Carbapenem-Only Combination Therapy against Multi-Drug Resistant Pseudomonas aeruginosa: Assessment of In Vitro and In Vivo Efficacy and Mode of Action
Abstract
1. Introduction
2. Results
2.1. A Carbapenemase-Producing Strain of P. aeruginosa Is Resistant to Four Carbapenem Antibiotics
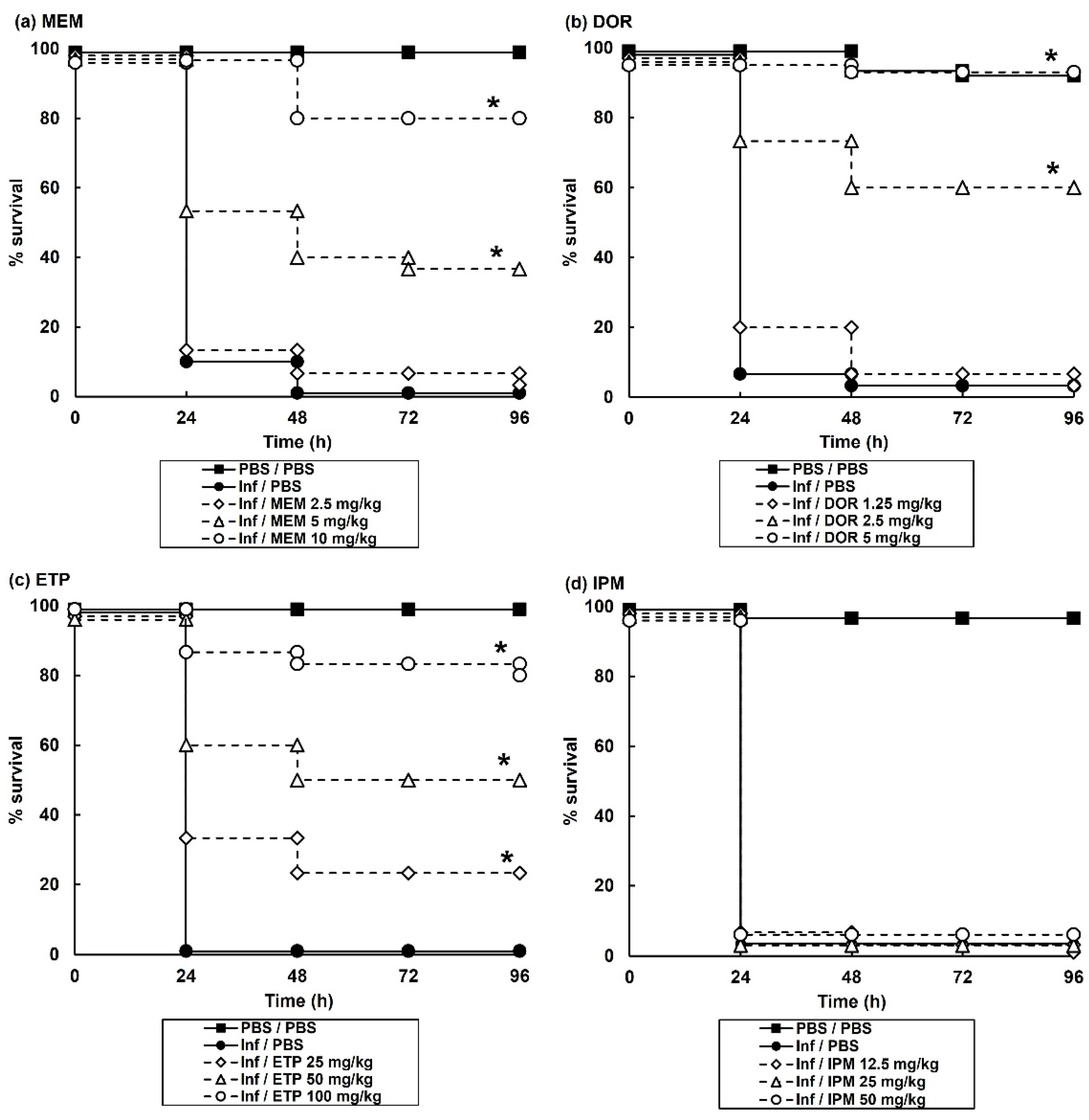
2.2. Carbapenem Monotherapy of G. mellonella Larvae Infected with P. aeruginosa NCTC13437 Reveals Antibiotic-Dependent Levels of Efficacy
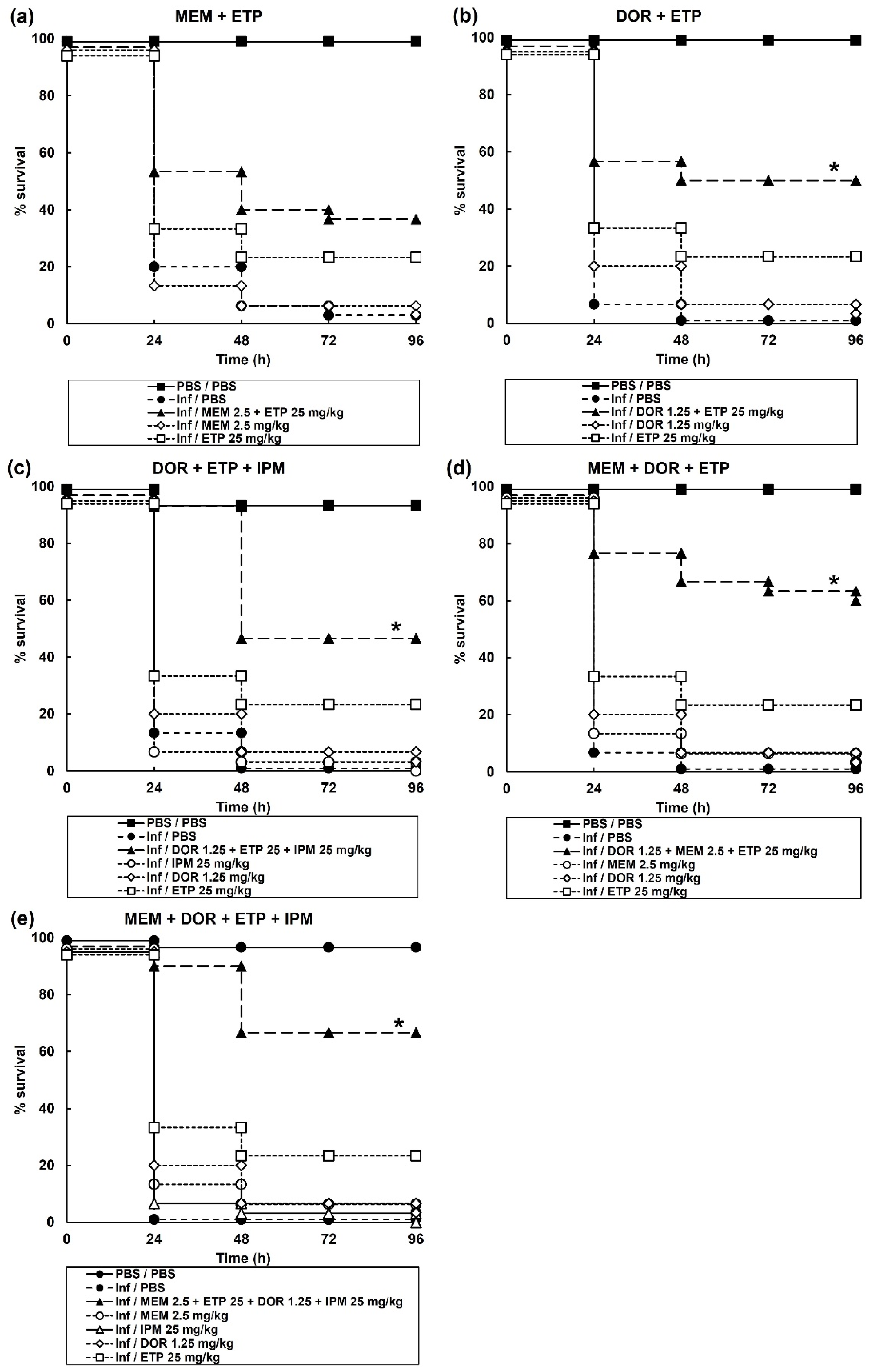
2.3. Treatment of G. mellonella Larvae Infected with P. aeruginosa with Combinations of Carbapenems Results in Enhanced Efficacy Compared to Monotherapies
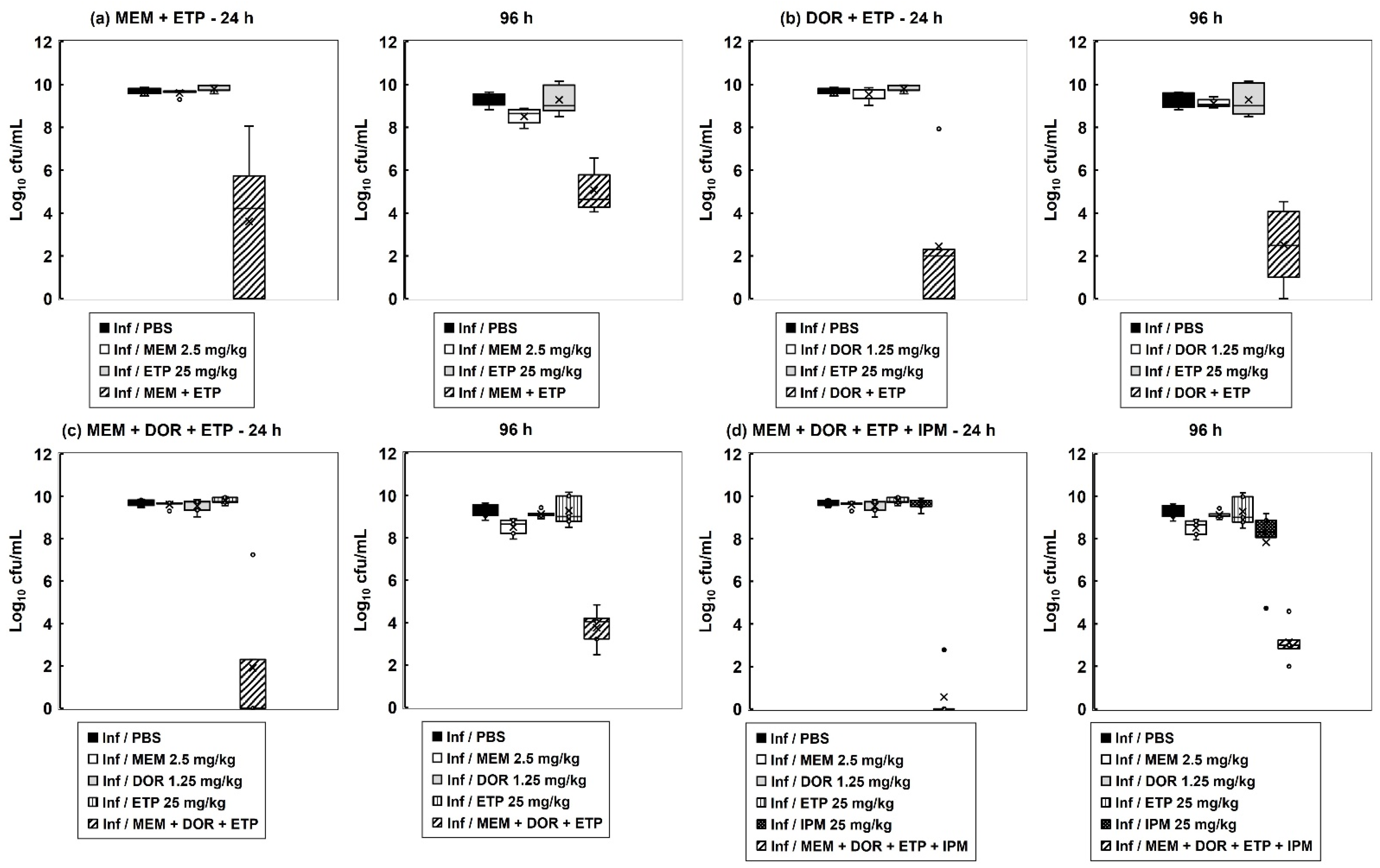
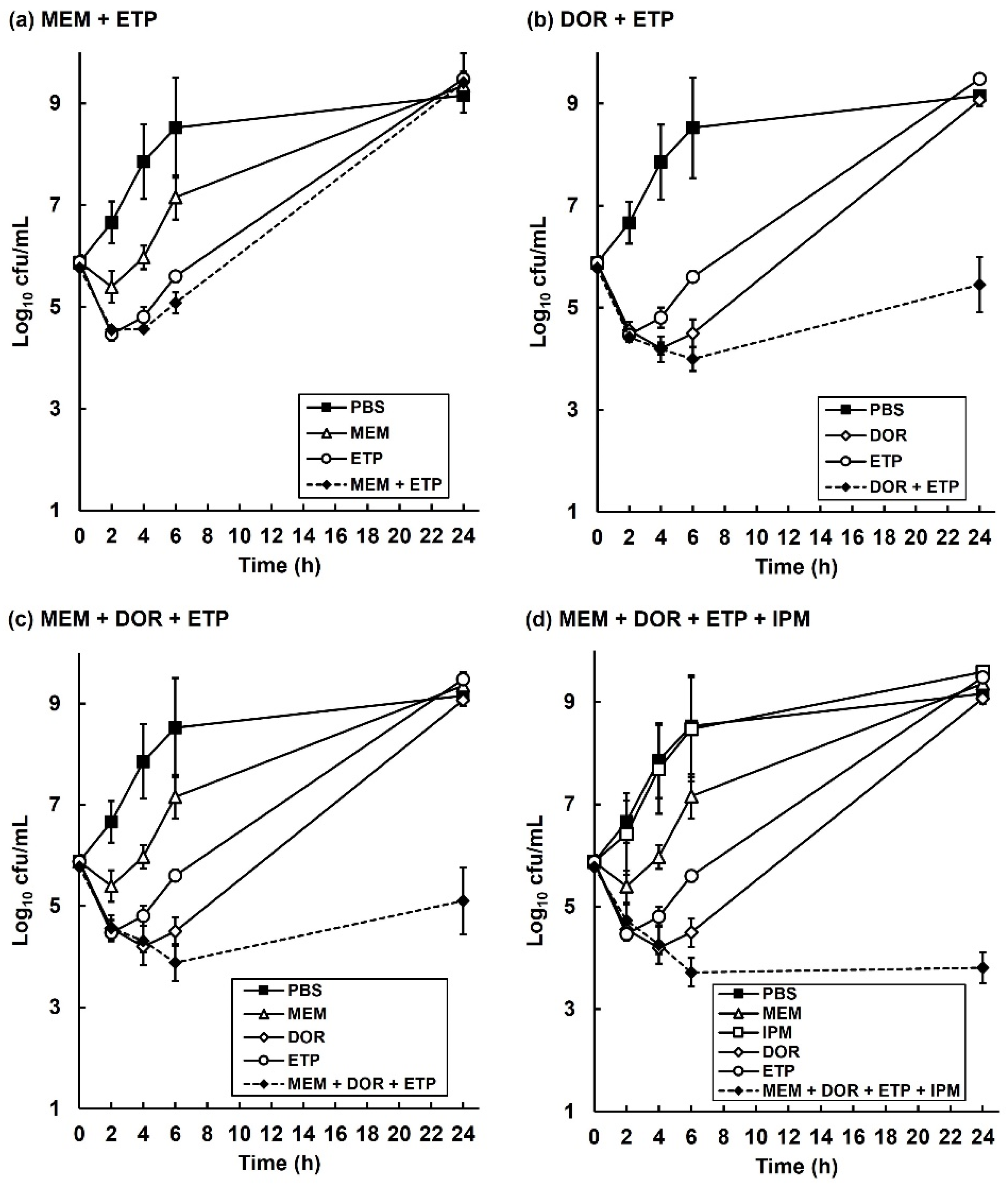
2.4. The Inhibitory Action of Carbapenem Combinations versus P. aeruginosa Is Bactericidal but Does Not Eliminate All Bacteria In Vitro
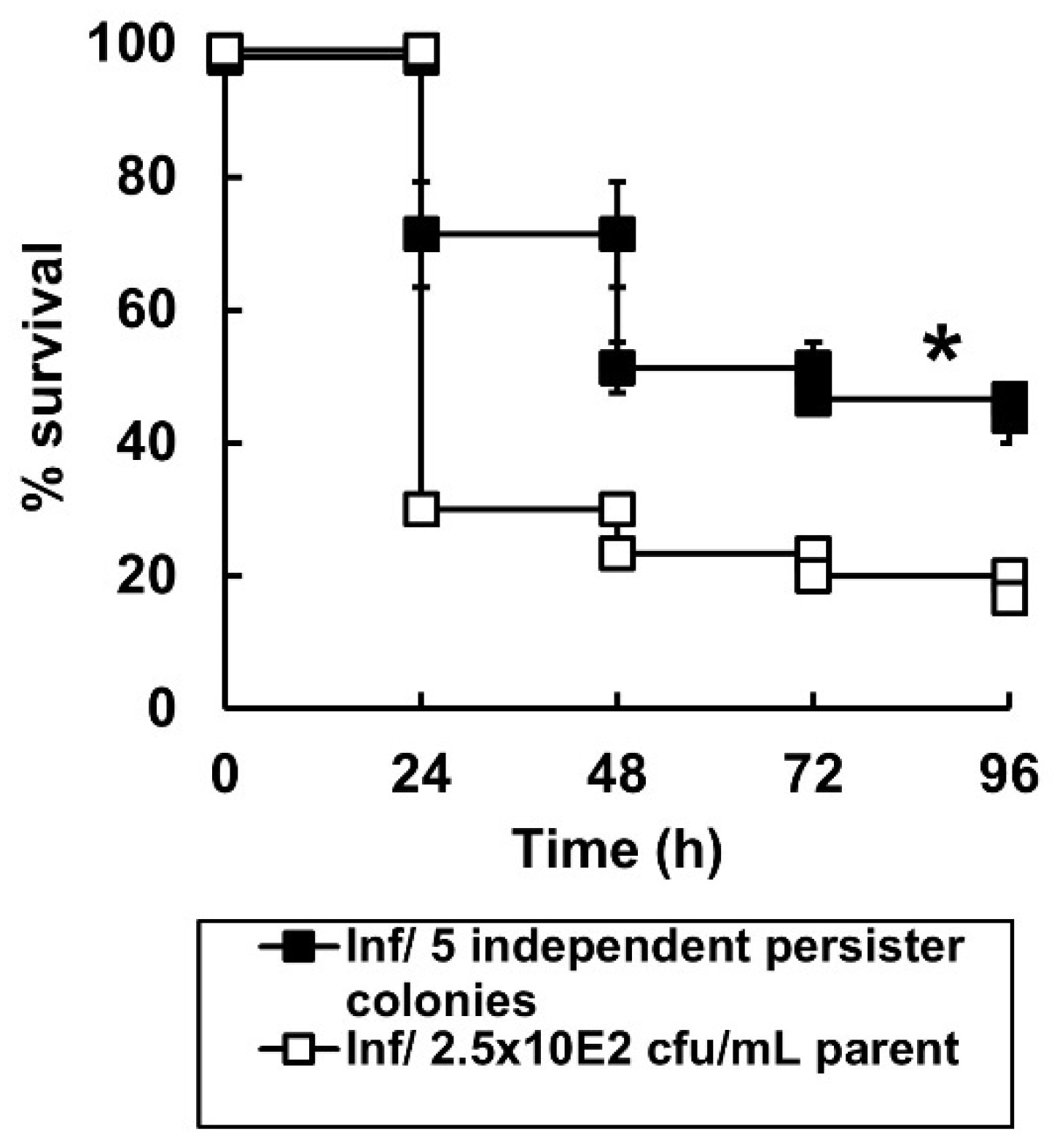
2.5. Surviving P. aeruginosa Cells Isolated from G. mellonella Larvae Exposed to Carbapenem Combination Therapy for 96 h Display a Persister Phenotype
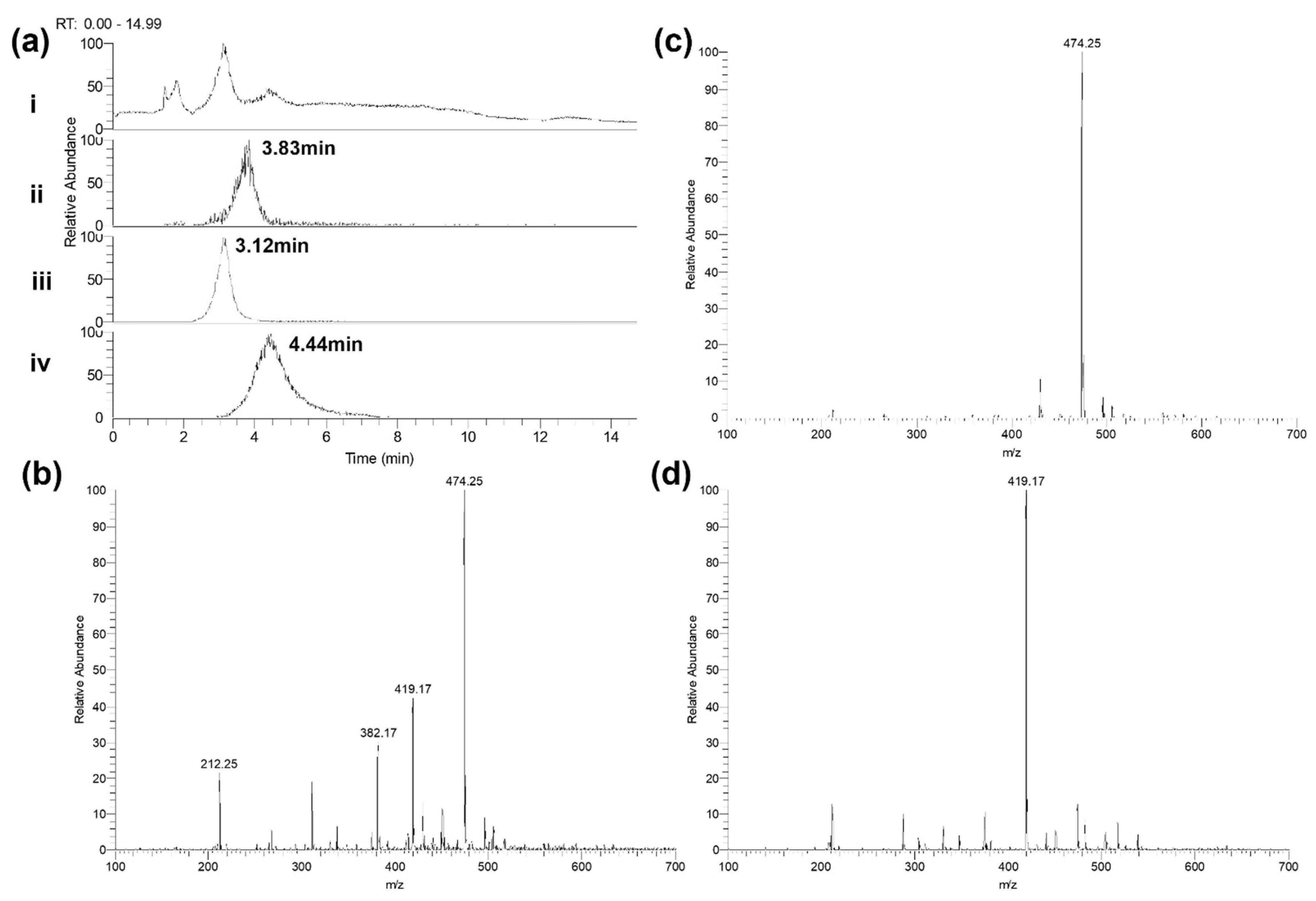
2.6. The Degradation of Individual Carbapenems in The Presence of Carbapenemase-Carrying P. aeruginosa NCTC13437 Occurs at Different Rates When in Combination with Other Carbapenems Than Alone
3. Discussion
4. Materials and Methods
4.1. Bacteria and Growth Media
4.2. Antibiotics and G. mellonella Larvae
4.3. Antibiotic Susceptibility Testing
4.4. G. mellonella Infection Model
4.5. Time-Kill Assay
4.6. Isolation and Characterisation of In Vivo Persister Cells
4.7. Quantification of Changes in Carbapenem Concentration in the Presence of P. aeruginosa NCTC13437 by Mass Spectrometry
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sader, H.S.; Farrell, D.J.; Flamm, R.K.; Jones, R.N. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalised with pneumonia in US and European hospitals: Results from the SENTRY Antimicrobial Surveillance Program, 2009–2012. Int. J. Antimicrob. Agents 2014, 43, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aeruginosa: An audacious pathogen with an adaptable arsenal of virulence factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotech. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Palavutitotai, N.; Jitmuang, A.; Tongsai, S.; Kiratisin, P.; Angkasekwinai, N. Epidemiology and risk factors of extensively drug-resistant Pseudomonas aeruginosa infections. PLoS ONE 2018, 13, e0193431. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, P.M.; Warren, R.E.; Livermore, D.M.; McNulty, C.A.M.; Enoch, D.A.; Otter, J.A.; Wilson, A.P.R. Treatment of infections caused by multidrug-resistant Gram-negative bacteria: Report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J. Antimic. Chemother. 2018, 73, 2–78. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet. Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe. 2017. Available online: https://www.ecdc.europa.eu/sites/portal/files/documents/AMR-surveillance-EARS-Net-2017.pdf (accessed on 22 September 2022).
- Lautenbach, E.; Weiner, M.G.; Nachamkin, I.; Bilker, W.B.; Sheridan, A.; Fishman, N.O. Imipenem resistance among Pseudomonas aeruginosa isolates: Risk factors for infection and impact of resistance on clinical and economic outcomes. Infect. Control Hosp. Epidemiol. 2006, 27, 893–900. [Google Scholar] [CrossRef]
- Chevalier, S.; Bouffartigues, E.; Bodilis, J.; Maillot, O.; Lesouhaitier, O.; Feuilloley, M.G.J.; Orange, N.; Dufour, A.; Cornelis, P. Structure, function and regulation of Pseudomonas aeruginosa porins. FEMS Microbiol. Rev. 2017, 41, 698–722. [Google Scholar] [CrossRef]
- Shigemura, K.; Osawa, K.; Kato, A.; Tokimatsu, I.; Arakawa, S.; Shirakawa, T.; Fujisawa, M. Association of overexpression of efflux pump genes with antibiotic resistance in Pseudomonas aeruginosa strains clinically isolated from urinary tract infection patients. J. Antibiot. 2015, 68, 568–572. [Google Scholar] [CrossRef]
- Botelho, J.; Grosso, F.; Peixe, L. Antibiotic resistance in Pseudomonas aeruginosa—Mechanisms, epidemiology and evolution. Drug Resist Updat. 2019, 44, 100640. [Google Scholar] [CrossRef]
- Carcione, D.; Siracusa, C.; Sulejmani, A.; Leoni, V.; Intra, J. Old and new Beta-lactamase inhibitors: Molecular structure, mechanism of action, and clinical use. Antibiotics 2021, 10, 995. [Google Scholar] [CrossRef]
- Bouza, E. The role of new carbapenem combinations in the treatment of multi-drug resistant Gram-negative infections. J. Antimic. Chemother. 2021, 76, iv38–iv45. [Google Scholar] [CrossRef] [PubMed]
- Rahme, C.; Butterfield, J.M.; Nicasio, A.M.; Lodise, T.P. Dual beta-lactam therapy for serious Gram-negative infections: Is it time to revisit? Diag. Microbiol. Infect. Dis. 2014, 80, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Siriyong, T.; Murray, R.M.; Bidgood, L.E.; Young, S.A.; Wright, F.; Parcell, B.J.; Voravuthikunchai, S.P.; Coote, P.J. Dual β-lactam combination therapy for multi-drug resistant Pseudomonas aeruginosa infection: Enhanced efficacy in vivo and comparison with monotherapies of penicillin-binding protein inhibition. Sci. Rep. 2019, 9, 1–13. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Clinical Breakpoints and Dosing of Antibiotics—Clinical Breakpoints—Bacteria, version 12. 2022. Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 22 September 2022).
- European Committee on Antimicrobial Susceptibility Testing. Ertapenem Rationale Document, version 2. 2021. Available online: http://eucast.org/rd (accessed on 22 September 2022).
- Khawcharoenporn, T.; Chuncharunee, A.; Maluangnon, C.; Taweesakulvashra, T.; Tiamsak, P. Active monotherapy and combination therapy for extensively drug-resistant Pseudomonas aeruginosa pneumonia. Int. J. Antimicrob. Agents 2018, 52, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Moya, B.; Chen, M.J.; Zavascki, A.P.; Tsai, H.; Tao, X.; Sutaria, D.S.; Louie, A.; Boyce, J.D.; Deveson Lucas, D.; et al. Comparable efficacy and better safety of double beta-lactam combination therapy versus beta-lactam plus aminoglycoside in gram-negatives: A meta-analysis of randomized, controlled trials. Antimicrob. Agents Chemother. 2019, 63, e00425-19. [Google Scholar] [CrossRef] [PubMed]
- Bulik, C.C.; Nicolau, D.P. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2011, 55, 3002–3004. [Google Scholar] [CrossRef]
- Cebrero-Cangueiro, T.; Nordmann, P.; Carretero-Ledesma, M.; Pachon, J.; Pachom-Ibanez, M.E. Efficacy of dual carbapenem treatment in a murine sepsis model of infection due to carbapenemase-producing Acinetobacter baumannii. J. Antimicrob. Chemother. 2021, 76, 680–683. [Google Scholar] [CrossRef]
- Giamarellou, H.; Galani, L.; Baziaka, F.; Karaiskos, I. Effectiveness of a double carbapenem regimen for infections in humans due to carbapenemase producing pandrug-resistant Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2013, 57, 2388–2390. [Google Scholar] [CrossRef]
- Oliva, A.; Gizzi, F.; Mascellino, M.T.; Cipolla, A.; D’Abramo, A.; D’Agostino, C.; Trinchieri, V.; Russo, G.; Tierno, F.; Iannetta, M.; et al. Bactericidal and synergistic activity of double-carbapenem regimen for infections caused by carbapenemase-producing Klebsiella pneumoniae. Clin. Microbiol. Infect. 2016, 22, 147–153. [Google Scholar] [CrossRef]
- De Pascale, G.; Martucci, G.; Montini, L.; Panarello, G.; Cutuli, S.L.; Di Carlo, D.; Di Gravio, V.; Di Stefano, R.; Capitanio, G.; Vallecoccia, M.S.; et al. Double carbapenem as a rescue strategy for the treatment of severe carbapenemase-producing Klebsiella pneumoniae infections: A two-centre, matched case-control study. Crit. Care 2017, 21, 173. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Wang, J.; Wang, R.; Cai, Y. Double-carbapenem therapy in the treatment of multidrug resistant Gram-negative bacterial infections: A systematic review and meta-analysis. BMC Infect. Dis. 2020, 20, 408. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Gilchrist, M.; Heard, K.; Hamilton, R.; Sneddon, J. Treating infections caused by carbapenemase-producing Enterobacterales (CPE): A pragmatic approach to antimicrobial stewardship on behalf of the UKCPA Pharmacy Infection Network (PIN). JAC Antimicrob. Resist. 2020, 2, dlaa075. [Google Scholar] [CrossRef] [PubMed]
- Abdelraouf, K.; Reyes, S.; Nicolau, D.P. The paradoxical in vivo activity of β-lactams against metallo-β-lactamase-producing Enterobacterales is not restricted to carbapenems. J. Antimic. Chemother. 2021, 76, 684–691. [Google Scholar] [CrossRef]
- Mashni, O.; Nazer, L.; Le, J. Critical review of double-carbapenem therapy for the treatment of carbapenemase-producing Klebsiella pneumoniae. Ann. Pharm. 2019, 53, 70–81. [Google Scholar] [CrossRef]
- Poirel, L.; Kieffer, N.; Nordmann, P. In vitro evaluation of dual carbapenem combinations against carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2016, 71, 156–161. [Google Scholar] [CrossRef]
- Fredborg, M.; Sondergaard, T.E.; Wang, M. Synergistic activities of meropenem double and triple combinations against carbapenemase-producing Enterobacteriaceae. Diagn Microbiol. Infect. Dis. 2017, 88, 355–360. [Google Scholar] [CrossRef]
- Oliva, A.; Scorzolini, L.; Cipolla, A.; Mascellino, M.T.; Cancelli, F.; Castaldi, D.; D’Abramo, A.; D’Agostino, C.; Russo, G.; Ciardi, M.R.; et al. In vitro evaluation of different antimicrobial combinations against carbapenemase-producing Klebsiella pneumoniae: The activity of the double-carbapenem regimen is related to meropenem MIC value. J. Antimicrob. Chemother. 2017, 72, 1981–1984. [Google Scholar] [CrossRef]
- Wiskirchen, D.E.; Crandon, J.L.; Nicolau, D.P. Impact of various conditions on the efficacy of dual carbapenem therapy against KPC-producing Klebsiella pneumoniae. Int. J. Antimicrob. Agents 2013, 41, 582–585. [Google Scholar] [CrossRef]
- Anderson, K.F.; Lonsway, D.R.; Rasheed, J.K.; Biddle, J.; Jensen, B.; McDougal, L.K.; Carey, R.B.; Thompson, A.; Stocker, S.; Limbago, B.; et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J. Clin. Microbiol. 2007, 45, 2723–2725. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019, 17, 441–448. [Google Scholar] [CrossRef]
- Gollan, B.; Grabe, G.; Michaux, C.; Helaine, S. Bacterial persisters and infection: Past, present and progressing. Annu. Rev. Microbiol. 2019, 73, 359–385. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.R.; Burns, J.L.; Lory, S.; Lewis, K. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J. Bacteriol. 2010, 192, 6191–6199. [Google Scholar] [CrossRef] [PubMed]
- Santi, I.; Manfredi, P.; Maffel, E.; Egli, A.; Jenal, U. Evolution of antibiotic tolerance shapes resistance development in chronic Pseudomonas aeruginosa infections. Ther. Prev. 2021, 12, e04382-20. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Kou, S.H.; Xie, R.; Van Nieuwenhze, M.S.; Qu, J.; Peng, B.; Zheng, J. Non-walled spherical Acinetobacter baumannii is an important type of persister upon β-lactam antibiotic treatment. Emerg. Microbes Infect. 2020, 9, 1149–1159. [Google Scholar] [CrossRef]
- Woodford, N.; Zhang, J.; Kaufmann, M.E.; Yarde, S.; del Mar Tomas, M.; Faris, C.; Vardhan, M.S.; Dawson, S.; Cotterill, S.L.; Livermore, D.M. Detection of Pseudomonas aeruginosa isolates producing VEB-type extended-spectrum β-lactamases in the United Kingdom. J. Antimic. Chemother. 2008, 62, 1265–1268. [Google Scholar] [CrossRef]
- Hill, L.; Veli, N.; Coote, P.J. Evaluation of Galleria mellonella larvae for measuring the efficacy and pharmacokinetics of antibiotic therapies against Pseudomonas aeruginosa infection. Int. J. Antimicrob. Agents 2014, 43, 254–261. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Survival probabilities (the Kaplan-Meier method). Brit. Med. J. 1998, 317, 1572. [Google Scholar] [CrossRef]
- Bland, J.M. The logrank test. Brit. Med. J. 2004, 328, 1073. [Google Scholar] [CrossRef]
- Krezdorn, J.; Adams, S.; Coote, P.J. A Galleria mellonella infection model reveals double and triple antibiotic combination therapies with enhanced efficacy versus a multidrug-resistant strain of Pseudomonas aeruginosa. J. Med. Microbiol. 2014, 63, 945–955. [Google Scholar] [CrossRef]
- Adamson, D.H.; Krikstopaityte, V.; Coote, P.J. Enhanced efficacy of putative efflux pump inhibitor/antibiotic combination treatments versus MDR strains of Pseudomonas aeruginosa in a Galleria mellonella in vivo infection model. J. Antimicrob. Chemother. 2015, 70, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, S.; Wichmann, C.; Bremer-Streck, S.; Hagel, S.; Kiehntopf, M. Simultaneous quantification of nine antimicrobials by LC-MS/MS for therapeutic drug monitoring in critically ill patients. Drug Monit. 2019, 41, 29–37. [Google Scholar] [CrossRef] [PubMed]

| MIC (mg/L) | |||||
|---|---|---|---|---|---|
| Strain | Resistance Mechanism | MEM | DOR | ETP | IPM |
| P. aeruginosa NCTC10662 | None | 2 | 1 | 16 | 8 |
| P. aeruginosa NCTC13437 | VEB1 and VIM10 | 64 | 32–64 | >256 | >256 |
| Therapy | Antibiotic(s) or PBS Control | Dose (mg/kg) | % Survival In Vivo 96 h p.i |
|---|---|---|---|
| Sham treatment | PBS | 10 μL PBS | 0 |
| Monotherapy | MEM | 2.5 | 3.3 |
| DOR | 1.25 | 3.3 | |
| ETP | 25 | 23.3 | |
| IPM | 25 | 0 | |
| Dual combination therapy | MEM + DOR | 2.5 + 1.25 | 0 |
| MEM + ETP | 2.5 + 25 | 36.7 | |
| MEM + IPM | 2.5 + 25 | 5 | |
| DOR + ETP | 1.25 + 25 | 50 * | |
| DOR + IPM | 1.25 + 25 | 13.3 | |
| ETP + IPM | 25 + 25 | 0 | |
| Triple combination therapy | MEM + DOR +ETP | 2.5 + 1.25 + 25 | 60 * |
| MEM + DOR + IPM | 2.5 + 1.25 + 25 | 0 | |
| MEM + ETP + IPM | 2.5 + 25 + 25 | 26.7 | |
| DOR + ETP + IPM | 1.25 + 25 + 25 | 46.7 * | |
| Quadruple combination therapy | MEM + DOR + ETP + IPM | 2.5 + 1.25 + 25 + 25 | 66.7 * |
| Pseudomonas aeruginosa NCTC13437 | Growth Rate (Optical Density 600 nm/h) | % Reduction in Growth Rate |
|---|---|---|
| Parent strain | 0.73 ± 0.17 | N/A |
| Persister colony 1 | 0.34 ± 0.08 | 53 |
| Persister colony 2 | 0.39 ± 0.03 | 47 |
| Persister colony 3 | 0.33 ± 0.01 | 55 |
| Persister colony 4 | 0.39 ± 0.05 | 47 |
| Persister colony 5 | 0.38 ± 0.24 | 48 |
| Carbapenem Treatment | Rate of Carbapenem Degradation (mg/L min−1) | Change in Rate of Carbapenem Degradation (%) | ||||
|---|---|---|---|---|---|---|
| MEM | DOR | ETP | MEM | DOR | ETP | |
| Carbapenem alone | −0.0953 | −0.0092 | −0.2671 | - | - | - |
| MEM + ETP | −0.1275 | N/A | −0.1722 | +34 | N/A | −36 |
| DOR + ETP | N/A | −0.0058 | −0.0914 | N/A | −37 | −66 |
| MEM + DOR + ETP | −0.0777 | −0.0048 | −0.1642 | −18 | −48 | −39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mackay, B.; Parcell, B.J.; Shirran, S.L.; Coote, P.J. Carbapenem-Only Combination Therapy against Multi-Drug Resistant Pseudomonas aeruginosa: Assessment of In Vitro and In Vivo Efficacy and Mode of Action. Antibiotics 2022, 11, 1467. https://doi.org/10.3390/antibiotics11111467
Mackay B, Parcell BJ, Shirran SL, Coote PJ. Carbapenem-Only Combination Therapy against Multi-Drug Resistant Pseudomonas aeruginosa: Assessment of In Vitro and In Vivo Efficacy and Mode of Action. Antibiotics. 2022; 11(11):1467. https://doi.org/10.3390/antibiotics11111467
Chicago/Turabian StyleMackay, Brendan, Benjamin J. Parcell, Sally L. Shirran, and Peter J. Coote. 2022. "Carbapenem-Only Combination Therapy against Multi-Drug Resistant Pseudomonas aeruginosa: Assessment of In Vitro and In Vivo Efficacy and Mode of Action" Antibiotics 11, no. 11: 1467. https://doi.org/10.3390/antibiotics11111467
APA StyleMackay, B., Parcell, B. J., Shirran, S. L., & Coote, P. J. (2022). Carbapenem-Only Combination Therapy against Multi-Drug Resistant Pseudomonas aeruginosa: Assessment of In Vitro and In Vivo Efficacy and Mode of Action. Antibiotics, 11(11), 1467. https://doi.org/10.3390/antibiotics11111467





