Sarecycline Demonstrates Clinical Effectiveness against Staphylococcal Infections and Inflammatory Dermatoses: Evidence for Improving Antibiotic Stewardship in Dermatology
Abstract
:1. Introduction
2. Results
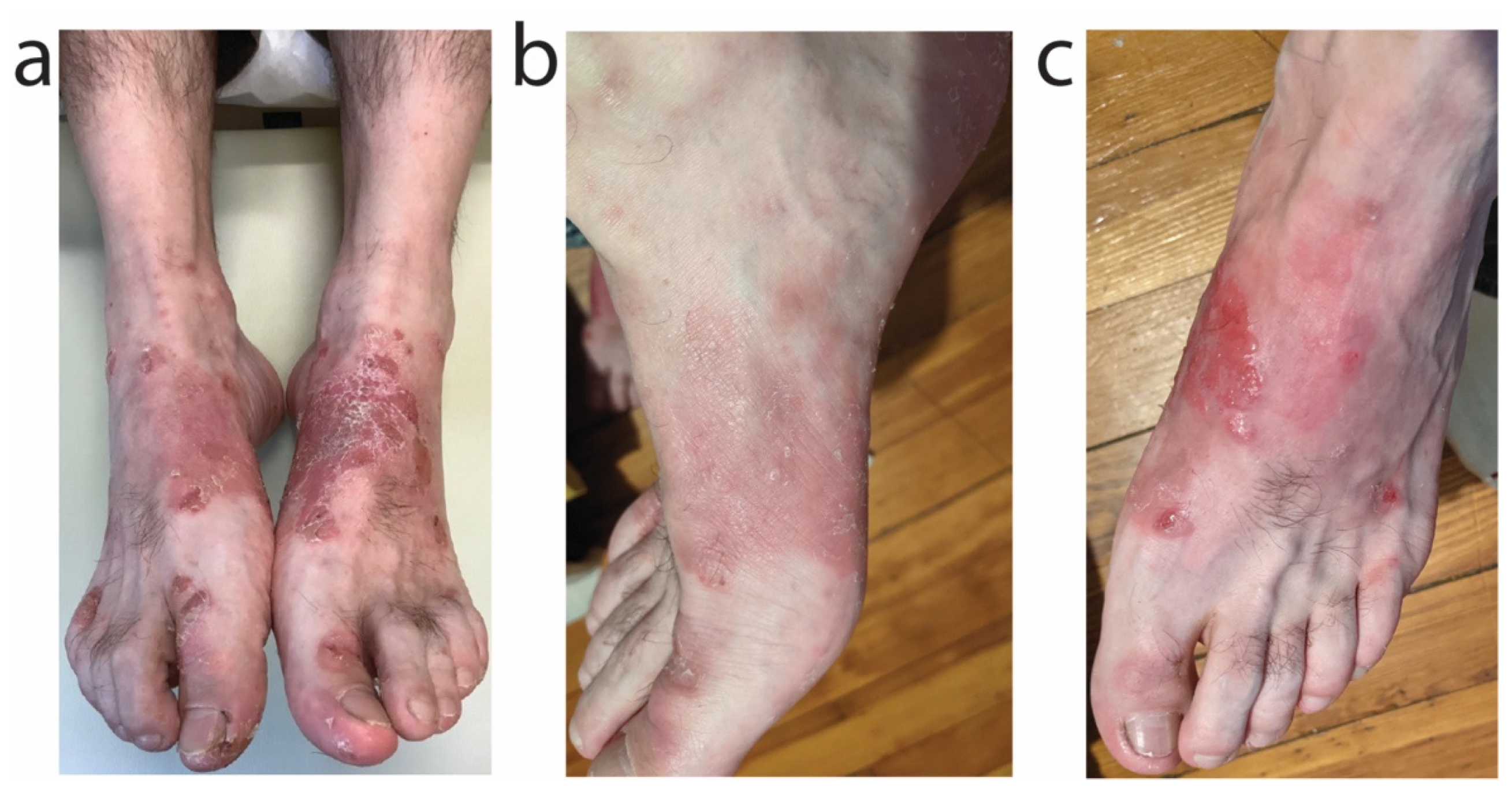
2.1. Patient 1
2.2. Patient 2
2.3. Patient 3
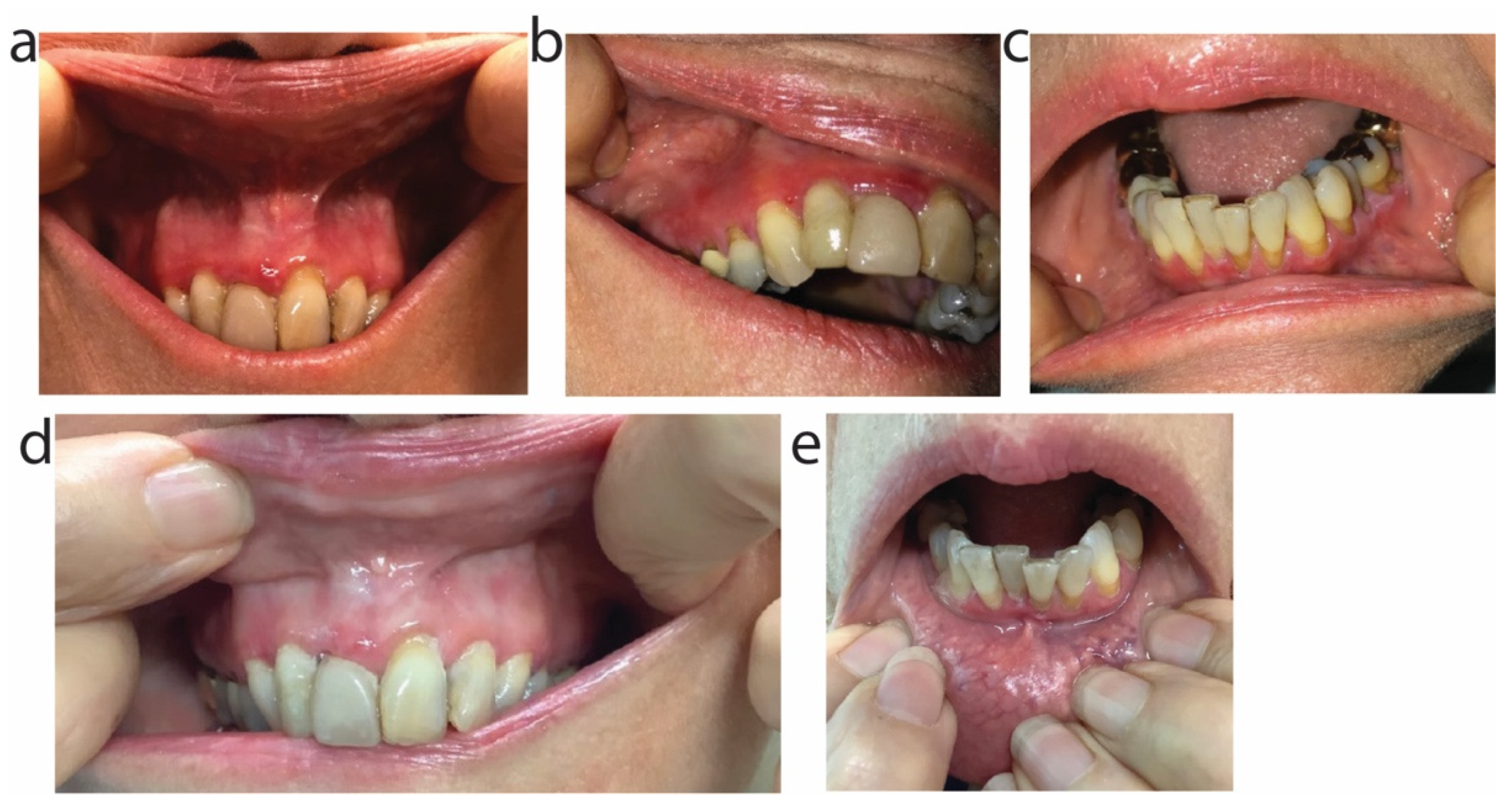
2.4. Patient 4
2.5. Patient 5
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Del Rosso, J.Q.; Webster, G.F.; Rosen, T.; Thiboutot, D.; Leyden, J.J.; Gallo, R.; Walker, C.; Zhanel, G.; Eichenfield, L. Status Report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: Part 1: Antibiotic Prescribing Patterns, Sources of Antibiotic Exposure, Antibiotic Consumption and Emergence of Antibiotic Resistance, Impact of Alterations in Antibiotic Prescribing, and Clinical Sequelae of Antibiotic Use. J. Clin. Aesthetic Dermatol. 2016, 9, 18–24. [Google Scholar]
- Graber, E.M. Treating acne with the tetracycline class of antibiotics: A review. Dermatol. Rev. 2021, 2, 321–330. [Google Scholar] [CrossRef]
- US Food and Drug Administration Approved Prescribing Information. In DORYX® (Doxycycline hyclate) Delayed-Release Tablets, 75 mg, 100 mg and 150 mg for Oral Use; Mayne Pharma International Pty. Ltd.: Salisbury, Australia, 2008.
- US Food and Drug Administration Approved Prescribing Information. In Minocin (Minocycline hydrochloride) for Oral Use; Triax Pharmaceuticals, LLC: Cranford, NJ, USA, 2010.
- Griffin, M.O.; Ceballos, G.; Villarreal, F.J. Tetracycline compounds with non-antimicrobial organ protective properties: Possible mechanisms of action. Pharmacol. Res. 2011, 63, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Madke, B.; Kabra, P.; Singh, A.L. Anti-inflammatory and Immunomodulatory Effects of Antibiotics and Their Use in Dermatology. Indian J. Dermatol. 2016, 61, 469–481. [Google Scholar] [CrossRef]
- Perret, L.J.; Tait, C.P. Non-antibiotic properties of tetracyclines and their clinical application in dermatology. Australas. J. Dermatol. 2014, 55, 111–118. [Google Scholar] [CrossRef]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2015, 6, 1543. [Google Scholar] [CrossRef] [Green Version]
- Dreno, B.; Thiboutot, D.; Gollnick, H.; Bettoli, V.; Kang, S.; Leyden, J.J.; Shalita, A.; Torres, V. Antibiotic stewardship in dermatology: Limiting antibiotic use in acne. Eur. J. Dermatol. 2014, 24, 330–334. [Google Scholar] [CrossRef]
- Alkhawaja, E.; Hammadi, S.; Abdelmalek, M.; Mahasneh, N.; Alkhawaja, B.; Abdelmalek, S.M. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: A cross sectional study. BMC Dermatol. 2020, 20, 17. [Google Scholar] [CrossRef]
- Sheffer-Levi, S.; Rimon, A.; Lerer, V.; Shlomov, T.; Coppenhagen-Glazer, S.; Rakov, C.; Zeiter, T.; Nir-Paz, R.; Hazan, R.; Molho-Pessach, V. Antibiotic Susceptibility of Cutibacterium acnes Strains Isolated from Israeli Acne Patients. Acta Derm. Venereol. 2020, 100, adv00295. [Google Scholar] [CrossRef]
- Aoki, S.; Nakase, K.; Hayashi, N.; Nakaminami, H.; Noguchi, N. Increased prevalence of doxycycline low-susceptible Cutibacterium acnes isolated from acne patients in Japan caused by antimicrobial use and diversification of tetracycline resistance factors. J. Dermatol. 2021, 48, 1365–1371. [Google Scholar] [CrossRef]
- Collaborators, A.R. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Wistrand-Yuen, E.; Knopp, M.; Hjort, K.; Koskiniemi, S.; Berg, O.G.; Andersson, D.I. Evolution of high-level resistance during low-level antibiotic exposure. Nat. Commun. 2018, 9, 1599. [Google Scholar] [CrossRef] [Green Version]
- Stanton, I.C.; Murray, A.K.; Zhang, L.; Snape, J.; Gaze, W.H. Evolution of antibiotic resistance at low antibiotic concentrations including selection below the minimal selective concentration. Commun. Biol. 2020, 3, 467. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; DePristo, M.A.; Collins, J.J. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol. Cell 2010, 37, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef] [Green Version]
- Healthcare Infection Control Practices Advisory Committee. Antibiotic Stewardship Statement for Antibiotic Guidelines–The Recommendations of the Healthcare Infection Control Practices Advisory Committee (HICPAC); Healthcare Infection Control Practices Advisory Committee: Atlanta, GA, USA, 2016.
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973.e933. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, J.S.; Hoffstad, O.; Margolis, D.J. Duration of oral tetracycline-class antibiotic therapy and use of topical retinoids for the treatment of acne among general practitioners (GP): A retrospective cohort study. J. Am. Acad. Dermatol. 2016, 75, 1142–1150.e1141. [Google Scholar] [CrossRef]
- Barbieri, J.S.; Bhate, K.; Hartnett, K.P.; Fleming-Dutra, K.E.; Margolis, D.J. Trends in Oral Antibiotic Prescription in Dermatology, 2008 to 2016. JAMA Dermatol. 2019, 155, 290–297. [Google Scholar] [CrossRef]
- Lee, Y.H.; Liu, G.; Thiboutot, D.M.; Leslie, D.L.; Kirby, J.S. A retrospective analysis of the duration of oral antibiotic therapy for the treatment of acne among adolescents: Investigating practice gaps and potential cost-savings. J. Am. Acad. Dermatol. 2014, 71, 70–76. [Google Scholar] [CrossRef]
- Deeks, E.D. Sarecycline: First Global Approval. Drugs 2019, 79, 325–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaul, G.; Saxena, D.; Dasgupta, A.; Chopra, S. Sarecycline hydrochloride for the treatment of acne vulgaris. Drugs Today 2019, 55, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Drug Approval Package: Seysara (Sarecycline). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/209521Orig1s000TOC.cfm (accessed on 10 April 2022).
- Nguyen, F.; Starosta, A.L.; Arenz, S.; Sohmen, D.; Dönhöfer, A.; Wilson, D.N. Tetracycline antibiotics and resistance mechanisms. Biol. Chem. 2014, 395, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Batool, Z.; Lomakin, I.B.; Polikanov, Y.S.; Bunick, C.G. Sarecycline interferes with tRNA accommodation and tethers mRNA to the 70S ribosome. Proc. Natl. Acad. Sci. USA 2020, 117, 20530–20537. [Google Scholar] [CrossRef]
- Bunick, C.G.; Keri, J.; Tanaka, S.K.; Furey, N.; Damiani, G.; Johnson, J.L.; Grada, A. Antibacterial Mechanisms and Efficacy of Sarecycline in Animal Models of Infection and Inflammation. Antibiotics 2021, 10, 439. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Long, L.; Bunick, C.G.; Del Rosso, J.Q.; Gamal, A.; Tyring, S.K.; McCormick, T.S.; Grada, A. Sarecycline Demonstrated Reduced Activity Compared to Minocycline against Microbial Species Representing Human Gastrointestinal Microbiota. Antibiotics 2022, 11, 324. [Google Scholar] [CrossRef]
- Zhanel, G.; Critchley, I.; Lin, L.Y.; Alvandi, N. Microbiological Profile of Sarecycline, a Novel Targeted Spectrum Tetracycline for the Treatment of Acne Vulgaris. Antimicrob. Agents Chemother. 2019, 63, e01297-18. [Google Scholar] [CrossRef] [Green Version]
- Rusu, A.; Buta, E.L. The Development of Third-Generation Tetracycline Antibiotics and New Perspectives. Pharmaceutics 2021, 13, 2085. [Google Scholar] [CrossRef]
- Moore, A.; Green, L.J.; Bruce, S.; Sadick, N.; Tschen, E.; Werschler, P.; Cook-Bolden, F.E.; Dhawan, S.S.; Forsha, D.; Gold, M.H.; et al. Once-Daily Oral Sarecycline 1.5 mg/kg/day Is Effective for Moderate to Severe Acne Vulgaris: Results from Two Identically Designed, Phase 3, Randomized, Double-Blind Clinical Trials. J. Drugs Dermatol. 2018, 17, 987–996. [Google Scholar] [CrossRef]
- Pariser, D.M.; Green, L.J.; Lain, E.L.; Schmitz, C.; Chinigo, A.S.; McNamee, B.; Berk, D.R. Safety and Tolerability of Sarecycline for the Treatment of Acne Vulgaris: Results from a Phase III, Multicenter, Open-Label Study and a Phase I Phototoxicity Study. J. Clin. Aesthetic Dermatol. 2019, 12, E53–E62. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Hekmatjah, J.; Kircik, L.H. Oral Tetracyclines and Acne: A Systematic Review for Dermatologists. J. Drugs Dermatol. 2020, 19, s6–s13. [Google Scholar] [PubMed]
- Jernberg, C.; Löfmark, S.; Edlund, C.; Jansson, J.K. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 2010, 156, 3216–3223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adjei, S.; Miller, A.; Temiz, L.; Tyring, S. A Rare Case of Empedobacter Brevis Cutaneous Infection Treated Successfully with Oral Sarecycline. J. Drugs Dermatol. 2022, 21, 201–202. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.; Del Rosso, J.Q.; Graber, E.; Bunick, C.G.; Stein Gold, L.; Moore, A.Y.; Baldwin, H.; Obagi, Z.; Damiani, G.; Carrothers, T.; et al. Sarecycline treatment for acne vulgaris: Rationale for weight-based dosing and limited impact of food intake on clinical efficacy. Dermatol. Ther. 2022, 35, e15275. [Google Scholar] [CrossRef] [PubMed]
- Jedlowski, P.M.; Jedlowski, M.F. Case/noncase analysis of the FDA Adverse Events Reporting System suggests higher reporting odds of photosensitivity and esophageal symptoms for sarecycline than of those for other tetracyclines. J. Am. Acad. Dermatol. 2022; in press. [Google Scholar] [CrossRef]
- US Food and Drug Administration approved prescribing information. In SEYSARA® (Sarecycline) Tablets for Oral Use (Package Insert); Almirall LLC: Barcelona, Spain, 2020.
- Goldberg, I.; Shirazi, I.; Brenner, S. In vitro interferon-gamma release test in patients with drug-induced pemphigus. Isr. Med. Assoc. J. 2008, 10, 424–427. [Google Scholar] [PubMed]
- Leyden, J.J.; Sniukiene, V.; Berk, D.R.; Kaoukhov, A. Efficacy and Safety of Sarecycline, a Novel, Once-Daily, Narrow Spectrum Antibiotic for the Treatment of Moderate to Severe Facial Acne Vulgaris: Results of a Phase 2, Dose-Ranging Study. J. Drugs Dermatol. 2018, 17, 333–338. [Google Scholar] [PubMed]
- Del Rosso, J.Q.; Stein Gold, L.; Baldwin, H.; Harper, J.C.; Zeichner, J.; Obagi, S.; Graber, E.; Jimenez, X.; Vicente, F.H.; Grada, A. Management of Truncal Acne with Oral Sarecycline: Pooled Results from Two Phase-3 Clinical Trials. J. Drugs Dermatol. 2021, 20, 634–640. [Google Scholar] [CrossRef]
- Graber, E.; Kay, C.R. Successful Treatment of Periorificial Dermatitis with Novel Narrow Spectrum Sarecycline. J. Drugs Dermatol. 2021, 20, 98–100. [Google Scholar] [CrossRef]
- Rosso, J.Q.; Draelos, Z.D.; Effron, C.; Kircik, L.H. Oral Sarecycline for Treatment of Papulopustular Rosacea: Results of a Pilot Study of Effectiveness and Safety. J. Drugs Dermatol. 2021, 20, 426–431. [Google Scholar] [CrossRef]
- Deckers, I.E.; Prens, E.P. An Update on Medical Treatment Options for Hidradenitis Suppurativa. Drugs 2016, 76, 215–229. [Google Scholar] [CrossRef]
- van Straalen, K.R.; Tzellos, T.; Guillem, P.; Benhadou, F.; Cuenca-Barrales, C.; Daxhelet, M.; Daoud, M.; Efthymiou, O.; Giamarellos-Bourboulis, E.J.; Jemec, G.B.E.; et al. The efficacy and tolerability of tetracyclines and clindamycin plus rifampicin for the treatment of hidradenitis suppurativa: Results of a prospective European cohort study. J. Am. Acad. Dermatol. 2021, 85, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Grantham, H.J.; Stocken, D.D.; Reynolds, N.J. Doxycycline: A first-line treatment for bullous pemphigoid? Lancet 2017, 389, 1586–1588. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.X.; Wang, X.; Shan, Y.; Li, S.Z.; Xu, Q.; Jin, H.Z.; Zuo, Y.G. Efficacy and safety of tetracyclines for pemphigoid: A systematic review and meta-analysis. Arch. Dermatol. Res. 2022, 314, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Morgado-Carrasco, D.; Riquelme-Mac Loughlin, C.; Fustà-Novell, X.; Iranzo, P. Doxycycline, a Well-Tolerated, Economic, and Effective Alternative for the First-Line Treatment of Bullous Pemphigoid. Actas Dermo-Sifiliogr. 2018, 109, 549–550. [Google Scholar] [CrossRef]
- Williams, H.C.; Wojnarowska, F.; Kirtschig, G.; Mason, J.; Godec, T.R.; Schmidt, E.; Chalmers, J.R.; Childs, M.; Walton, S.; Harman, K.; et al. Doxycycline versus prednisolone as an initial treatment strategy for bullous pemphigoid: A pragmatic, non-inferiority, randomised controlled trial. Lancet 2017, 389, 1630–1638. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, E.; Rashid, H.; Marzano, A.V.; Lamberts, A.; Di Zenzo, G.; Diercks, G.F.H.; Alberti-Violetti, S.; Barry, R.J.; Borradori, L.; Caproni, M.; et al. European Guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European Academy of Dermatology and Venereology-Part II. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1926–1948. [Google Scholar] [CrossRef]
- Juliandri, J.; Wang, X.; Liu, Z.; Zhang, J.; Xu, Y.; Yuan, C. Global rosacea treatment guidelines and expert consensus points: The differences. J. Cosmet. Dermatol. 2019, 18, 960–965. [Google Scholar] [CrossRef]
- Redd, T.K.; Seitzman, G.D. Ocular rosacea. Curr. Opin. Ophthalmol. 2020, 31, 503–507. [Google Scholar] [CrossRef]
- Bamford, J.T.; Gessert, C.E.; Renier, C.M.; Jackson, M.M.; Laabs, S.B.; Dahl, M.V.; Rogers, R.S. Childhood stye and adult rosacea. J. Am. Acad. Dermatol. 2006, 55, 951–955. [Google Scholar] [CrossRef]
- Driver, P.J.; Lemp, M.A. Meibomian gland dysfunction. Surv. Ophthalmol. 1996, 40, 343–367. [Google Scholar] [CrossRef]
- Brahe, C.; Peters, K. Fighting acne for the fighting forces. Cutis 2020, 106, 18–20, 22. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grada, A.; Ghannoum, M.A.; Bunick, C.G. Sarecycline Demonstrates Clinical Effectiveness against Staphylococcal Infections and Inflammatory Dermatoses: Evidence for Improving Antibiotic Stewardship in Dermatology. Antibiotics 2022, 11, 722. https://doi.org/10.3390/antibiotics11060722
Grada A, Ghannoum MA, Bunick CG. Sarecycline Demonstrates Clinical Effectiveness against Staphylococcal Infections and Inflammatory Dermatoses: Evidence for Improving Antibiotic Stewardship in Dermatology. Antibiotics. 2022; 11(6):722. https://doi.org/10.3390/antibiotics11060722
Chicago/Turabian StyleGrada, Ayman, Mahmoud A. Ghannoum, and Christopher G. Bunick. 2022. "Sarecycline Demonstrates Clinical Effectiveness against Staphylococcal Infections and Inflammatory Dermatoses: Evidence for Improving Antibiotic Stewardship in Dermatology" Antibiotics 11, no. 6: 722. https://doi.org/10.3390/antibiotics11060722
APA StyleGrada, A., Ghannoum, M. A., & Bunick, C. G. (2022). Sarecycline Demonstrates Clinical Effectiveness against Staphylococcal Infections and Inflammatory Dermatoses: Evidence for Improving Antibiotic Stewardship in Dermatology. Antibiotics, 11(6), 722. https://doi.org/10.3390/antibiotics11060722







