Cefiderocol: Systematic Review of Mechanisms of Resistance, Heteroresistance and In Vivo Emergence of Resistance
Abstract
:1. Introduction
2. Results
2.1. Role of β-Lactamases
2.1.1. NDM Metallo-β-Lactamases (MBL)
2.1.2. KPC Variants
2.1.3. Role of OXA-Type β-Lactamases
2.1.4. Role of PER-Type, SHV-Type, and BEL-Type ESBLs
2.1.5. Role of AmpC Variants
2.1.6. Reversal of Cefiderocol Susceptibility by β-Lactamase Inhibitors
2.2. Permeability Defects/Increased Efflux
2.2.1. Mutations Affecting Siderophore Receptors
2.2.2. Porin Mutations
2.2.3. Overexpression of Efflux Pumps
2.3. Target Modification and Other Genes Potentially Involved in Cefiderocol Resistance
2.4. Combination of Mechanisms Contribute to Cefiderocol Resistance
2.5. Heteroresistance (In Vitro Emergence of Resistant Subpopulations)
2.6. In Vivo Emergence of Resistance or Reduced Cefiderocol Susceptibility
3. Discussion
3.1. Overview of Mechanisms of Cefiderocol Resistance
3.2. Role of β-Lactamases in Cefiderocol Resistance
3.3. Cross-Resistance between Other Antibiotics and Cefiderocol
3.4. Importance of Heteroresistance and In Vivo Emergence of Resistance
3.5. Limitations
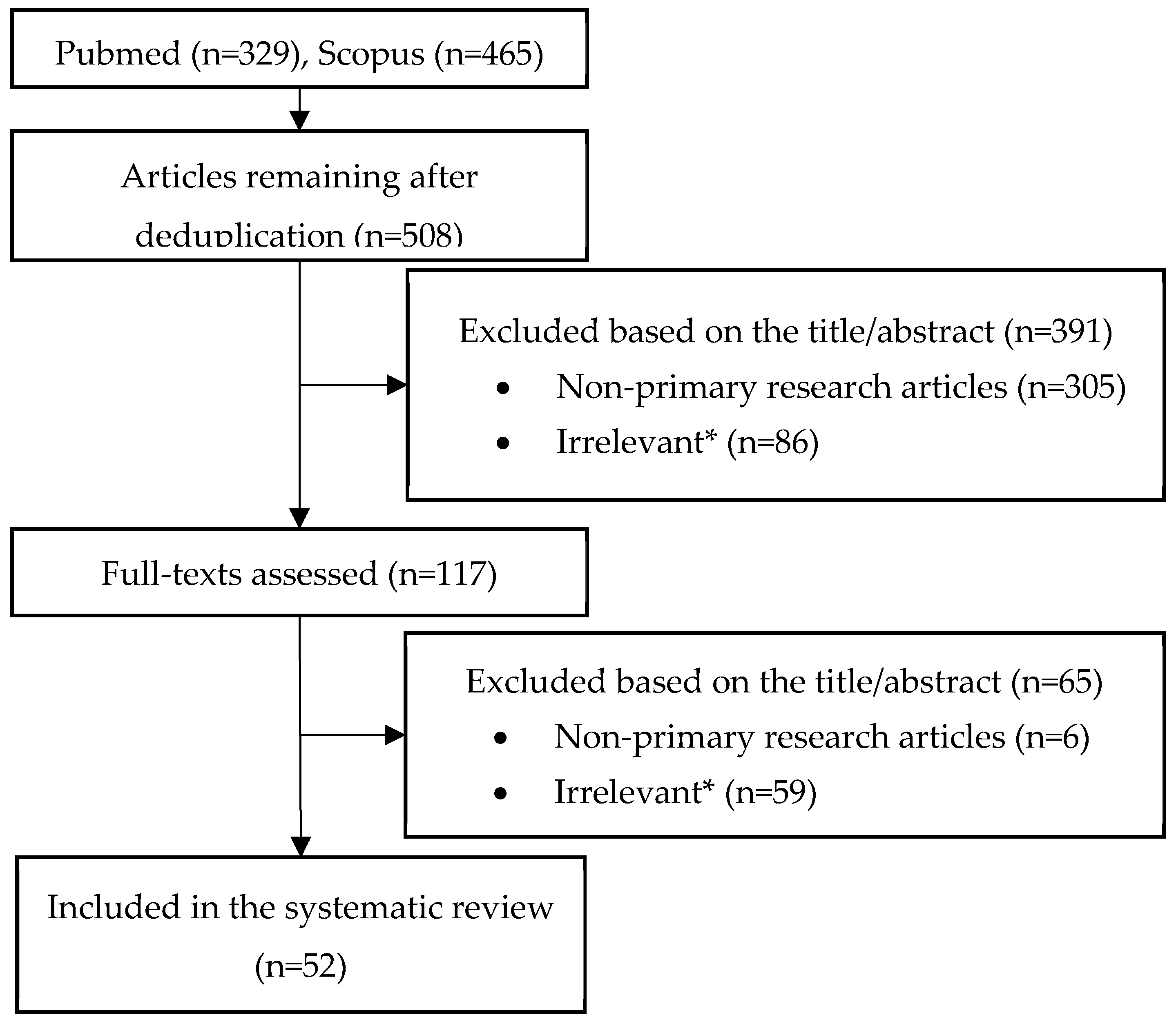
4. Materials and Methods
4.1. Search Strategy and Sources
4.2. Eligibility Criteria
4.3. Data Items
4.4. Assessment of the Evidence for the Reported Mechanisms of Resistance
4.5. Synthesis of Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| β-Lactamase | Organism(s) | Findings |
|---|---|---|
| NDM | Enterobacterales, A. baumannii |
|
| KPC-variants (conferring resistance to ceftazidime/avibactam) | K. pneumoniae, E. coli * |
|
| PER-type ESBL | A. baumannii, P. aeruginosa, E. coli * |
|
| SHV-type ESBL | K. pneumoniae, A. baumannii, E. coli * |
|
| AmpC variants | Enterobacter spp. P. aeruginosa * E. coli * |
|
| OXA-427 | Enterobacterales |
|
| SPM-1, VIM-2, AIM-1, GIM-1 (MBLs) | E. coli *, P. aeruginosa * |
|
| GES-6 | P. aeruginosa * |
|
| PDC-30 (P. aeruginosa cephalosporinase) | P. aeruginosa |
|
| ADC variants (cephalosporinase), OXA-66, (OXA 23) | A. baumannii |
Note: Based on isogenic mutants, introduction of OXA-23 in A. baumannii and E. coli was not shown to affect cefiderocol MIC [29,32,54,66]. Furthermore, cefiderocol has been shown to be stable against OXA-23 [66]. |
| BEL * | E. coli *, P. aeruginosa * |
|
| CTX-M-27 * | E. coli * | Introduction of CTX-M-27 in E. coli was associated with a 4- to 8-fold higher cefiderocol MIC (0.063–0.125→0.5) [29]. |
| Target Gene | Organism(s) | Findings |
|---|---|---|
| piuA, piuD, pirA | P. aeruginosa, A. baumannii |
|
| fecI ** | P. aeruginosa ** |
|
| cirA, fiu * | E. coli, K. pneumoniae, E. cloacae ** |
|
| fhuA, fepA, fecA **, fbpA **, efeO **, exbD ** | K. pneumoniae |
|
| tonB **, exbD **, smlat1148 **, cirA ** | S. maltophilia ** |
|
| Target Genes/Involved Porins/Efflux Pumps | Organism(s) | Findings |
|---|---|---|
| ompK35, ompK36, ompK37 (porins) | K. pneumoniae |
|
| ompC, ompF (porins) | Enterobacter spp. |
|
| oprD(porin) | P. aeruginosa |
|
| ChrA (heavy metal iron transporter), SugE (efflux pump) | K. pneumoniae | n = 7 cefiderocol-resistant (MIC > 2 mg/L) and CR K. pneumoniae clinical isolates were compared by WGS with n = 8 cefiderocol-susceptible CR K. pneumoniae isolates. ChrA expression was detected in five of seven cefiderocol-resistant isolates but only one of eight cefiderocol-susceptible isolates [37]. SugE expression was detected in two of seven cefiderocol-resistant isolates but none of the cefiderocol-susceptible isolates [37]. |
| mexR or nalD (repressors of MexAB–OprM efflux pump) * | P. aeruginosa * | Based on isogenic mutants: mutations in mexR or nalD leading to overexpression of the MexAB–OprM efflux pump were associated with 2-fold higher cefiderocol MIC (0.125→0.25 mg/L) in P. aeruginosa, while mutations in mexB or oprM (resulting in loss of function of the MexAB–OprM efflux pump) resulted in a 2-fold lower cefiderocol MIC [47]. |
| smeT ** | S. maltophilia ** | SmeT promoter mutation (resulting in overexpression of the efflux pump smeDEF) was detected in an in vitro derived cefiderocol-resistant mutant [60]. |
| AxyABM (efflux pump) * | A. xylosoxidans * | Overexpression of AxyABM was associated with a 3-fold higher cefiderocol MIC when comparing two isogenic A. xylosoxidans isolates (0.032→0.094 mg/L). Disruption of the efflux pump was associated with 2- to 23.5-fold lower cefiderocol MICs [51]. |
| Target Gene | Organism(s) | Findings |
|---|---|---|
| PBP-3 (=target of cefiderocol) | E. coli, A. baumannii |
|
| baeS (a sensor of a two-component regulation system) | K. pneumoniae | |
| envZ (a sensor of a two-component regulation system) ** | K. pneumoniae **, E. coli |
|
| yicM (putative membrane transport protein) | K. pneumoniae | Mutations in yicM were detected in two of six cefiderocol-resistant K. pneumoniae clinical isolates [37]. |
| tolQ (membrane transporter), smf-1 (affects fimbriae and surface adhesion) ** | S. maltophilia ** | tolQ and smf-1 mutations were each found in two separate in vitro derived mutants [60]. |
| PmrB, mcr-10 | A higher prevalence of colistin resistance (29% vs. 0%) was reported in cefiderocol-resistant than in susceptible K. pneumoniae clinical isolates [37]. Furthermore, PmrB mutations (known to be involved in cefiderocol resistance [69]) were identified in four of seven (57%) cefiderocol-resistant K. pneumoniae isolates, while the mcr-10 gene was identified in half (three of six) of cefiderocol-resistant E. cloacae isolates [37]. A reduction in the net negative charge (associated with cefiderocol resistance) could also affect cefiderocol, but future studies are necessary to confirm this hypothetical mechanism [37]. |
References
- Karakonstantis, S.; I Kritsotakis, E.; Gikas, A. Pandrug-resistant Gram-negative bacteria: A systematic review of current epidemiology, prognosis and treatment options. J. Antimicrob. Chemother. 2020, 75, 271–282. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Gikas, A.; Astrinaki, E.; Kritsotakis, E.I. Excess mortality due to pandrug-resistant Acinetobacter baumannii infections in hospitalized patients. J. Hosp. Infect. 2020, 106, 447–453. [Google Scholar] [CrossRef]
- Karakonstantis, S.; I Kritsotakis, E.; Gikas, A. Treatment options for K. pneumoniae, P. aeruginosa and A. baumannii co-resistant to carbapenems, aminoglycosides, polymyxins and tigecycline: An approach based on the mechanisms of resistance to carbapenems. Infection 2020, 48, 835–851. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Ioannou, P.; Samonis, G.; Kofteridis, D.P. Systematic Review of Antimicrobial Combination Options for Pandrug-Resistant Acinetobacter baumannii. Antibiotics 2021, 10, 1344. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Ioannou, P.; Kofteridis, D.D. In search for a synergistic combination against pandrug-resistant A. baumannii; methodological considerations. Infection 2022, 1–13. [Google Scholar] [CrossRef]
- Sato, T.; Yamawaki, K. Cefiderocol: Discovery, Chemistry, and In Vivo Profiles of a Novel Siderophore Cephalosporin. Clin. Infect. Dis. 2019, 69, S538–S543. [Google Scholar] [CrossRef] [Green Version]
- Aoki, T.; Yoshizawa, H.; Yamawaki, K.; Yokoo, K.; Sato, J.; Hisakawa, S.; Hasegawa, Y.; Kusano, H.; Sano, M.; Sugimoto, H.; et al. Cefiderocol (S-649266), A new siderophore cephalosporin exhibiting potent activities against Pseudomonas aeruginosa and other gram-negative pathogens including multi-drug resistant bacteria: Structure activity relationship. Eur. J. Med. Chem. 2018, 155, 847–868. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Hackel, M.A.; Takemura, M.; Yamano, Y.; Echols, R.; Sahm, D.F. In Vitro Susceptibility of Gram-Negative Pathogens to Cefiderocol in Five Consecutive Annual Multinational SIDERO-WT Surveillance Studies, 2014 to 2019. Antimicrob. Agents Chemother. 2022, 66, e0199021. [Google Scholar] [CrossRef]
- Candel, F.J.; Henriksen, A.S.; Longshaw, C.; Yamano, Y.; Oliver, A. In vitro activity of the novel siderophore cephalosporin, cefiderocol, in Gram-negative pathogens in Europe by site of infection. Clin. Microbiol. Infect. 2022, 28, 447.e1–447.e6. [Google Scholar] [CrossRef]
- Hackel, M.A.; Tsuji, M.; Yamano, Y.; Echols, R.; Karlowsky, J.A.; Sahm, D.F. In Vitro Activity of the Siderophore Cephalosporin, Cefiderocol, against a Recent Collection of Clinically Relevant Gram-Negative Bacilli from North America and Europe, Including Carbapenem-Nonsusceptible Isolates (SIDERO-WT-2014 Study). Antimicrob. Agents Chemother. 2017, 61, e00093-17. [Google Scholar] [CrossRef] [Green Version]
- Karlowsky, J.A.; Hackel, M.A.; Tsuji, M.; Yamano, Y.; Echols, R.; Sahm, D.F. In Vitro Activity of Cefiderocol, a Siderophore Cephalosporin, Against Gram-Negative Bacilli Isolated by Clinical Laboratories in North America and Europe in 2015-2016: SIDERO-WT-2015. Int. J. Antimicrob. Agents 2018, 53, 456–466. [Google Scholar] [CrossRef]
- Kazmierczak, K.M.; Tsuji, M.; Wise, M.G.; Hackel, M.; Yamano, Y.; Echols, R.; Sahm, D.F. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-β-lactamase-producing isolates (SIDERO-WT-2014 Study). Int. J. Antimicrob. Agents 2018, 53, 177–184. [Google Scholar] [CrossRef]
- Golden, A.R.; Adam, H.J.; Baxter, M.; Walkty, A.; Lagacé-Wiens, P.; Karlowsky, J.A.; Zhanel, G.G. In Vitro Activity of Cefiderocol, a Novel Siderophore Cephalosporin, against Gram-Negative Bacilli Isolated from Patients in Canadian Intensive Care Units. Diagn. Microbiol. Infect. Dis. 2020, 97, 115012. [Google Scholar] [CrossRef]
- Bianco, G.; Boattini, M.; Comini, S.; Iannaccone, M.; Bondi, A.; Cavallo, R.; Costa, C. In vitro activity of cefiderocol against ceftazidime-avibactam susceptible and resistant KPC-producing Enterobacterales: Cross-resistance and synergistic effects. Eur. J. Clin. Microbiol. 2021, 41, 63–70. [Google Scholar] [CrossRef]
- Jacob, A.-S.; Chong, G.-L.; Lagrou, K.; Depypere, M.; Desmet, S. No in vitro activity of cefiderocol against OXA-427-producing Enterobacterales. J. Antimicrob. Chemother. 2021, 76, 3317–3318. [Google Scholar] [CrossRef] [PubMed]
- Simner, P.J.; Beisken, S.; Bergman, Y.; E Posch, A.; E Cosgrove, S.; Tamma, P.D. Cefiderocol Activity Against Clinical Pseudomonas aeruginosa Isolates Exhibiting Ceftolozane-Tazobactam Resistance. Open Forum Infect. Dis. 2021, 8, ofab311. [Google Scholar] [CrossRef]
- Iregui, A.; Khan, Z.; Landman, D.; Quale, J. Activity of Cefiderocol Against Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumannii Endemic to Medical Centers in New York City. Microb. Drug Resist. 2020, 26, 722–726. [Google Scholar] [CrossRef] [Green Version]
- Morris, C.P.; Bergman, Y.; Tekle, T.; Fissel, J.A.; Tamma, P.D.; Simner, P.J. Cefiderocol Antimicrobial Susceptibility Testing against Multidrug-Resistant Gram-Negative Bacilli: A Comparison of Disk Diffusion to Broth Microdilution. J. Clin. Microbiol. 2020, 59, e01649-20. [Google Scholar] [CrossRef]
- Mushtaq, S.; Sadouki, Z.; Vickers, A.; Livermore, D.M.; Woodford, N. In Vitro Activity of Cefiderocol, a Siderophore Cephalosporin, against Multidrug-Resistant Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2020, 64, 12. [Google Scholar] [CrossRef]
- E Choby, J.; Ozturk, T.; Satola, S.W.; Jacob, J.T.; Weiss, D.S. Widespread cefiderocol heteroresistance in carbapenem-resistant Gram-negative pathogens. Lancet Infect. Dis. 2021, 21, 597–598. [Google Scholar] [CrossRef]
- Takemura, M.; Yamano, Y.; Matsunaga, Y.; Ariyasu, M.; Echols, R.; Nagata, T.D. 1266. Characterization of Shifts in Minimum Inhibitory Concentrations During Treatment with Cefiderocol or Comparators in the Phase 3 CREDIBLE-CR and APEKS-NP Studies. Open Forum Infect. Dis. 2020, 7, S649–S650. [Google Scholar] [CrossRef]
- Wunderink, R.G.; Matsunaga, Y.; Ariyasu, M.; Clevenbergh, P.; Echols, R.; Kaye, K.S.; Kollef, M.; Menon, A.; Pogue, J.M.; Shorr, A.F.; et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): A randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2020, 21, 213–225. [Google Scholar] [CrossRef]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef]
- Klein, S.; Boutin, S.; Kocer, K.; O Fiedler, M.; Störzinger, D.; A Weigand, M.; Tan, B.; Richter, D.; Rupp, C.; Mieth, M.; et al. Rapid Development of Cefiderocol Resistance in Carbapenem-resistant Enterobacter cloacae During Therapy Is Associated With Heterogeneous Mutations in the Catecholate Siderophore Receptor cirA. Clin. Infect. Dis. 2021, 74, 905–908. [Google Scholar] [CrossRef]
- Simner, P.J.; Mostafa, H.H.; Bergman, Y.; Ante, M.; Tekle, T.; Adebayo, A.; Beisken, S.; Dzintars, K.; Tamma, P.D. Progressive Development of Cefiderocol Resistance in Escherichia coli During Therapy is Associated With an Increase in blaNDM-5 Copy Number and Gene Expression. Clin. Infect. Dis. 2021, ciab888. [Google Scholar] [CrossRef]
- Meschiari, M.; Volpi, S.; Faltoni, M.; Dolci, G.; Orlando, G.; Franceschini, E.; Menozzi, M.; Sarti, M.; Del Fabro, G.; Fumarola, B.; et al. Real-life experience with compassionate use of cefiderocol for difficult-to-treat resistant Pseudomonas aeruginosa (DTR-P) infections. JAC-Antimicrob. Resist. 2021, 3, dlab188. [Google Scholar] [CrossRef]
- Ballesté-Delpierre, C.; Ramírez, Á.; Muñoz, L.; Longshaw, C.; Roca, I.; Vila, J. Assessment of In Vitro Cefiderocol Susceptibility and Comparators against an Epidemiologically Diverse Collection of Acinetobacter baumannii Clinical Isolates. Antibiotics 2022, 11, 187. [Google Scholar] [CrossRef]
- Bao, J.; Xie, L.; Ma, Y.; An, R.; Gu, B.; Wang, C. Proteomic and Transcriptomic Analyses Indicate Reduced Biofilm-Forming Abilities in Cefiderocol-Resistant Klebsiella pneumoniae. Front. Microbiol. 2022, 12, 778190. [Google Scholar] [CrossRef]
- Kohira, N.; Hackel, M.A.; Ishioka, Y.; Kuroiwa, M.; Sahm, D.F.; Sato, T.; Maki, H.; Yamano, Y. Reduced susceptibility mechanism to cefiderocol, a siderophore cephalosporin, among clinical isolates from a global surveillance programme (SIDERO-WT-2014). J. Glob. Antimicrob. Resist. 2020, 22, 738–741. [Google Scholar] [CrossRef]
- Yamano, Y.; Ishibashi, N.; Kuroiwa, M.; Takemura, M.; Sheng, W.-H.; Hsueh, P.-R. Characterisation of cefiderocol-non-susceptible Acinetobacter baumannii isolates from Taiwan. J. Glob. Antimicrob. Resist. 2021, 28, 120–124. [Google Scholar] [CrossRef]
- Sato, T.; Ito, A.; Ishioka, Y.; Matsumoto, S.; Rokushima, M.; Kazmierczak, K.M.; Hackel, M.; Sahm, D.F.; Yamano, Y. Escherichia coli strains possessing a four amino acid YRIN insertion in PBP3 identified as part of the SIDERO-WT-2014 surveillance study. JAC-Antimicrob. Resist. 2020, 2, dlaa081. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Shields, R.K.; Doi, Y.; Takemura, M.; Echols, R.; Matsunaga, Y.; Yamano, Y. Mechanisms of Reduced Susceptibility to Cefiderocol Among Isolates from the CREDIBLE-CR and APEKS-NP Clinical Trials. Microb. Drug Resist. 2022, 28, 398–407. [Google Scholar] [CrossRef] [PubMed]
- E Choby, J.; Ozturk, T.; Satola, S.W.; Jacob, J.T.; Weiss, D.S. Does cefiderocol heteroresistance explain the discrepancy between the APEKS-NP and CREDIBLE-CR clinical trial results? Lancet Microbe 2021, 2, 648–649. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Leonildi, A.; Della Sala, L.; Vecchione, A.; Barnini, S.; Farcomeni, A.; Menichetti, F. Cefiderocol-Compared to Colistin-Based Regimens for the Treatment of Severe Infections Caused by Carbapenem-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2022, 66, 0214221. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Nicastro, M.; Leonildi, A.; Vecchione, A.; Casella, C.; Forfori, F.; Malacarne, P.; Guarracino, F.; Barnini, S.; et al. Cefiderocol as Rescue Therapy for Acinetobacter baumannii and Other Carbapenem-resistant Gram-negative Infections in Intensive Care Unit Patients. Clin. Infect. Dis. 2021, 72, 2021–2024. [Google Scholar] [CrossRef]
- Gatti, M.; Bartoletti, M.; Cojutti, P.G.; Gaibani, P.; Conti, M.; Giannella, M.; Viale, P.; Pea, F. A descriptive case series of pharmacokinetic/pharmacodynamic target attainment and microbiological outcome in critically ill patients with documented severe extensively drug-resistant Acinetobacter baumannii bloodstream infection and/or ventilator-associated pneumonia treated with cefiderocol. J. Glob. Antimicrob. Resist. 2021, 27, 294–298. [Google Scholar] [CrossRef]
- Simner, P.J.; Beisken, S.; Bergman, Y.; Ante, M.; Posch, A.E.; Tamma, P.D. Defining Baseline Mechanisms of Cefiderocol Resistance in the Enterobacterales. Microb. Drug Resist. 2022, 28, 161–170. [Google Scholar] [CrossRef]
- Perez, C.G.; Maillart, E.; Deyi, V.M.; Huang, T.-D.; Kamgang, P.; Dernier, Y.; Clevenbergh, P. Compassionate use of cefiderocol in a pancreatic abscess and emergence of resistance. Infect. Dis. Now 2020, 51, 399–401. [Google Scholar] [CrossRef]
- Kufel, W.D.; Steele, J.M.; Riddell, S.W.; Jones, Z.; Shakeraneh, P.; Endy, T.P. Cefiderocol for treatment of an empyema due to extensively drug-resistant Pseudomonas aeruginosa: Clinical observations and susceptibility testing considerations. IDCases 2020, 21, e00863. [Google Scholar] [CrossRef]
- Nurjadi, D.; Kocer, K.; Chanthalangsy, Q.; Klein, S.; Heeg, K.; Boutin, S. New Delhi Metallo-Beta-Lactamase Facilitates the Emergence of Cefiderocol Resistance in Enterobacter cloacae. Antimicrob. Agents Chemother. 2022, 66, 2. [Google Scholar] [CrossRef]
- Hobson, C.A.; Cointe, A.; Jacquier, H.; Choudhury, A.; Magnan, M.; Courroux, C.; Tenaillon, O.; Bonacorsi, S.; Birgy, A. Cross-resistance to cefiderocol and ceftazidime–avibactam in KPC β-lactamase mutants and the inoculum effect. Clin. Microbiol. Infect. 2021, 27, 1172.e7–1172.e10. [Google Scholar] [CrossRef] [PubMed]
- Tiseo, G.; Falcone, M.; Leonildi, A.; Giordano, C.; Barnini, S.; Arcari, G.; Carattoli, A.; Menichetti, F. Meropenem-Vaborbactam as Salvage Therapy for Ceftazidime-Avibactam-, Cefiderocol-Resistant ST-512 Klebsiella pneumoniae–Producing KPC-31, a D179Y Variant of KPC-3. Open Forum Infect. Dis. 2021, 8, ofab141. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Iovleva, A.; Kline, E.G.; Kawai, A.; McElheny, C.L.; Doi, Y. Clinical Evolution of AmpC-Mediated Ceftazidime-Avibactam and Cefiderocol Resistance in Enterobacter cloacae Complex Following Exposure to Cefepime. Clin. Infect. Dis. 2020, 71, 2713–2716. [Google Scholar] [CrossRef] [PubMed]
- Streling, A.P.; Al Obaidi, M.M.; Lainhart, W.D.; Zangeneh, T.; Khan, A.; Dinh, A.Q.; Hanson, B.; A Arias, C.; Miller, W.R. Evolution of Cefiderocol Non-Susceptibility in Pseudomonas aeruginosa in a Patient Without Previous Exposure to the Antibiotic. Clin. Infect. Dis. 2021, 73, 4472–4474. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Landman, D.; Quale, J. Relationship of TonB-dependent receptors with susceptibility to cefiderocol in clinical isolates of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2022, 77, 1282–1285. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Nishikawa, T.; Ishii, R.; Kuroiwa, M.; Ishioka, Y.; Kurihara, N.; Sakikawa, I.; Ota, T.; Rokushima, M.; Tsuji, M.; et al. 696. Mechanism of Cefiderocol high MIC mutants obtained in non-clinical FoR studies. Open Forum Infect. Dis. 2018, 5, S251. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Sato, T.; Ota, M.; Takemura, M.; Nishikawa, T.; Toba, S.; Kohira, N.; Miyagawa, S.; Ishibashi, N.; Matsumoto, S.; et al. In Vitro Antibacterial Properties of Cefiderocol, a Novel Siderophore Cephalosporin, against Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2018, 62, e01454-17. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Nishikawa, T.; Ota, M.; Ito-Horiyama, T.; Ishibashi, N.; Sato, T.; Tsuji, M.; Yamano, Y. Stability and low induction propensity of cefiderocol against chromosomal AmpC β-lactamases of Pseudomonas aeruginosa and Enterobacter cloacae. J. Antimicrob. Chemother. 2018, 73, 3049–3052. [Google Scholar] [CrossRef]
- Kawai, A.; McElheny, C.L.; Iovleva, A.; Kline, E.G.; Sluis-Cremer, N.; Shields, R.K.; Doi, Y. Structural Basis of Reduced Susceptibility to Ceftazidime-Avibactam and Cefiderocol in Enterobacter cloacae Due to AmpC R2 Loop Deletion. Antimicrob. Agents Chemother. 2020, 64, 7. [Google Scholar] [CrossRef]
- Luscher, A.; Moynié, L.; Auguste, P.S.; Bumann, D.; Mazza, L.; Pletzer, D.; Naismith, J.H.; Köhler, T. TonB-Dependent Receptor Repertoire of Pseudomonas aeruginosa for Uptake of Siderophore-Drug Conjugates. Antimicrob. Agents Chemother. 2018, 62, e00097-18. [Google Scholar] [CrossRef] [Green Version]
- Magallon, A.; Amoureux, L.; Garrigos, T.; Sonois, M.; Varin, V.; Neuwirth, C.; Bador, J. Role of AxyABM overexpression in acquired resistance in Achromobacter xylosoxidans. J. Antimicrob. Chemother. 2022, 77, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Kaminski, M.; Landman, D.; Quale, J. Cefiderocol Resistance in Acinetobacter baumannii: Roles of β-Lactamases, Siderophore Receptors, and Penicillin Binding Protein 3. Antimicrob. Agents Chemother. 2020, 64, e01221-20. [Google Scholar] [CrossRef] [PubMed]
- Rolston, K.V.I.; Gerges, B.; Shelburne, S.; Aitken, S.L.; Raad, I.; Prince, R.A. Activity of Cefiderocol and Comparators against Isolates from Cancer Patients. Antimicrob. Agents Chemother. 2020, 64, e01955-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirel, L.; Sadek, M.; Nordmann, P. Contribution of PER-Type and NDM-Type β-Lactamases to Cefiderocol Resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, e0087721. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Sadek, M.; Kusaksizoglu, A.; Nordmann, P. Co-resistance to ceftazidime-avibactam and cefiderocol in clinical isolates producing KPC variants. Eur. J. Clin. Microbiol. 2022, 41, 677–680. [Google Scholar] [CrossRef]
- Poirel, L.; de la Rosa, J.-M.O.; Sadek, M.; Nordmann, P. Impact of Acquired Broad-Spectrum β-Lactamases on Susceptibility to Cefiderocol and Newly Developed β-Lactam/β-Lactamase Inhibitor Combinations in Escherichia coli and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2022, 66, e0003922. [Google Scholar] [CrossRef]
- McElheny, C.L.; Fowler, E.L.; Iovleva, A.; Shields, R.K.; Doi, Y. In Vitro Evolution of Cefiderocol Resistance in an NDM-Producing Klebsiella pneumoniae Due to Functional Loss of CirA. Microbiol. Spectr. 2021, 9, e0177921. [Google Scholar] [CrossRef]
- Gill, C.M.; Abdelraouf, K.; Oota, M.; Nakamura, R.; Kuroiwa, M.; Gahara, Y.; Takemura, M.; Yamano, Y.; Nicolau, D.P. Discrepancy in sustained efficacy and resistance emergence under human-simulated exposure of cefiderocol against Stenotrophomonas maltophilia between in vitro chemostat and in vivo murine infection models. J. Antimicrob. Chemother. 2021, 76, 2615–2621. [Google Scholar] [CrossRef]
- Price, T.K.; Davar, K.; Contreras, D.; Ward, K.W.; Garner, O.B.; Simner, P.J.; Yang, S.; Chandrasekaran, S. Case Report and Genomic Analysis of Cefiderocol-Resistant Escherichia coli Clinical Isolates. Am. J. Clin. Pathol. 2021, 157, 257–265. [Google Scholar] [CrossRef]
- Werth, B.J.; Ashford, N.K.; Penewit, K.; Waalkes, A.; Holmes, E.A.; Bryan, A.; Salipante, S.J. Evolution of cefiderocol resistance in Stenotrophomonas maltophilia using in vitro serial passage techniques. JAC-Antimicrob. Resist. 2022, 4, dlac011. [Google Scholar] [CrossRef]
- Zhang, Q.; Neidig, N.; Chu, T.-Y.; Divoky, C.; Carpenter, J.; Lee-Hsiao, C.; Threatt, H.; Sultana, R.; Bush, K. In vitro antibacterial activity of cefiderocol against recent multidrug-resistant carbapenem-nonsusceptible Enterobacterales isolates. Diagn. Microbiol. Infect. Dis. 2022, 103, 115651. [Google Scholar] [CrossRef] [PubMed]
- Longshaw, C.; Manissero, D.; Tsuji, M.; Echols, R.; Yamano, Y. In vitro activity of the siderophore cephalosporin, cefiderocol, against molecularly characterized, carbapenem-non-susceptible Gram-negative bacteria from Europe. JAC-Antimicrob. Resist. 2020, 2, dlaa060. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Mutakabbir, J.C.; Nguyen, L.; Maassen, P.T.; Stamper, K.C.; Kebriaei, R.; Kaye, K.S.; Castanheira, M.; Rybak, M.J. In Vitro Antibacterial Activity of Cefiderocol against Multidrug-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, e0264620. [Google Scholar] [CrossRef]
- Lan, P.; Lu, Y.; Chen, Z.; Wu, X.; Hua, X.; Jiang, Y.; Zhou, J.; Yu, Y. Emergence of High-Level Cefiderocol Resistance in Carbapenem-Resistant Klebsiella pneumoniae from Bloodstream Infections in Patients with Hematologic Malignancies in China. Microbiol. Spectr. 2022, 10, e0008422. [Google Scholar] [CrossRef] [PubMed]
- Bogaerts, P.; Naas, T.; Saegeman, V.; Bonnin, R.A.; Schuermans, A.; Evrard, S.; Bouchahrouf, W.; Jove, T.; Tande, D.; de Bolle, X.; et al. OXA-427, a new plasmid-borne carbapenem-hydrolysing class D β-lactamase in Enterobacteriaceae. J. Antimicrob. Chemother. 2017, 72, 2469–2477. [Google Scholar] [CrossRef] [Green Version]
- Poirel, L.; Kieffer, N.; Nordmann, P. Stability of cefiderocol against clinically significant broad-spectrum oxacillinases. Int. J. Antimicrob. Agents 2018, 52, 866–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stainton, S.M.; Monogue, M.L.; Tsuji, M.; Yamano, Y.; Echols, R.; Nicolau, D.P. Efficacy of Humanized Cefiderocol Exposures over 72 Hours against a Diverse Group of Gram-Negative Isolates in the Neutropenic Murine Thigh Infection Model. Antimicrob. Agents Chemother. 2019, 63, e0104018. [Google Scholar] [CrossRef] [Green Version]
- Karakonstantis, S.; Saridakis, I. Colistin heteroresistance in Acinetobacter spp.: Systematic review and meta-analysis of the prevalence and discussion of the mechanisms and potential therapeutic implications. Int. J. Antimicrob. Agents 2020, 56, 106065. [Google Scholar] [CrossRef]
- Karakonstantis, S. A systematic review of implications, mechanisms, and stability of in vivo emergent resistance to colistin and tigecycline in Acinetobacter baumannii. J. Chemother. 2020, 33, 1–11. [Google Scholar] [CrossRef]
- Andersson, D.I.; Nicoloff, H.; Hjort, K. Mechanisms and clinical relevance of bacterial heteroresistance. Nat. Rev. Genet. 2019, 17, 479–496. [Google Scholar] [CrossRef]
- Bassetti, M.; Echols, R.; Koren, A.; Karas, A.; Longshaw, C.; Yamano, Y.; Nagata, T.D. Placing in-vitro heteroresistance in the context of clinical results. Lancet Infect. Dis. 2021, 21, 908–909. [Google Scholar] [CrossRef]
- El-Halfawy, O.M.; Valvano, M.A. Antimicrobial Heteroresistance: An Emerging Field in Need of Clarity. Clin. Microbiol. Rev. 2015, 28, 191–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brukner, I.; Oughton, M. A Fundamental Change in Antibiotic Susceptibility Testing Would Better Prevent Therapeutic Failure: From Individual to Population-Based Analysis. Front. Microbiol. 2020, 11, 1820. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Larsson, J.; Hjort, K.; Elf, J.; Andersson, D.I. The highly dynamic nature of bacterial heteroresistance impairs its clinical detection. Commun. Biol. 2021, 4, 521. [Google Scholar] [CrossRef]
- Bassetti, M.; Ariyasu, M.; Binkowitz, B.; Nagata, T.D.; Echols, R.M.; Matsunaga, Y.; Toyoizumi, K.; Doi, Y. Designing A Pathogen-Focused Study To Address The High Unmet Medical Need Represented By Carbapenem-Resistant Gram-Negative Pathogens-The International, Multicenter, Randomized, Open-Label, Phase 3 CREDIBLE-CR Study. Infect. Drug Resist. 2019, 12, 3607–3623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, C.; Pimentel, C.; Pasteran, F.; Tuttobene, M.R.; Subils, T.; Escalante, J.; Nishimura, B.; Arriaga, S.; Carranza, A.; Mezcord, V.; et al. Human Serum Proteins and Susceptibility of Acinetobacter baumannii to Cefiderocol: Role of Iron Transport. Biomedicines 2022, 10, 600. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [Green Version]
- Mc Gann, P.; Geringer, M.R.; Hall, L.R.; Lebreton, F.; Markelz, E.; Kwak, Y.I.; Johnson, S.; Ong, A.C.; Powell, A.; Tekle, T.; et al. Pan-drug resistant Providencia rettgeri contributing to a fatal case of COVID-19. J. Med. Microbiol. 2021, 70, 001406. [Google Scholar] [CrossRef]
- Oinuma, K.I.; Suzuki, M.; Sakiyama, A.; Tsubouchi, T.; Saeki, K.; Sato, K.; Niki, M.; Yamada, K.; Shibayama, K.; Kakeya, H.; et al. Genomic characterization of triple-carbapenemase-producing Acinetobacter baumannii. JAC-Antimicrob. Resist. 2021, 3, dlab191. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakonstantis, S.; Rousaki, M.; Kritsotakis, E.I. Cefiderocol: Systematic Review of Mechanisms of Resistance, Heteroresistance and In Vivo Emergence of Resistance. Antibiotics 2022, 11, 723. https://doi.org/10.3390/antibiotics11060723
Karakonstantis S, Rousaki M, Kritsotakis EI. Cefiderocol: Systematic Review of Mechanisms of Resistance, Heteroresistance and In Vivo Emergence of Resistance. Antibiotics. 2022; 11(6):723. https://doi.org/10.3390/antibiotics11060723
Chicago/Turabian StyleKarakonstantis, Stamatis, Maria Rousaki, and Evangelos I. Kritsotakis. 2022. "Cefiderocol: Systematic Review of Mechanisms of Resistance, Heteroresistance and In Vivo Emergence of Resistance" Antibiotics 11, no. 6: 723. https://doi.org/10.3390/antibiotics11060723
APA StyleKarakonstantis, S., Rousaki, M., & Kritsotakis, E. I. (2022). Cefiderocol: Systematic Review of Mechanisms of Resistance, Heteroresistance and In Vivo Emergence of Resistance. Antibiotics, 11(6), 723. https://doi.org/10.3390/antibiotics11060723








