Overcoming Methicillin-Resistance Staphylococcus aureus (MRSA) Using Antimicrobial Peptides-Silver Nanoparticles
Abstract
1. Introduction
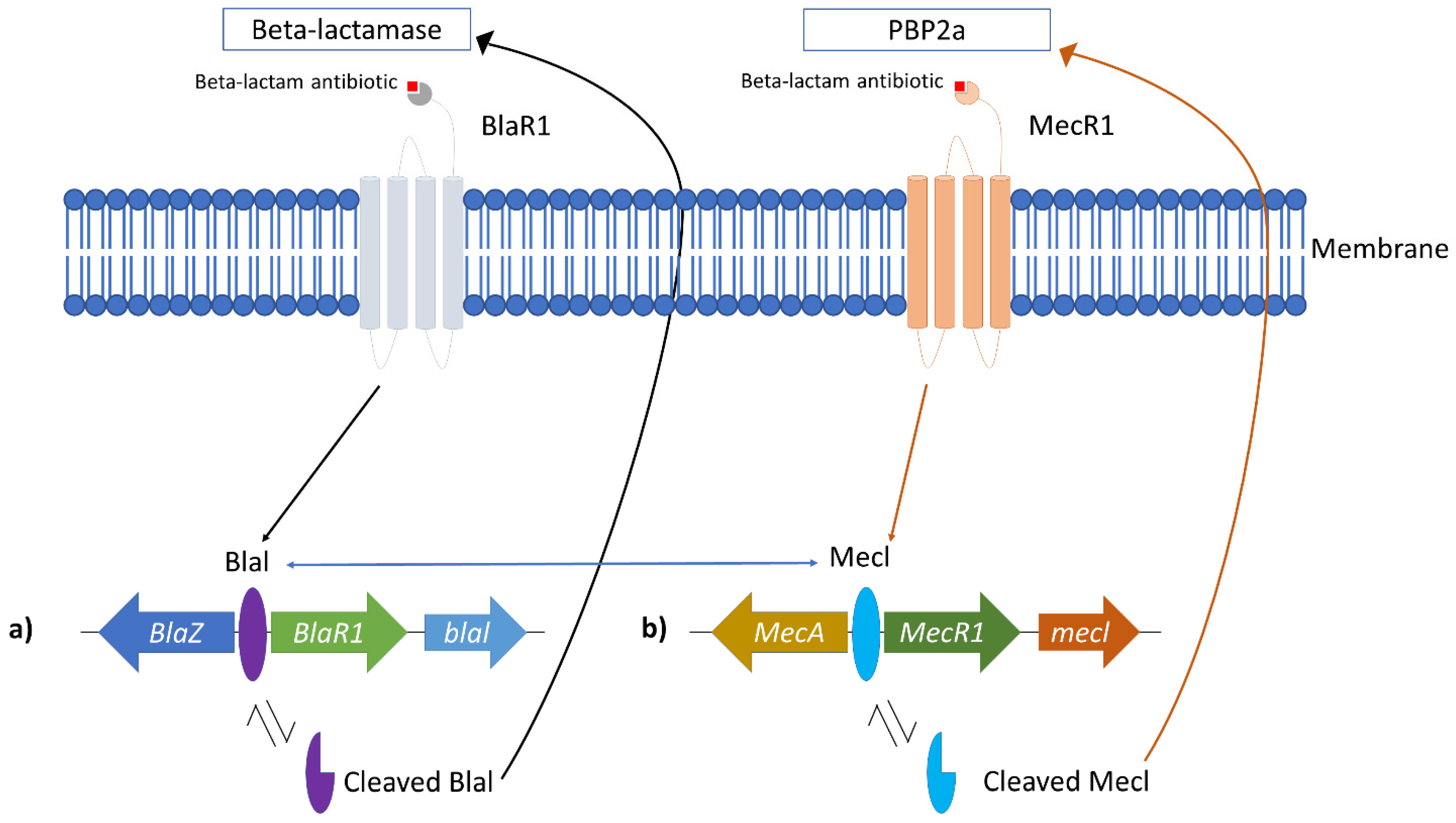
2. Methicillin-Resistant Staphylococcus aureus
3. Antimicrobial Peptides (AMPs)
4. Silver Nanoparticles (AgNPs)
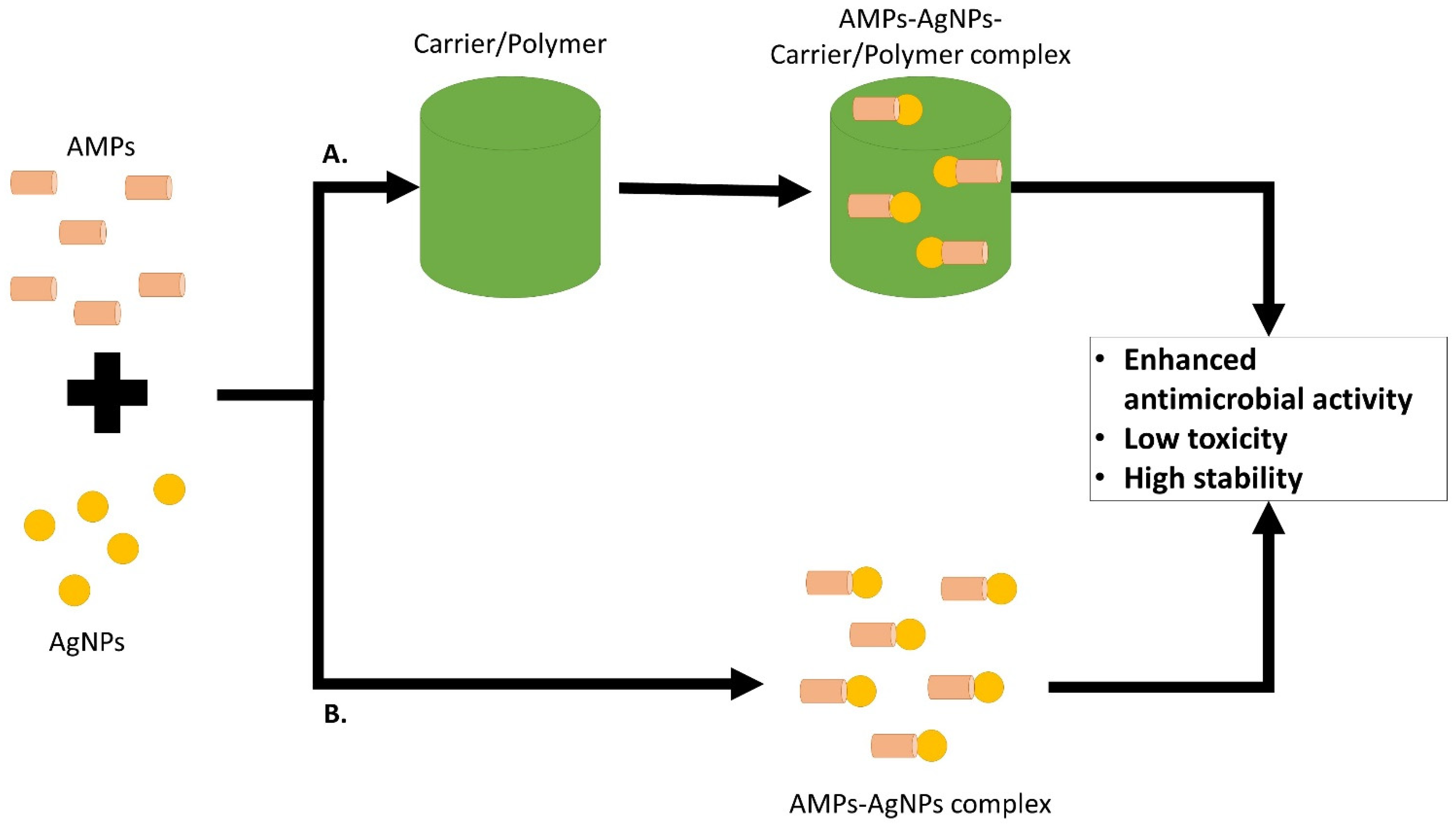
5. AMP and AgNPs Combination on MRSA or MSSA
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, Present and Future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Rigol, S. A Brief History of Antibiotics and Select Advances in Their Synthesis. J. Antibiot. 2018, 71, 153–184. [Google Scholar] [CrossRef]
- Abraham, E.P.; Chain, E. An Enzyme from Bacteria Able to Destroy Penicillin. Nature 1940, 146, 837. [Google Scholar] [CrossRef]
- Dixit, A.; Kumar, N.; Kumar, S.; Trigun, V. Antimicrobial Resistance: Progress in the Decade since Emergence of New Delhi Metallo-β-Lactamase in India. Indian J. Community Med. Off. Publ. Indian Assoc. Prev. Soc. Med. 2019, 44, 4–8. [Google Scholar] [CrossRef]
- Lv, J.; Deng, S.; Zhang, L. A Review of Artificial Intelligence Applications for Antimicrobial Resistance. Biosaf. Health 2021, 3, 22–31. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Kirmusaolu, S. MRSA and MSSA: The Mechanism of Methicillin Resistance and the Influence of Methicillin Resistance on Biofilm Phenotype of Staphylococcus Aureus. In The Rise of Virulence and Antibiotic Resistance in Staphylococcus aureus; Enany, S., Crotty Alexander, L.E., Eds.; InTech: Hong Kong, China, 2017; ISBN 978-953-51-2983-7. [Google Scholar]
- Vestergaard, M.; Frees, D.; Ingmer, H. Antibiotic Resistance and the MRSA Problem. Microbiol. Spectr. 2019, 7, 18. [Google Scholar] [CrossRef]
- Nandhini, P.; Kumar, P.; Mickymaray, S.; Alothaim, A.S.; Somasundaram, J.; Rajan, M. Recent Developments in Methicillin-Resistant Staphylococcus Aureus (MRSA) Treatment: A Review. Antibiotics 2022, 11, 606. [Google Scholar] [CrossRef]
- King, D.T.; Sobhanifar, S.; Strynadka, N.C.J. The Mechanisms of Resistance to β-Lactam Antibiotics. In Handbook of Antimicrobial Resistance; Berghuis, A., Matlashewski, G., Wainberg, M.A., Sheppard, D., Eds.; Springer: New York, NY, USA, 2017; pp. 177–201. ISBN 978-1-4939-0693-2. [Google Scholar]
- Pandey, N.; Cascella, M. Beta Lactam Antibiotics. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kırmusaoğlu, S.; Gareayaghi, N.; Kocazeybek, S.B. Introductory Chapter: The Action Mechanisms of Antibiotics and Antibiotic Resistance. In Antimicrobials, Antibiotic Resistance, Antibiofilm Strategies and Activity Methods; Kırmusaoğlu, S., Ed.; IntechOpen: Hong Kong, China, 2019; ISBN 978-1-78985-789-4. [Google Scholar]
- Cameron, D.R.; Jiang, J.-H.; Kostoulias, X.; Foxwell, D.J.; Peleg, A.Y. Vancomycin Susceptibility in Methicillin-Resistant Staphylococcus Aureus Is Mediated by YycHI Activation of the WalRK Essential Two-Component Regulatory System. Sci. Rep. 2016, 6, 30823. [Google Scholar] [CrossRef]
- Ahmed, M.O.; Baptiste, K.E. Vancomycin-Resistant Enterococci: A Review of Antimicrobial Resistance Mechanisms and Perspectives of Human and Animal Health. Microb. Drug Resist. 2018, 24, 590–606. [Google Scholar] [CrossRef]
- Stogios, P.J.; Savchenko, A. Molecular Mechanisms of Vancomycin Resistance. Protein Sci. Publ. Protein Soc. 2020, 29, 654–669. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.M.; Goodman, A.L.; Horner, C.; Jenkins, A.; Brown, E.M. Treatment of Methicillin-Resistant Staphylococcus Aureus (MRSA): Updated Guidelines from the UK. JAC-Antimicrob. Resist. 2021, 3, dlaa114. [Google Scholar] [CrossRef] [PubMed]
- Montravers, P.; Eckmann, C. Cotrimoxazole and Clindamycin in Skin and Soft Tissue Infections. Curr. Opin. Infect. Dis. 2021, 34, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Ciandrini, E.; Morroni, G.; Arzeni, D.; Kamysz, W.; Neubauer, D.; Kamysz, E.; Cirioni, O.; Brescini, L.; Baffone, W.; Campana, R. Antimicrobial Activity of Different Antimicrobial Peptides (AMPs) against Clinical Methicillin-Resistant Staphylococcus aureus (MRSA). Curr. Top. Med. Chem. 2018, 18, 2116–2126. [Google Scholar] [CrossRef]
- Ansari, M.A.; Alzohairy, M.A. One-Pot Facile Green Synthesis of Silver Nanoparticles Using Seed Extract of Phoenix Dactylifera and Their Bactericidal Potential against MRSA. Evid. Based Complement. Altern. Med. 2018, 2018, 1860280. [Google Scholar] [CrossRef]
- Baharin, N.H.Z.; Mokhtar, N.F.K.; Desa, M.N.M.; Gopalsamy, B.; Zaki, N.N.M.; Yuswan, M.H.; Muthanna, A.; Dzaraly, N.D.; Abbasiliasi, S.; Hashim, A.M.; et al. The Characteristics and Roles of Antimicrobial Peptides as Potential Treatment for Antibiotic-Resistant Pathogens: A Review. PeerJ 2021, 9, e12193. [Google Scholar] [CrossRef]
- Patrulea, V.; Borchard, G.; Jordan, O. An Update on Antimicrobial Peptides (AMPs) and Their Delivery Strategies for Wound Infections. Pharmaceutics 2020, 12, 840. [Google Scholar] [CrossRef]
- Benfield, A.H.; Henriques, S.T. Mode-of-Action of Antimicrobial Peptides: Membrane Disruption vs. Intracellular Mechanisms. Front. Med. Technol. 2020, 2, 610997. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial Peptides as Therapeutic Agents: Opportunities and Challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992. [Google Scholar] [CrossRef]
- Frimodt-Møller, J.; Campion, C.; Nielsen, P.E.; Løbner-Olesen, A. Translocation of Non-Lytic Antimicrobial Peptides and Bacteria Penetrating Peptides across the Inner Membrane of the Bacterial Envelope. Curr. Genet. 2022, 68, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Moravej, H.; Moravej, Z.; Yazdanparast, M.; Heiat, M.; Mirhosseini, A.; Moosazadeh Moghaddam, M.; Mirnejad, R. Antimicrobial Peptides: Features, Action, and Their Resistance Mechanisms in Bacteria. Microb. Drug Resist. 2018, 24, 747–767. [Google Scholar] [CrossRef] [PubMed]
- Seyfi, R.; Kahaki, F.A.; Ebrahimi, T.; Montazersaheb, S.; Eyvazi, S.; Babaeipour, V.; Tarhriz, V. Antimicrobial Peptides (AMPs): Roles, Functions and Mechanism of Action. Int. J. Pept. Res. Ther. 2020, 26, 1451–1463. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial Peptides: Mechanism of Action, Activity and Clinical Potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Rosman, N.S.R.; Masimen, M.A.A.; Harun, N.A.; Idris, I.; Ismail, W.I.W. Biogenic Silver Nanoparticles (AgNPs) from Marphysa Moribidii Extract: Optimization of Synthesis Parameters. Int. J. Technol. 2021, 12, 635. [Google Scholar] [CrossRef]
- Huq, M.A.; Ashrafudoulla, M.; Rahman, M.M.; Balusamy, S.R.; Akter, S. Green Synthesis and Potential Antibacterial Applications of Bioactive Silver Nanoparticles: A Review. Polymers 2022, 14, 742. [Google Scholar] [CrossRef]
- Rosman, N.S.R.; Harun, N.A.; Idris, I.; Ismail, W.I.W. Eco-Friendly Silver Nanoparticles (AgNPs) Fabricated by Green Synthesis Using the Crude Extract of Marine Polychaete, Marphysa Moribidii: Biosynthesis, Characterisation, and Antibacterial Applications. Heliyon 2020, 6, e05462. [Google Scholar] [CrossRef]
- Lee, S.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yang, Y.; Duan, W.; Qu, X.; Wu, J. Synergistic and On-Demand Release of Ag-AMPs Loaded on Porous Silicon Nanocarriers for Antibacteria and Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 16127–16141. [Google Scholar] [CrossRef] [PubMed]
- Salouti, M.; Mirzaei, F.; Shapouri, R.; Ahangari, A. Synergistic Antibacterial Activity of Plant Peptide MBP-1 and Silver Nanoparticles Combination on Healing of Infected Wound Due to Staphylococcus Aureus. Jundishapur J. Microbiol. 2016, 9, e27997. [Google Scholar] [CrossRef] [PubMed]
- Enright, M.C.; Robinson, D.A.; Randle, G.; Feil, E.J.; Grundmann, H.; Spratt, B.G. The Evolutionary History of Methicillin-Resistant Staphylococcus Aureus (MRSA). Proc. Natl. Acad. Sci. USA 2002, 99, 7687–7692. [Google Scholar] [CrossRef]
- Peacock, S.J.; Paterson, G.K. Mechanisms of Methicillin Resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organisation: Geneva, Switzerland, 2017. [Google Scholar]
- Centers for Disease Control and Prevention (U.S.). Antibiotic Resistance Threats in the United States, 2019; Centers for Disease Control and Prevention (U.S.): Atlanta, GS, USA, 2019.
- Hibbitts, A.; O’Leary, C. Emerging Nanomedicine Therapies to Counter the Rise of Methicillin-Resistant Staphylococcus aureus. Materials 2018, 11, 321. [Google Scholar] [CrossRef]
- Siddiqui, A.H.; Koirala, J. Methicillin Resistant Staphylococcus aureus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and Resistance Mechanisms of Antibiotics: A Guide for Clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The Antimicrobial Peptides and Their Potential Clinical Applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Kang, J.; Dietz, M.J.; Li, B. Antimicrobial Peptide LL-37 Is Bactericidal against Staphylococcus Aureus Biofilms. PLoS ONE 2019, 14, e0216676. [Google Scholar] [CrossRef]
- Garbacz, K.; Kamysz, W.; Piechowicz, L. Activity of Antimicrobial Peptides, Alone or Combined with Conventional Antibiotics, against Staphylococcus aureus Isolated from the Airways of Cystic Fibrosis Patients. Virulence 2017, 8, 94–100. [Google Scholar] [CrossRef]
- Rodrigues de Almeida, N.; Catazaro, J.; Krishnaiah, M.; Singh Chhonker, Y.; Murry, D.J.; Powers, R.; Conda-Sheridan, M. Understanding Interactions of Citropin 1.1 Analogues with Model Membranes and Their Influence on Biological Activity. Peptides 2019, 119, 170119. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, O.; Cirioni, O.; Goteri, G.; Lucarini, G.; Kamysz, E.; Kamysz, W.; Orlando, F.; Rizzetto, G.; Molinelli, E.; Morroni, G.; et al. Efficacy of Cathelicidin LL-37 in an MRSA Wound Infection Mouse Model. Antibiotics 2021, 10, 1210. [Google Scholar] [CrossRef] [PubMed]
- Demirci, M.; Yigin, A.; Demir, C. Efficacy of Antimicrobial Peptide LL-37 against Biofilm Forming Staphylococcus Aureus Strains Obtained from Chronic Wound Infections. Microb. Pathog. 2022, 162, 105368. [Google Scholar] [CrossRef] [PubMed]
- Bedlovičová, Z.; Salayová, A. Green-Synthesized Silver Nanoparticles and Their Potential for Antibacterial Applications. In Bacterial Pathogenesis and Antibacterial Control; Kırmusaoğlu, S., Ed.; InTech: Hong Kong, China, 2018; ISBN 978-1-78923-160-1. [Google Scholar]
- Vigneswari, S.; Amelia, T.S.M.; Hazwan, M.H.; Mouriya, G.K.; Bhubalan, K.; Amirul, A.-A.A.; Ramakrishna, S. Transformation of Biowaste for Medical Applications: Incorporation of Biologically Derived Silver Nanoparticles as Antimicrobial Coating. Antibiotics 2021, 10, 229. [Google Scholar] [CrossRef]
- de Alwis Weerasekera, H.; Griffith, M.; Alarcon, E.I. Biomedical Uses of Silver Nanoparticles: From Roman Wine Cups to Biomedical Devices. In Silver Nanoparticle Applications; Engineering Materials; Springer: Cham, Switzerland, 2015; pp. 93–125. ISBN 978-3-319-11261-9. [Google Scholar]
- Prabhu, S.; Poulose, E.K. Silver Nanoparticles: Mechanism of Antimicrobial Action, Synthesis, Medical Applications, and Toxicity Effects. Int. Nano Lett. 2012, 2, 32–42. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver Nanoparticles: A New View on Mechanistic Aspects on Antimicrobial Activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial Mechanism of Silver Nanoparticles in Pseudomonas aeruginosa: Proteomics Approach. Metallomics 2018, 10, 557–564. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of Enhanced Antibacterial Effects of Novel Silver Nanoparticles. Nanotechnology 2007, 18, 225103–225112. [Google Scholar] [CrossRef]
- López-Heras, M.; Theodorou, I.G.; Leo, B.F.; Ryan, M.P.; Porter, A.E. Towards Understanding the Antibacterial Activity of Ag Nanoparticles: Electron Microscopy in the Analysis of the Materials-Biology Interface in the Lung. Environ. Sci. Nano 2015, 2, 312–326. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The Effect of Charge at the Surface of Silver Nanoparticles on Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria: A Preliminary Study. J. Nanomater. 2015, 2015, 720654. [Google Scholar] [CrossRef]
- Auer, G.K.; Weibel, D.B. Bacterial Cell Mechanics. Biochemistry 2017, 56, 3710–3724. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver Nanoparticles as Antimicrobial Agent: A Case Study on E. coli as a Model for Gram-Negative Bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Salvioni, L.; Galbiati, E.; Collico, V.; Alessio, G.; Avvakumova, S.; Corsi, F.; Tortora, P.; Prosperi, D.; Colombo, M. Negatively Charged Silver Nanoparticles with Potent Antibacterial Activity and Reduced Toxicity for Pharmaceutical Preparations. Int. J. Nanomed. 2017, 12, 2517–2530. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Sato, M.; Sato, Y.; Ando, N.; Takayama, T.; Fujita, M.; Ishihara, M. Synthesis and Application of Silver Nanoparticles (AgNPs) for the Prevention of Infection in Healthcare Workers. Int. J. Mol. Sci. 2019, 20, 3620. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential Antibacterial Mechanism of Silver Nanoparticles and the Optimization of Orthopedic Implants by Advanced Modification Technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver Nanoparticles: The Powerful Nanoweapon against Multidrug-Resistant Bacteria: Activity of Silver Nanoparticles against MDR Bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Kumar, N.; Das, S.; Jyoti, A.; Kaushik, S. Synergistic Effect of Silver Nanoparticles with Doxycycline against Klebsiella pneumonia. Int. J. Pharm. Pharm. Sci. 2016, 8, 183–186. [Google Scholar]
- Liao, C.; Li, Y.; Tjong, S. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Xu, H.; Qu, F.; Xu, H.; Lai, W.; Andrew Wang, Y.; Aguilar, Z.P.; Wei, H. Role of Reactive Oxygen Species in the Antibacterial Mechanism of Silver Nanoparticles on Escherichia coli O157:H7. Biomet. Int. J. Role Met. Ions Biol. Biochem. Med. 2012, 25, 45–53. [Google Scholar] [CrossRef]
- Salah, R.; Karmy, M.; Abdelraouf, A.; Kotb, S. Evaluation of the Bactericidal Effect of Silver Nanoparticles against Methicillin Resistant Staphylococcus Aureus (MRSA) and Methicillin Sensitive Staphylococcus Aureus (MSSA) Strains Isolated from Mastitic Milk of Small Ruminants and Their Surrounding Environment in Aswan. J. Vet. Med. Res. 2021, 27, 143–151. [Google Scholar] [CrossRef]
- Ansari, M.; Khan, H.; Khan, A.; Cameotra, S.; Alzohairy, M. Anti-Biofilm Efficacy of Silver Nanoparticles against MRSA and MRSE Isolated from Wounds in a Tertiary Care Hospital. Indian J. Med. Microbiol. 2015, 33, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Sedrizadeh-Bami, S.; Kariminik, A.; Ranjbar, M. Synthesis and Characterization of Silver Nanoparticles with Ultrasound-Assisted Reverse Micelles Method and Their Antibacterial Effects on Methicillin-Resistant Staphylococcus Aureus Isolates. Avicenna J. Clin. Microbiol. Infect. 2020, 7, 99–103. [Google Scholar] [CrossRef]
- Paredes, D.; Ortiz, C.; Torres, R. Synthesis, Characterization, and Evaluation of Antibacterial Effect of Ag Nanoparticles against Escherichia coli O157:H7 and Methicillin-Resistant Staphylococcus aureus (MRSA). Int. J. Nanomed. 2014, 9, 1717–1729. [Google Scholar] [CrossRef][Green Version]
- Wady, A.F.; Machado, A.L.; Foggi, C.C.; Zamperini, C.A.; Zucolotto, V.; Moffa, E.B.; Vergani, C.E. Effect of a Silver Nanoparticles Solution on Staphylococcus aureus and Candida spp. J. Nanomater. 2014, 2014, 545279. [Google Scholar] [CrossRef]
- Das, B.; Dash, S.K.; Mandal, D.; Ghosh, T.; Chattopadhyay, S.; Tripathy, S.; Das, S.; Dey, S.K.; Das, D.; Roy, S. Green Synthesized Silver Nanoparticles Destroy Multidrug Resistant Bacteria via Reactive Oxygen Species Mediated Membrane Damage. Arab. J. Chem. 2017, 10, 862–876. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Khalil, M.I.; Al-Zaban, M.I. Novel Biogenic Silver Nanoparticle-Induced Reactive Oxygen Species Inhibit the Biofilm Formation and Virulence Activities of Methicillin-Resistant Staphylococcus Aureus (MRSA) Strain. Front. Bioeng. Biotechnol. 2020, 8, 433. [Google Scholar] [CrossRef]
- Zhen, J.-B.; Kang, P.-W.; Zhao, M.-H.; Yang, K.-W. Silver Nanoparticle Conjugated Star PCL- b -AMPs Copolymer as Nanocomposite Exhibits Efficient Antibacterial Properties. Bioconjug. Chem. 2020, 31, 51–63. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Sun, P.; Zhang, N.; Zhao, Y.; Qin, S.; Zhao, Y. Antimicrobial Peptide-Modified Silver Nanoparticles for Enhancing the Antibacterial Efficacy. RSC Adv. 2020, 10, 38746–38754. [Google Scholar] [CrossRef]
- Zharkova, M.S.; Golubeva, O.Y.; Orlov, D.S.; Vladimirova, E.V.; Dmitriev, A.V.; Tossi, A.; Shamova, O.V. Silver Nanoparticles Functionalized with Antimicrobial Polypeptides: Benefits and Possible Pitfalls of a Novel Anti-Infective Tool. Front. Microbiol. 2021, 12, 750556. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Wang, H.; Zhu, M.; Feng, W.; Liang, G. Enhanced Antibacterial and Anti-Biofilm Activities of Antimicrobial Peptides Modified Silver Nanoparticles. Int. J. Nanomed. 2021, 16, 4831–4846. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Duan, W.; Wo, F.; Wu, J. Two-Dimensional Fluorescent Strategy Based on Porous Silicon Quantum Dots for Metal-Ion Detection and Recognition. ACS Appl. Nano Mater. 2019, 2, 6110–6115. [Google Scholar] [CrossRef]
- Chen, X.; Wo, F.; Jin, Y.; Tan, J.; Lai, Y.; Wu, J. Drug-Porous Silicon Dual Luminescent System for Monitoring and Inhibition of Wound Infection. ACS Nano 2017, 11, 7938–7949. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tang, H.; Bian, X.; Ma, K.; Chang, J.; Fu, X.; Zhang, C. Calcium Silicate Accelerates Cutaneous Wound Healing with Enhanced Re-Epithelialization through EGF/EGFR/ERK-Mediated Promotion of Epidermal Stem Cell Functions. Burns Trauma 2021, 9, tkab029. [Google Scholar] [CrossRef] [PubMed]
- Awad, K.; Ahuja, N.; Fiedler, M.; Peper, S.; Wang, Z.; Aswath, P.; Brotto, M.; Varanasi, V. Ionic Silicon Protects Oxidative Damage and Promotes Skeletal Muscle Cell Regeneration. Int. J. Mol. Sci. 2021, 22, 497. [Google Scholar] [CrossRef]
- Bassous, N.J.; Webster, T.J. The Binary Effect on Methicillin-Resistant Staphylococcus aureus of Polymeric Nanovesicles Appended by Proline-Rich Amino Acid Sequences and Inorganic Nanoparticles. Small 2019, 15, 1804247. [Google Scholar] [CrossRef]
- Gao, J.; Na, H.; Zhong, R.; Yuan, M.; Guo, J.; Zhao, L.; Wang, Y.; Wang, L.; Zhang, F. One Step Synthesis of Antimicrobial Peptide Protected Silver Nanoparticles: The Core-Shell Mutual Enhancement of Antibacterial Activity. Colloids Surf. B Biointerfaces 2020, 186, 110704. [Google Scholar] [CrossRef]
- Zhao, X.; Kuipers, O.P. Synthesis of Silver-Nisin Nanoparticles with Low Cytotoxicity as Antimicrobials against Biofilm-Forming Pathogens. Colloids Surf. B Biointerfaces 2021, 206, 111965. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Lim, T.-P.; Leong, D.T.; Xie, J. Antimicrobial Cluster Bombs: Silver Nanoclusters Packed with Daptomycin. ACS Nano 2016, 10, 7934–7942. [Google Scholar] [CrossRef]
- Ye, Z.; Sang, T.; Li, K.; Fischer, N.G.; Mutreja, I.; Echeverría, C.; Kumar, D.; Tang, Z.; Aparicio, C. Hybrid Nanocoatings of Self-Assembled Organic-Inorganic Amphiphiles for Prevention of Implant Infections. Acta Biomater. 2022, 140, 338–349. [Google Scholar] [CrossRef]
- Golubeva, O.Y.; Shamova, O.V.; Orlov, D.S.; Pazina, T.Y.; Boldina, A.S.; Drozdova, I.A.; Kokryakov, V.N. Synthesis and Study of Antimicrobial Activity of Bioconjugates of Silver Nanoparticles and Endogenous Antibiotics. Glass Phys. Chem. 2011, 37, 78–84. [Google Scholar] [CrossRef]
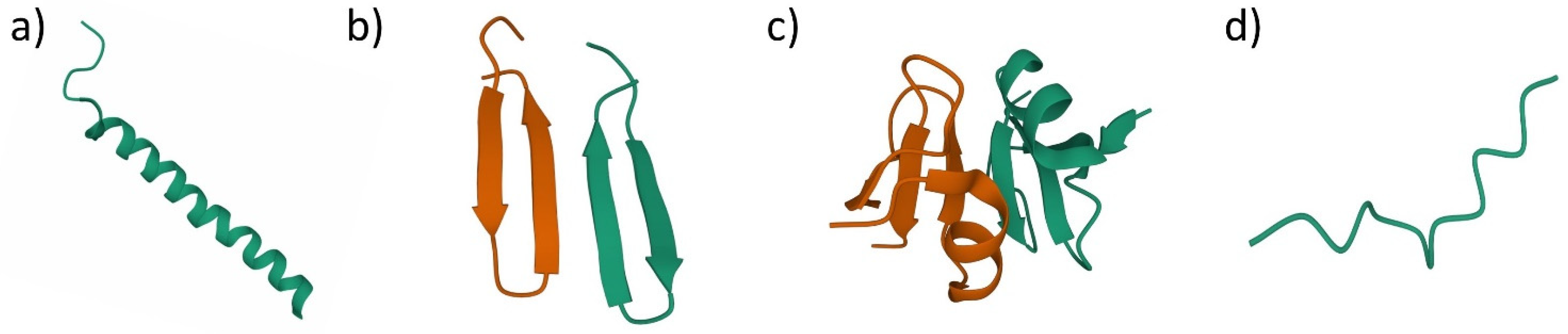


| AMPs Type(s) | AMPs Origin/Type | Amino Acid Sequence | Antibacterial Activity on Staphylococcus aureus | Ref. |
|---|---|---|---|---|
| Temporin A | Isolated from frog skin secretion, Rana temporaria | FLPLIGRVLSGIL-NH2 | Effective toward methicillin-susceptible S. aureus (MSSA) and MRSA. Exhibit MIC value of 4 µg/mL once tested on surgical wound isolated MRSA. | [18] |
| Exhibit MIC values of 16–64 µg/mL once tested on 215 isolates of MSSA and MRSA | [47] | |||
| Cecropin A-melittin hybrid peptide [CA(1–7)M(2–9)NH2] | Hybrid peptide derived from cecropin A and melittin partial sequence | KWKLFKKIGAVLKVL-NH2 | Effective towards MRSA. Exhibit MIC value of 8 µg/mL once tested on skin lesion isolated MRSA. | [18] |
| Exhibit MIC values of 4 mg/mL to 32 mg/mL once tested on 215 isolates of MSSA and MRSA | [47] | |||
| Citropin 1.1 | Isolated from frog’s dorsal and submental glands Litoria citropa | GLFDVIKKVASVIGGL-NH (2) | Exhibit MIC value of 16–64 mg/mL once tested on 215 isolates of MSSA and MRSA | [47] |
| Effective towards MRSA. Exhibit MIC value of 16 µg/mL once tested on wound, deep wound and skin lesion isolated MRSA. | [18] | |||
| Exhibit MIC value of 32 µg/mL once tested on MRSA strain JE2 | [48] | |||
| Cathelicidin LL-37 | Human derived cathelicidin AMPs | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | Effectively reduces infection once tested on MRSA infected wound on mice in comparison to the antibiotic groups (teicoplanin). | [49] |
| Exhibit MIC values on biofilm forming MSSA and MRSA (isolated from chronic wound) 89.6 mg/L and 132.3 mg/L, respectively. Inhibit the growth by affecting quorum sensing and biofilm gene expression. | [50] |
| Silver Nanoparticles’ Properties | Antibacterial Action | Ref. |
|---|---|---|
| Spherical shape with the size ranges from 8.55 to 20.3 nm | Exhibit MIC value 8.125 µg/mL on MRSA. It said the AgNPs inhibit MRSA by adhering and penetrating the cell by interacting with vital cellular compounds. | [70] |
| Spherical AgNPs with the size range from 5–10 nm | Exhibit MIC value ranging from 11.25 µg/mL to 45 µg/mL on MRSA. AgNPs disrupt the biofilm formed by MRSA once visualised using a scanning electron microscope. | [71] |
| Spherical AgNPs with the size 150 nm that are determined by dynamic light scattering | Showed inhibition on disk diffusion assay and exhibited MIC value at 0.015 mg/mL on all tested MRSA strains. | [72] |
| Spherical AgNPs with the size range <100 nm (Three different AgNP sizes used in the experiment. AgNPs 1:36 nm, AgNPs 2:113 nm and AgNPs 3:78 nm) | Smaller AgNPs (AgNPs 1:36 nm) showed higher MRSA inhibition due to higher AgNP contact rate with bacteria based on a disk diffusion assay. MIC value of MRSA upon interaction with AgNPs is 0.50 μg/mL. | [73] |
| Spherical AgNPs with diameter of 9 nm | Exhibit MIC value of 1.95 µg/mL on MRSA (ATCC 33591) | [74] |
| Spherical AgNPs with size range of 16–18 nm | Inhibit MRSA growth at MIC value of 8 μg/mL and AgNPs cause the accumulation of ROS, which led to irreversible oxidative damage on MRSA. | [75] |
| Spherical AgNPs with size range of 4.5 to 26 nm | Disk diffusion assay showed an inhibition zone of 23.7 ± 0.08 mm in comparison to ampicillin treatment (26.7 ± 0.33 mm). AgNPs also exhibits MIC value of 1.2 mg/mL. ROS accumulation contributed to MRSA membrane disruption and led to cell death. | [76] |
| AMP Type | Product Combination | Antibacterial Properties | Ref. |
|---|---|---|---|
| Nisin (antibacterial peptide produced by the Lactococcus lactis, which is commonly used as food preservative) | Silver-nisin nanoparticles (Ag-nisin NP) | Exhibit MIC value of 4 mg/L on MRSA in comparison to silver nitrate (16 mg/L) and nisin (4 mg/L) alone. Inhibit MRSA growth by destroying the biofilm. Ag-nisin NP showed lower cytotoxicity on human skin fibroblasts (Hs 44.Fs, ATCC® CRL7024™) and human kidney epithelium cell line (HEK) compared to silver nitrate. | [87] |
| Daptomycin (clinically approved AMPs for medical usage) | Daptomycin-silver nanoclusters (D−AgNCs) | Complex exhibits the highest inhibitory effect against S. aureus in comparison to the controls (daptomycin or AgNCs alone). Inhibit growth by inducing DNA damage and ROS generation. | [88] |
| GL13K (amphiphilic AMPs that was developed from BPIFA2 (human salivary protein) | AgNP-dGL13K complexes (AMPs and AgNPs coated with etched Titanium (eTi) for stable nanostructure) | Exhibit excellent antibacterial properties on MRSA through in vitro and in vivo rat models. | [89] |
| G-Bac3.4 (amino acid sequence: CRFRLPFRRPPIRIHPP PFYPPFRPFL–NH2) | Bioconjugate G-Bac3.4 with silver nanoparticles | These bioconjugate AMPs and AgNPs exhibit antimicrobial action by internalising into MRSA and inhibiting the growth. | [90] |
| MBP-1 (plant antimicrobial peptide) | MBP-1 and silver nanoparticles combination | The MIC of MBP-1 is 0.6 mg/mL while MIC for silver nanoparticles were 6.25 and 12.5 mg/L. MIC of silver nanoparticles and MBP-1 combination was found to be 3.125 mg/mL and 6.25 mg/mL, respectively, on S. aureus. Faster wound healing can be observed on rats infected with S. aureus. | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masimen, M.A.A.; Harun, N.A.; Maulidiani, M.; Ismail, W.I.W. Overcoming Methicillin-Resistance Staphylococcus aureus (MRSA) Using Antimicrobial Peptides-Silver Nanoparticles. Antibiotics 2022, 11, 951. https://doi.org/10.3390/antibiotics11070951
Masimen MAA, Harun NA, Maulidiani M, Ismail WIW. Overcoming Methicillin-Resistance Staphylococcus aureus (MRSA) Using Antimicrobial Peptides-Silver Nanoparticles. Antibiotics. 2022; 11(7):951. https://doi.org/10.3390/antibiotics11070951
Chicago/Turabian StyleMasimen, Mohammad Asyraf Adhwa, Noor Aniza Harun, M. Maulidiani, and Wan Iryani Wan Ismail. 2022. "Overcoming Methicillin-Resistance Staphylococcus aureus (MRSA) Using Antimicrobial Peptides-Silver Nanoparticles" Antibiotics 11, no. 7: 951. https://doi.org/10.3390/antibiotics11070951
APA StyleMasimen, M. A. A., Harun, N. A., Maulidiani, M., & Ismail, W. I. W. (2022). Overcoming Methicillin-Resistance Staphylococcus aureus (MRSA) Using Antimicrobial Peptides-Silver Nanoparticles. Antibiotics, 11(7), 951. https://doi.org/10.3390/antibiotics11070951







