Epidemiology of Candidemia and Fluconazole Resistance in an ICU before and during the COVID-19 Pandemic Era
Abstract
:1. Introduction
2. Methods
2.1. Study Setting and Design
2.2. Definitions
2.3. Species Identification and Antifungal Susceptibility Testing
2.4. Statistical Analysis
3. Results
3.1. COVID-19 Candidemia Cohort
3.2. Comparison between COVID-19 Candidemia Cohort and the Pre-COVID-19 Cohorts
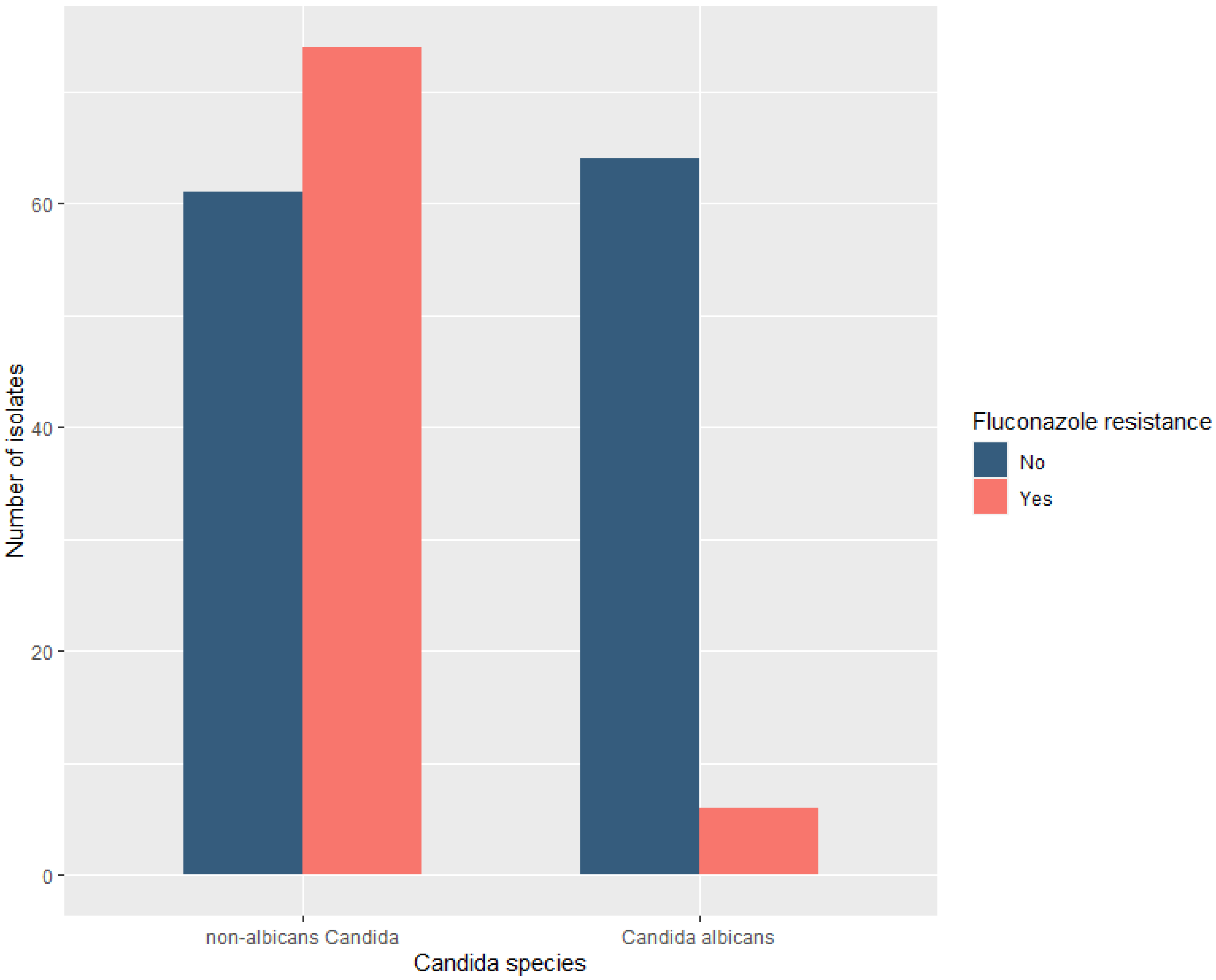
3.3. Candida Species Distribution and Fluconazole Resistance
3.4. Factors Associated with Non-Albicans Candidemia
3.5. Factors Associated with Fluconazole Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report–51. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10 (accessed on 30 January 2022).
- World Health Organization. Coronavirus Disease (COVID-19) Pandemic. 2021. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019?gclid=Cj0KCQiAhs79BRD0ARIsAC6XpaXhxHeN64r7-j5rvv0ZDtNGxNkA0e2EWCAUr8QWWj-qi_PPrXOljroaAjXBEALw_wcB (accessed on 30 January 2022).
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Battaglini, D.; Ball, L.; Brunetti, I.; Bruzzone, B.; Codda, G.; Crea, F.; De Maria, A.; Dentone, C.; Di Biagio, A.; et al. Bloodstream infections in critically ill patients with COVID-19. Eur. J. Clin. Investig. 2020, 50, e13319. [Google Scholar] [CrossRef] [PubMed]
- Shukla, B.S.; Warde, P.R.; Knott, E.; Arenas, S.; Pronty, D.; Ramirez, D.; Rego, A.; Levy, M.; Zak, M.; Parekh, D.J.; et al. Bloodstream infection risk, incidence, and deaths for hospitalized patients during coronavirus disease pandemic. Emerg. Infect. Dis. 2021, 27, 2588–2594. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.R.; Weiner-Lastinger, L.M.; Dudeck, M.A.; Fike, L.V.; Kuhar, D.T.; Edwards, J.R.; Pollock, D.; Benin, A. Impact of COVID-19 pandemic on central-line-associated bloodstream infections during the early months of 2020, National Healthcare Safety Network. Infect. Control. Hosp. Epidemiol. 2021, 15, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kokkoris, S.; Papachatzakis, I.; Gavrielatou, E.; Ntaidou, T.; Ischaki, E.; Malachias, S.; Vrettou, C.; Nichlos, C.; Kanavou, A.; Zervakis, D.; et al. ICU-acquired bloodstream infections in critically ill patients with COVID-19. J. Hosp. Infect. 2021, 107, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Cona, A.; Tavelli, A.; Renzelli, A.; Varisco, B.; Bai, F.; Tesoro, D.; Za, A.; Biassoni, C.; Battaglioli, L.; Allegrini, M.; et al. Incidence, risk factors and impact on clinical outcomes of bloodstream infections in patients hospitalised with COVID-19: A prospective cohort study. Antibiotics 2021, 10, 1031. [Google Scholar] [CrossRef]
- Segala, F.V.; Bavaro, D.F.; Di Gennaro, F.; Salvati, F.; Marotta, C.; Saracino, A.; Murri, R.; Fantoni, M. Impact of SARS-CoV-2 Epidemic on Antimicrobial Resistance: A Literature Review. Viruses 2021, 13, 2110. [Google Scholar] [CrossRef]
- Mastrangelo, A.; Germinario, N.; Ferrante, M.; Frangi, C.; Voti, R.L.; Muccini, C.; Ripa1, M.; On behalf of COVID-BioB Study Group. Candidemia in COVID-19 patients: Incidence and characteristics in a prospective cohort compared to historical non-COVID-19 controls. Clin. Infect. Dis. 2021, 73, e2838–e2839. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- White, P.L.; Dhillon, R.; Cordey, A. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin. Infect. Dis. 2021, 73, e1634–e1644. [Google Scholar] [CrossRef] [PubMed]
- Seagle, E.E.; Jackson, B.R.; Lockhart, S.R.; Georgacopoulos, O.; Nunnally, N.S.; Roland, J.; Barter, D.M.; Johnston, H.L.; Czaja, C.A.; Kayalioglu, H.; et al. The landscape of candidemia during the COVID-19 pandemic. Clin. Infect. Dis. 2022, 74, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Bishburg, E.; Okoh, A.; Nagarakanti, S.R.; Lindner, M.; Migliore, C.; Patel, P. Fungemia in COVID-19 ICU patients, a single medical center experience. J. Med. Virol. 2021, 93, 2810–2814. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Barreiros, G.; Guimaraes, L.F.; Deriquehem, V.A.S.; Castineiras, A.C.; Nouer, S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses 2021, 64, 152–156. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Lortholary, O.; Renaudat, C.; Sitbon, K.; Madec, Y.; Denoeud-Ndam, L.; Wolff, M.; Fontanet, A.; Bretagne, S.; Dromer, F.; Dromer, F. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010). Intensive Care Med. 2014, 40, 1303–1312. [Google Scholar] [CrossRef]
- Goemaere, B.; Becker, P.; Van Wijngaerden, E.; Maertens, J.; Spriet, I.; Hendrickx, M.; Lagrou, K. Increasing candidaemia incidence from 2004 to 2015 with a shift in epidemiology in patients preexposed to antifungals. Mycoses 2018, 61, 127–133. [Google Scholar] [CrossRef]
- Siopi, M.; Tarpatzi, A.; Kalogeropoulou, E.; Damianidou, S.; Vasilakopoulou, A.; Vourli, S.; Pournaras, S.; Meletiadis, J. Epidemiological trends of fungemia in Greece with a focus on candidemia during the recent financial crisis: A 10-year survey in a tertiary care academic hospital and review of literature. Antimicrob. Agents Chemother. 2020, 64, e01516–e01519. [Google Scholar] [CrossRef]
- Guinea, J. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014, 20, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Knaus, W.; Draper, E.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Vincent, J.L.; de Mendonça, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.; Sprung, C.; Colardyn, F.; Serge, B. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Alhazzani, W.; Evans, L.; Alshamsi, F.; Møller, M.H.; Ostermann, M.; Prescott, H.C.; Arabi, Y.M.; Loeb, M.; Ng Gong, M.; Fan, E.; et al. Surviving Sepsis Campaign Guidelines on the Management of Adults With Coronavirus Disease 2019 (COVID-19) in the ICU: First Update. Crit. Care Med. 2021, 49, e219–e234. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Antonelli, M.; Cuenca-Estrella, M.; Dimopoulos, G.; Einav, S.; De Waele, J.; Garnacho-Montero, J.; Kanj, S.S.; Machado, F.R.; Montravers, P.; et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019, 45, 789–805. [Google Scholar] [CrossRef] [PubMed]
- Macauley, P.; Epelbaum, O. Epidemiology and mycology of Candidaemia in non-oncological medical intensive care unit patients in a tertiary center in the United States: Overall analysis and comparison between non-COVID-19 and COVID-19 cases. Mycoses 2021, 64, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Riche, C.V.W.; Cassol, R.; Pasqualotto, A.C. Is the frequency of candidemia increasing in COVID-19 patients receiving corticosteroids? J. Fungi 2020, 6, 286. [Google Scholar] [CrossRef]
- Mulet Bayona, J.V.; Tormo Palop, N.; Salvador García, C.; Fuster Escrivá, B.; Chanzá Aviñó, M.; Ortega García, P.; Gimeno Cardona, C. Impact of the SARS-CoV-2 pandemic in candidaemia, invasive aspergillosis and antifungal consumption in a tertiary hospital. J. Fungi 2021, 7, 440. [Google Scholar] [CrossRef]
- Rajni, E.; Singh, A.; Tarai, B.; Jain, K.; Shankar, R.; Pawar, K.; Mamoria, V.; Chowdhary, A. A high frequency of Candida auris blood stream infections in Coronavirus disease 2019 patients admitted to intensive care units, Northwestern India: A Case Control Study. Open Forum Infect. Dis. 2021, 8, ofab452. [Google Scholar] [CrossRef]
- Meyer, E.; Geffers, C.; Gastmeier, P.; Schwab, F. No increase in primary nosocomial candidemia in 682 German intensive care units during 2006 to 2011. Eurosurveillance 2013, 18, 20505. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20505 (accessed on 30 January 2022). [CrossRef]
- The Recovery Collaborative Group. Dexamethazone in hospitalized patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Pratikaki, M.; Platsouka, E.; Sotiropoulou, C.; Douka, E.; Paramythiotou, E.; Kaltsas, P.; Kotanidou, A.; Paniara, O.; Roussos, C.; Routsi, C. Epidemiology, risk factors for and outcome of candidaemia among non-neutropenic patients in a Greek intensive care unit. Mycoses 2011, 54, 154–161. [Google Scholar] [CrossRef]
- Mamali, V.; Siopi, M.; Charpantidis, S.; Samonis, G.; Tsakris, A.; Vrioni, G. On Behalf of the Candi-Candi network.increasing incidence and shifting epidemiology of candidemia in Greece: Results from the first nationwide 10-year survey. J. Fungi 2022, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Azzam Lopez, A.; Diez-Remesal, Y.; Castro, N.M.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Agrifoglio, A.; Cachafeiro, L.; Figueira, J.C.; Añón, J.M.; de Lorenzo, A.G. Critically ill patients with COVID-19 and candidaemia: We must keep this in mind. J. Mycol. Med. 2020, 30, 10101. [Google Scholar] [CrossRef]
- Berrouane, Y.F.; Herwaldt, L.A.; Pfaller, M.A. Trends in antifungal use and epidemiology of nosocomial yeast infections in a university hospital. J. Clin. Microbiol. 1999, 37, 531–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirayama, T.; Miyazaki, T.; Ito, Y.; Wakayama, M.; Shibuya, K.; Yamashita, K.; Takazono, T.; Saijo, T.; Shimamura, S.; Yamamoto, K.; et al. Virulence assessment of six major pathogenic Candida species in the mouse model of invasive candidiasis caused by fungal translocation. Sci. Rep. 2020, 10, 3814. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variables | Pre-COVID-19 Cohorts | COVID-19 Cohort | p-Value | |
|---|---|---|---|---|
| 2005–2008 n = 66 | 2012–2015 n = 77 | 2019–2021 n = 62 | ||
| Age, median (IQR) | 67 (21) | 63 (31) | 69 (15.8) | 0.001 |
| males, n (%) | 45 (68.1) | 46 (59.7) | 45 (72.4) | 0.27 |
| APACHE II score on ICU admission, median (IQR) | 19 (8.8) | 20 (10) | 15 (7) | <0.001 |
| SOFA score on ICU admission, median (IQR) | 9 (4) | 10 (5) | 8 (3) | 0.001 |
| SOFA score on candidemia day, median (IQR) | 8.5 (6) | 7 (5) | 11 (6) | 0.001 |
| Delta SOFA score, median (IQR) | 0 (4) | −1 (3.5) | 3 (6) | <0.001 |
| ICU admission diagnosis Medical, n (%) Surgical, n (%) | 22 (33) 44 (66.7) | 40 (53.3) 35 (46.7) | 62 (100) 0 (0) | <0.001 |
| Co-morbidities Diabetes mellitus, n (%) Current malignancy, n (%) | 5 (7.6) 6 (9.1) | 3 (3.9) 5 (6.5) | 16 (25.8) 5 (8.1) | <0.001 0.99 |
| Hospital stay before candidemia onset, days, median (IQR) | 24 (18) | 30 (41.8) | 28.5 (19.5) | 0.15 |
| ICU stay before candidemia onset, days, median (IQR) | 15.5 (19.8) | 25 (34.5) | 22 (18.2) | 0.69 |
| ICU length of stay, days, median (IQR) | 35.5 (34.5) | 49 (51) | 34.5 (39.8) | 0.029 |
| Presence of shock on candidemia day, n (%) | 34 (52.3) | 30 (46.1) | 45 (75) | 0.001 |
| Incidence of candidemia, (%) | 3.8 | 4.2 | 10.3 | <0.001 |
| Mortality, n (%) | 42 (63.6) | 35 (46.7) | 35 (56.5) | 0.92 |
| Pre-COVID-19 Cohorts | COVID-19 Cohort | p | ||
|---|---|---|---|---|
| 2005–2008 n = 66 | 2012–2015 n = 77 | 2020–2021 n = 62 | ||
| Candidaspecies | ||||
| C. albicans, n (%) | 26 (39.4) | 29 (37.7) | 15 (24.2) | 0.069 |
| non-albicans Candida, n (%) | 40 (60.6) | 48 (62.3) | 47 (75.8) | 0.069 |
| C. parapsilosis | 28 (70) | 36 (75) | 31 (66) | 0.16 |
| C. glabrata | 8 (20) | 6 (12.5) | 5 (10.6) | 1 |
| C. tropicalis | 2 (5) | 3 (6.3) | 1 (2) | 0.77 |
| C. krusei | 0 (0) | 1 (2) | 0 (0) | 1 |
| C. auris | 0 (0) | 0 (0) | 9 (19) | <0.001 |
| other Candida species | 2 (5) | 2 | 1 | |
| Fluconazole resistance | ||||
| Fluconazole-resistant, n (%) | 21 (31.8) | 29 (37.7) | 30/62 (48.4) | 0.098 |
| C. albicans | 4/26 (15.4) | 1/29 (3.4) | 2/15 (13.3) | |
| C. parapsilosis | 10/28 (35.7) | 20/36 (55.6) | 17/31 (48.6) | |
| C. glabrata | 7/8 (87.5) | 2/6 (33.3) | 2/5 (40) | |
| C. tropicalis | 0/2 (0) | 0/3 (0) | 0/1 (0) | |
| C. krusei | NA | 1/1(100) | NA | |
| C. auris | NA | NA | 9/9 (100) | |
| Patients with Candidemia, n = 205 | ||||
|---|---|---|---|---|
| Candida albicans Species, n = 70 | Non-albicans Candida Species, n = 135 | OR (95% CI) | p-Value | |
| Univariate analysis | ||||
| Age (years) ‡ | 63.0 (22.0) | 67.0 (21.0) | 0.98 (0.97–1.01) b | 0.19 |
| Gender (Female), n (%) | 24 (34.3%) | 45 (33.3%) | 1.04 (0.56–1.91) | 0.89 |
| ICU stay before candidaemia onset, days ‡ | 15.0 (19.2) | 23.0 (24.5) | 0.98 (0.9–1.00) c | 0.08 |
| Hospital stay before candidaemia onset, days ‡ | 23 (25) | 29.5 (24) | 0.99 (0.98–1.01) c | 0.17 |
| Delta SOFA | −0.32 ± 4.07 | 1.10 ± 4.15 | 0.91 (0.84–0.98) d | 0.03 |
| ICU length of stay, days ‡ | 39.0 (36.5) | 38.0 (37.0) | 0.99 (0.99–1.01) c | 0.90 |
| Diagnosis (surgical), n (%) | 30 (43.5%) | 49 (36.6%) | 1.33 (0.73–2.41) | 0.33 |
| Presence of shock on candidemia day, n (%) | 36 (54.5%) | 73 (58.9%) | 0.83 (0.45–1.53) | 0.56 |
| COVID-19 | 15 (24.2%) | 47 (75.8%) | 0.51 (0.25–0.98) | 0.049 |
| Multivariate analysis a | ||||
| ICU stay before candidemia onset, days | 0.97 (0.95–1.00) c | 0.08 | ||
| Delta SOFA | 0.74 (0.60–0.89) d | 0.002 | ||
| Presence of shock on candidemia day | 6.89 (2.2–25.0) | 0.001 | ||
| Patients with Candidemia, n = 205 | ||||
|---|---|---|---|---|
| Fluconazole-Resistant Species, n = 80 | Fluconazole-Susceptible Species, n = 125 | OR (95% CI) | p-Value | |
| Univariate analysis | ||||
| Age (years) ‡ | 67.0 (20.8) | 65.5 (22.5) | 1.01 (0.98–1.03) b | 0.22 |
| Gender (Female), n (%) | 29 (36.2%) | 40 (32.0%) | 1.20 (0.66–2.17) | 0.53 |
| ICU stay before candidemia onset, days ‡ | 26.0 (22.5) | 16 (21.0) | 1.02 (1.01–1.04) c | 0.003 |
| Hospital stay before candidemia onset, days ‡ | 33.0 (27.0) | 23.0 (22.8) | 1.01 (1.00–1.03) c | 0.03 |
| Delta SOFA | 0.49 ± 4.64 | 0.68 ± 3.87 | 0.98 (0.91–1.06) d | 0.76 |
| ICU length of stay, days ‡ | 43.0 (48.0) | 36.0 (37.0) | 1.01 (0.99–1.02) c | 0.09 |
| Diagnosis (surgical), n (%) | 30 (38.0%) | 49 (39.5%) | 0.93 (0.52–1.66) | 0.82 |
| Presence of shock on candidemia day, n (%) | 37 (52.9%) | 72 (60.0%) | 0.74 (0.41–1.35) | 0.33 |
| COVID-19 | 30 (48.4%) | 32 (51.6%) | 1.74 (0.95–3.20) | 0.07 |
| Multivariate analysis a | ||||
| ICU stay before candidemia onset | 1.03 (1.01–1.06) | 0.003 | ||
| Presence of shock on candidemia day | 0.23 (0.07–0.64) | 0.006 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Routsi, C.; Meletiadis, J.; Charitidou, E.; Gkoufa, A.; Kokkoris, S.; Karageorgiou, S.; Giannopoulos, C.; Koulenti, D.; Andrikogiannopoulos, P.; Perivolioti, E.; et al. Epidemiology of Candidemia and Fluconazole Resistance in an ICU before and during the COVID-19 Pandemic Era. Antibiotics 2022, 11, 771. https://doi.org/10.3390/antibiotics11060771
Routsi C, Meletiadis J, Charitidou E, Gkoufa A, Kokkoris S, Karageorgiou S, Giannopoulos C, Koulenti D, Andrikogiannopoulos P, Perivolioti E, et al. Epidemiology of Candidemia and Fluconazole Resistance in an ICU before and during the COVID-19 Pandemic Era. Antibiotics. 2022; 11(6):771. https://doi.org/10.3390/antibiotics11060771
Chicago/Turabian StyleRoutsi, Christina, Joseph Meletiadis, Efstratia Charitidou, Aikaterini Gkoufa, Stelios Kokkoris, Stavros Karageorgiou, Charalampos Giannopoulos, Despoina Koulenti, Petros Andrikogiannopoulos, Efstathia Perivolioti, and et al. 2022. "Epidemiology of Candidemia and Fluconazole Resistance in an ICU before and during the COVID-19 Pandemic Era" Antibiotics 11, no. 6: 771. https://doi.org/10.3390/antibiotics11060771
APA StyleRoutsi, C., Meletiadis, J., Charitidou, E., Gkoufa, A., Kokkoris, S., Karageorgiou, S., Giannopoulos, C., Koulenti, D., Andrikogiannopoulos, P., Perivolioti, E., Argyropoulou, A., Vasileiadis, I., Vrioni, G., & Paramythiotou, E. (2022). Epidemiology of Candidemia and Fluconazole Resistance in an ICU before and during the COVID-19 Pandemic Era. Antibiotics, 11(6), 771. https://doi.org/10.3390/antibiotics11060771











