Synergistic Action of AMX Associated with 1,8-Cineole and Its Effect on the ESBL Enzymatic Resistance Mechanism
Abstract
1. Introduction
2. Results
2.1. Determination of MIC and PIC Values
2.2. The Checkerboard Assay
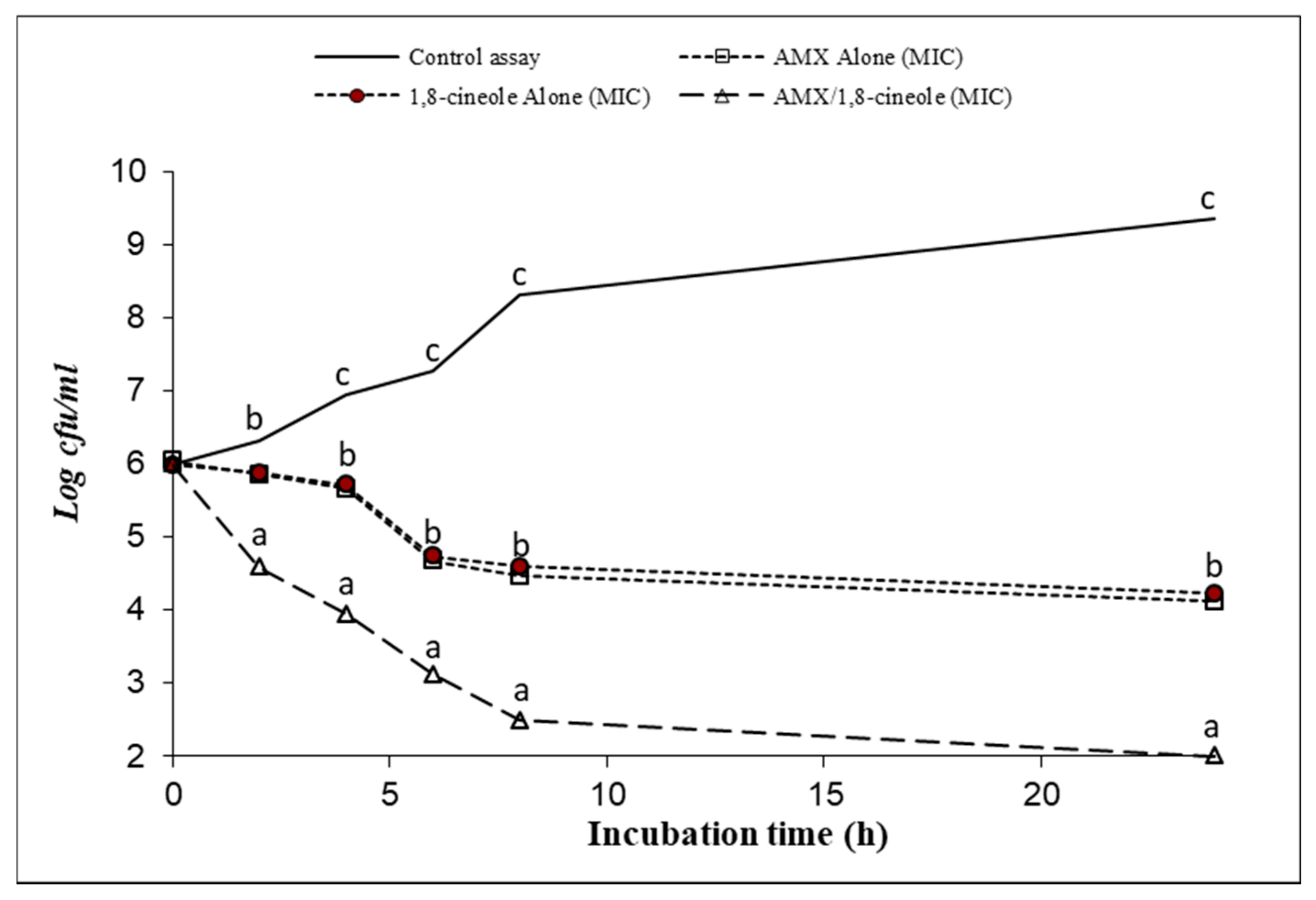
2.3. Time–kill Assay
2.4. Enzymatic Assay
3. Discussion
4. Materials and Methods
4.1. Culture Media
4.2. Bacterial Strains
4.3. Antibacterial Agents
4.4. ß-Lactamase Enzyme
4.5. Determination of Partial Inhibitory Concentrations (PIC) of AMX and 1,8-Cineole Using a Microplate Assay Technique
4.6. Checkerboard Assay
4.7. Time–Kill Assay
4.8. Enzyme Assay
4.9. Data Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhusal, R.P.; Barr, J.J.; Subedi, D.A. Metabolic perspective into antimicrobial tolerance and resistance. Lancet Microbe 2022, 3, e160–e161. [Google Scholar] [CrossRef]
- Taati, M.M.; Amirmozafari, N.; Shariati, A.; Hallajzadeh, M.; Mirkalantari, S.; Khoshbayan, A.; Masjedian, J.F. How Phages Overcome the Challenges of Drug Resistant Bacteria in Clinical Infections. Infect. Drug Resist. 2020, 13, 45–61. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Resistance: Global Report on Surveillance. 2014. Available online: https://apps.who.int/iris/bitstream/handle/10665/112647/WHO_HSE_PED_AIP_?sequence=1 (accessed on 1 April 2022).
- Gautret, P.; Lagier, J.C.; Parola, P.; Meddeb, L.; Sevestre, J.; Mailhe, M.; Doudier, B.; Aubry, C.; Amrane, S.; Seng, P.; et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med. Infect. Dis. 2020, 34, 101663. [Google Scholar] [CrossRef]
- Lanckohr, C. Rational use of antibiotics in the era of multi-resistance. Anästh. Intensivmed. 2022, 63, 26–33. [Google Scholar] [CrossRef]
- Mansour, T.S.; Bradford, P.A.; Venkatesan, A.M. Recent Developments in β-Lactamases and Inhibitors. Annu. Rep. Med. Chem. 2008, 43, 247–267. [Google Scholar] [CrossRef]
- Tansawai, U.; Walsh, T.R.; Niumsup, P.R. Extended spectrum ß-lactamase-producing Escherichia coli among backyard poultry farms, farmers, and environments in Thailand. Poult. Sci. 2019, 98, 2622–2631. [Google Scholar] [CrossRef] [PubMed]
- Dembele, R.; Kaboré, W.A.; Soulama, I.; Traoré, O.; Ouédraogo, N.; Konaté, A.; Guessennd, N.K.; N’Golo, D.C.; Sanou, A.; Serme, S.; et al. Molecular Characterization of β-Lactamase Producing Genes and Integrons in Diarrheagenic Escherichia Coli from Diarrheal Children Less than Five Years of Age in Ouagadougou, Burkina Faso. Res. Square, 2021; accepted. Available online: https://www.researchsquare.com (accessed on 1 June 2022).
- Chaves, T.P.; Pinheiro, R.E.E.; Melo, E.S.; Soares, M.J.D.S.; Souza, J.S.N.; de Andrade, T.B.; de Lemos, T.L.G.; Coutinho, H.D. Essential oil of Eucalyptus camaldulensis Dehn potentiates β-lactam activity against Staphylococcus aureus and Escherichia coli resistant strains. Ind. Crop. Prod. 2018, 112, 70–74. [Google Scholar] [CrossRef]
- Das, T.; Nandy, S.; Mukherjee, A.; Nongdam, P.; Dey, A. Plant Essential Oils for Combating Antimicrobial Resistance via Re-potentiating the Fading Antibiotic Arsenal. Antimicrob. Resist. 2022, 419–485. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Lim, S.H.E.; Hu, C.P.; Yiap, B.C. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomed 2013, 20, 710–713. [Google Scholar] [CrossRef]
- Hriouech, S.; Akhmouch, A.A.; Mzabi, A.; Chefchaou, H.; Tanghort, M.; Oumokhtar, B.; Chami, N.; Remmal, A. The Antistaphylococcal Activity of Amoxicillin/Clavulanic Acid, Gentamicin, and 1,8-Cineole Alone or in Combination and Their Efficacy through a Rabbit Model of Methicillin-Resistant Staphylococcus aureus Osteomyelitis. Evid.-Based Compl. Altern. Med. 2020, 2020, 4271017. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, A.; Miguel, S.; Ribeiro, M.; Coutinho, P.; Silva, L.; Duarte, A.P.; Ferreira, S. Thymus zygis Essential Oil: Phytochemical Characterization, Bioactivity Evaluation and Synergistic Effect with Antibiotics against Staphylococcus aureus. J. Antibiot. 2022, 11, 146. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Synergistic interactions of plant essential oils with antimicrobial agents: A new antimicrobial therapy. Crit. Rev. Food Sci. Nutr. 2022, 62, 1740–1751. [Google Scholar] [CrossRef] [PubMed]
- Nafis, A.; Iriti, M.; Ouchari, L.; El Otmani, F.; Marraiki, N.; Elgorban, A.M.; Syed, A.; Mezrioui, N.; Hassani, L.; Custódio, L. New Insight into the Chemical Composition, Antimicrobial and Synergistic Effects of the Moroccan Endemic Thymus atlanticus (Ball) Roussine Essential Oil in Combination with Conventional Antibiotics. Molecules 2021, 26, 5850. [Google Scholar] [CrossRef] [PubMed]
- Aelenei, P.; Miron, A.; Trifan, A.; Bujor, A.; Gille, E.; Aprotosoaie, A.C. Essential Oils and Their Components as Modulators of Antibiotic Activity against Gram-Negative Bacteria. Medicines 2016, 3, 19. [Google Scholar] [CrossRef]
- Karumathil, D.P.; Nair, M.S.; Gaffney, J.; Kollanoor-Johny, A.; Venkitanarayanan, K. Trans-Cinnamaldehyde and Eugenol Increase Acinetobacter baumannii Sensitivity to Beta-Lactam Antibiotics. Front. Microbiol. 2018, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Dumlupinar, B.; Celik, D.D.; Karatoprak, G.Ş.; Soyoğul Gürer, Ü. Synergy between Pelargonium endlicherianum essential oil and conventional antibiotics against Neisseria meningitidis and Haemophilus influenzae. S. Afr. J. Bot. 2022, 146, 243–253. [Google Scholar] [CrossRef]
- Dumlupinar, B.; Celik, D.D.; Gürer, Ü.S.; Demirci, B.; Gürbüz, B.; Kurtulus, E.M. Synergic potential of Pelargonium endlicherianum Fenzl. Essential oil and antibiotic combinations against Klebsiella pneumoniae. S. Afr. J. Bot. 2020, 135, 117–126. [Google Scholar] [CrossRef]
- Tang, S.S.; Apisarnthanarak, A.; Hsu, L.Y. Mechanisms of β-lactam antimicrobial resistance and epidemiology of major community- and healthcare-associated multidrug-resistant bacteria. Adv. Drug Delivery Rev. 2014, 78, 3–13. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold. Spring. Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef]
- Fernandes, R.; Amador, P.; Prudêncio, C. β-Lactams: Chemical structure, mode of action and mechanisms of resistance. Rev. Med. Microbiol. 2013, 24, 7–17. [Google Scholar] [CrossRef]
- Oteo, J.; Campos, J.; Lázaro, E.; Cuevas, Ó.; García-Cobos, S.; Pérez-Vázquez, M.; De Abajo, F.J.; Spanish Members of EARSS, S.M. Increased Amoxicillin–Clavulanic Acid Resistance in Escherichia coli Blood Isolates, Spain. Emerg. Infect. Dis. 2008, 14, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Rodrıguez-Villodres, A.; Gil-Marqués, M.L.; Alvarez-Marın, R.; Bonnin, R.A.; Pachón-Ibánez, M.E.; Aguilar-Guisado, M.; Naas, T.; Aznar, J.; Pachón, J.; Lepe, J.A.; et al. Extended-spectrum resistance to β-lactams/β-lactamase inhibitors (ESRI) evolved from low-level resistant Escherichia coli. J. Antimicrob. Chemother. 2020, 75, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Özel, Y.; Yilmaz, U.; Ünlü, M.; Ünlü, G.V. Antibacterial Activity and Synergistic Interaction of Various Essential Oil Components and Antibiotics. Mikrobiyoloji Bulteni. 2022, 56, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Remmal, A.; Akhmouch, A.A. Pharmaceutical Formulation Comprising Cineole and Amoxicillin. 2019. Patent. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=US250863563&docAn=16306262 (accessed on 1 June 2022).
- Remmal, A. Pharmaceutical Composition Comprising an Anti-Bacteral Agent and an Active Ingredient Selected from Carveol, Thymol, Eugenol, Borneol and Carvacrol. 2006. Patent. Available online: https://patents.google.com/patent/WO2006120567A2/en (accessed on 1 June 2022).
- Remmal, A.; Bouchikhi, T.; Rhayour, K.; Ettayebi, M.; Tantaoui-Elaraki, A. Improved Method for the Determination of Antimicrobial Activity of Essential Oils in Agar Medium. J. Essent. Oil Res. 1993, 5, 179–184. [Google Scholar] [CrossRef]
- Casey, J.T.; O’Cleirigh, C.; Walsh, P.K.; O’Shea, D.G. Development of a robust microtiter plate-based assay method for assessment of bioactivity. J. Microbiol. Methods 2004, 58, 327–334. [Google Scholar] [CrossRef]
- Matsumura, S.O.; Louie, L.; Louie, M.; Simor, A.E. Synergy Testing of Vancomycin-Resistant Enterococcus faecium against Quinupristin-Dalfopristin in Combination with Other Antimicrobial Agents. Antimicrob Agents Chemother. 1999, 43, 2776–2779. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomed 2010, 17, 1061–1066. [Google Scholar] [CrossRef]
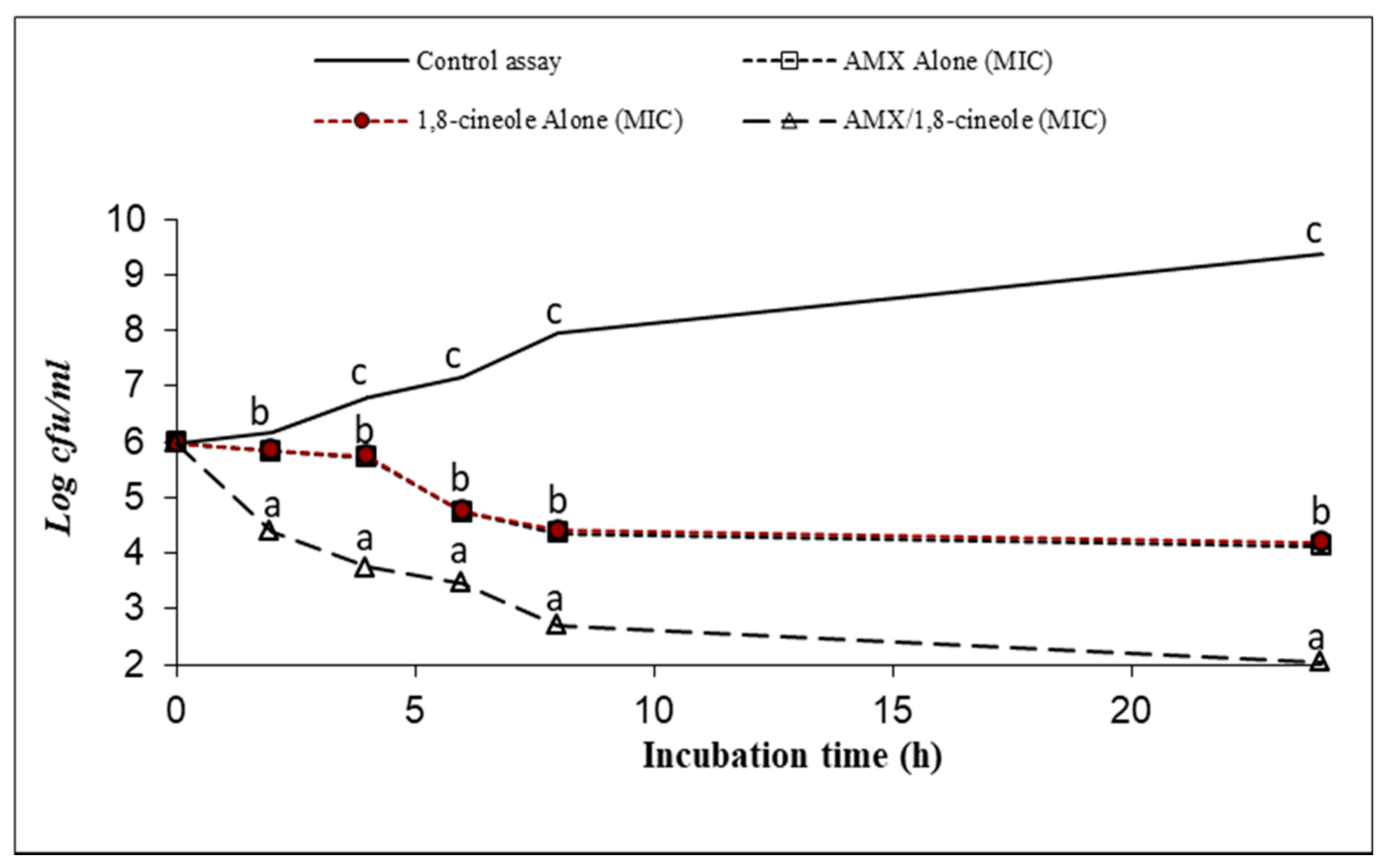
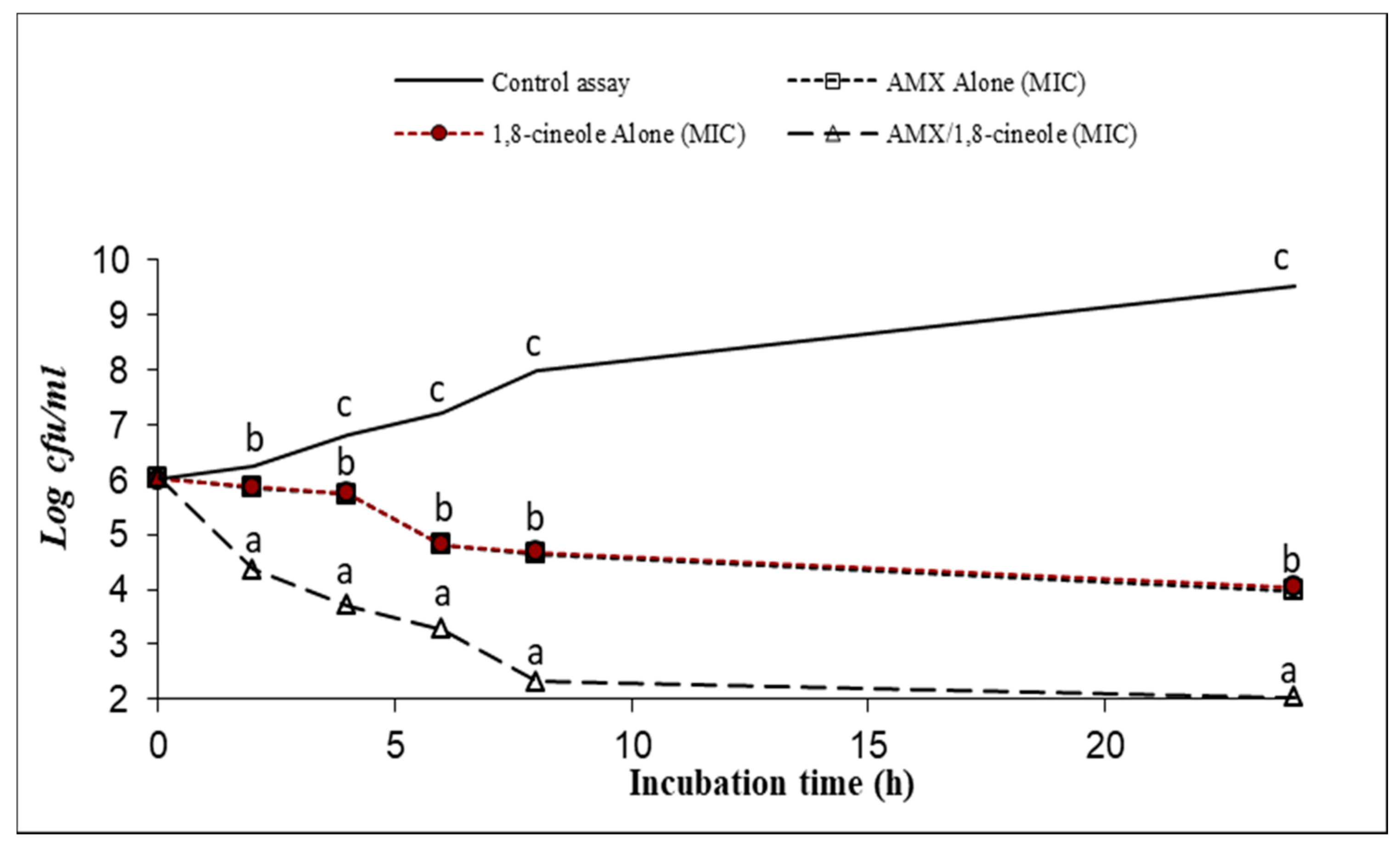
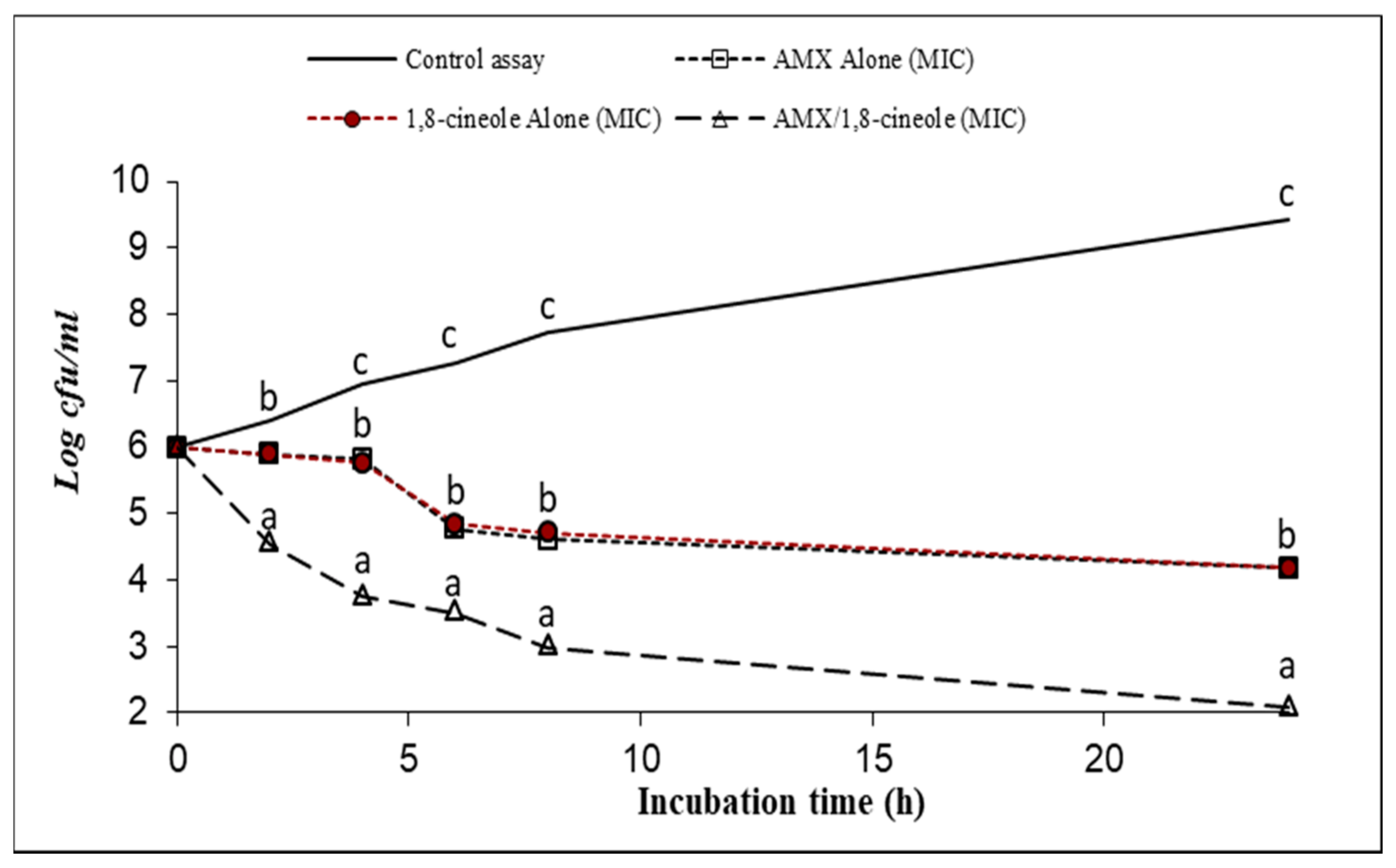
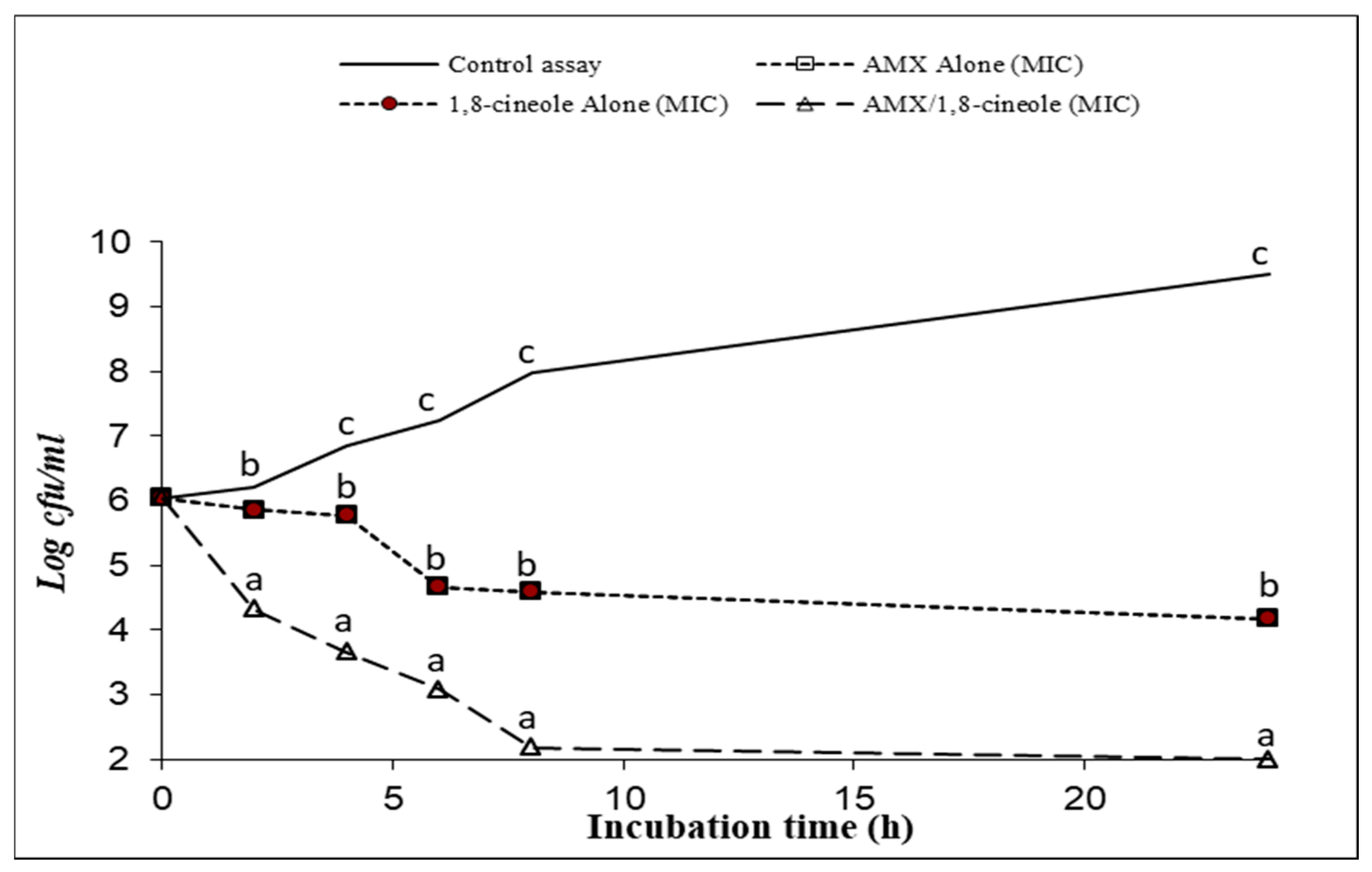

| Strains | IC50 (mg/L) | IC25 (mg/L) | |
|---|---|---|---|
| E. coli | P956 | 1.1 ± 0.25 | 0.7 ± 0.14 |
| P933 | 1.3 ± 0.24 | 0.5 ± 0.10 | |
| P7847 | 1.5 ± 0.13 | 0.8 ± 0.17 | |
| K. pneumoniae | H1878 | 1.6 ± 0.17 | 0.9 ± 0.09 |
| H2001 | 1.3 ± 0.12 | 0.6 ± 0.14 | |
| H1893 | 1.7 ± 0.09 | 0.8 ± 0.12 | |
| Strains | IC40 (mg/L) | IC30 (mg/L) | IC20 (mg/L) | |
|---|---|---|---|---|
| E. coli | P956 | 6.2 ± 0.20 | 2.6 ± 0.12 | 1.2 ± 0.16 |
| P933 | 6.8 ± 0.14 | 2.3 ± 0.09 | 1.3 ± 0.24 | |
| P7847 | 6.5 ± 0.10 | 2.7 ± 0.12 | 1.2 ± 0.11 | |
| K. pneumoniae | H1878 | 7.1 ± 0.12 | 2.9 ± 0.12 | 0.4 ± 0.12 |
| H2001 | 6.9 ± 0.12 | 3.1 ± 0.10 | 0.7 ± 0.09 | |
| H1893 | 6.8 ± 0.09 | 2.7 ± 0.09 | 0.6 ± 0.12 | |
| Strains | AMX/1,8-Cineole IC 50%/IC 40% | AMX/1,8-Cineole IC 50%/IC 30% | AMX/1,8-Cineole IC 50%/IC 20% | |
|---|---|---|---|---|
| E. coli | P956 | 100.0 ± 0.0 | 92.5 ± 0.56 | 80.4 ± 0.69 |
| P933 | 100.0 ± 0.0 | 95.2 ± 0.75 | 81.5 ± 0.59 | |
| P7847 | 100.0 ± 0.0 | 90.8 ± 1.29 | 79.4 ± 0.47 | |
| K. pneumoniae | H1878 | 100.0 ± 0.0 | 90.2 ± 0.68 | 82.8 ± 0.94 |
| H2001 | 100.0 ± 0.0 | 93.7 ± 0.80 | 81.2 ± 0.46 | |
| H1893 | 100.0 ± 0.0 | 90.5 ± 0.25 | 85.2 ± 0.41 | |
| Strains | AMX/1,8-Cineole IC 25%/IC 40% | AMX/1,8-Cineole IC 25%/IC 30% | AMX/1,8-Cineole IC 25%/IC 20% | |
|---|---|---|---|---|
| E. coli | P956 | 72.0 ± 0.38 | 62.1 ± 0.22 | 55.3 ± 0.75 |
| P933 | 73.1 ± 0.45 | 59.4 ± 0.32 | 51.2 ± 0.48 | |
| P7847 | 70.1 ± 0.46 | 63.3 ± 0.36 | 53.4 ± 0.60 | |
| K. pneumoniae | H1878 | 74.4 ± 0.28 | 61.2 ± 0.26 | 56.3 ± 0.38 |
| H2001 | 72.4 ± 0.25 | 60.2 ± 0.77 | 53.9 ± 0.67 | |
| H1893 | 71.1 ± 0.29 | 64.4 ± 0.34 | 52.6 ± 0.35 | |
| Strains | MIC Alone (mg/L) | MIC Combined (mg/L) | FIC-Index | Result | |||
|---|---|---|---|---|---|---|---|
| AMX | 1,8-Cineole | AMX | 1,8-Cineole | ||||
| E. coli | P956 | 50 | 100 | 1.1 | 6.2 | 0.08 | Synergy |
| P933 | 50 | 100 | 1.3 | 6.8 | 0.09 | Synergy | |
| P7847 | 50 | 100 | 1.5 | 6.5 | 0.10 | Synergy | |
| K. pneumoniae | H1878 | 50 | 100 | 1.6 | 7.1 | 0.10 | Synergy |
| H2001 | 50 | 100 | 1.3 | 6.9 | 0.10 | Synergy | |
| H1893 | 50 | 100 | 1.7 | 6.8 | 0.10 | Synergy | |
| % Inhibition | ||
|---|---|---|
| Positive controls | AMX | 100% ± 0.0 a |
| 1,8-cineole | 0% ± 0.0 c | |
| AMX + ß-lactamase | 0% ± 0.0 c | |
| ß-lactamase + 1,8-cineole + AMX | 83.4% ± 1.1 b | |
| MHB | Inocula 1 | AMX 2 | ß-lactamase 3 | 1,8-cineole 4 | ||
|---|---|---|---|---|---|---|
| Positive controls | PC1 | 1000 µL | – | – | – | – |
| PC2 | 970 µL | 30 µL | – | – | – | |
| PC3 | 940 µL | 30 µL | 30 µL | – | – | |
| PC4 | 940 µL | 30 µL | – | – | 30 µL | |
| PC5 | 930 µL | 30 µL | 30 µL | 10 µL | – | |
| Assay | 900 µL | 30 µL | 30 µL | 10 µL | 30 µL | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhmouch, A.A.; Hriouech, S.; Mzabi, A.; Tanghort, M.; Chefchaou, H.; Remmal, A.; Chami, N. Synergistic Action of AMX Associated with 1,8-Cineole and Its Effect on the ESBL Enzymatic Resistance Mechanism. Antibiotics 2022, 11, 1002. https://doi.org/10.3390/antibiotics11081002
Akhmouch AA, Hriouech S, Mzabi A, Tanghort M, Chefchaou H, Remmal A, Chami N. Synergistic Action of AMX Associated with 1,8-Cineole and Its Effect on the ESBL Enzymatic Resistance Mechanism. Antibiotics. 2022; 11(8):1002. https://doi.org/10.3390/antibiotics11081002
Chicago/Turabian StyleAkhmouch, Ahmed Amin, Soukayna Hriouech, Aouatef Mzabi, Mariam Tanghort, Hanane Chefchaou, Adnane Remmal, and Najat Chami. 2022. "Synergistic Action of AMX Associated with 1,8-Cineole and Its Effect on the ESBL Enzymatic Resistance Mechanism" Antibiotics 11, no. 8: 1002. https://doi.org/10.3390/antibiotics11081002
APA StyleAkhmouch, A. A., Hriouech, S., Mzabi, A., Tanghort, M., Chefchaou, H., Remmal, A., & Chami, N. (2022). Synergistic Action of AMX Associated with 1,8-Cineole and Its Effect on the ESBL Enzymatic Resistance Mechanism. Antibiotics, 11(8), 1002. https://doi.org/10.3390/antibiotics11081002






