–2017, between 17 and 39% were MDR, dependent on hospital location [4]. In the NHSN, reported rates of resistance in VABP P. aeruginosa were generally higher than rates observed in our study (aminoglycosides 13.6–29.9%, carbapenems 26.3–61.4%, extended-spectrum cephalosporin 25.9–44.7%, fluoroquinolones 26.7–45.8%, and piperacillin/tazobactam 21.7–34.8%) [4]. Rates of resistance may have been lower if the NHSN data included isolates from CAP. Similarly, of 258 patients with P. aeruginosa nosocomial pneumonia from 3 hospitals in the US, 20.5% were MDR, and reported resistance rates for each class of antibiotics were higher than those we observed (aminoglycosides 19.2%, carbapenems 21%, extended-spectrum cephalosporin 18.6%, fluoroquinolones 24.1%, and piperacillin/tazobactam 17.4%) [14]. Twenty-years of surveillance data (1997–2016) from the worldwide SENTRY Antimicrobial Surveillance Program also demonstrated higher rates of resistance than we observed in our cohort [46]. The overall rate of MDR clinical P. aeruginosa isolates was 24.9%, with the highest rates among those with pneumonia (27.7%) [46]. Isolates with MDR phenotypes were less common in North America (18.9%) than other regions worldwide, but still higher than the rate we observed.
Despite general recommendations, there are limited comparative data to support the most optimal agent/s to use for treatment of P. aeruginosa pneumonia, especially those due to resistant phenotypes. More comparative clinical data is needed to identify if there are preferred antibiotic agents overall for P. aeruginosa pneumonia or if there are clinical scenarios in which certain agents may be preferred [34,42]. Additionally, more data is needed to identify when combination therapy may be most beneficial to patients with P. aeruginosa pneumonia and what antibiotic combinations are associated with the highest effectiveness and safety. Additional data may also be important to determine if there is a need to tailor antibiotic regimens based on the risk or presence of MDR isolates. These gaps in knowledge need to be filled with strong real-world clinical data for guidelines and hospital protocols to outline optimal therapy for patients with serious P. aeruginosa infections. The high treatment heterogeneity we observed, even despite clinicians commonly using recommended agents, highlights the continued uncertainty surrounding optimal treatment of P. aeruginosa infections and the need for clearer, more detailed evidence-based specific treatment guidelines based on patient risk factors and/or local epidemiology.
There are limitations to this retrospective observational study. We were unable to distinguish between true infection versus colonization as clinical signs and symptoms and signs of pneumonia were not captured. However, 72% of patients had a pneumonia diagnosis during the admission. It is possible that the treatment captured may have been for another infection or for a concomitant and/or polymicrobial infection. Furthermore, we were also unable to classify the type of pneumonia (HABP/VABP/vHABP versus CAP) diagnosed. Given the differential burden of pseudomonal HABP/VABP/vHABP and CAP on mortality as identified in previous studies [17], stratification of these diagnoses could help inform the clinical outcomes seen in our cohort. The reasons for changes in antibiotic therapy are unknown. We measured the time from culture collection to antibiotic change as opposed to time from culture results, including pathogen identification and antimicrobial susceptibilities. Potential reasons for change include possible antibiotic de-escalation or escalation based on pathogen and/or susceptibly results, clinical improvement or worsening of clinical signs and symptoms, or care transitions (such a preparatory to discharge, discontinue intravenous antibiotics for oral).
Our estimate of heterogeneity is likely an underestimate, as it did not include changes in route (for example, intravenous to oral changes) or dose, antibiotics received in other non-VA settings (non-VA nursing home or non-VA hospital), inhaled antibiotics, or post-discharge antibiotics. Similarly, outcomes were only captured among those who were treated in VA facilities and not those who sought treatment outside the VA. Susceptibility testing across the VA Healthcare system is not uniform which may lead to inconsistencies in determining antibiotic resistance and MDR. However, accepted definitions of the CDC NHSN were used to identify MDR phenotypes [47]. While several demographics and clinical characteristics of the study cohort were described, previous antibiotic exposures, one of the main risk factors for resistant pseudomonal infections, were not assessed. The generalizability of this study is limited to patients admitted to VA hospitals. Heterogeneity among VA hospitals may be lower than other non-VA hospitals, as the VA is the nation’s largest integrated healthcare system and while there are no national VA guidelines for treatment of P. aeruginosa pneumonia, resources and education are often shared among local VA facilities through national antibiotic stewardship resources and initiatives, such as the national VA Antibiotic Stewardship Task Force.
4. Materials and Methods
4.1. Study Population
Our study included hospitalized patients ≥18 years old with P. aeruginosa isolated from respiratory tract cultures (sputum and/or bronchoalveolar lavage) collected between January 2015 and April 2018 in the national VA Healthcare System. The culture could be collected anytime during admission and only the first hospitalization identified during the study period was selected for inclusion. This study was approved by the Institutional Review Board and Research and Development Committee of the VA Providence Healthcare System.
4.2. Exposure Mapping
We utilized exposure mapping to identify all antibiotics received on each day, from three days prior to the culture collection date until discharge, or 30 days from culture for longer hospital stays. With exposure mapping, all antibiotic exposures are captured, which allows assessment of combination therapy, duration of therapy, and changes in therapy. Antibiotic exposures were captured from barcode medication administration records and pharmacy dispensings, which include antibiotics given in the emergency department.
4.3. Treatment Heterogeneity
Measures of heterogeneity included number of changes in therapy and time to first change for each patient. Time to first change was assessed to capture initial change from empiric to targeted therapy after initial culture results are communicated to the treating clinician. Treatment patterns were built from daily antibiotic exposures and duration of each antibiotic exposure, and therefore captures all changes in antibiotic treatments day to day. Dose changes and changes from intravenous to oral forms of the same antibiotic were not considered changes in therapy. Exposure mapping was carried out for (1) all antibiotics, (2) any initial antibiotic/s and then at least one anti-pseudomonal antibiotic at the first change in therapy (aminoglycosides: amikacin, gentamicin, tobramycin; carbapenems: imipenem, meropenem, doripenem; extended-spectrum cephalosporins: cefepime, ceftazidime; fluoroquinolones: ciprofloxacin, levofloxacin; piperacillin/tazobactam; aztreonam; polymyxins: colistin, polymyxin B; ceftazidime/avibactam; ceftolozane/tazobactam), (3) any initial antibiotic/s and then only anti-pseudomonal antibiotics after first change, and (4) only assessing anti-pseudomonal antibiotic classes.
The definition of a common, or shared, treatment pattern was at least two patients hospitalized with positive P. aeruginosa respiratory tract cultures and treated with the same antibiotic exposures for the same duration. Therefore, unique treatment patterns were those where only a single patient had a specific treatment pattern, assessed either at the antibiotic or as antibiotic class level and accounting for duration. Non-unique treatment patterns were defined as those where multiple admissions had the same pattern of antibiotic exposures and durations. For example, the following was a treatment pattern shared by several patients: piperacillin/tazobactam plus vancomycin for three days, which was changed to only piperacillin/tazobactam for five days. In contrast, this was an example of a unique treatment pattern which was only identified in one patient: amoxicillin/clavulanate for one day, which was changed to piperacillin/tazobactam for six days and then changed to levofloxacin and piperacillin/tazobactam for one day.
4.4. Clinical Outcomes
Outcomes assessed included length of stay (from admission and culture collection to discharge), mortality (inpatient and 30-day from culture), readmission within 30 days of discharge, persistent positive P. aeruginosa culture, and P. aeruginosa reinfection within 30 days of discharge. Persistent positive P. aeruginosa culture was defined as a positive sputum and/or bronchoalveolar lavage for P. aeruginosa after 7 days of treatment. P. aeruginosa reinfection was defined as a positive sputum and/or bronchoalveolar lavage culture for P. aeruginosa after discharge.
4.5. Statistical Analysis
Outcomes were compared between patients who had changes in therapy and no changes in therapy, and among those who had no changes in therapy, between those treated with monotherapy and combination therapy. Proportions of categorical variables were compared between groups using Chi-square tests (one comparison with expected cell count <5 utilized Fisher’s exact test, as indicted in Table 3) and the non-parametric Wilcoxon test when comparing medians of continuous variables. Statistical significance was considered at a p-value < 0.05. Analyses were completed with SAS (Version 9.2; SAS Institute, Inc.: Cary, NC, USA).
5. Conclusions
In our retrospective cohort study of 5300 MDR P. aeruginosa infections, treatment heterogeneity was high, ranging from 97.2% in patients receiving any initial antibiotic/s with a change to at least one anti-pseudomonal antibiotic, to 50.5% when assessing anti-pseudomonal antibiotic classes, irrespective of other antibiotic received. Real-world evidence is needed to help support and inform the development of treatment protocols and antibiotic stewardship programs based on clinical and patient outcome data, which is currently lacking in the presence of such treatment heterogeneity.
Author Contributions
Conception and design of the study: A.R.C., L.A.P. Data generation: A.R.C., E.C.P., V.L. Analysis and/or interpretation of the data: A.R.C., H.J.A., J.X.L., E.C.P., V.L., L.A.P. Preparation or critical revision of the manuscript: A.R.C., H.J.A., J.X.L., E.C.P., V.L., L.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work: including the APC, was funded, in part, by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Institutional Review Board Statement
This study was approved by the Institutional Review Board and Research and Development Committee of the VA Providence Healthcare System (RDC-2018-001, approval date 10 January 2018).
Informed Consent Statement
Patient consent was waived due as this was a retrospective study of existing health records.
Data Availability Statement
The study data may be made available upon reasonable request and approval by the Department of Veterans Affairs.
Acknowledgments
Views expressed are those of the authors and do not necessarily reflect the position or policy of the United States Department of Veterans Affairs. This material is based upon work supported, in part, by the Office of Research and Development, Department of Veterans Affairs.
Conflicts of Interest
A.R.C. has received research funding from AbbVie, Gilead, Merck, and Shionogi, and has been a speaker/advisor for Merck. L.A.P. was an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA at the time the study was conducted. No other conflicts to report.
References
- Fujii, A.; Seki, M.; Higashiguchi, M.; Tachibana, I.; Kumanogoh, A.; Tomono, K. Community-acquired, hospital-acquired, and healthcare-associated pneumonia caused by Pseudomonas aeruginosa. Respir. Med Case Rep. 2014, 12, 30–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadikot, R.T.; Blackwell, T.S.; Christman, J.W.; Prince, A.S. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005, 171, 1209–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sader, H.S.; Castanheira, M.; Arends, S.J.R.; Goossens, H.; Flamm, R.K. Geographical and temporal variation in the frequency and antimicrobial susceptibility of bacteria isolated from patients hospitalized with bacterial pneumonia: Results from 20 years of the SENTRY Antimicrobial Surveillance Program (1997–2016). J. Antimicrob. Chemother. 2019, 74, 1595–1606. [Google Scholar] [CrossRef]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-Resistant Pathogens Associated with Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, D.J.; Rutala, W.A.; Sickbert-Bennett, E.E.; Samsa, G.P.; Brown, V.; Niederman, M.S. Microbiology of ventilator-associated pneumonia compared with that of hospital-acquired pneumonia. Infect. Control Hosp. Epidemiol. 2007, 28, 825–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiner-Lastinger, L.M.; Abner, S.; Edwards, J.R.; Kallen, A.J.; Karlsson, M.; Magill, S.S.; Pollock, D.; See, I.; Soe, M.M.; Walters, M.S.; et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control Hosp. Epidemiol. 2020, 41, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, V.D.; Bat-Erdene, I.; Gupta, D.; Belkebir, S.; Rajhans, P.; Zand, F.; Myatra, S.N.; Afeef, M.; Tanzi, V.L.; Muralidharan, S.; et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012–2017: Device-associated module. Am. J. Infect. Control 2020, 48, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.H.; Saha, B.K.; Ananthakrishnan, R.; Chopra, A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection 2021, 49, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Strateva, T.; Yordanov, D. Pseudomonas aeruginosa-A phenomenon of bacterial resistance. J. Med. Microbiol. 2009, 58 Pt 9, 1133–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raman, G.; Avendano, E.E.; Chan, J.; Merchant, S.; Puzniak, L. Risk factors for hospitalized patients with resistant or multidrug-resistant Pseudomonas aeruginosa infections: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2018, 7, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CDC. Antibiotic Resistance Threats in the United States; CDC: Atlanta, GA, USA, 2013. [Google Scholar]
- CDC. Antibiotic Resistance Threats in the United States; CDC: Atlanta, GA, USA, 2019. [Google Scholar]
- Micek, S.T.; Wunderink, R.G.; Kollef, M.H.; Chen, C.; Rello, J.; Chastre, J.; Antonelli, M.; Welte, T.; Clair, B.; Ostermann, H.; et al. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: Impact of multidrug resistance. Crit. Care 2015, 19, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pena, C.; Gomez-Zorrilla, S.; Oriol, I.; Tubau, F.; Domínguez, M.A.; Pujol, M.; Ariza, J. Impact of multidrug resistance on Pseudomonas aeruginosa ventilator-associated pneumonia outcome: Predictors of early and crude mortality. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Troyano, A.; Sibila, O. The respiratory threat posed by multidrug resistant Gram-negative bacteria. Respirology 2017, 22, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Micek, S.T.; Kollef, M.H.; Torres, A.; Chen, C.; Rello, J.; Chastre, J.; Antonelli, M.; Welte, T.; Clair, B.; Ostermann, H.; et al. Pseudomonas aeruginosa nosocomial pneumonia: Impact of pneumonia classification. Infect. Control Hosp. Epidemiol. 2015, 36, 1190–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagon, J.Y.; Chastre, J.; Hance, A.J.; Montravers, P.; Novara, A.; Gibert, C. Nosocomial pneumonia in ventilated patients: A cohort study evaluating attributable mortality and hospital stay. Am. J. Med. 1993, 94, 281–288. [Google Scholar] [CrossRef]
- Montravers, P.; Fagon, J.Y.; Chastre, J.; Lecso, M.; Dombret, M.C.; Trouillet, J.-L.; Gibert, C. Follow-up protected specimen brushes to assess treatment in nosocomial pneumonia. Am. Rev. Respir. Dis. 1993, 147, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Cilloniz, C.; Gabarrus, A.; Ferrer, M.; de la Bellacasa, J.P.; Rinaudo, M.; Mensa, J.; Niederman, M.S.; Torres, A. Community-Acquired Pneumonia Due to Multidrug- and Non-Multidrug-Resistant Pseudomonas aeruginosa. Chest 2016, 150, 415–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of Extended-Spectrum beta-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2021, 72, e169–e183. [Google Scholar]
- Kanj, S.S.; Bassetti, M.; Kiratisin, P.; Rodrigues, C.; Villegas, M.V.; Yu, Y.; van Duin, D. Clinical data from studies involving novel antibiotics to treat multidrug-resistant Gram-negative bacterial infections. Int. J. Antimicrob. Agents 2022. [Google Scholar] [CrossRef]
- Caffrey, A.R.; Babcock, Z.R.; Lopes, V.V.; Timbrook, T.T.; LaPlante, K.L. Heterogeneity in the treatment of bloodstream infections identified from antibiotic exposure mapping. Pharmacoepidemiol. Drug Saf. 2019, 28, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Mauri, M.; Elli, T.; Caviglia, G.; Uboldi, G.; Azzi, M. RAWGraphs: A Visualisation Platform to Create Open Outputs. In Proceedings of the CHItaly ’17: 12th Biannual Conference on Italian SIGCHI Chapter, Cagliari, Italy, 18–20 September 2017; Volume 28, pp. 1–5. [Google Scholar]
- Lister, P.D.; Wolter, D.J.; Hanson, N.D. Antibacterial-resistant Pseudomonas aeruginosa: Clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aloush, V.; Navon-Venezia, S.; Seigman-Igra, Y.; Cabili, S.; Carmeli, Y. Multidrug-resistant Pseudomonas aeruginosa: Risk factors and clinical impact. Antimicrob. Agents Chemother. 2006, 50, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmeli, Y.; Troillet, N.; Karchmer, A.W.; Samore, M.H. Health and economic outcomes of antibiotic resistance in Pseudomonas aeruginosa. Arch. Intern. Med. 1999, 159, 1127–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimatatac, E.L.; Alejandria, M.M.; Montalban, C.; Pineda, C.; Ang, C.; Delino, R. Clinical outcomes and costs of care of antibiotic resistant Pseudomonas aeruginosa infections. Philipp. J. Microbiol. Infect. Dis. 2003, 32, 159–167. [Google Scholar]
- Gasink, L.B.; Fishman, N.O.; Weiner, M.G.; Nachamkin, I.; Bilker, W.B.; Lautenbach, E. Fluoroquinolone-resistant Pseudomonas aeruginosa: Assessment of risk factors and clinical impact. Am. J. Med. 2006, 119, e519–e525. [Google Scholar] [CrossRef] [PubMed]
- Joo, E.J.; Kang, C.I.; Ha, Y.E.; Park, S.Y.; Kang, S.J.; Wi, Y.M.; Lee, N.Y.; Chung, D.R.; Peck, K.R.; Song, J.H. Impact of inappropriate empiric antimicrobial therapy on outcome in Pseudomonas aeruginosa bacteraemia: A stratified analysis according to sites of infection. Infection 2011, 39, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.J.; Sorensen, J.; Jephson, A.; Mecham, I.; Dean, N.C. Broad-spectrum antibiotic use and poor outcomes in community-onset pneumonia: A cohort study. Eur. Respir. J. 2019, 54, 1900057. [Google Scholar] [CrossRef] [PubMed]
- Hiensch, R.; Poeran, J.; Saunders-Hao, P.; Adams, V.; Powell, C.A.; Glasser, A.; Mazumdar, M.; Patel, G. Impact of an electronic sepsis initiative on antibiotic use and health care facility-onset Clostridium difficile infection rates. Am. J. Infect. Control 2017, 45, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Teshome, B.F.; Vouri, S.M.; Hampton, N.; Kollef, M.H.; Micek, S.T. Duration of Exposure to Antipseudomonal β-Lactam Antibiotics in the Critically Ill and Development of New Resistance. Pharmacotherapy 2019, 39, 261–270. [Google Scholar] [CrossRef]
- O’Donnell, J.N.; Bidell, M.R.; Lodise, T.P. Approach to the Treatment of Patients with Serious Multidrug-Resistant Pseudomonas aeruginosa Infections. Pharmacotherapy 2020, 40, 952–969. [Google Scholar] [CrossRef] [PubMed]
- Kuti, E.L.; Patel, A.A.; Coleman, C.I. Impact of inappropriate antibiotic therapy on mortality in patients with ventilator-associated pneumonia and blood stream infection: A meta-analysis. J. Crit. Care 2008, 23, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Muscedere, J.G.; Shorr, A.F.; Jiang, X.; Day, A.; Heyland, D.K.; Canadian Critical Care Trials, G. The adequacy of timely empiric antibiotic therapy for ventilator-associated pneumonia: An important determinant of outcome. J. Crit. Care. 2012, 27, 322.e7–322.e14. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [PubMed]
- Parker, C.M.; Kutsogiannis, J.; Muscedere, J.; Cook, D.; Dodek, P.; Day, A.G.; Heyland, D.K. Ventilator-associated pneumonia caused by multidrug-resistant organisms or Pseudomonas aeruginosa: Prevalence, incidence, risk factors, and outcomes. J. Crit. Care. 2008, 23, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Montero, M.; Sala, M.; Riu, M.; Belvis, F.; Salvado, M.; Grau, S.; Horcajada, J.P.; Alvarez-Lerma, F.; Terradas, R.; Orozco-Levi, M.; et al. Risk factors for multidrug-resistant Pseudomonas aeruginosa acquisition. Impact of antibiotic use in a double case-control study. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Pogue, J.M.; Kaye, K.S.; Bonomo, R.A.; Perez, F. Reply to Vena et al. Clin. Infect. Dis. 2020, 71, 1801–1802. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, E.; Bax, H.I.; Verkaik, N.J.; van Westreenen, M. An Update on Eight “New” Antibiotics against Multidrug-Resistant Gram-Negative Bacteria. J. Clin. Med. 2021, 10, 1068. [Google Scholar] [CrossRef]
- El Solh, A.A.; Alhajhusain, A. Update on the treatment of Pseudomonas aeruginosa pneumonia. J. Antimicrob. Chemother. 2009, 64, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamma, P.D.; Cosgrove, S.E.; Maragakis, L.L. Combination therapy for treatment of infections with gram-negative bacteria. Clin. Microbiol. Rev. 2012, 25, 450–470. [Google Scholar] [CrossRef] [Green Version]
- Bassetti, M.; Peghin, M.; Vena, A.; Giacobbe, D.R. Treatment of Infections Due to MDR Gram-Negative Bacteria. Front. Med. 2019, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Pogue, J.M.; Zhou, Y.; Kanakamedala, H.; Cai, B. 842. Impact of Active Treatment of Carbapenem-Resistant Acinetobacter baumannii Infections in US Hospitals Between 2014 and 2019. Open Forum. Infect. Dis. 2020, 7, S462. [Google Scholar] [CrossRef]
- Shortridge, D.; Gales, A.C.; Streit, J.M.; Huband, M.D.; Tsakris, A.; Jones, R.N. Geographic and Temporal Patterns of Antimicrobial Resistance in Pseudomonas aeruginosa Over 20 Years from the SENTRY Antimicrobial Surveillance Program, 1997–2016. Open Forum. Infect. Dis. 2019, 6, S63–S68. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Antimicrobial-Resistant Phenotype Definitions. Published 2020. Available online: https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/phenotype_definitions.pdf (accessed on 13 May 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).