New Drugs for the Treatment of Pseudomonas aeruginosa Infections with Limited Treatment Options: A Narrative Review
Abstract
:1. Introduction
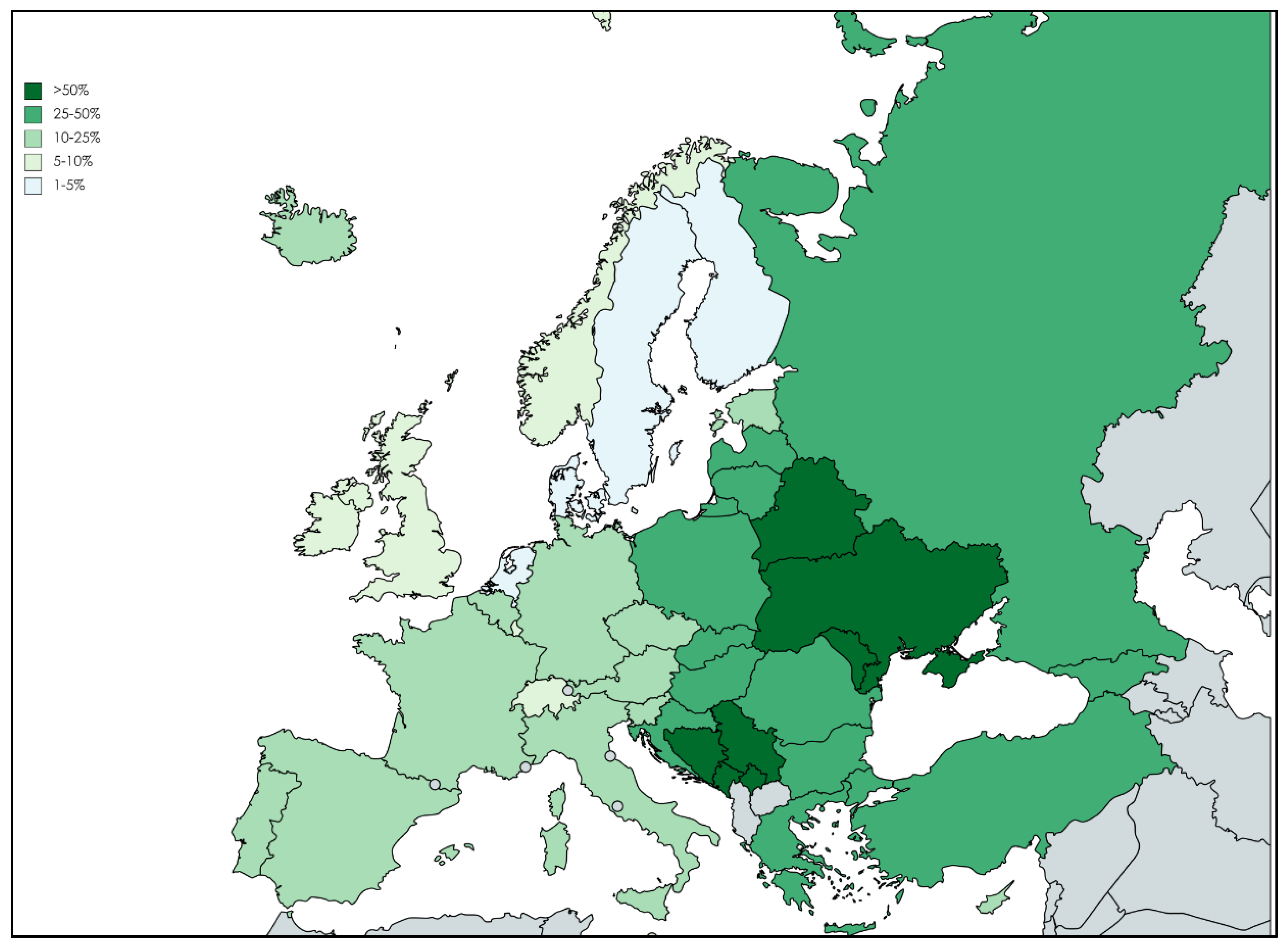
2. Epidemiology
3. Ceftolozane-Tazobactam
4. Ceftazidime-Avibactam
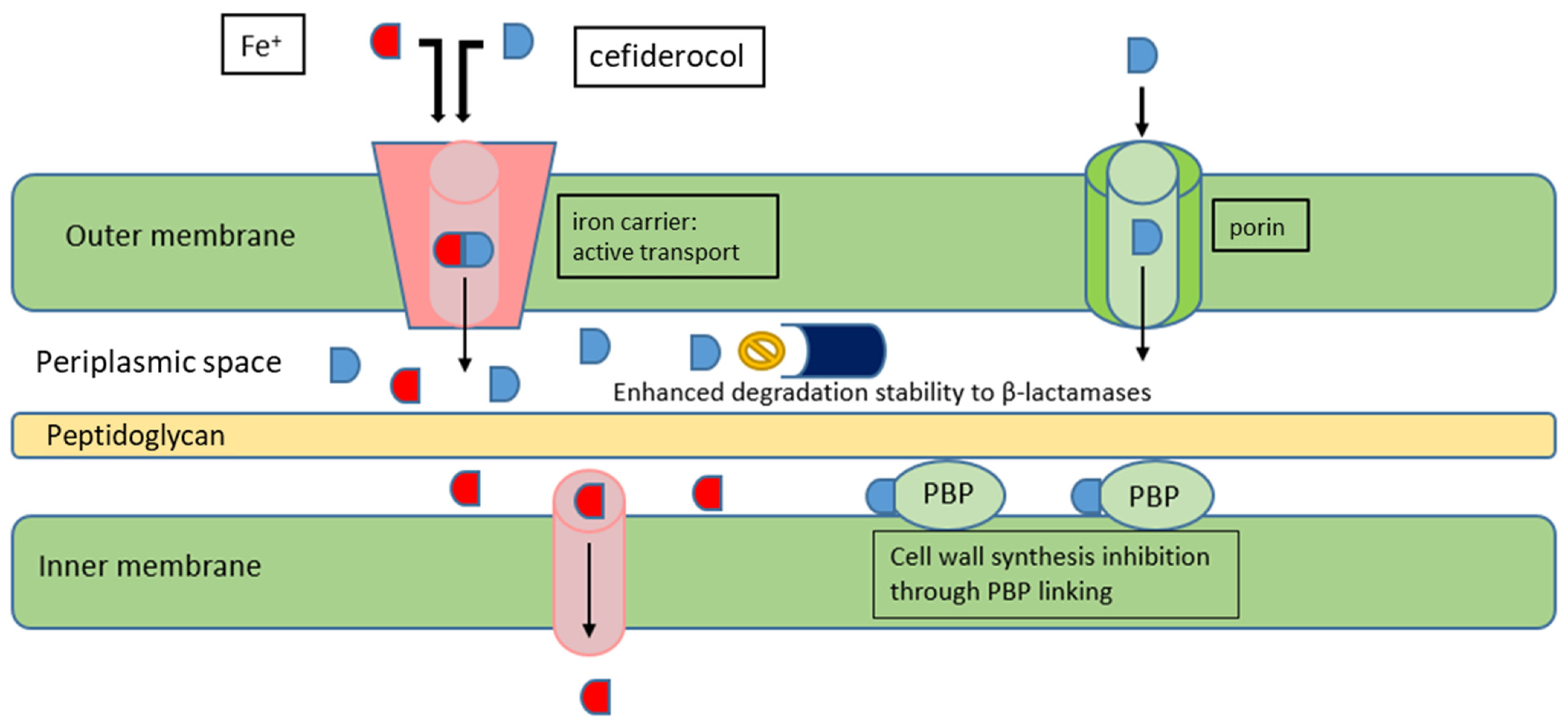
5. Cefiderocol
6. Imipenem-Cilastatin-Relebactam
7. Meropenem-Vaborbactam
8. New β-Lactamase Inhibitor Combinations
8.1. Cefepime-Taniborbactam
8.2. Cefepime-Zidebactam
8.3. Meropenem-Nacubactam
9. Plazomicin
10. Fosfomycin: Combination Strategy
11. Treatment Strategies
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. Difficult-to-Treat Resistance in Gram-negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-line Agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar] [CrossRef] [Green Version]
- Behzadi, P.; Baráth, Z.; Gajdács, M. It’s Not Easy Being Green: A Narrative Review on the Microbiology, Virulence and Therapeutic Prospects of Multidrug-Resistant Pseudomonas aeruginosa. Antibiotics 2021, 10, 42. [Google Scholar] [CrossRef]
- WHO World Health Organization. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 30 March 2022).
- Healthcare-Associated Infections Acquired in Intensive Care Units. Annual Epidemiological Report for 2017; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2019; Available online: https://www.ecdc.europa.eu/en/publications-data/healthcare-associated-infections-intensive-care-units-annual-epidemiological-1 (accessed on 30 March 2022).
- Russotto, V.; Cortegiani, A.; Raineri, S.M.; Giarratano, A. Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. J. Intensive Care 2015, 3, 54. [Google Scholar] [CrossRef] [Green Version]
- WHO Regional Office for Europe/European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2022–2020 Data; WHO Regional Office for Europe: Copenhagen, Denmark, 2022; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/ECDC-WHO-AMR-report.pdf (accessed on 30 March 2022).
- Del Barrio-Tofiño, E.; López-Causapé, C.; Oliver, A. Pseudomonas aeruginosa epidemic high-risk clones and their association with horizontally-acquired β-lactamases: 2020 update. Int. J. Antimicrob. Agents 2020, 56, 106196. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Resistance & Patient Safety Portal. Available online: https://arpsp.cdc.gov/profile/antibiotic-resistance/mdr-pseudomonas-aeruginosa (accessed on 30 March 2022).
- Jarlier, V.; Diaz Högberg, L.; Heuer, O.E.; Campos, J.; Eckmanns, T.; Giske, C.G.; Grundmann, H.; Johnson, A.P.; Kahlmeter, G.; Monen, J.; et al. Strong correlation between the rates of intrinsically antibiotic-resistant species and the rates of acquired resistance in Gram-negative species causing bacteraemia, EU/EEA, 2016. Eurosurveillance 2019, 24, 1800538. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial consumption. In Annual Epidemiological Report for 2019; ECDC, Ed.; ECDC: Stockholm, Sweden, 2020; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Antimicrobial-consumption-in-the-EU-Annual-Epidemiological-Report-2019.pdf (accessed on 30 March 2022).
- López-Jácome, L.E.; Fernández-Rodríguez, D.; Franco-Cendejas, R.; Camacho-Ortiz, A.; Morfin-Otero, M.D.R.; Rodríguez-Noriega, E.; Ponce-de-León, A.; Ortiz-Brizuela, E.; Rojas-Larios, F.; Velázquez-Acosta, M.D.C.; et al. Increment Antimicrobial Resistance During the COVID-19 Pandemic: Results from the Invifar Network. Microb. Drug Resist. 2022, 28, 338–345. [Google Scholar] [PubMed]
- Kunz Coyne, A.J.; El Ghali, A.; Holger, D.; Rebold, N.; Rybak, M.J. Therapeutic Strategies for Emerging Multidrug-Resistant Pseudomonas aeruginosa. Infect. Dis. Ther. 2022, 11, 661–682. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Burden of AMR Collaborative Group. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Tabak, Y.P.; Merchant, S.; Ye, G.; Vankeepuram, L.; Gupta, V.; Kurtz, S.G.; Puzniak, L.A. Incremental clinical and economic burden of suspected respiratory infections due to multi-drug-resistant Pseudomonas aeruginosa in the United States. J. Hosp. Infect. 2019, 103, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Bassetti, M.; De Rosa, F.G.; Del Bono, V.; Grossi, P.A.; Menichetti, F.; Pea, F.; Rossolini, G.M.; Tumbarello, M.; Viale, P.; et al. Ceftolozane/tazobactam: Place in therapy. Expert Rev. Anti. Infect. Ther. 2018, 16, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Murano, K.; Yamanaka, T.; Toda, A.; Ohki, H.; Okuda, S.; Kawabata, K.; Hatano, K.; Takeda, S.; Akamatsu, H.; Itoh, K.; et al. Structural requirements for the stability of novel cephalosporins to AmpC beta-lactamase based on 3D-structure. Bioorg. Med. Chem. 2008, 16, 2261–2275. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Bonomo, R.A. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: Second-generation β-Lactam/β-Lactamase Inhibitor Combinations. Clin. Infect. Dis. 2016, 63, 234–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haidar, G.; Philips, N.J.; Shields, R.K.; Snyder, D.; Cheng, S.; Potoski, B.A.; Doi, Y.; Hao, B.; Press, E.G.; Cooper, V.S.; et al. Ceftolozane-Tazobactam for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Infections: Clinical Effectiveness and Evolution of Resistance. Clin. Infect. Dis. 2017, 65, 110–120. [Google Scholar] [CrossRef]
- Rubio, A.M.; Kline, E.G.; Jones, C.E.; Chen, L.; Kreiswirth, B.N.; Nguyen, M.H.; Clancy, C.J.; Cooper, V.S.; Haidar, G.; Van Tyne, D.; et al. In Vitro Susceptibility of Multidrug-Resistant Pseudomonas aeruginosa following Treatment-Emergent Resistance to Ceftolozane-Tazobactam. Antimicrob. Agents Chemother. 2021, 65, e00084-21. [Google Scholar] [CrossRef]
- Fraile-Ribot, P.A.; Mulet, X.; Cabot, G.; Del Barrio-Tofiño, E.; Juan, C.; Pérez, J.L.; Oliver, A. In Vivo Emergence of Resistance to Novel Cephalosporin-β-Lactamase Inhibitor Combinations through the Duplication of Amino Acid D149 from OXA-2 β-Lactamase (OXA-539) in Sequence Type 235 Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2017, 61, e01117-17. [Google Scholar] [CrossRef] [Green Version]
- Arca-Suárez, J.; Fraile-Ribot, P.; Vázquez-Ucha, J.C.; Cabot, G.; Martínez-Guitián, M.; Lence, E.; González-Bello, C.; Beceiro, A.; Rodríguez-Iglesias, M.; Galán-Sánchez, F.; et al. Challenging Antimicrobial Susceptibility and Evolution of Resistance (OXA-681) during Treatment of a Long-Term Nosocomial Infection Caused by a Pseudomonas aeruginosa ST175 Clone. Antimicrob. Agents Chemother. 2019, 63, e01110-19. [Google Scholar] [CrossRef] [Green Version]
- Arca-Suárez, J.; Lasarte-Monterrubio, C.; Rodiño-Janeiro, B.K.; Cabot, G.; Vázquez-Ucha, J.C.; Rodríguez-Iglesias, M.; Galán-Sánchez, F.; Beceiro, A.; González-Bello, C.; Oliver, A.; et al. Molecular mechanisms driving the in vivo development of OXA-10-mediated resistance to ceftolozane/tazobactam and ceftazidime/avibactam during treatment of XDR Pseudomonas aeruginosa infections. J. Antimicrob. Chemother. 2021, 76, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Mills, J.C.; Farrell, D.J.; Jones, R.N. Mutation-driven β-lactam resistance mechanisms among contemporary ceftazidime-nonsusceptible Pseudomonas aeruginosa isolates from U.S. hospitals. Antimicrob. Agents Chemother. 2014, 58, 6844–6850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livermore, D.M.; Mushtaq, S.; Meunier, D.; Hopkins, K.L.; Hill, R.; Adkin, R.; Chaudhry, A.; Pike, R.; Staves, P.; Woodford, N. BSAC Resistance Surveillance Standing Committee. Activity of ceftolozane/tazobactam against surveillance and ‘problem’ Enterobacteriaceae, Pseudomonas aeruginosa and non-fermenters from the British Isles. J. Antimicrob. Chemother. 2017, 72, 2278–2289. [Google Scholar] [CrossRef] [Green Version]
- Gomis-Font, M.A.; Pitart, C.; Del Barrio-Tofiño, E.; Zboromyrska, Y.; Cortes-Lara, S.; Mulet, X.; Marco, F.; Vila, J.; López-Causapé, C.; Oliver, A. Emergence of Resistance to Novel Cephalosporin-β-Lactamase Inhibitor Combinations through the Modification of the Pseudomonas aeruginosa MexCD-OprJ Efflux Pump. Antimicrob. Agents Chemother. 2021, 65, e0008921. [Google Scholar] [CrossRef]
- Velez Perez, A.L.; Schmidt-Malan, S.M.; Kohner, P.C.; Karau, M.J.; Greenwood-Quaintance, K.E.; Patel, R. In vitro activity of ceftolozane/tazobactam against clinical isolates of Pseudomonas aeruginosa in the planktonic and biofilm states. Diagn. Microbiol. Infect. Dis. 2016, 85, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Junyent, J.; Benavent, E.; Sierra, Y.; El Haj, C.; Soldevila, L.; Torrejón, B.; Rigo-Bonnin, R.; Tubau, F.; Ariza, J.; Murillo, O. Efficacy of ceftolozane/tazobactam, alone and in combination with colistin, against multidrug-resistant Pseudomonas aeruginosa in an in vitro biofilm pharmacodynamic model. Int. J. Antimicrob. Agents 2019, 53, 612–619. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; Umeh, O.; Steenbergen, J.; Yuan, G.; Darouiche, R.O. Ceftolozane-tazobactam compared with levofloxacin in the treatment of complicated urinary-tract infections, including pyelonephritis: A randomised, double-blind, phase 3 trial (ASPECT-cUTI). Lancet 2015, 385, 1949–1956. [Google Scholar] [CrossRef]
- Solomkin, J.; Hershberger, E.; Miller, B.; Popejoy, M.; Friedland, I.; Steenbergen, J.; Yoon, M.; Collins, S.; Yuan, G.; Barie, P.S.; et al. Ceftolozane/Tazobactam Plus Metronidazole for Complicated Intra-abdominal Infections in an Era of Multidrug Resistance: Results From a Randomized, Double-Blind, Phase 3 Trial (ASPECT-cIAI). Clin. Infect. Dis. 2015, 60, 1462–1471. [Google Scholar] [CrossRef] [Green Version]
- Kollef, M.H.; Nováček, M.; Kivistik, Ü.; Réa-Neto, Á.; Shime, N.; Martin-Loeches, I.; Timsit, J.F.; Wunderink, R.G.; Bruno, C.J.; Huntington, J.A.; et al. Ceftolozane-tazobactam versus meropenem for treatment of nosocomial pneumonia (ASPECT-NP): A randomised, controlled, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2019, 19, 1299–1311. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2021, 72, 1109–1116. [Google Scholar]
- Gallagher, J.C.; Satlin, M.J.; Elabor, A.; Saraiya, N.; McCreary, E.K.; Molnar, E.; El-Beyrouty, C.; Jones, B.M.; Dixit, D.; Heil, E.L.; et al. Ceftolozane-Tazobactam for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Infections: A Multicenter Study. Open Forum Infect. Dis. 2018, 5, ofy280. [Google Scholar] [CrossRef]
- Pogue, J.M.; Kaye, K.S.; Veve, M.P.; Patel, T.S.; Gerlach, A.T.; Davis, S.L.; Puzniak, L.A.; File, T.M.; Olson, S.; Dhar, S.; et al. Ceftolozane/Tazobactam vs Polymyxin or Aminoglycoside-based Regimens for the Treatment of Drug-resistant Pseudomonas aeruginosa. Clin. Infect. Dis. 2020, 71, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Carvalhaes, C.G.; Duncan, L.R.; Flamm, R.K.; Shortridge, D. Susceptibility trends of ceftolozane/tazobactam and comparators when tested against European Gram-negative bacterial surveillance isolates collected during 2012–2018. J. Antimicrob. Chemother. 2020, 75, 2907–2913. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, S.; García-Castillo, M.; Bou, G.; Calvo, J.; Cercenado, E.; Delgado, M.; Pitart, C.; Mulet, X.; Tormo, N.; Mendoza, D.L.; et al. Activity of ceftolozane/tazobactam against Pseudomonas aeruginosa and Enterobacterales isolates recovered from intensive care unit patients in Spain: The SUPERIOR multicentre study. Int. J. Antimicrob. Agents 2019, 53, 682–688. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Trinh, T.D.; Zasowski, E.J.; Lagnf, A.M.; Simon, S.P.; Bhatia, S.; Melvin, S.M.; Steed, M.E.; Finch, N.A.; Morrisette, T.; et al. Real-World Experience with Ceftolozane-Tazobactam for Multidrug-Resistant Gram-Negative Bacterial Infections. Antimicrob. Agents Chemother. 2020, 64, e02291-19. [Google Scholar] [CrossRef]
- Sader, H.S.; Duncan, L.R.; Doyle, T.B.; Castanheira, M. Antimicrobial activity of ceftazidime/avibactam, ceftolozane/tazobactam and comparator agents against Pseudomonas aeruginosa from cystic fibrosis patients. JAC Antimicrob. Resist. 2021, 3, dlab126. [Google Scholar] [CrossRef]
- Sader, H.S.; Carvalhaes, C.G.; Shortridge, D.; Castanheira, M. Comparative activity of newer β-lactam/β-lactamase inhibitor combinations against Pseudomonas aeruginosa from patients hospitalized with pneumonia in European medical centers in 2020. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 319–324. [Google Scholar] [CrossRef]
- Bassetti, M.; Castaldo, N.; Cattelan, A.; Mussini, C.; Righi, E.; Tascini, C.; Menichetti, F.; Mastroianni, C.M.; Tumbarello, M.; Grossi, P.; et al. Ceftolozane/tazobactam for the treatment of serious Pseudomonas aeruginosa infections: A multicentre nationwide clinical experience. Int. J. Antimicrob. Agents 2019, 53, 408–415. [Google Scholar] [CrossRef]
- Balandin, B.; Ballesteros, D.; Ruiz de Luna, R.; López-Vergara, L.; Pintado, V.; Sancho-González, M.; Soriano-Cuesta, C.; Pérez-Pedrero, M.J.; Asensio-Martín, M.J.; Fernández-Simón, I.; et al. Multicenter study of ceftolozane/tazobactam for treatment of Pseudomonas aeruginosa infections in critically ill patients. Int. J. Antimicrob. Agents 2021, 57, 106270. [Google Scholar] [CrossRef]
- Fernández-Cruz, A.; Alba, N.; Semiglia-Chong, M.A.; Padilla, B.; Rodríguez-Macías, G.; Kwon, M.; Cercenado, E.; Chamorro-de-Vega, E.; Machado, M.; Pérez-Lago, L.; et al. A Case-Control Study of Real-Life Experience with Ceftolozane-Tazobactam in Patients with Hematologic Malignancy and Pseudomonas aeruginosa Infection. Antimicrob. Agents Chemother. 2019, 63, e02340-18. [Google Scholar] [CrossRef] [Green Version]
- Gatti, M.; Pea, F. Continuous versus intermittent infusion of antibiotics in Gram-negative multidrug-resistant infections. Curr. Opin. Infect. Dis. 2021, 34, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorlí, L.; Luque, S.; Gómez-Zorrilla, S.; Benito, N.; Grau, S. Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections. Clin. Microbiol. Rev. 2019, 32, e00031-19. [Google Scholar] [CrossRef] [PubMed]
- Daikos, G.L.; da Cunha, C.A.; Rossolini, G.M.; Stone, G.G.; Baillon-Plot, N.; Tawadrous, M.; Irani, P. Review of Ceftazidime-Avibactam for the Treatment of Infections Caused by Pseudomonas aeruginosa. Antibiotics 2021, 10, 1126. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Giske, C.G.; Grāmatniece, A.; Abodakpi, H.; Tam, V.H.; Leibovici, L. New β-Lactam-β-Lactamase Inhibitor Combinations. Clin. Microbiol. Rev. 2020, 34, e00115-20. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; Sobel, J.D.; Newell, P.; Armstrong, J.; Huang, X.; Stone, G.G.; Yates, K.; Gasink, L.B. Ceftazidime-avibactam Versus Doripenem for the Treatment of Complicated Urinary Tract Infections, Including Acute Pyelonephritis: RECAPTURE, a Phase 3 Randomized Trial Program. Clin. Infect. Dis. 2016, 63, 754–762. [Google Scholar] [CrossRef] [Green Version]
- Carmeli, Y.; Armstrong, J.; Laud, P.J.; Newell, P.; Stone, G.; Wardman, A.; Gasink, L.B. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): A randomised, pathogen-directed, phase 3 study. Lancet Infect. Dis. 2016, 16, 661–673. [Google Scholar]
- Mazuski, J.E.; Gasink, L.B.; Armstrong, J.; Broadhurst, H.; Stone, G.G.; Rank, D.; Llorens, L.; Newell, P.; Pachl, J. Efficacy and Safety of Ceftazidime-Avibactam Plus Metronidazole Versus Meropenem in the Treatment of Complicated Intra-abdominal Infection: Results From a Randomized, Controlled, Double-Blind, Phase 3 Program. Clin. Infect. Dis. 2016, 62, 1380–1389. [Google Scholar] [CrossRef]
- Qin, X.; Tran, B.G.; Kim, M.J.; Wang, L.; Nguyen, D.A.; Chen, Q.; Song, J.; Laud, P.J.; Stone, G.G.; Chow, J.W. A randomised, double-blind, phase 3 study comparing the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem for complicated intra-abdominal infections in hospitalised adults in Asia. Int. J. Antimicrob. Agents 2017, 49, 579–588. [Google Scholar] [CrossRef]
- Torres, A.; Zhong, N.; Pachl, J.; Timsit, J.F.; Kollef, M.; Chen, Z.; Song, J.; Taylor, D.; Laud, P.J.; Stone, G.G.; et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): A randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect. Dis. 2018, 18, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Stone, G.G.; Newell, P.; Gasink, L.B.; Broadhurst, H.; Wardman, A.; Yates, K.; Chen, Z.; Song, J.; Chow, J.W. Clinical activity of ceftazidime/avibactam against MDR Enterobacteriaceae and Pseudomonas aeruginosa: Pooled data from the ceftazidime/avibactam Phase III clinical trial programme. J. Antimicrob. Chemother. 2018, 73, 2519–2523. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Trinh, T.D.; Zasowski, E.J.; Lagnf, A.M.; Bhatia, S.; Melvin, S.M.; Steed, M.E.; Simon, S.P.; Estrada, S.J.; Morrisette, T.; et al. Real-World Experience With Ceftazidime-Avibactam for Multidrug-Resistant Gram-Negative Bacterial Infections. Open Forum Infect. Dis. 2019, 6, ofz522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbella, L.; Boán, J.; San-Juan, R.; Fernández-Ruiz, M.; Carretero, O.; Lora, D.; Hernández-Jiménez, P.; Ruiz-Ruigómez, M.; Rodríguez-Goncer, I.; Silva, J.T.; et al. Effectiveness of ceftazidime-avibactam for the treatment of infections due to Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2022, 59, 106517. [Google Scholar] [CrossRef] [PubMed]
- Vena, A.; Giacobbe, D.R.; Castaldo, N.; Cattelan, A.; Mussini, C.; Luzzati, R.; Rosa, F.G.; Del Puente, F.; Mastroianni, C.M.; Cascio, A.; et al. Clinical Experience with Ceftazidime-Avibactam for the Treatment of Infections due to Multidrug-Resistant Gram-Negative Bacteria Other than Carbapenem-Resistant Enterobacterales. Antibiotics 2020, 9, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soriano, A.; Carmeli, Y.; Omrani, A.S.; Moore, L.S.P.; Tawadrous, M.; Irani, P. Ceftazidime-Avibactam for the Treatment of Serious Gram-Negative Infections with Limited Treatment Options: A Systematic Literature Review. Infect. Dis. Ther. 2021, 10, 1989–2034. [Google Scholar] [CrossRef]
- Nichols, W.W.; de Jonge, B.L.; Kazmierczak, K.M.; Karlowsky, J.A.; Sahm, D.F. In Vitro Susceptibility of Global Surveillance Isolates of Pseudomonas aeruginosa to Ceftazidime-Avibactam (INFORM 2012 to 2014). Antimicrob. Agents Chemother. 2016, 60, 4743–4749. [Google Scholar] [CrossRef] [Green Version]
- Piérard, D.; Stone, G.G. In vitro antimicrobial susceptibility of clinical respiratory isolates to ceftazidime-avibactam and comparators (2016–2018). BMC Infect. Dis. 2021, 21, 600. [Google Scholar] [CrossRef]
- Winkler, M.L.; Papp-Wallace, K.M.; Hujer, A.M.; Domitrovic, T.N.; Hujer, K.M.; Hurless, K.N.; Tuohy, M.; Hall, G.; Bonomo, R.A. Unexpected challenges in treating multidrug-resistant Gram-negative bacteria: Resistance to ceftazidime-avibactam in archived isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2015, 59, 1020–1029. [Google Scholar] [CrossRef] [Green Version]
- Buehrle, D.J.; Shields, R.K.; Chen, L.; Hao, B.; Press, E.G.; Alkrouk, A.; Potoski, B.A.; Kreiswirth, B.N.; Clancy, C.J.; Nguyen, M.H. Evaluation of the In Vitro Activity of Ceftazidime-Avibactam and Ceftolozane-Tazobactam against Meropenem-Resistant Pseudomonas aeruginosa Isolates. Antimicrob. Agents Chemother. 2016, 60, 3227–3231. [Google Scholar] [CrossRef] [Green Version]
- Zamudio, R.; Hijazi, K.; Joshi, C.; Aitken, E.; Oggioni, M.R.; Gould, I.M. Phylogenetic analysis of resistance to ceftazidime/avibactam, ceftolozane/tazobactam and carbapenems in piperacillin/tazobactam-resistant Pseudomonas aeruginosa from cystic fibrosis patients. Int. J. Antimicrob. Agents 2019, 53, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Sid Ahmed, M.A.; Khan, F.A.; Hadi, H.A.; Skariah, S.; Sultan, A.A.; Salam, A.; Al Khal, A.L.; Söderquist, B.; Ibrahim, E.B.; Omrani, A.S.; et al. Association of blaVIM-2, blaPDC-35, blaOXA-10, blaOXA-488 and blaVEB-9 β-Lactamase Genes with Resistance to Ceftazidime-Avibactam and Ceftolozane-Tazobactam in Multidrug-Resistant Pseudomonas aeruginosa. Antibiotics 2022, 11, 130. [Google Scholar] [CrossRef]
- Gatti, M.; Cojutti, P.G.; Pascale, R.; Tonetti, T.; Laici, C.; Dell’Olio, A.; Siniscalchi, A.; Giannella, M.; Viale, P.; Pea, F. Assessment of a PK/PD Target of Continuous Infusion Beta-Lactams Useful for Preventing Microbiological Failure and/or Resistance Development in Critically Ill Patients Affected by Documented Gram-Negative Infections. Antibiotics 2021, 10, 1311. [Google Scholar] [CrossRef]
- Yamano, Y. In Vitro Activity of Cefiderocol Against a Broad Range of Clinically Important Gram-negative Bacteria. Clin. Infect. Dis. 2019, 69 (Suppl. 7), S544–S551. [Google Scholar] [CrossRef] [Green Version]
- Hackel, M.A.; Tsuji, M.; Yamano, Y.; Echols, R.; Karlowsky, J.A.; Sahm, D.F. In Vitro Activity of the Siderophore Cephalosporin, Cefiderocol, against Carbapenem-Nonsusceptible and Multidrug-Resistant Isolates of Gram-Negative Bacilli Collected Worldwide in 2014 to 2016. Antimicrob. Agents Chemother. 2018, 62, e01968-17. [Google Scholar] [CrossRef] [Green Version]
- Shortridge, D.; Streit, J.M.; Mendes, R.; Castanheira, M. In Vitro Activity of Cefiderocol against U.S. and European Gram-Negative Clinical Isolates Collected in 2020 as Part of the SENTRY Antimicrobial Surveillance Program. Microbiol. Spectr. 2022, e02712-21. [Google Scholar] [CrossRef]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef]
- Wunderink, R.G.; Matsunaga, Y.; Ariyasu, M.; Clevenbergh, P.; Echols, R.; Kaye, K.S.; Kollef, M.; Menon, A.; Pogue, J.M.; Shorr, A.F.; et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): A randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2021, 21, 213–225. [Google Scholar] [CrossRef]
- Timsit, J.F.; Paul, M.; Shields, R.K.; Echols, R.; Baba, T.; Yamano, Y.; Portsmouth, S. Cefiderocol for the Treatment of Infections Due To Metallo-Beta-Lactamase-Producing Pathogens in the CREDIBLE-CR And APEKS-NP Phase 3 Randomized Studies. Clin. Infect. Dis. 2022, ciac078. [Google Scholar] [CrossRef]
- Paterson, D.L.; Kinoshita, M.; Baba, T.; Echols, R.; Portsmouth, S. Outcomes with Cefiderocol Treatment in Patients with Bacteraemia Enrolled into Prospective Phase 2 and Phase 3 Randomised Clinical Studies. Infect. Dis. Ther. 2022, 11, 853–870. [Google Scholar] [CrossRef]
- Zingg, S.; Nicoletti, G.J.; Kuster, S.; Junker, M.; Widmer, A.; Egli, A.; Hinic, V.; Sendi, P.; Battegay, M.; Bättig, V.; et al. Cefiderocol for Extensively Drug-Resistant Gram-Negative Bacterial Infections: Real-world Experience from a Case Series and Review of the Literature. Open Forum Infect. Dis. 2020, 7, ofaa185. [Google Scholar] [CrossRef]
- Meschiari, M.; Volpi, S.; Faltoni, M.; Dolci, G.; Orlando, G.; Franceschini, E.; Menozzi, M.; Sarti, M.; Del Fabro, G.; Fumarola, B.; et al. Real-life experience with compassionate use of cefiderocol for difficult-to-treat resistant Pseudomonas aeruginosa (DTR-P) infections. JAC Antimicrob. Resist. 2021, 3, dlab188. [Google Scholar] [CrossRef]
- Bavaro, D.F.; Belati, A.; Diella, L.; Stufano, M.; Romanelli, F.; Scalone, L.; Stolfa, S.; Ronga, L.; Maurmo, L.; Dell’Aera, M.; et al. Cefiderocol-Based Combination Therapy for “Difficult-to-Treat” Gram-Negative Severe Infections: Real-Life Case Series and Future Perspectives. Antibiotics 2021, 10, 652. [Google Scholar] [CrossRef]
- Bleibtreu, A.; Dortet, L.; Bonnin, R.A.; Wyplosz, B.; Sacleux, S.C.; Mihaila, L.; Dupont, H.; Junot, H.; Bunel, V.; Grall, N.; et al. Susceptibility Testing Is Key for the Success of Cefiderocol Treatment: A Retrospective Cohort Study. Microorganisms 2021, 9, 282. [Google Scholar] [CrossRef]
- Yao, J.; Wang, J.; Chen, M.; Cai, Y. Cefiderocol: An Overview of Its in-vitro and in-vivo Activity and Underlying Resistant Mechanisms. Front. Med. 2021, 8, 741940. [Google Scholar] [CrossRef]
- Streling, A.P.; Al Obaidi, M.M.; Lainhart, W.D.; Zangeneh, T.; Khan, A.; Dinh, A.Q.; Hanson, B.; Arias, C.A.; Miller, W.R. Evolution of Cefiderocol Non-Susceptibility in Pseudomonas aeruginosa in a Patient Without Previous Exposure to the Antibiotic. Clin. Infect. Dis. 2021, 73, e4472–e4474. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Ciacco, E.; Girmenia, C.; Pea, F.; Rossolini, G.M.; Sotgiu, G.; Tascini, C.; Tumbarello, M.; Viale, P.; Bassetti, M. ISGRI-SITA (Italian Study Group on Resistant Infections of the Italian Society of Anti-infective Therapy). Evaluating Cefiderocol in the Treatment of Multidrug-Resistant Gram-Negative Bacilli: A Review of the Emerging Data. Infect. Drug Resist. 2020, 13, 4697–4711. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Nicastro, M.; Leonildi, A.; Vecchione, A.; Casella, C.; Forfori, F.; Malacarne, P.; Guarracino, F.; Barnini, S.; et al. Cefiderocol as Rescue Therapy for Acinetobacter baumannii and Other Carbapenem-resistant Gram-negative Infections in Intensive Care Unit Patients. Clin. Infect. Dis. 2021, 72, 2021–2024. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Lawrence, C.K.; Adam, H.; Schweizer, F.; Zelenitsky, S.; Zhanel, M.; Lagacé-Wiens, P.R.S.; Walkty, A.; Denisuik, A.; Golden, A.; et al. Imipenem-Relebactam and Meropenem-Vaborbactam: Two Novel Carbapenem-β-Lactamase Inhibitor Combinations. Drugs 2018, 78, 65–98. [Google Scholar] [CrossRef]
- Young, K.; Painter, R.E.; Raghoobar, S.L.; Hairston, N.N.; Racine, F.; Wisniewski, D.; Balibar, C.J.; Villafania, A.; Zhang, R.; Sahm, D.F.; et al. In vitro studies evaluating the activity of imipenem in combination with relebactam against Pseudomonas aeruginosa. BMC Microbiol. 2019, 19, 150. [Google Scholar] [CrossRef]
- Livermore, D.M.; Warner, M.; Mushtaq, S. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2013, 68, 2286–2290. [Google Scholar] [CrossRef] [Green Version]
- Lob, S.H.; Karlowsky, J.A.; Young, K.; Motyl, M.R.; Hawser, S.; Kothari, N.D.; Sahm, D.F. In vitro activity of imipenem-relebactam against resistant phenotypes of Enterobacteriaceae and Pseudomonas aeruginosa isolated from intraabdominal and urinary tract infection samples-SMART Surveillance Europe 2015–2017. J. Med. Microbiol. 2020, 69, 207–217. [Google Scholar] [CrossRef]
- Livermore, D.M.; Jamrozy, D.; Mushtaq, S.; Nichols, W.W.; Young, K.; Woodford, N. AmpC β-lactamase induction by avibactam and relebactam. J. Antimicrob. Chemother. 2017, 72, 3342–3348. [Google Scholar] [CrossRef]
- Lapuebla, A.; Abdallah, M.; Olafisoye, O.; Cortes, C.; Urban, C.; Landman, D.; Quale, J. Activity of Imipenem with Relebactam against Gram-Negative Pathogens from New York City. Antimicrob. Agents Chemother. 2015, 59, 5029–5031. [Google Scholar] [CrossRef] [Green Version]
- Lob, S.H.; Karlowsky, J.A.; Young, K.; Motyl, M.R.; Hawser, S.; Kothari, N.D.; Gueny, M.E.; Sahm, D.F. Activity of imipenem/relebactam against MDR Pseudomonas aeruginosa in Europe: SMART 2015–17. J. Antimicrob. Chemother. 2019, 74, 2284–2288. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Lob, S.H.; Young, K.; Motyl, M.R.; Sahm, D.F. Activity of imipenem-relebactam against multidrug-resistant Pseudomonas aeruginosa from the United States-SMART 2015–2017. Diagn. Microbiol. Infect. Dis. 2019, 95, 212–215. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Lob, S.H.; Kazmierczak, K.M.; Young, K.; Motyl, M.R.; Sahm, D.F. In Vitro Activity of Imipenem-Relebactam against Clinical Isolates of Gram-Negative Bacilli Isolated in Hospital Laboratories in the United States as Part of the SMART 2016 Program. Antimicrob. Agents Chemother. 2018, 62, e00169-18. [Google Scholar] [CrossRef] [Green Version]
- Karlowsky, J.A.; Lob, S.H.; Kazmierczak, K.M.; Hawser, S.P.; Magnet, S.; Young, K.; Motyl, M.R.; Sahm, D.F. In vitro activity of imipenem/relebactam against Gram-negative ESKAPE pathogens isolated in 17 European countries: 2015 SMART surveillance programme. J. Antimicrob. Chemother. 2018, 73, 1872–1879. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Lob, S.H.; Young, K.; Motyl, M.R.; Sahm, D.F. Activity of imipenem/relebactam against Pseudomonas aeruginosa with antimicrobial-resistant phenotypes from seven global regions: SMART 2015–2016. J. Glob. Antimicrob. Resist. 2018, 15, 140–147. [Google Scholar] [CrossRef]
- Lob, S.H.; Hackel, M.A.; Kazmierczak, K.M.; Young, K.; Motyl, M.R.; Karlowsky, J.A.; Sahm, D.F. In Vitro Activity of Imipenem-Relebactam against Gram-Negative ESKAPE Pathogens Isolated by Clinical Laboratories in the United States in 2015 (Results from the SMART Global Surveillance Program). Antimicrob. Agents Chemother. 2017, 61, e02209-16. [Google Scholar] [CrossRef] [Green Version]
- Karlowsky, J.A.; Lob, S.H.; Raddatz, J.; DePestel, D.D.; Young, K.; Motyl, M.R.; Sahm, D.F. In Vitro Activity of Imipenem/Relebactam and Ceftolozane/Tazobactam Against Clinical Isolates of Gram-negative Bacilli With Difficult-to-Treat Resistance and Multidrug-resistant Phenotypes-Study for Monitoring Antimicrobial Resistance Trends, United States 2015–2017. Clin. Infect. Dis. 2021, 72, 2112–2120. [Google Scholar]
- Karlowsky, J.A.; Lob, S.H.; Kazmierczak, K.M.; Young, K.; Motyl, M.R.; Sahm, D.F. In vitro activity of imipenem/relebactam against Enterobacteriaceae and Pseudomonas aeruginosa isolated from intraabdominal and urinary tract infection samples: SMART Surveillance United States 2015–2017. J. Glob. Antimicrob. Resist. 2020, 21, 223–228. [Google Scholar] [CrossRef]
- Walkty, A.; Karlowsky, J.A.; Baxter, M.R.; Adam, H.J.; Golden, A.; Lagace-Wiens, P.; Zhanel, G.G. Canadian Antimicrobial Resistance Alliance (CARA). In vitro activity of imipenem-relebactam against various resistance phenotypes/genotypes of Enterobacterales and Pseudomonas aeruginosa isolated from patients across Canada as part of the CANWARD study, 2016–2019. Diagn. Microbiol. Infect. Dis. 2021, 101, 115418. [Google Scholar]
- Lob, S.H.; DePestel, D.D.; DeRyke, C.A.; Kazmierczak, K.M.; Young, K.; Motyl, M.R.; Sahm, D.F. Ceftolozane/Tazobactam and Imipenem/Relebactam Cross-Susceptibility Among Clinical Isolates of Pseudomonas aeruginosa From Patients With Respiratory Tract Infections in ICU and Non-ICU Wards-SMART United States 2017–2019. Open Forum Infect. Dis. 2021, 8, ofab320. [Google Scholar] [CrossRef]
- Gomis-Font, M.A.; Cabot, G.; López-Argüello, S.; Zamorano, L.; Juan, C.; Moyá, B.; Oliver, A. Comparative analysis of in vitro dynamics and mechanisms of ceftolozane/tazobactam and imipenem/relebactam resistance development in Pseudomonas aeruginosa XDR high-risk clones. J. Antimicrob. Chemother. 2022, dkab496. [Google Scholar] [CrossRef]
- Noel, A.R.; Bowker, K.E.; Attwood, M.; MacGowan, A.P. Antibacterial effect of imipenem/relebactam on aerobic Gram-negative bacilli: In vitro simulations of 7 or 14 day human exposures. J. Antimicrob. Chemother. 2019, 74, 1945–1951. [Google Scholar] [CrossRef]
- Reyes, S.; Abdelraouf, K.; Nicolau, D.P. In vivo activity of human-simulated regimens of imipenem alone and in combination with relebactam against Pseudomonas aeruginosa in the murine thigh infection model. J. Antimicrob. Chemother. 2020, 75, 2197–2205. [Google Scholar] [CrossRef]
- Titov, I.; Wunderink, R.G.; Roquilly, A.; Rodríguez Gonzalez, D.; David-Wang, A.; Boucher, H.W.; Kaye, K.S.; Losada, M.C.; Du, J.; Tipping, R.; et al. A Randomized, Double-blind, Multicenter Trial Comparing Efficacy and Safety of Imipenem/Cilastatin/Relebactam Versus Piperacillin/Tazobactam in Adults With Hospital-acquired or Ventilator-associated Bacterial Pneumonia (RESTORE-IMI 2 Study). Clin. Infect. Dis. 2021, 73, e4539–e4548. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Approves New Treatment for Complicated Urinary Tract and Complicated Intra-abdominal Infections. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-complicated-urinary-tract-and-complicated-intra-abdominal-infections (accessed on 30 March 2022).
- European Medicines Agency. Recarbrio: EPAR-Product Information. Available online: https://www.ema.europa.eu/en/documents/product-information/recarbrio-epar-product-information_en.pdf (accessed on 30 March 2022).
- Motsch, J.; Murta de Oliveira, C.; Stus, V.; Köksal, I.; Lyulko, O.; Boucher, H.W.; Kaye, K.S.; File, T.M.; Brown, M.L.; Khan, I.; et al. RESTORE-IMI 1: A Multicenter, Randomized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs Colistin Plus Imipenem in Patients With Imipenem-nonsusceptible Bacterial Infections. Clin. Infect. Dis. 2020, 70, 1799–1808. [Google Scholar] [CrossRef] [Green Version]
- Rebold, N.; Morrisette, T.; Lagnf, A.M.; Alosaimy, S.; Holger, D.; Barber, K.; Justo, J.A.; Antosz, K.; Carlson, T.J.; Frens, J.J.; et al. Early Multicenter Experience with Imipenem-Cilastatin-Relebactam for Multidrug-Resistant Gram-Negative Infections. Open Forum Infect. Dis. 2021, 8, ofab554. [Google Scholar] [CrossRef]
- Noval, M.; Banoub, M.; Claeys, K.C.; Heil, E. The Battle Is on: New Beta-Lactams for the Treatment of Multidrug-Resistant Gram-Negative Organisms. Curr. Infect. Dis. Rep. 2020, 22, 1. [Google Scholar] [CrossRef]
- Lapuebla, A.; Abdallah, M.; Olafisoye, O.; Cortes, C.; Urban, C.; Quale, J.; Landman, D. Activity of Meropenem Combined with RPX7009, a Novel β-Lactamase Inhibitor, against Gram-Negative Clinical Isolates in New York City. Antimicrob. Agents Chemother. 2015, 59, 4856–4860. [Google Scholar] [CrossRef] [Green Version]
- Novelli, A.; Del Giacomo, P.; Rossolini, G.M.; Tumbarello, M. Meropenem/vaborbactam: A next generation β-lactam β-lactamase inhibitor combination. Expert Rev. Anti. Infect. Ther. 2020, 18, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Sabet, M.; Tarazi, Z.; Griffith, D.C. Activity of Meropenem-Vaborbactam against Pseudomonas aeruginosa and Acinetobacter baumannii in a Neutropenic Mouse Thigh Infection Model. Antimicrob. Agents Chemother. 2018, 63, e01665-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalhaes, C.G.; Shortridge, D.; Sader, H.S.; Castanheira, M. Activity of Meropenem-Vaborbactam against Bacterial Isolates Causing Pneumonia in Patients in U.S. Hospitals during 2014 to 2018. Antimicrob. Agents Chemother. 2020, 64, e02177-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shortridge, D.; Carvalhaes, C.; Deshpande, L.; Castanheira, M. Activity of meropenem/vaborbactam and comparators against Gram-negative isolates from Eastern and Western European patients hospitalized with pneumonia including ventilator-associated pneumonia (2014–19). J. Antimicrob. Chemother. 2021, 76, 2600–2605. [Google Scholar] [CrossRef]
- Carsenti-Etesse, H.; Cavallo, J.D.; Roger, P.M.; Ziha-Zarifi, I.; Plesiat, P.; Garrabé, E.; Dellamonica, P. Effect of beta-lactam antibiotics on the in vitro development of resistance in Pseudomonas aeruginosa. Clin. Microbiol. Infect. 2001, 7, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Hocquet, D.; Nordmann, P.; El Garch, F.; Cabanne, L.; Plésiat, P. Involvement of the MexXY-OprM efflux system in emergence of cefepime resistance in clinical strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 1347–1351. [Google Scholar] [CrossRef] [Green Version]
- Livermore, D.M.; Williams, R.J.; Lindridge, M.A.; Slack, R.C.; Williams, J.D. Pseudomonas aeruginosa isolates with modified beta-lactamase inducibility: Effects on beta-lactam sensitivity. Lancet 1982, 1, 1466–1467. [Google Scholar] [CrossRef]
- Jean, S.S.; Ko, W.C.; Lu, M.C.; Lee, W.S.; Hsueh, P.R. Multicenter surveillance of in vitro activities of cefepime-zidebactam, cefepime-enmetazobactam, omadacycline, eravacycline, and comparator antibiotics against Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumannii complex causing bloodstream infection in Taiwan, 2020. Expert Rev. Anti. Infect. Ther. 2022, 1–13. [Google Scholar] [CrossRef]
- Mushtaq, S.; Vickers, A.; Doumith, M.; Ellington, M.J.; Woodford, N.; Livermore, D.M. Activity of β-lactam/taniborbactam (VNRX-5133) combinations against carbapenem-resistant Gram-negative bacteria. J. Antimicrob. Chemother. 2021, 76, 160–170. [Google Scholar] [CrossRef]
- Hamrick, J.C.; Docquier, J.D.; Uehara, T.; Myers, C.L.; Six, D.A.; Chatwin, C.L.; John, K.J.; Vernacchio, S.F.; Cusick, S.M.; Trout, R.E.L.; et al. VNRX-5133 (Taniborbactam), a Broad-Spectrum Inhibitor of Serine- and Metallo-β-Lactamases, Restores Activity of Cefepime in Enterobacterales and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2020, 64, e01963-19. [Google Scholar] [CrossRef] [Green Version]
- Kloezen, W.; Melchers, R.J.; Georgiou, P.C.; Mouton, J.W.; Meletiadis, J. Activity of Cefepime in Combination with the Novel β-Lactamase Inhibitor Taniborbactam (VNRX-5133) against Extended-Spectrum-β-Lactamase-Producing Isolates in In Vitro Checkerboard Assays. Antimicrob. Agents Chemother. 2021, 65, e02338-20. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, M.; García-Castillo, M.; Ruiz-Garbajosa, P.; Bou, G.; Siller-Ruiz, M.; Pitart, C.; Gracia-Ahufinger, I.; Mulet, X.; Pascual, Á.; Tormo, N.; et al. In vitro activity of cefepime-taniborbactam against carbapenemase producing Enterobacterales and Pseudomonas aeruginosa isolates recovered in Spain. Antimicrob. Agents Chemother. 2022, aac0216121. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; Paranos, P.; Georgiou, P.C.; Vourli, S.; Antonopoulou, S.; Michelaki, A.; Vagiakou, E.; Pournaras, S. In vitro comparative activity of the new beta-lactamase inhibitor taniborbactam with cefepime or meropenem against Klebsiella pneumoniae and cefepime against Pseudomonas aeruginosa metallo-beta-lactamase-producing clinical isolates. Int. J. Antimicrob. Agents 2021, 58, 106440. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Safety and Pharmacokinetics of VNRX-5133 in the Epithelial Lining Fluid of Healthy Adult Subjects. Available online: https://clinicaltrials.gov/ct2/show/NCT03870490 (accessed on 30 March 2022).
- ClinicalTrials.gov. VNRX-5133 with VNRX-5022 in Subjects with Varying Degrees of Renal Impairment. Available online: https://clinicaltrials.gov/ct2/show/NCT03690362 (accessed on 30 March 2022).
- ClinicalTrials.gov. VNRX-5133 SAD/MAD Safety and PK in Healthy Adult Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT02955459 (accessed on 30 March 2022).
- ClinicalTrials.gov. VNRX-5133 Drug-Drug Interaction in Healthy Adult Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT03332732 (accessed on 30 March 2022).
- ClinicalTrials.gov. Safety and Efficacy Study of Cefepime/VNRX-5133 in Patients with Complicated Urinary Tract Infections. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03840148 (accessed on 30 March 2022).
- Lasko, M.J.; Nicolau, D.P.; Asempa, T.E. Clinical exposure-response relationship of cefepime/taniborbactam against Gram-negative organisms in the murine complicated urinary tract infection model. J. Antimicrob. Chemother. 2022, 77, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.S.; Legakis, N.J.; Skalidis, T.; Loannidis, A.; Goumenopoulos, C.; Joshi, P.R.; Shrivastava, R.; Palwe, S.R.; Periasamy, H.; Patel, M.V.; et al. In vitro activity of cefepime/zidebactam (WCK 5222) against recent Gram-negative isolates collected from high resistance settings of Greek hospitals. Diagn. Microbiol. Infect. Dis. 2021, 100, 115327. [Google Scholar] [CrossRef]
- Almarzoky Abuhussain, S.S.; Avery, L.M.; Abdelraouf, K.; Nicolau, D.P. In Vivo Efficacy of Humanized WCK 5222 (Cefepime-Zidebactam) Exposures against Carbapenem-Resistant Acinetobacter baumannii in the Neutropenic Thigh Model. Antimicrob. Agents Chemother. 2018, 63, e01931-18. [Google Scholar] [CrossRef] [Green Version]
- Mullane, E.M.; Avery, L.M.; Nicolau, D.P. Comparative Evaluation of the In Vitro Activities of WCK 5222 (Cefepime-Zidebactam) and Combination Antibiotic Therapies against Carbapenem-Resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2020, 64, e01669-19. [Google Scholar] [CrossRef]
- Avery, L.M.; Abdelraouf, K.; Nicolau, D.P. Assessment of the In Vivo Efficacy of WCK 5222 (Cefepime-Zidebactam) against Carbapenem-Resistant Acinetobacter baumannii in the Neutropenic Murine Lung Infection Model. Antimicrob. Agents Chemother. 2018, 62, e00948-18. [Google Scholar] [CrossRef] [Green Version]
- Sader, H.S.; Castanheira, M.; Huband, M.; Jones, R.N.; Flamm, R.K. WCK 5222 (Cefepime-Zidebactam) Antimicrobial Activity against Clinical Isolates of Gram-Negative Bacteria Collected Worldwide in 2015. Antimicrob. Agents Chemother. 2017, 61, e00072-17. [Google Scholar] [CrossRef] [Green Version]
- Karlowsky, J.A.; Hackel, M.A.; Bouchillon, S.K.; Sahm, D.F. In vitro activity of WCK 5222 (cefepime-zidebactam) against worldwide collected Gram-negative bacilli not susceptible to carbapenems. Antimicrob. Agents Chemother. 2020, 64, e01432-20. [Google Scholar]
- Mushtaq, S.; Garello, P.; Vickers, A.; Woodford, N.; Livermore, D.M. Activity of cefepime/zidebactam (WCK 5222) against ‘problem’ antibiotic-resistant Gram-negative bacteria sent to a national reference laboratory. J. Antimicrob. Chemother. 2021, 76, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y.; Yin, D.; Zheng, Y.; Wu, S.; Zhu, D.; Hu, F.; on behalf of the China Antimicrobial Surveillance Network (CHINET) Study Group. In vitro activity of cefepime-zidebactam, ceftazidime-avibactam, and other comparators against clinical isolates of Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumannii: Results from China Antimicrobial Surveillance Network (CHINET) in 2018. Antimicrob. Agents Chemother. 2021, 65, e01726-20. [Google Scholar]
- Bhagwat, S.S.; Periasamy, H.; Takalkar, S.S.; Palwe, S.R.; Khande, H.N.; Patel, M.V. The Novel β-Lactam Enhancer Zidebactam Augments the In Vivo Pharmacodynamic Activity of Cefepime in a Neutropenic Mouse Lung Acinetobacter baumannii Infection Model. Antimicrob. Agents Chemother. 2019, 63, e02146-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moya, B.; Barcelo, I.M.; Bhagwat, S.; Patel, M.; Bou, G.; Papp-Wallace, K.M.; Bonomo, R.A.; Oliver, A. WCK 5107 (zidebactam) and WCK 5153 are novel inhibitors of PBP2 showing potent “β-lactam enhancer” activity against Pseudomonas aeruginosa, including multidrug-resistant metallo-β-lactamase-producing high-risk clones. Antimicrob. Agents Chemother. 2017, 61, e02529-16. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov. Study of Cefepime-zidebactam (FEP-ZID) in Complicated Urinary Tract Infection (cUTI) or Acute Pyelonephritis (AP). Available online: https://clinicaltrials.gov/ct2/show/NCT04979806 (accessed on 30 March 2022).
- Isler, B.; Harris, P.; Stewart, A.G.; Paterson, D.L. An update on cefepime and its future role in combination with novel beta-lactamase inhibitors for MDR Enterobacterales and Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2021, 76, 550–560. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, X.; Song, Y.; Ren, H.; Tian, Z.; Liang, Q.; Jin, Y.; Bai, F.; Cheng, Z.; Feng, J.; et al. Molecular Characterization of WCK 5222 (Cefepime/Zidebactam)-Resistant Mutants Developed from a Carbapenem-Resistant Pseudomonas aeruginosa Clinical Isolate. Microbiol. Spectr. 2022, 10, e0267821. [Google Scholar] [CrossRef]
- Barceló, I.; Cabot, G.; Palwe, S.; Joshi, P.; Takalkar, S.; Periasamy, H.; Cortés-Lara, S.; Zamorano, L.; Sánchez-Diener, I.; Moya, B.; et al. In vitro evolution of cefepime/zidebactam (WCK 5222) resistance in Pseudomonas aeruginosa: Dynamics, mechanisms, fitness trade-off and impact on in vivo efficacy. J. Antimicrob. Chemother. 2021, 76, 2546–2557. [Google Scholar] [CrossRef]
- World Health Organization. 2019 Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline. Available online: https://apps.who.int/iris/bitstream/handle/10665/330420/9789240000193-eng.pdf (accessed on 11 April 2022).
- Asempa, T.E.; Motos, A.; Abdelraouf, K.; Bissantz, C.; Zampaloni, C.; Nicolau, D.P. Meropenem-nacubactam activity against AmpC-overproducing and KPC-expressing Pseudomonas aeruginosa in a neutropenic murine lung infection model. Int. J. Antimicrob. Agents 2020, 55, 105838. [Google Scholar] [CrossRef]
- Bouza, E. The role of new carbapenem combinations in the treatment of multidrug-resistant Gram-negative infections. J. Antimicrob. Chemother. 2021, 76 (Suppl. 4), iv38–iv45. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. ZEMDRI® Prescribing Information. Available online: https://zemdri.com/assets/pdf/Prescribing-Information.pdf (accessed on 30 March 2022).
- Saravolatz, L.D.; Stein, G.E. Plazomicin: A New Aminoglycoside. Clin. Infect. Dis. 2020, 70, 704–709. [Google Scholar] [CrossRef]
- Golla, V.K.; Piselli, C.; Kleinekathöfer, U.; Benz, R. Permeation of Fosfomycin through the Phosphate-Specific Channels OprP and OprO of Pseudomonas aeruginosa. J. Phys. Chem. B 2022, 126, 1388–1403. [Google Scholar] [CrossRef] [PubMed]
- Monogue, M.L.; Nicolau, D.P. Antibacterial activity of ceftolozane/tazobactam alone and in combination with other antimicrobial agents against MDR Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2018, 73, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Cuba, G.T.; Rocha-Santos, G.; Cayô, R.; Streling, A.P.; Nodari, C.S.; Gales, A.C.; Pignatari, A.C.C.; Nicolau, D.P.; Kiffer, C.R.V. In vitro synergy of ceftolozane/tazobactam in combination with fosfomycin or aztreonam against MDR Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2020, 75, 1874–1878. [Google Scholar] [CrossRef] [PubMed]
- Papp-Wallace, K.M.; Zeiser, E.T.; Becka, S.A.; Park, S.; Wilson, B.M.; Winkler, M.L.; D’Souza, R.; Singh, I.; Sutton, G.; Fouts, D.E.; et al. Ceftazidime-Avibactam in Combination With Fosfomycin: A Novel Therapeutic Strategy Against Multidrug-Resistant Pseudomonas aeruginosa. J. Infect. Dis. 2019, 220, 666–676. [Google Scholar] [CrossRef] [Green Version]
- Mikhail, S.; Singh, N.B.; Kebriaei, R.; Rice, S.A.; Stamper, K.C.; Castanheira, M.; Rybak, M.J. Evaluation of the Synergy of Ceftazidime-Avibactam in Combination with Meropenem, Amikacin, Aztreonam, Colistin, or Fosfomycin against Well-Characterized Multidrug-Resistant Klebsiella pneumoniae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 63, e00779-19. [Google Scholar] [CrossRef] [Green Version]
- Avery, L.M.; Sutherland, C.A.; Nicolau, D.P. Prevalence of in vitro synergistic antibiotic interaction between fosfomycin and nonsusceptible antimicrobials in carbapenem-resistant Pseudomonas aeruginosa. J. Med. Microbiol. 2019, 68, 893–897. [Google Scholar] [CrossRef]
- Gatti, M.; Viaggi, B.; Rossolini, G.M.; Pea, F.; Viale, P. An Evidence-Based Multidisciplinary Approach Focused on Creating Algorithms for Targeted Therapy of Infection-Related Ventilator-Associated Complications (IVACs) Caused by Pseudomonas aeruginosa and Acinetobacter baumannii in Critically Ill Adult Patients. Antibiotics 2021, 11, 33. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of AmpC β-lactamase-Producing Enterobacterales, Carbapenem-Resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia Infections. Clin. Infect. Dis. 2021, ciab1013. [Google Scholar] [CrossRef]
- Kalil, A.C. Antibiotic Combination Therapy for Patients with Gram-Negative Septic Shock. Crit. Care Med. 2017, 45, 1933–1936. [Google Scholar] [CrossRef]
- Albiero, J.; Mazucheli, J.; Barros, J.P.D.R.; Szczerepa, M.M.D.A.; Nishiyama, S.A.B.; Carrara-Marroni, F.E.; Sy, S.; Fidler, M.; Sy, S.K.B.; Tognim, M.C.B. Pharmacodynamic Attainment of the Synergism of Meropenem and Fosfomycin Combination against Pseudomonas aeruginosa Producing Metallo-β-Lactamase. Antimicrob. Agents Chemother. 2019, 63, e00126-19. [Google Scholar] [CrossRef] [Green Version]
- Ulloa, E.R.; Sakoulas, G. Azithromycin: An Underappreciated Quinolone-Sparing Oral Treatment for Pseudomonas aeruginosa Infections. Antibiotics 2022, 11, 515. [Google Scholar] [CrossRef] [PubMed]
| Anti-Pseudomonals in Clinical Use | Main Resistance Mechanisms |
|---|---|
| Ceftolozane-tazobactam | AmpC structural mutations, β-lactam target modification (PBP) [21,22,47], OprD mutation and efflux pumps upregulation [28], MBL productions [27], OXA-2 and OXA-10 mutations [23,24,25] |
| Ceftazidime-avibactam | OprD mutation and efflux pumps upregulation [28,47,62,63,64], AmpC structural mutations, β-lactam target modification (PBP) [22,28,47], OXA-2 and OXA-10 mutations [24,25,65], MBL production [61] |
| Cefiderocol | Mutations in major iron transport pathways, possible AmpC mutations [79] mutations in β-lactamases [78] |
| Meropenem-vaborbactam | Porin mutations, efflux pump upregulation, MBL and OXA production [108] |
| Imipenem-cilastatin-relebactam | MBL and GES carbapenemases [85], mutations in MexB or in ParS [98] |
| Plazomicin | 16S rRNA methyltransferases (i.e. Rmt or Arm) [145] |
| Drug | Clinical Dosage | Comments |
|---|---|---|
| Ceftolozane-tazobactam | 1.5 g (ceftolozane 1 g/tazobactam 0.5 g) intravenous every 8 h over 1 h 3 g (ceftolozane 2 g/tazobactam 1 g) intravenous every 8 h over 1 h for HAP/VAP | Extended infusion (over 3 h) 1.5 g or 3 g every 8 h is recommended [46] Renal adjustment with CrCl < 50 mL/min |
| Ceftazidime-avibactam | 2.5 g (ceftazidime 2 g/avibactam 0.5 g) intravenous every 8 h over 2 h | Extended infusion (over 3 h) 2.5 g every 8 h is recommended [46] Renal adjustment with CrCl < 50 mL/min |
| Cefiderocol | 2 g intravenous every 8 h over 3 h | Renal adjustment with CrCl < 60 mL/min |
| Imipenem-cilastatin-relebactam | 1.25 g (imipenem 500 mg/cilastatin 500 mg/relebactam 250 mg) intravenous every 6 h over 30 min | Renal adjustment with CrCl < 90 mL/min |
| Meropenem-vaborbactam | 4 g (meropenem 2 g/vaborbactam 2 g) intravenous every 8 h over 3 h | Renal adjustment with CrCl < 50 mL/min |
| Plazomicin | 15 mg/kg every 24 h over 30 min | Renal adjustment with CrCl < 60 mL/min |
| Fosfomycin | 6–8 g loading dose intravenous, followed by 16 g/day [152] | Renal adjustment with CrCl < 40 mL/min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losito, A.R.; Raffaelli, F.; Del Giacomo, P.; Tumbarello, M. New Drugs for the Treatment of Pseudomonas aeruginosa Infections with Limited Treatment Options: A Narrative Review. Antibiotics 2022, 11, 579. https://doi.org/10.3390/antibiotics11050579
Losito AR, Raffaelli F, Del Giacomo P, Tumbarello M. New Drugs for the Treatment of Pseudomonas aeruginosa Infections with Limited Treatment Options: A Narrative Review. Antibiotics. 2022; 11(5):579. https://doi.org/10.3390/antibiotics11050579
Chicago/Turabian StyleLosito, Angela Raffaella, Francesca Raffaelli, Paola Del Giacomo, and Mario Tumbarello. 2022. "New Drugs for the Treatment of Pseudomonas aeruginosa Infections with Limited Treatment Options: A Narrative Review" Antibiotics 11, no. 5: 579. https://doi.org/10.3390/antibiotics11050579
APA StyleLosito, A. R., Raffaelli, F., Del Giacomo, P., & Tumbarello, M. (2022). New Drugs for the Treatment of Pseudomonas aeruginosa Infections with Limited Treatment Options: A Narrative Review. Antibiotics, 11(5), 579. https://doi.org/10.3390/antibiotics11050579






